(C) 2012 Ivan Antonović. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Terrestrial isopods were studied in the Dubravica peat bog and surrounding forest in the northwestern Croatia. Sampling was conducted using pitfall traps over a two year period. Studied peat bog has a history of drastically decrease in area during the last five decades mainly due to the process of natural succession and changes in the water level. A total of 389 isopod individuals belonging to 8 species were captured. Species richness did not significantly differ between bog, edge and surrounding forest. High species richness at the bog is most likely the result of progressive vegetation succession, small size of the bog and interspecific relationships, such as predation. With spreading of Molinia grass on the peat bog, upper layers of Sphagnum mosses become less humid and probably more suitable for forest species that slowly colonise bog area. The highest diversity was found at the edge mainly due to the edge effect and seasonal immigration, but also possibly due to high abundance and predator pressure of the Myrmica ants and lycosid spiders at the bog site. The most abundant species were Trachelipus rathkii and Protracheoniscus politus, in the bog area and in the forest, respectively. Bog specific species were not recorded and the majority of the species collected belong to the group of tyrphoneutral species. However, Hyloniscus adonis could be considered as a tyrphoxenous species regarding its habitat preferences. Most of collected isopod species are widespread eurytopic species that usually inhabit various habitats and therefore indicate negative successive changes or degradation processes in the peat bog.
Edge, seasonal dynamics, pitfall trapping, predation, Trachelipus rathkii, Protracheoniscus politus, Hyloniscus adonis, tyrphoxenous species
Peat bogs are a type of wetlands characterized by a high water table, low levels of nutrients, low pH values, and are dominated by Sphagnum mosses (
These unique ecosystems often consist of specialized flora and fauna, largely limited only to these habitats. Although most of the peat matrix is dead, it contains a great variety of living organisms that contribute in decomposing (
As members of the soil macrofauna community, isopods play an important role in the processes and soil formation (
The objectives of this study were: (1) to determine the terrestrial isopod assemblages and their spatial distribution in the peat bog remnant and surrounding forest, (2) to determine which basic ecological groups inhabit the peat bog and adjacent forest (3) to analyze the seasonal dynamics of the dominant isopod species and (4) to asses possible influence of soil temperature and humidity on isopod abundance.
Methods Study areaPeat bog Dubravica is located in the Northwestern part of Croatia, in Hrvatsko zagorje Region (45°57.430'N, 14°44.470'E). It is situated in sessile oak and hornbeam forest (ass. Epimedio-Carpinetum betuli (Ht.1938) Borh. 1963) at an altitude of l60 m asl. Since 1966 it has been protected as a Botanical Reserve and it is a potential NATURA 2000 site.
According to
Three sites were selected in and around the bog area, situated in different vegetational associations (Figure 1). The site B is located in the centre of the bog and the bog vegetation belongs to the Rhynchosporetum albae W. Koch, 1926 association. The ground layer was dominated by typical bog plant species: common sundew (Drosera rotundifolia L.), white beak-sedge (Rhynchospora alba (L.) Vahl.) and Sphagnum mosses (Sphagnum subsecundum Ness.). Site E is located on the edge of the bog and sessile oak and hornbeam forest. The edge is covered with rather strong Rubus sp. plants, which are opportunistically taking the open area on the edge zones. In order to avoid edge effect, site F is located 60 m from the edge into the forest (
Sampling sites in the Dubravica bog and surrounding forest. Site B - bog Site E - edge Site F - forest.
Isopods were collected using pitfall traps during the carbide beetle study and sampling was conducted from May to November 2008 and from May to November 2009. Five plastic traps per site were placed (volume 0.3 dm3) 5 m apart. They were half filled with a saturated solution of sodium chloride with a few drops of detergent to break the surface tension of the liquid. A styrofoam roof was placed above each trap to protect them from rain. The traps were emptied once a month. The collected specimens were kept in 75% ethyl-alcohol with glycerol. The individuals were identified to the species level, apart from Armadillidium sp. and Oniscidae sp., due to lack of adult male individuals. All individuals were identified by authors (I. A. and J. B.), with the exception of Hyloniscus adonis, which was identified by Stefano Taiti (Istituto per lo Studio degli Ecosistemi, Consiglio Nazionale delle Ricerche).
Soil analysisBasic pedophysiological properties of the soil were determined using standard methods (
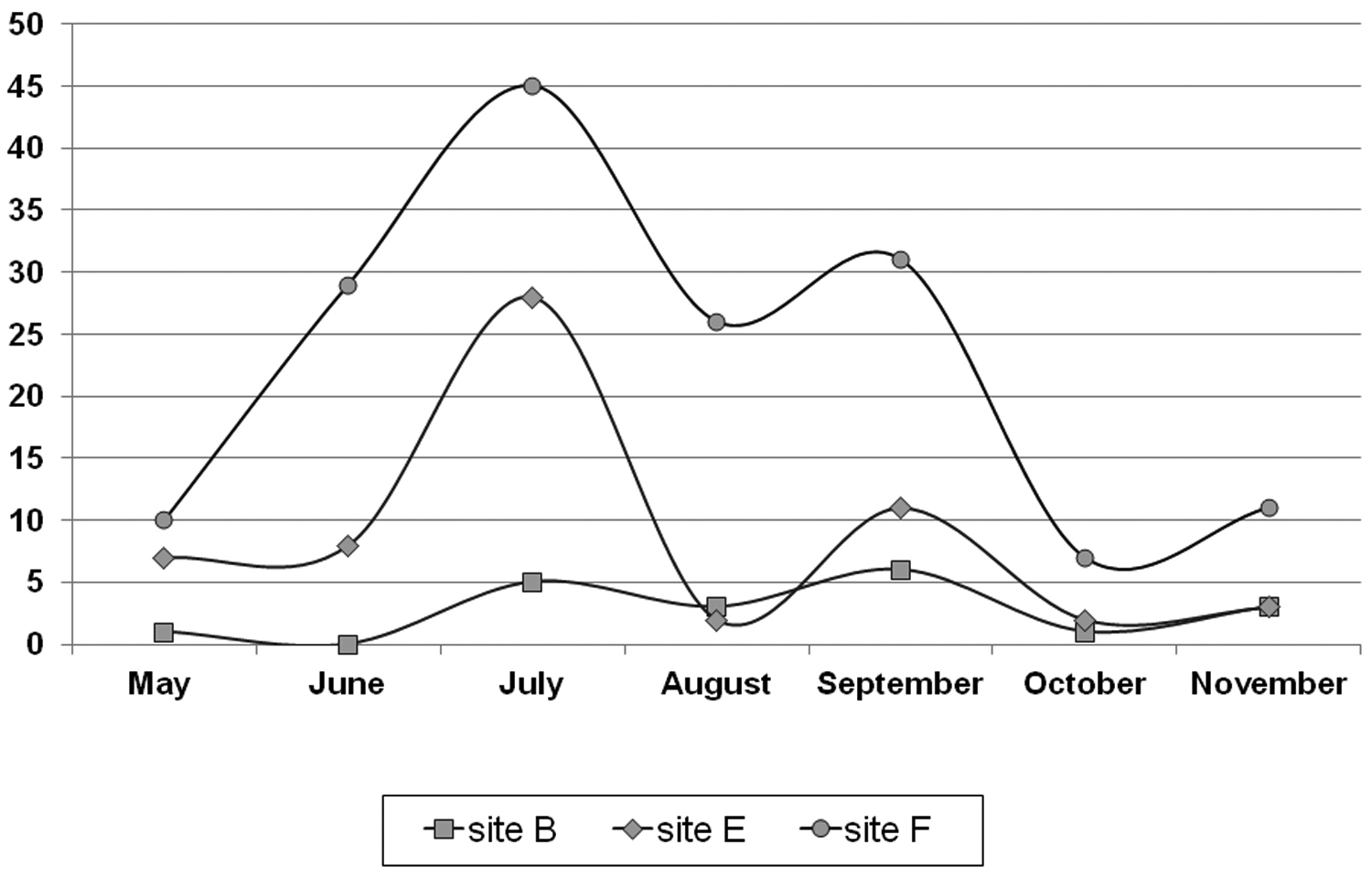
Soil analysis showed that pH values were very low at each sampling site, while calcium carbonate concentration was lowest in the forest, but higher at the edge and at the bog site (Table 1). Soil humidity was highest (sometimes ≥80%) at the bog during most of the sampling period. In addition, comparable results were observed at the edge, where soil humidity was also high (≥70%) (Figure 2). Soil humidity was particularly low in the forest (18–33%) in both study years. Oscillations of the soil temperature were most expressed at the edge (during both study years), especially during summer. At the bog an intermediate pattern was recorded (between edge and forest). Conversely, the lowest temperatures were measured at the bog site (during autumn and winter). Differences in soil humidity were observed in autumn between two study years, most likely due to higher amount of rain in 2009.
Vegetational and pedagogical properties of studied sites in the Dubravica peat bog. Site B – bog; Site E – edge; Site F – forest.
| Environmental variable | Site B | Site E | Site F |
|---|---|---|---|
| Vegetation analysis | |||
| Plant association | Rhynchosporetum albae W. Koch | - | Epimedio-Carpinetum betuli (Ht 38) Both. 63 |
| Vegetation height/m | 1 | 0.2-0.5 | 20 |
| Vegetation density | high | low | middle, thick layer of litter |
| Tree layer, dominant plant species | - | - | 95% Carpinus betulus, Quercus robur |
| Frutescent layer, dominant plant species | 0% | 50% Rubus sp. | 5% Carpinus betulus |
| Herbaceous layer, dominant plant species | 100% Sphagnum subsecundum, Molinia caerulea | 10% Molinia caerulea | 80% Epimedium alpinum |
| Soil analysis | |||
| Soil type | peat | - | stagnosol |
| pH (H20) | 4.47 | 4.18 | 4.09 |
| pH (KCl) | 3.92 | 3.55 | 3.57 |
| Humus (Tjurin) % | 7.0 | 6.3 | 2.0 |
| P2O5 mg/100 g | 0.8 | 1.7 | 0.4 |
| CaCO3 % | 0.235 | 0.226 | 0.127 |
| C/N | 17.2 | 15.6 | 13.6 |
Soil temperature and soil humidity at the Dubravica bog and adjacent forest during 2008 and 2009. Temperature values are presented with different symbols and humidity values with bars. Site B – bog; Site E – edge; Site F – forest.
Activity-density was compared between two years, using Mann-Whiney U test and Spearman correlation. To calculate the diversity of the isopod assemblages we used Simpson (1-λ’) and Shannon-Wiener indices (H’). Evenness was estimated using Pielou’s evenness (J). Similarity between sites was calculated using the Bray-Curtis similarity coefficient, calculated on square root transformed and standardized abundance data (traps total catches). Non-metric MDS (multidimensional scaling) was constructed on Bray-Curtis similarity. The analyses were carried out using the PRIMER program v.6 (
ni = number of individuals in habitat; n: number of individuals in all habitats; N: number of habitats, Hi: habitat i, Hi’: all habitats except habitat i. The values of this index range from -1 to +1. The maximum value, +1 shows that all individuals of the given species were in the given habitat, whereas -1 indicates that no individuals of the species were recorded in the habitat, and 0 indicates that there was an average number of individuals in the habitat (
During both study years, a total of 389 isopod individuals belonging to 8 species were captured (Table 2). Species richness and activity density were both lowest at the bog (over the whole study period). Overall, the highest number of individuals was recorded in the forest. ANOVA indicated significant difference in activity density between sites (d.f. = 2.42, F = 3.604, p<0.05). Post-hoc test separated activity density in the bog from values in the forest (Tukey HSD, p<0.05), while other combinations were not significantly different (Tukey HSD, p>0.05). Overall, activity density was positively correlated between the two years of the study. Moreover, no significant difference of the latter between 2008 and 2009 was recorded for site B (Mann-Whitney U-test, p=0.327), in contrast to sites E and F (Mann-Whitney U-test, p=0.013 and p=0.007). Spearman’s rank correlation coefficient was statistically significant for site B (Rs=0.83, p=0.022), marginally significant for site E (Rs=0.73, p=0.10) and not significant for site F (Rs=0.55, p=0.20). Therefore, data for both years were pooled for subsequent analyses.
Isopod species recorded at Dubravica bog and adjacent forest with indices of diversity and evenness. Site B – bog; Site E – edge; Site F – forest; Tn – tyrphoneutral species Tx – tyrphoxenous species; % – percent share of total individuals per site.
| Species name | site B | % | site E | % | site F | % | Ecological group |
|---|---|---|---|---|---|---|---|
| Armadillidium carniolense Verhoeff, 1901 | 8 | 12.7 | 28 | 19.2 | 3 | 1.7 | Tn |
| Armadillidium sp. | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | Tn |
| Ligidium germanicum Verhoeff, 1901 | 6 | 9.5 | 3 | 2.1 | 3 | 1.7 | Tn |
| Oniscidae sp. | 0 | 0.0 | 2 | 1.4 | 0 | 0 | Tn |
| Protracheoniscus politus C. Koch, 1841 | 19 | 30.2 | 61 | 41.8 | 159 | 88.3 | Tn |
| Trachelipus rathkii Brandt, 1883 | 28 | 44.4 | 20 | 13.7 | 3 | 1.7 | Tn |
| Trachelipus ratzeburgi Brandt, 1883 | 1 | 1.6 | 17 | 11.6 | 8 | 4.4 | Tn |
| Hyloniscus adonis Verhoeff, 1927 | 1 | 1.6 | 15 | 10.3 | 3 | 1.7 | Tn (possible Tx) |
| Number of isopod species (S) | 6 | 7 | 7 | ||||
| Number of isopod individuals (N) | 63 | 146 | 180 | ||||
| Simpson index (1-λ’) | 0.697 | 0.750 | 0.218 | ||||
| Shannon-Wiener index (H’) | 1.339 | 1.576 | 0.549 | ||||
| Pielou’s evenness index (J’) | 0.748 | 0.810 | 0.283 |
Soil temperature was positively correlated with the isopod total monthly catch at all three studied sites (Spearman’s rank correlation; site B: N=14, Rs=0.594, p=0.025; site E: N=13, Rs=0.619, p=0.024; site F: N=14, Rs= 0.558, p=0.038). On the contrary, soil humidity negatively correlated with the isopod total monthly catch in the bog and forest samples, but the correlations were not statistically significant (Spearman’s rank correlation; site B: N=14, Rs= -0.246, p=0.397; site F: N=14, Rs= -0.372, p=0.190). However, in edge samples correlations between soil humidity and the total monthly catch was positive, but also statistically not significant (Spearman’s rank correlation; site E: N=13, Rs=0.077, p=0.802).
Protracheoniscus politus C. Koch was generally the most abundant species (61.4% of the total catch). Together with following species; Trachelipus rathkii Brandt (13.1%), Armadillidium carniolense Verhoeff (10%) and Trachelipus ratzeburgi Brandt (6.7%) it constituted 91.2% of the total catch. Trachelipus rathkii was the most abundant species at the bog (44.4% of the catch), while its abundance decreased at the edge, and was the lowest in the forest (reaching only 1.7%). Three following species; Protracheoniscus politus (30.2%), Armadillidium carniolense (12.7%) and Ligidium germanicum Verhoeff (9.5%) also accounted for a larger proportion of the catch at the bog. Protracheoniscus politus (41.8%) and Armadillidium carniolense (19.2%) were the most frequent species at the edge and Protracheoniscus politus was particularly abundant in the forest (88.3%). Hyloniscus adonis Verhoeff, had the highest abundance at the edge, while only 1 specimen was found at the bog. Majority of isopod taxa found at the bog are tyrphoneutral. According to diversity and evenness indices, the greatest and lowest diversity was recorded at the edge and in the forest, respectively.
Seasonal activityActivity density of dominant Protracheoniscus politus was expressed as the total number of individuals caught monthly and plotted against time (Figure 3). The maximum seasonal activity was observed in July at both forest and edge sites. During August, the number of individuals decreased at all studied sites, whereas an increase was observed in September. Overall, low number of individuals was caught at the bog, and this was insufficient to observe seasonal dynamics. Seasonal activity density of Protracheoniscus politus did positively correlate with soil temperature at the edge and forest sites (Spearman’s rank correlation; site E: N=14, Rs=0.093, p=0.751; site F: N=14, Rs=0.427, p=0.128), and negatively at the bog (Spearman’s rank correlation; site B: N=14, Rs= -0.168, p=0.565). However, the correlations were not statistically significant. Additionally, Spearman’s rank correlation coefficient was positive between soil humidity and seasonal activity density of Protracheoniscus politus at the bog and edge (site B: N=14, Rs=0.025, p=0.932; site E: N=14, Rs=0.033, p=0.910, respectively) and negative in the forest (site F: N=14, Rs= -0.461, p=0.096). Also, correlations were not statistically significant.
Protracheoniscus politus seasonal activity at three studied sites with different vegetation. Site B – bog; Site E – edge; Site F – forest; Y-axis shows total number of monthly caught individuals.
The habitat fidelity values of collected isopod species are shown in Table 3. According to these results, two dominant species, Protracheoniscus politus and Trachelipus rathkii, preferred different habitat types. Protracheoniscus politus mostly inhabited forest (site F) with a high habitat fidelity value (+ 0.6), while positive habitat fidelity value was calculated for Trachelipus rathkii (+ 0.4) at the bog site. Typical hygrophylic species, Ligidium germanicum and Hyloniscus adonis also showed quite different habitat preferences. Ligidium germanicum occurred mostly at the bog area (+ 0.33), while Hyloniscus adonis preferred the edge site (+ 0.77). Armadillidium carniolense and Trachelipus ratzeburgi seemed both to prefer edge site with a higher habitat fidelity values in contrast to the values calculated for the other sites.
The habitat fidelity values of dominant isopod species in each of the three habitats. Site B – bog; Site E – edge; Site F – forest.
| ∑ N | site B | site E | site F | Habitat selection index | |
|---|---|---|---|---|---|
| Protracheoniscus politus | 239 | - 0.7 | - 0.05 | + 0.6 | 1.35 |
| Trachelipus rathkii | 51 | + 0.4 | + 0.1 | - 0.8 | 1.3 |
| Armadillidium carniolense | 39 | - 0.32 | + 0.67 | - 0.73 | 1.72 |
| Trachelipus ratzeburgi | 26 | - 0.88 | + 0.54 | - 0.06 | 1.48 |
| Hyloniscus adonis | 19 | - 0.8 | + 0.77 | - 0.47 | 2.04 |
| Ligidium germanicum | 12 | + 0.33 | - 0.2 | - 0.2 | 0.73 |
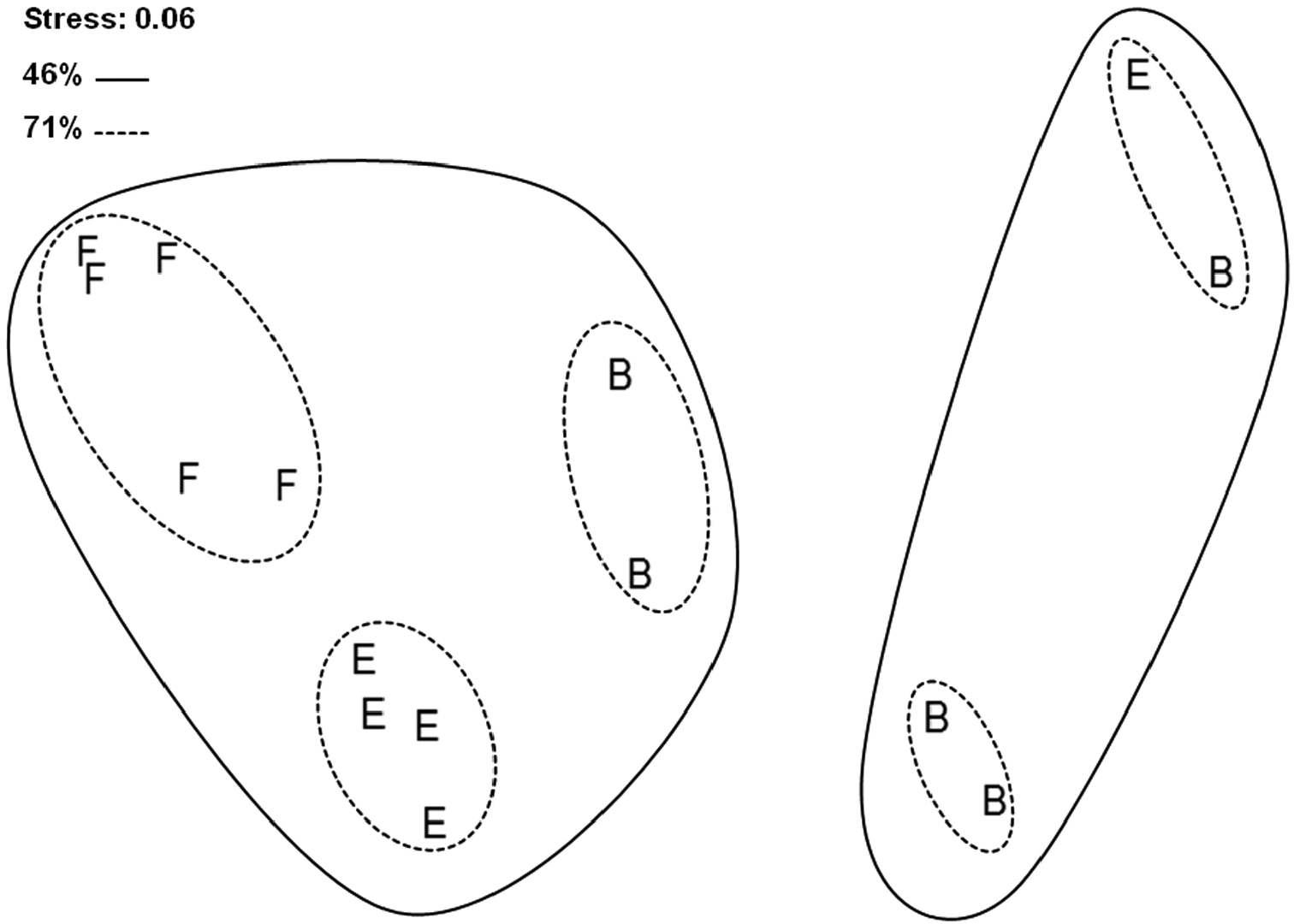
Non-metric MDS ordination based on Bray-Curtis similarity index, with superimposed results of cluster analysis (group-average linking), shows generally high degree of similarity between studied sites (i.e. they all cluster at 46% similarity level; Figure 4). In particular, high degree of similarity is obvious for forest and edge sites that cluster together at 71% similarity level. Forest replicate samples grouped strongly together at the same similarity level, whereas, most of the edge replicate samples clustered together at 80% similarity level. On the contrary, bog replicate samples did not form a distinct group and they were combined with edge samples.
nMDS ordination of studied sites and Bray-Curtis similarities with superimposed results of cluster analysis. Site B – bog; Site E – edge; Site F – forest.
Current study shows that isopod species richness of the isolated bog was surprisingly high and it did not considerably differ from the species richness of the edge or adjacent forest. Contrary to this,
The greatest diversity of isopods in the current study was found exactly in the edge mainly due to the edge effect. According to
In our study, isopod activity density significantly differed between the bog and surrounding habitat (forest), being the lowest at the bog. This is in accordance with data from studies on other ground dwelling arthropods in peat bogs, e.g. carabid beetles (
The harsh environmental conditions, such as daily and annual temperature differences and low pH values (Figure 2; Table 1) might also have affected the isopod species richness and activity at the bog (
Except above mentioned grounds, predation might also influence isopod activity density at the bog. High population density of lycosid spiders (
Trachelipus rathkii was the dominant species at the bog. Its abundance decreased towards the forest where the soil humidity was lower comparing to other two sites. Furthermore, its habitat fidelity value was highest at the bog (Table 3). As a widely distributed and common isopod species, Trachelipus rathkii inhabits different biotopes, including extreme ones (
Although species from the family Trichoniscidae, including Hyloniscus spp. are highly hygrophylic, and usually inhabit wetlands, species Hyloniscus adonis is not abundant on the bog, but surprisingly on the edge. Due to bog’s inclination towards the edge, high, but relatively even soil humidity was recorded throughout the sampling period at the edge site. Hence, availability of microhabitats with optimum ecological conditions for this species, but also lower predation pressure from ants and lycosid spiders could explain its highest abundance at the edge.
Among typical central European species, rather rare and less known isopod Ligidium germanicum was present at all study sites. It is highly hygrophylic species (
At all studied sites, Protracheoniscus politus was one of the most frequent and numerous species. It was dominant species in the forest where the soil humidity content was considerably lower. It is a xerophilous species that prefers dryer habitats (
Within the current and previous studies (e.g.
This study reveals that vegetation succession has a strong impact on community composition of fauna inhabiting the peat bog. With overgrowth of the peat bog by Myrmica caerulea grass, water level has significantly decreased. Therefore, the bog area becomes dryer, shaded and more suitable for forest species. Although there were no previous studies on isopod fauna, the presence of forest species indicates such changes in this habitat. Peat bog size and interspecific relationships, such as predation, also affected isopod species richness, activity density and diversity. Typical tyrphobiontic and tyrphophilous species were not observed, but further studies implying additional sampling methods should provide more detailed insight into isopod faunistics and ecology. In order to preserve suitable microclimatic conditions and biodiversity of the bog, management practices, like mowing, are required.
We are very grateful to Ivan Tuf, Martin Zimmer and Ferenc Vilisics for providing us some papers, to Stefano Taiti for identification of Hyloniscus, to Renata Matoničkin Kepčija who helped us with statistical analysis, to Ana Previšić for English corrections and to anonymous reviewers for their critical comments and suggestions.