(C) 2011 Diana M. P. Galassi. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The family Phyllognathopodidae (Crustacea, Copepoda, Harpacticoida) is heavily affected by the floating taxonomic status of the type-genus Phyllognathopus. A revision of the different character states displayed by members of the family is presented, and new phylogenetically informative characters are described, enlarging the analysis to the remaining genera of the family, Parbatocamptus and Allophyllognathopus. Phyllognathopus viguieri (Maupas, 1892) and Parbatocamptus jochenmartensi Dumont and Maas, 1988 are redescribed in detail, and Phyllognathopus inexspectatus sp. n. is described from ground water in Italy. The new genus Neophyllognathopus is established to accommodate Phyllognathopus bassoti Rouch, 1972, originally collected from Long Island (Papua - New Guinea), and subsequently recorded also from the Bantayan Island (Philippines), and from the Indian subcontinent. The new genus is presently monotypic and is easily defined by the unique construction and morphology of leg 5 in both male and female, of male leg 6, and by the peculiar ornamentation of male third and fourth urosomites. Biogeographical and ecological considerations are presented for members of the family.
Harpacticoida, Phyllognathopodidae, taxonomy, Phyllognathopus, Parbatocamptus, Neophyllognathopus bassoti comb. n.
The harpacticoid family Phyllognathopodidae exhibits a low diversity, currently containing 13 species according to
The family is undoubtedly monophyletic, being instantly
recognizable by the unique phyllopodial lamelliform maxilliped, in
conjunction with the first pedigerous somite not being incorporated into
the cephalosome. Despite the low diversification in the family, the
taxonomic history of the type-genus Phyllognathopus has been and still is highly controversial (
Recent discoveries of new phyllognathopodid representatives in Italian ground water, together with the re-examination of different species and populations coming from several localities world-wide, allowed a re-analysis of key-taxa within the family, the description of a new stygobiotic species of Phyllognathopus and the establishment of a new taxonomic rank for Phyllognathopus bassoti Rouch, 1972, assigned herein to the new genus Neophyllognathopus.
Material and methodsSpecimenswere collected from hyporheic habitats by using the Bou-Rouch method (
Additional material, preserved on slides, was loaned by
the Natural History Museum (London), the Museum National d’Histoire
Naturelle (Paris), the National Museum of Natural History, Smithsonian
Museum, Washington, D.C. (U.S.A.), the Senckenberg Museum (Germany).
The descriptive terminology of
Family PHYLLOGNATHOPODIDAE Gurney, 1932
Phyllognathopodidae. Habitus slender, with no clear demarcation between prosome and urosome. Integumental dorsal window on cephalosome not confirmed for all members of the genus. Integument with surface pits, moderately sclerotized. Cephalosome rounded; rostrum clearly articulated to cephalosome. First pedigerous somite free. P5-bearing somite with large paired pores laterodorsally. Anal operculum plain or ornamented by fine spinules or extruded in strong spinular processes. Sexual dimorphism in antennule, P5, P6, urosomal segmentation and ornamentation. Female first and second abdominal somites fused forming genital double-somite. Anal somite with paired sensilla on dorsal side. Male urosome consisting of 6 segments. Caudal rami sub-quadrate, or longer than wide, with incomplete setal pattern (6 setae). Dorsal seta inserted on distal third of caudal ramus. Setae III and V variable in morphology among species. Antennule: 8-segmented in female, basically 10-segmented in male, although a suture line marking original segmentation between former segments 10 and 11 may be still discernible in some species; geniculation between segments 7 and 8; segment 9 discrete. Long tube-pores on segments 1 and 2 in both sexes. Antenna: armature of the second endopodal segment consisting of 10 elements. Exopod 1-segmented, with 3 lateral and 2 apical setae. Mandible: mandibular palp biramous, basis unarmed; exopod with 1 apical and 1 inner setae; endopod with 1 inner, 1 subapical and 2 apical setae. Armature of maxillule and maxilla as in Phyllognathopus viguieri. Maxilliped: phyllopodial, lamelliform, 1-segmented. Trace of ancestral 2-segmented condition marked by the presence of outer incision; armature consisting of 10 elements.
P1-P3 with praecoxa and 3-segmented exopods and endopods. P4 small-sized, praecoxa missing, with 3- or 2- or 1-segmented exopod and 2- or 1-segmented endopod. Female P5 free, with clear articulation to P5-bearing somite; right and left legs distinct; intercoxal sclerite absent; baseoendopod and exopod coalescent, feeble incision marking original segmentation between them; endopodal lobe not pronounced, bearing 2 apical setae. Exopodal lobe fully incorporated into baseoendopod, not pronounced; basipodal outer seta present. Female P6 present, right and left legs represented by small chitinous lamellar plates, each leg bearing 1 normal seta or a stout spine with rounded tip. Male P5 free, with clear articulation to P5-bearing somite; right and left legs coalescent, intercoxal sclerite absent. Exopod discrete, but sometimes incorporated to basis. Endopod 1-segmented, normally conformed, cylindrical, bearing 1 leaf-like seta, alternatively transformed in a curved and stout element bearing 1 bipinnate seta, inserted on posterior surface of the endopod, close to its articulation to basis. Male P6 present; symmetrical, right and left legs coalescent along their medial margin, forming a continuous lamellar plate; each leg bearing 2 inner spines of different length and 1 outer seta.
http://species-id.net/wiki/Phyllognathopus_viguieri
Figs 1–911 ♀♀ and 2 ♂♂, completely dissected and mounted in polyvinyl lactophenol, S. Anna D’Alfaedo, Progno di Valpantena (Verona, Italy), hyporheic habitat, 25.06.2002, E. Gattone coll.; 1 ♀, karstic spring in the hydrogeological basin of Rio Biondo, Progno di Valpantena (Verona, Italy), karstic habitat, 7. 07. 2003, B. Fiasca coll.; 3 ♀♀, Lake Bracciano (Latium, Italy), interstitial habitat, 27.05.02, V. Cottarelli coll.; 10 ♀♀ and 5 ♂♂, Avisio floodplain (Trento, northern Italy), hyporheic habitat, 30.05.2006, T. Di Lorenzo coll.; 3 ♀♀ and 1 ♂, Oignin stream (French Jura Mountains), hyporheic habitat, 30.07.2002, M.-J. Dole-Olivier coll.; 2 ♀♀ and 1 ♂, Ariège floodplain (France), P. Dumas coll.; 1 ♀, Lac Léman (France), slide code MNHN - Cp922, Paris; 2 ♂♂, S. Pierre, (France), slide code MNHN - Cp456, Paris; 3 ♂♂ and 2 ♀♀, Ruhr floodplain (Germany), T. Glatzel coll.; 6 ♀♀ and 1 ♂, R. Krishna, India, Y. Ranga Reddy coll.; 1 ♀, in pitcher of Nepenthes mirabilis (Hong Kong), B. Coker det., slide code 1982.329, Natural History Museum, London; 2 ♀♀ deposited at the Smithsonian Institution, Washington D.C., code USMN 251806, 204501; 1 ♂, code USMN 204500; 1 ♂ (juvenile), code USMN 204501.
Phyllognathopus cf. viguieri A. 2 ♂♂, 2 ♀♀, Madagascar, vial code, MNHN - Cp910, Paris, B. Dussart coll..
Phyllognathopus cf. viguieri B. 1 ♀, slide code 66/52, freshwater well, Mindoro Island, Philippines, 17.8.1992, V. Cottarelli coll.
Phyllognathopus viguieri ?. 10 ♀♀ and 5 ♂♂, Andhra Loyola College Campus, Vijaya-Woda, Andhra Pradesh, India, Y. Ranga Reddy coll..
Phyllognathopus viguieri menzeli. 1♀, vial code USNM 150192, labelled: Pacific Ocean, Mariana Islands, Guam, 19 November 1970, Watkins R.L. coll. (remaining material in the vial: 17 ♀♀).
Phyllognathopus viguieri menzeli 2. ♂♂, vial code USNM 150193, labelled: Pacific Ocean, Mariana Islands, Guam, 1 April 1971, Belk and Watkins R.L. coll. (remaining material in the vial: 8 specimens, of which several copepodids).
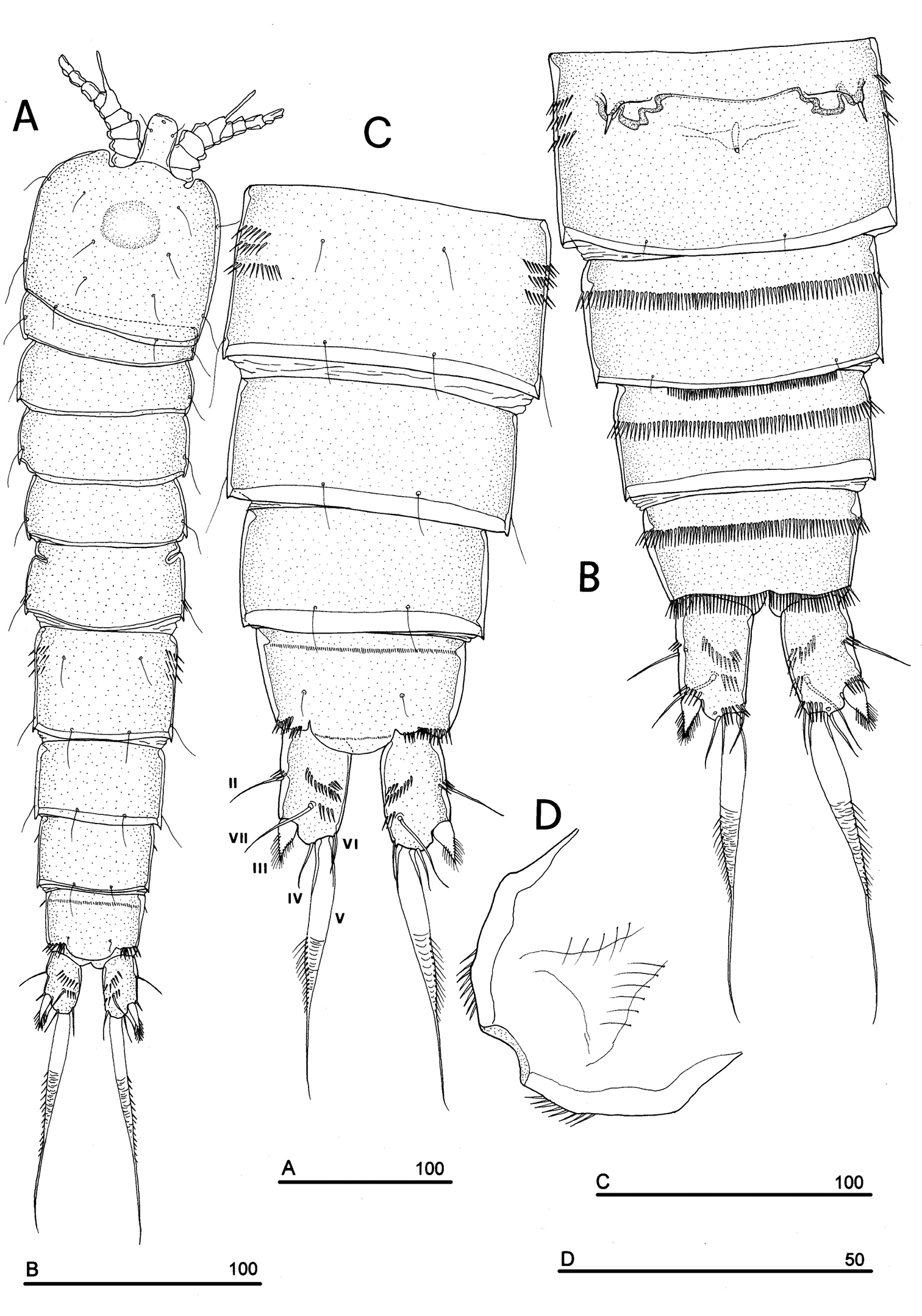
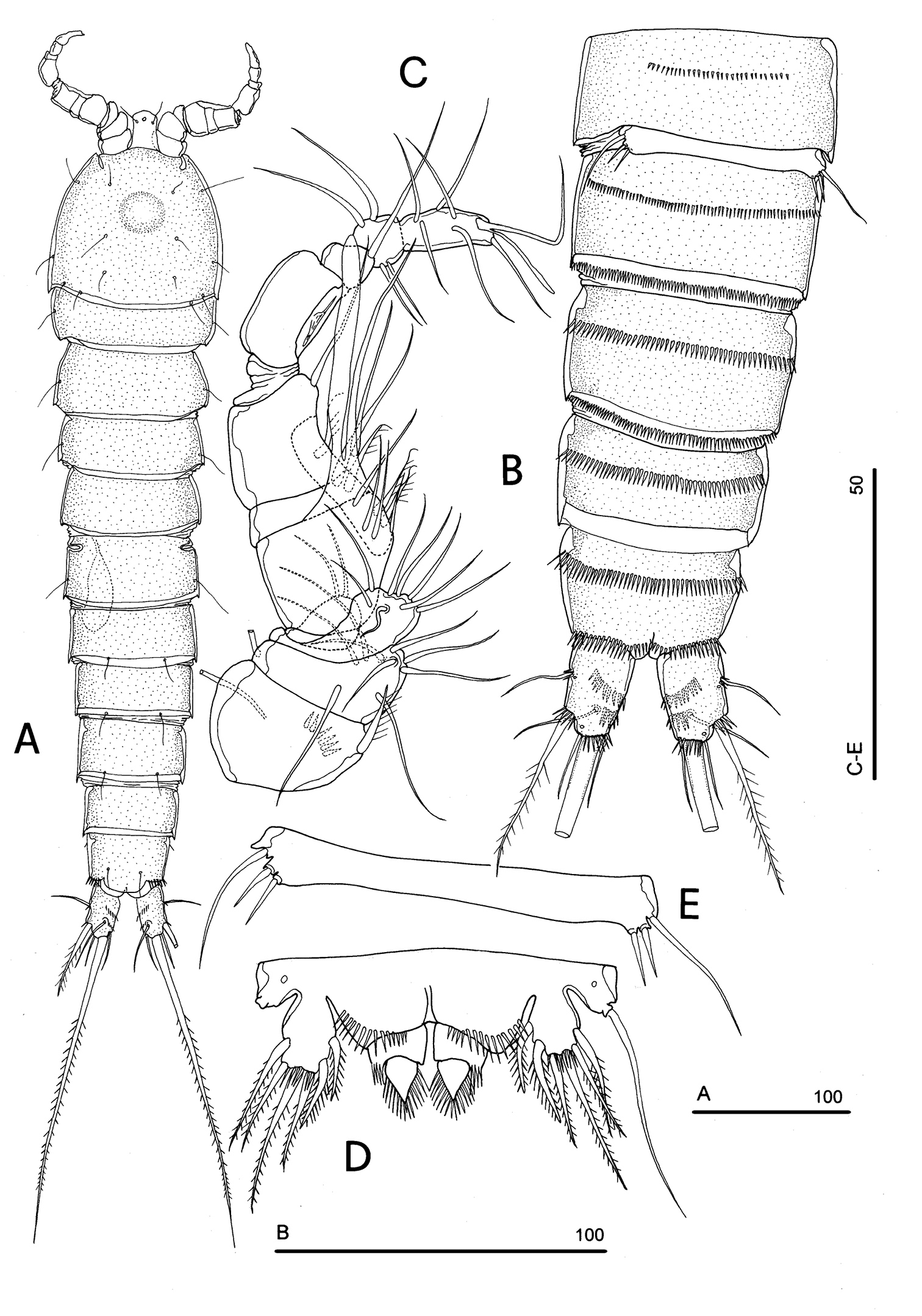
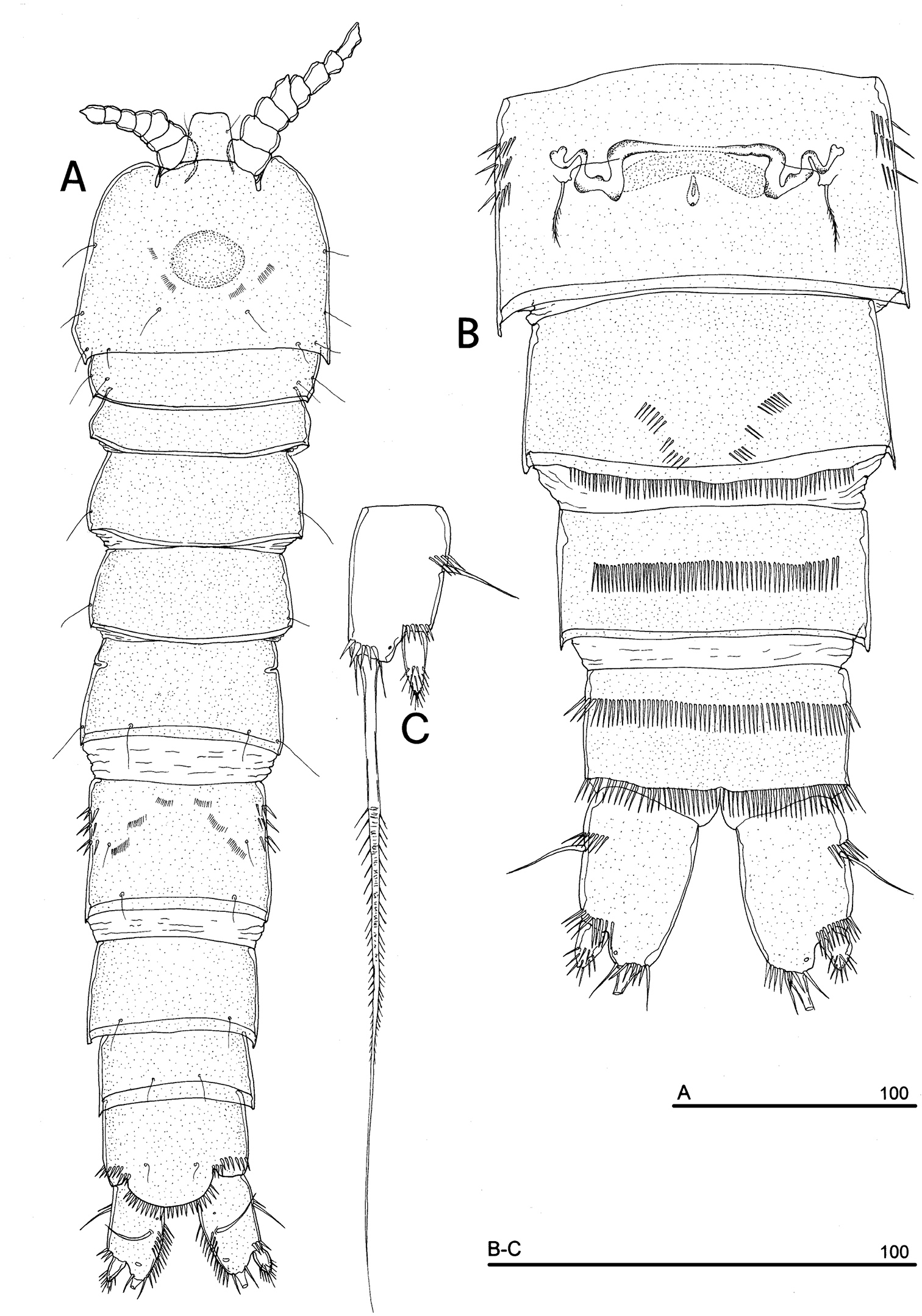
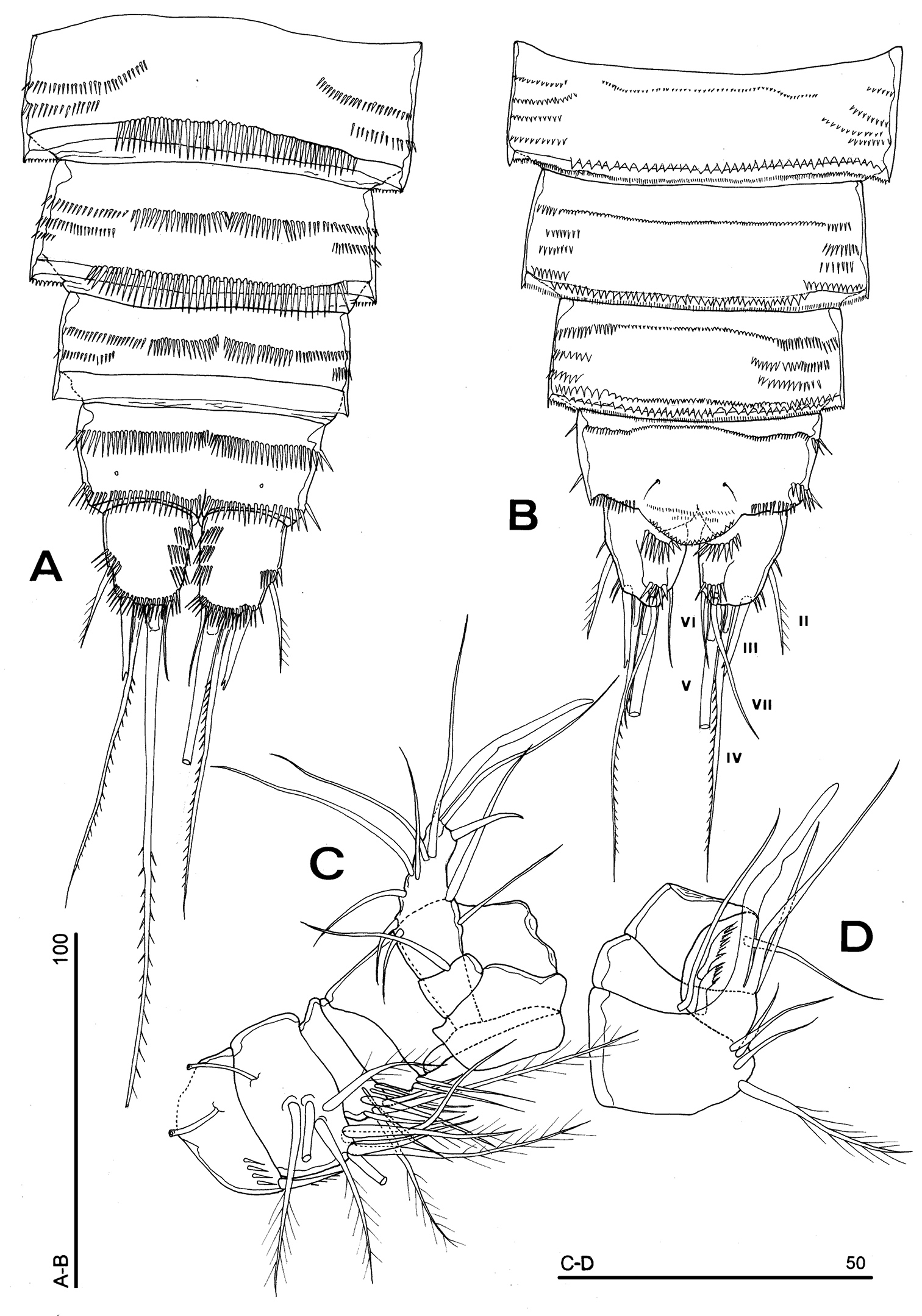
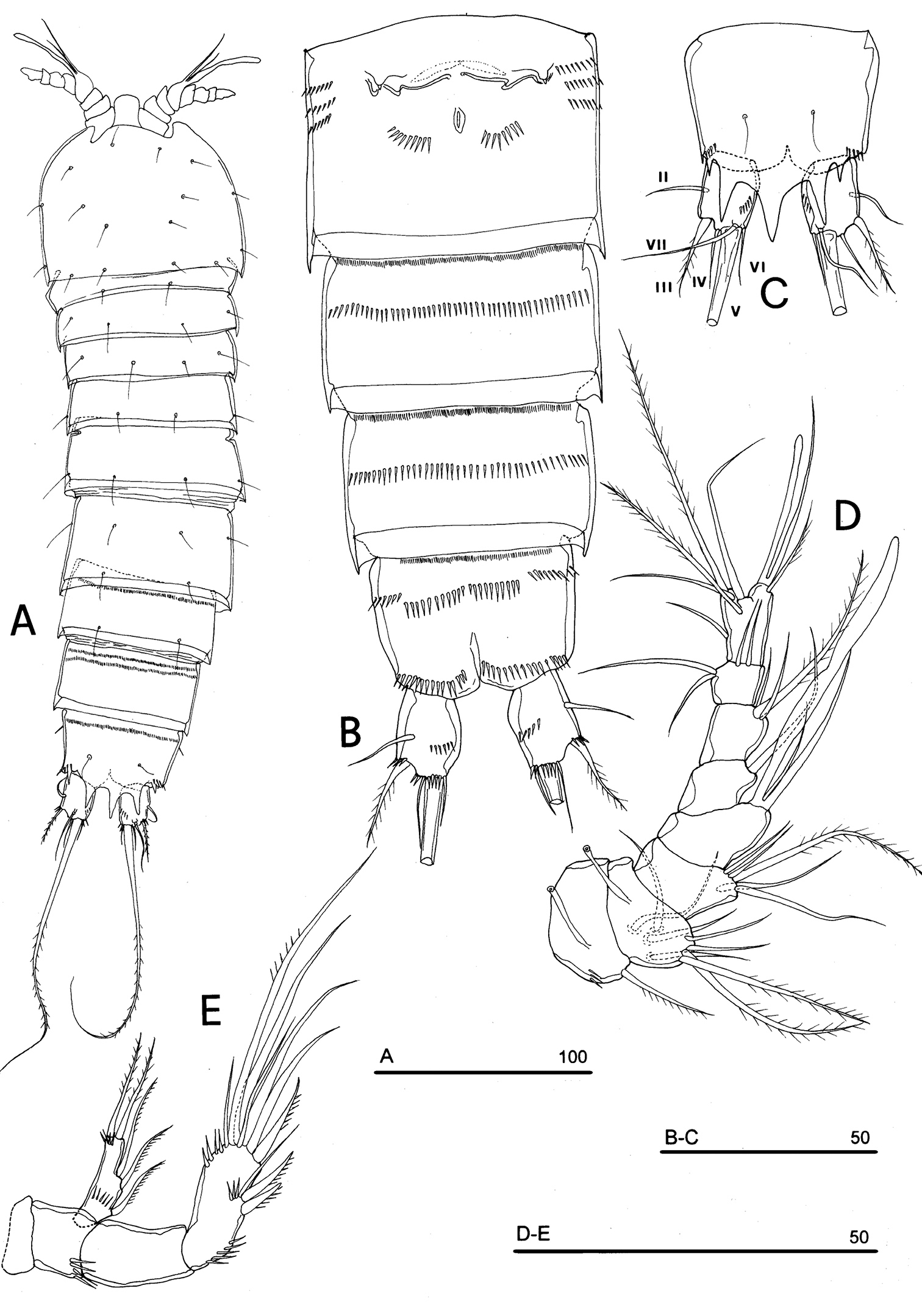
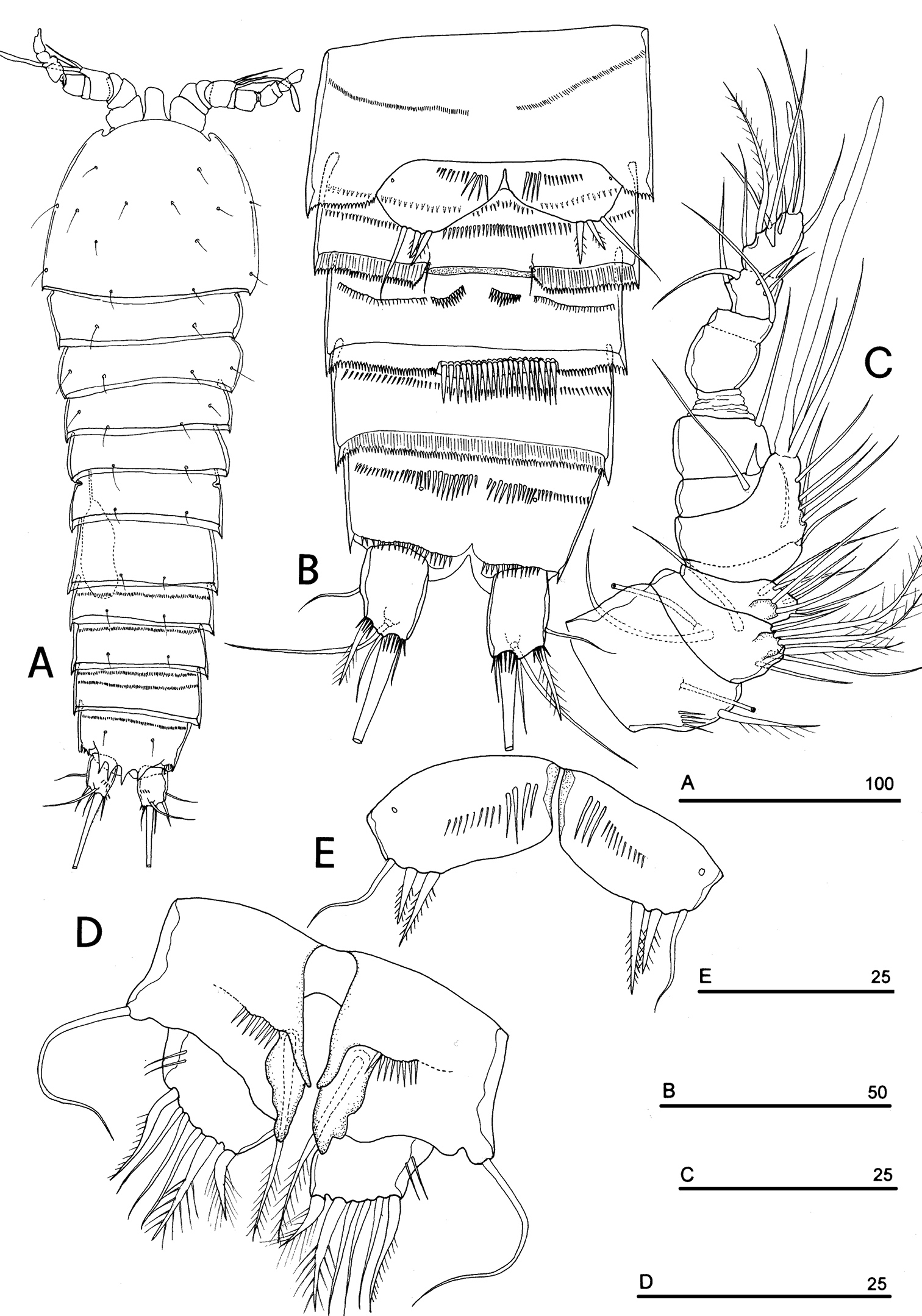
FEMALE. Body length, measured from tip of rostrum to posterior margin of caudal rami, from 400 to 600 µm (mean = 439 µm; n = 27). Habitus slender, no clear demarcation between prosome and urosome. Integument with surface pits, moderately sclerotized as in Fig. 1A. Cephalosome sub-quadrate, with a dorsal rounded protuberance, hardly observable, plausibly referable to a dorsal integumental window. Setule pattern as in Fig. 1A. Rostrum elongate, subrectangular in shape, clearly articulated to the cephalosome; two dorsal sensilla laterally inserted on its distal third, and one pore apically. Cephalosome and both thoracic and abdominal somites with cuticular ornamentation apparently represented by reduced number of paired sensilla (Fig. 1A). First pedigerous somite free. Hyaline frills of cephalosome, somites bearing P1-P4 and urosome dorsally smooth. Urosomites with smooth hyaline frill ventrally, except third urosomite (Figs 1B, 2A). Last three urosomites with spinular fringe on proximal third ventrally; anal somite with distal continuous spinule row. Anal somite with paired sensilla on dorsal side only (Fig. 1C), and two short spinule rows close to the anal operculum. Anal operculum rounded, only slightly protruding beyond insertion line of caudal rami (Figs 1C, 2B). P5-bearing somite with large paired pores laterodorsally and paired spinule rows laterally inserted on distal third of somite (Fig. 1A). Genital double-somite with three lateral spinule rows. Female genital field located between first and second third of genital double-somite. Genital apparatus apparently simplified; copulatory pore located halfway of genital double-somite (Figs 1B, 3A). Seminal receptacles located laterally and condensed close to the lamellar P6 (Fig. 3B).
Phyllognathopus viguieri (Maupas, 1892) (♀). A habitus, dorsal view B abdomen, ventral view C abdomen, dorsal view D labrum (scale bars in μm).
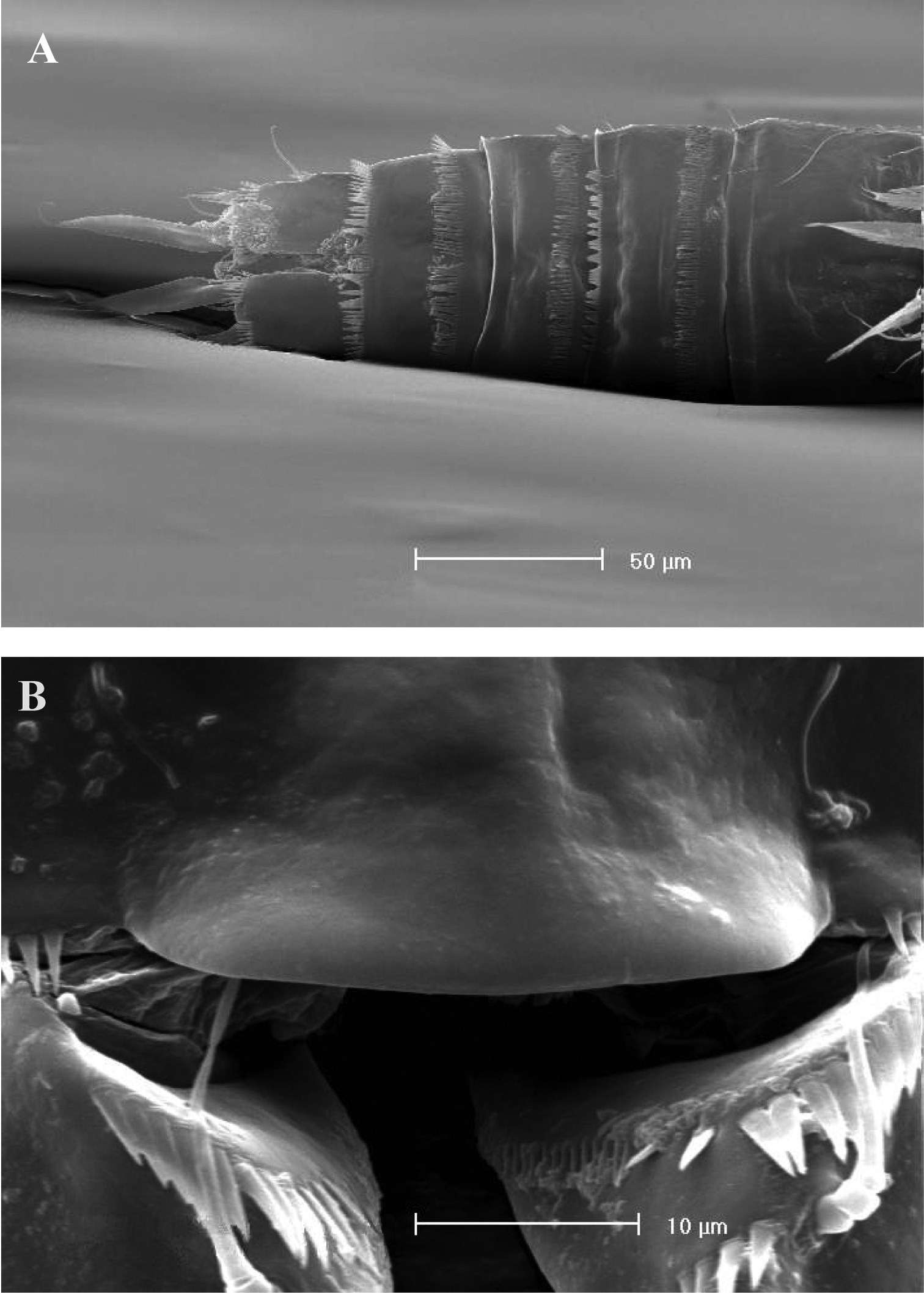
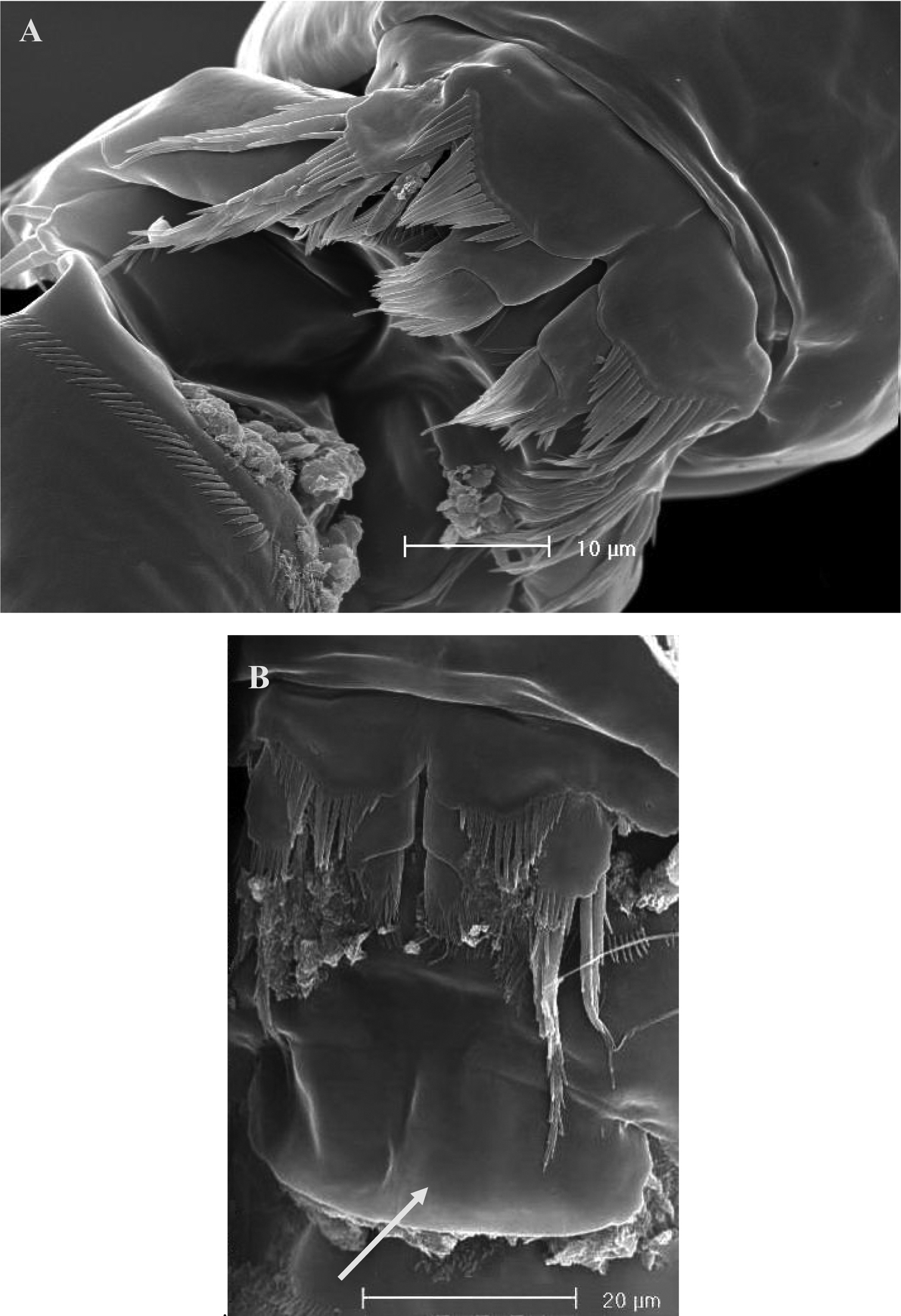
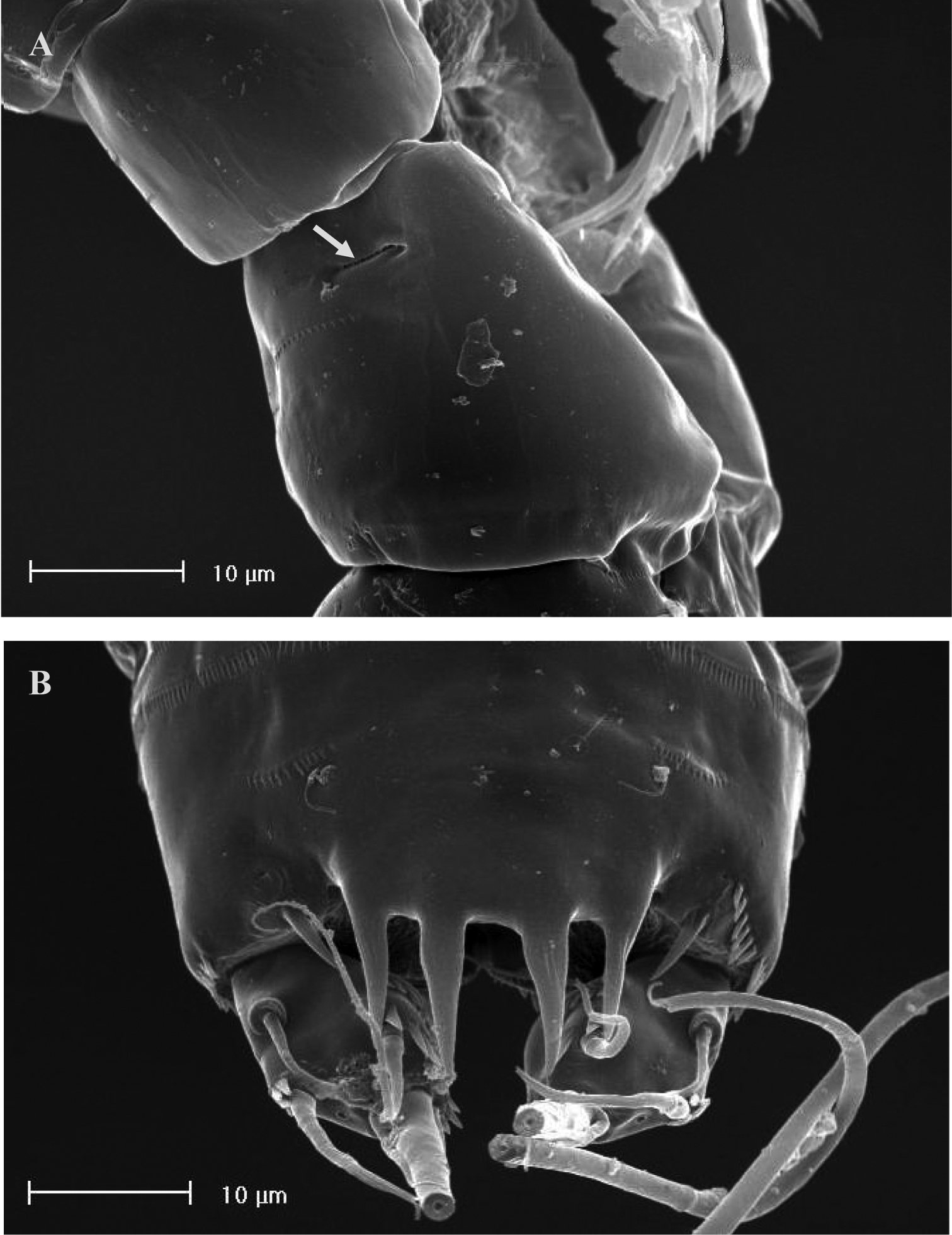
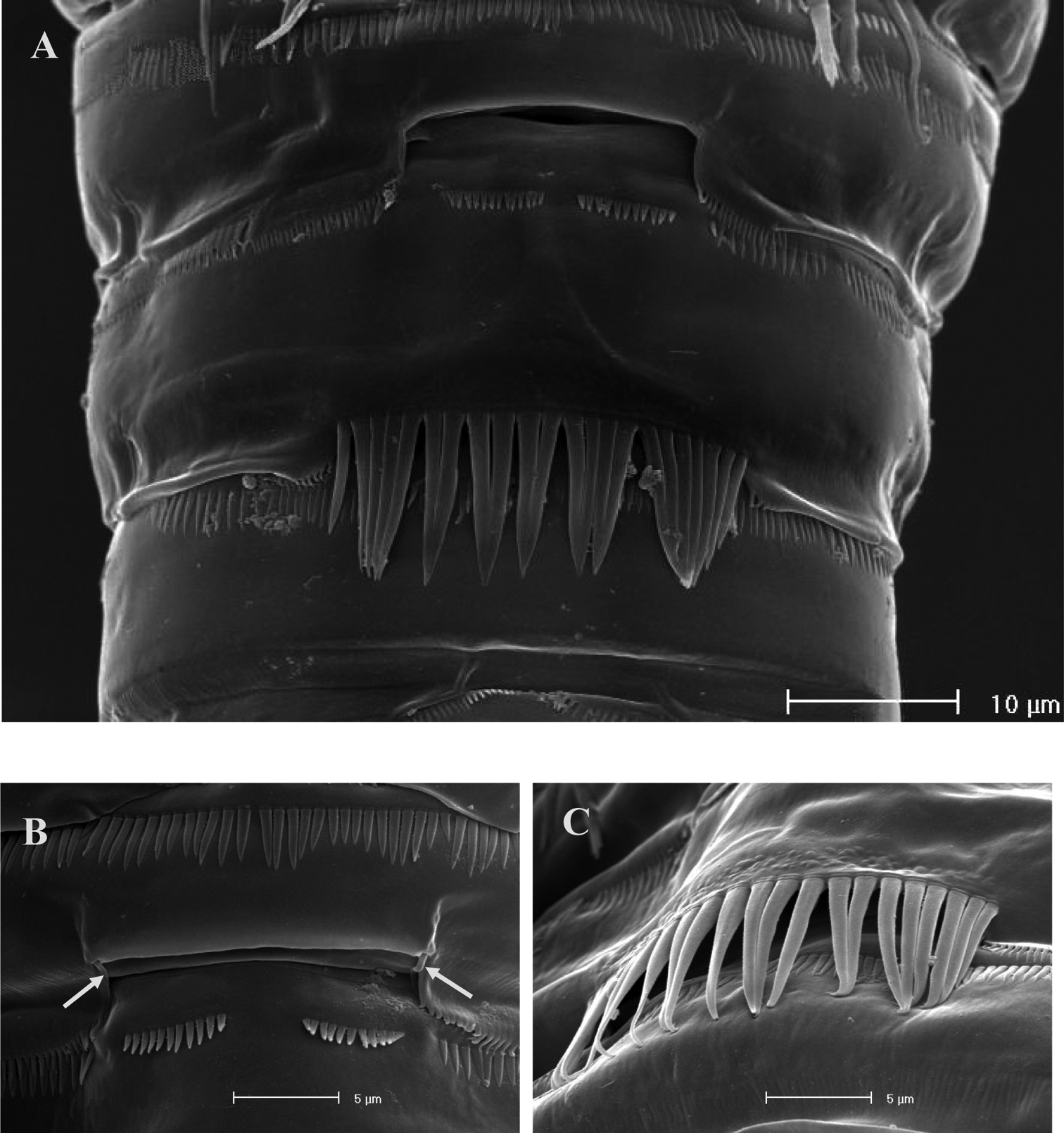
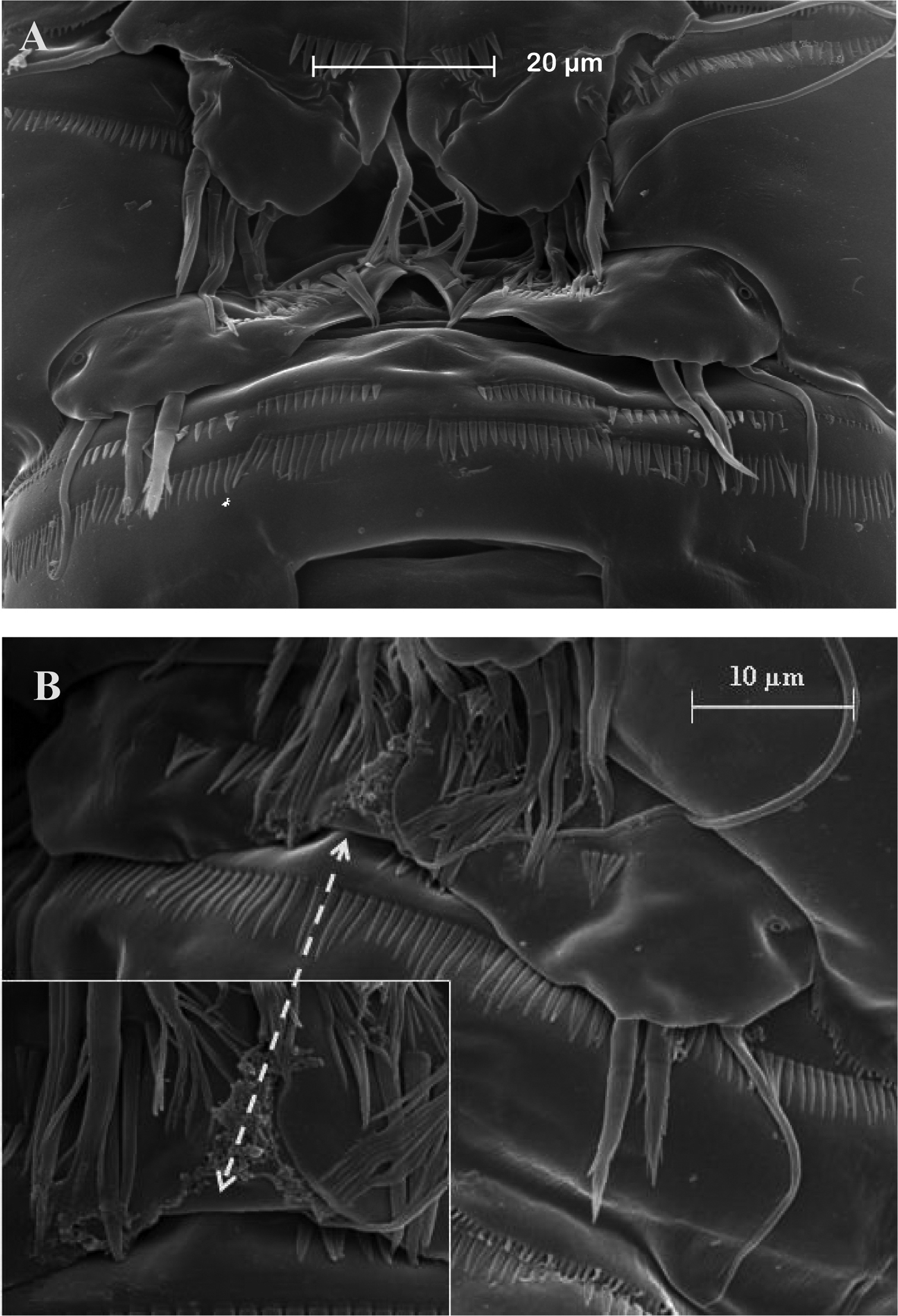
SEM micrographs of Phyllognathopus viguieri (Maupas, 1892) (♀). A ventral surface of urosome (first urosomite omitted) B anal operculum.
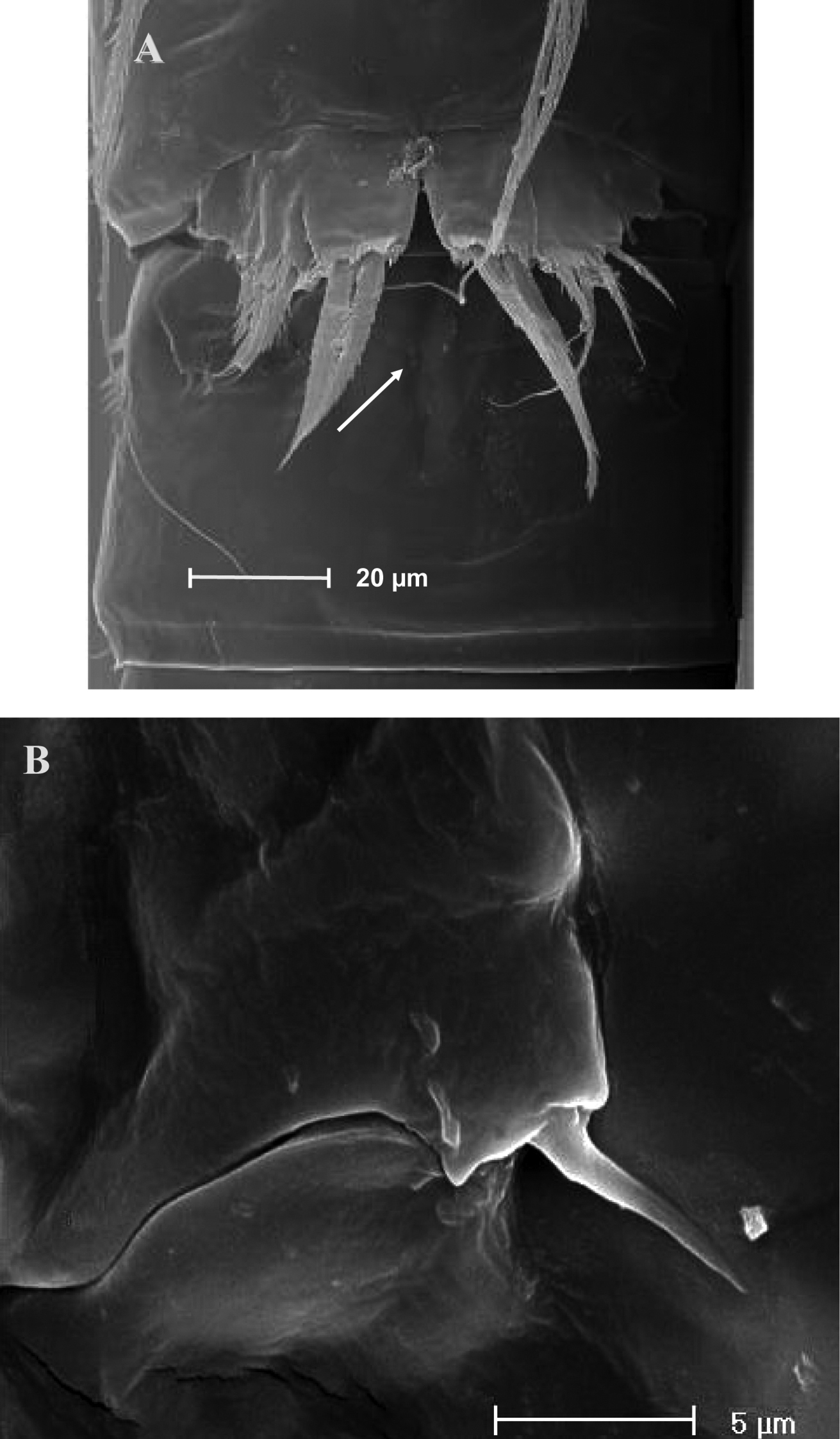
SEM micrographs of Phyllognathopus viguieri (Maupas, 1892) (♀). A P5 and genital double-somite (copulatory pore arrowed) B P6.
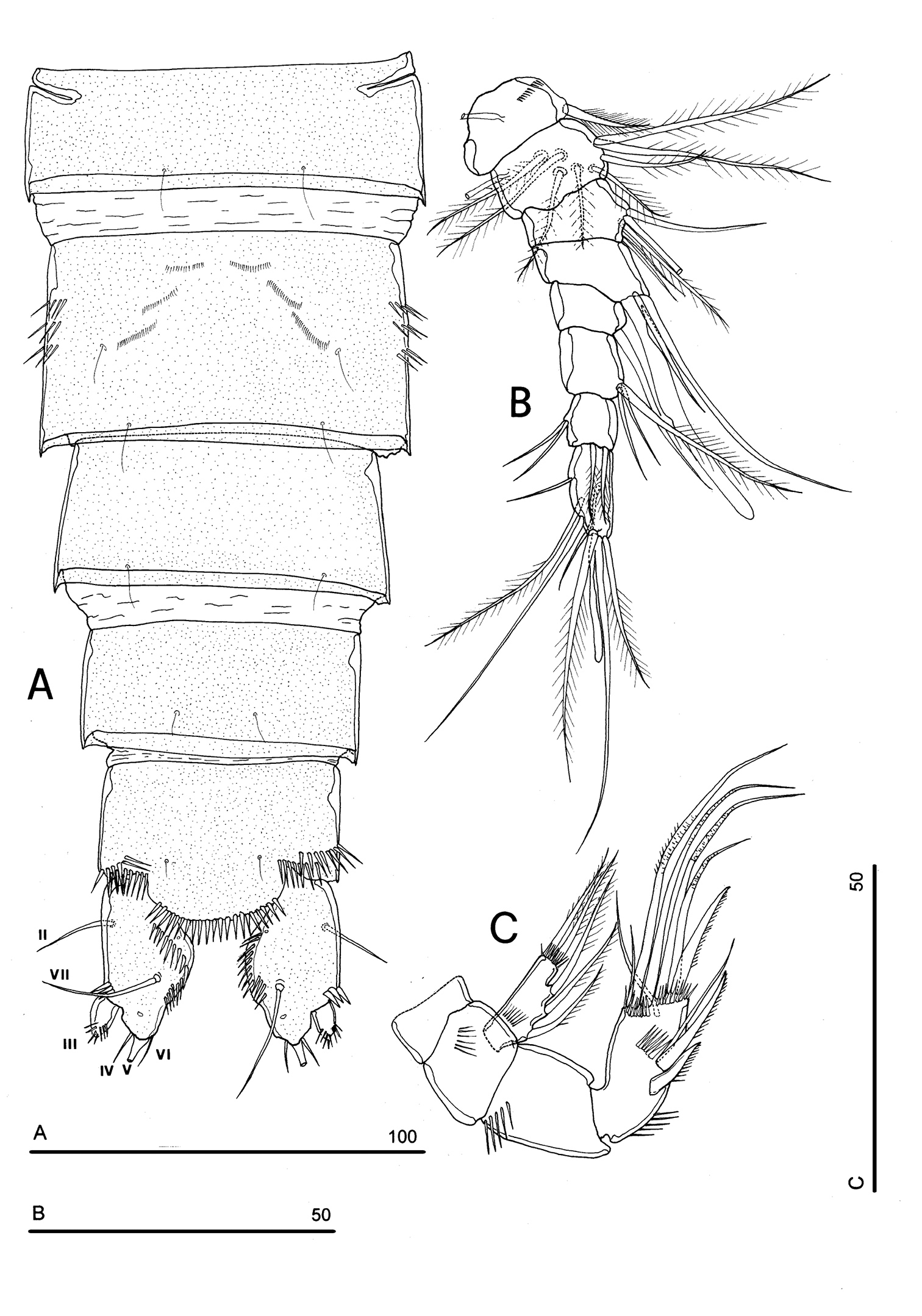
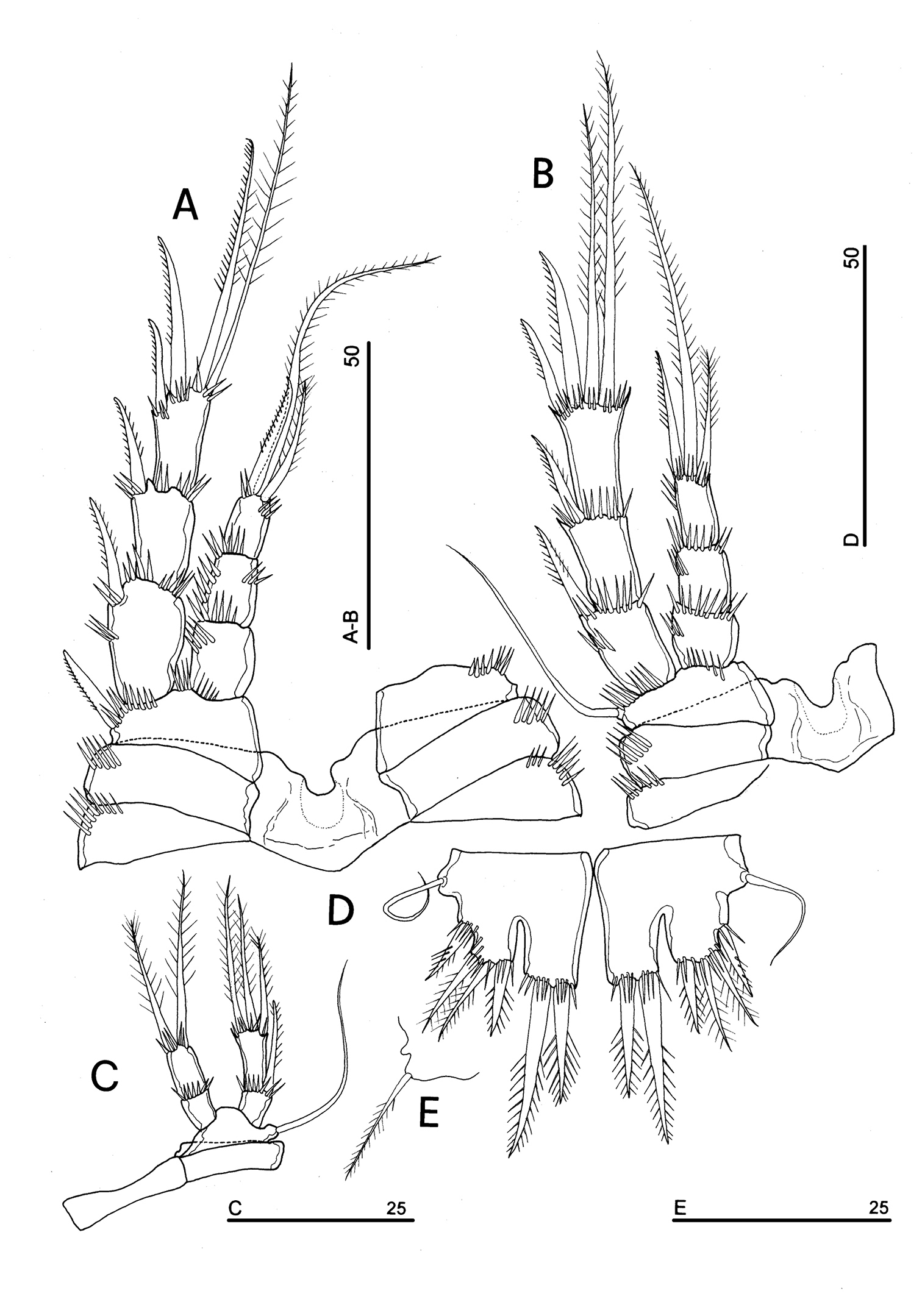
Caudal rami rectangular, parallel, distinctly longer than wide (length/width ratio about 1.7), with incomplete setal pattern (6 setae) (Fig. 1B–C). Anterolateral accessory seta (I) absent, anterolateral seta (II) smooth, inserted at half of caudal ramus; posterolateral seta (III) inserted on distal third of ramus, transformed in a large and stout spiniform element. Outer terminal seta (IV) very short, thin, and naked, shorter than caudal ramus, inner terminal seta (V) unipinnate and relatively short, without articulation at base, with very enlarged proximal part tapering in a subtle tip; terminal accessory seta (VI) slightly shorter than outer terminal seta, thin and naked; dorsal seta (VII) inserted on distal third of caudal ramus, about as long as caudal ramus. Three spinule rows inserted dorsolaterally and two spinule rows inserted at distal margin of caudal ramus ventrally. Two pores located close to the insertion of setae II and IV, ventrally.
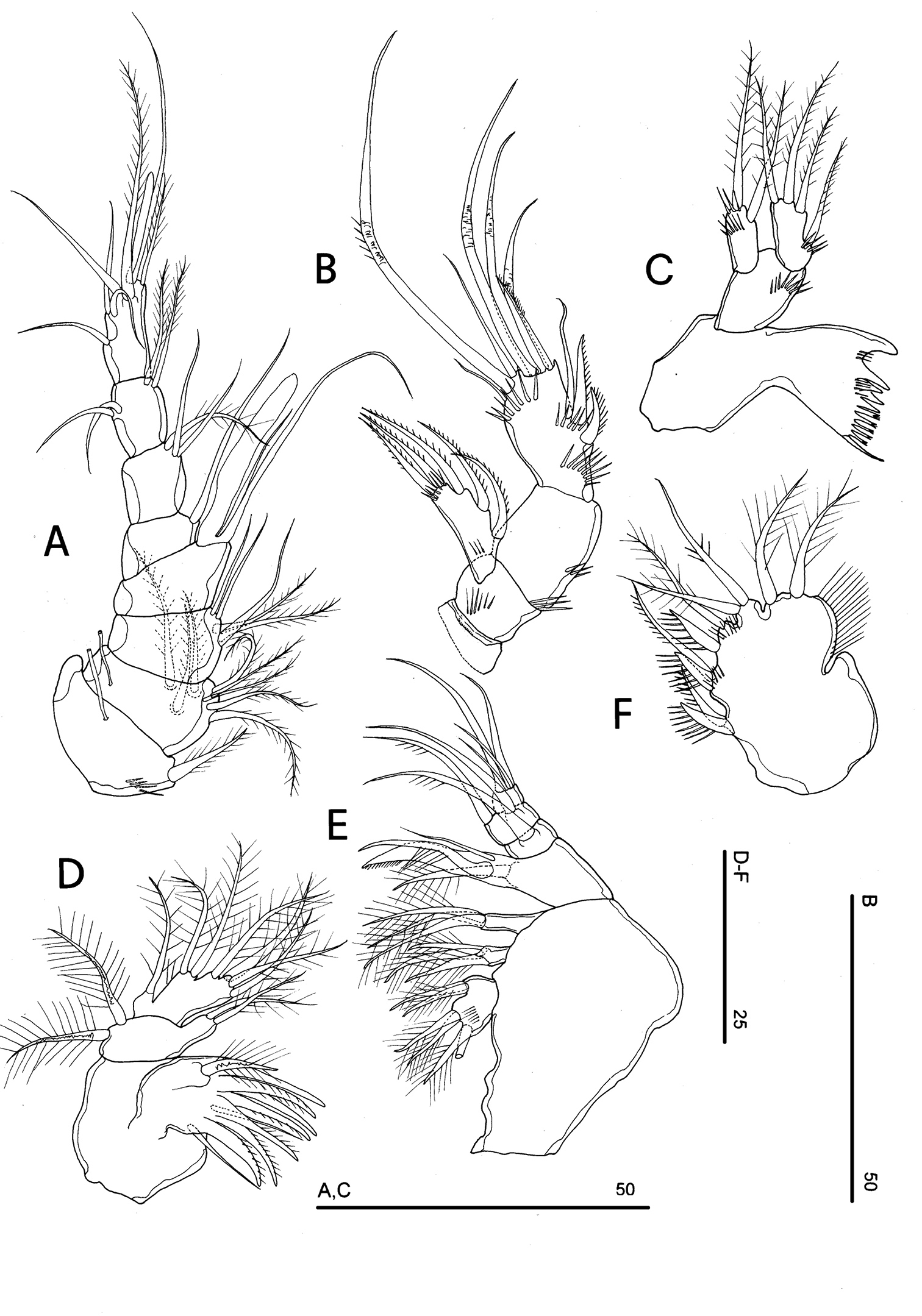
Antennule (Fig. 4A): short, 8-segmented. Segment 1 with 1 spinule row. Both segments 1 and 2 bearing long and flaccid tube-pores. Armature formula: 1-[1], 2-[8], 3-[5], 4-[1 + (1+ae)], 5-[1], 6-[3], 7-[4], 8-[6 + (1+ae)]. Aesthetasc on segment 4 large, reaching about the proximal part of the penultimate antennulary segment.
Antenna (Fig. 4B): coxa unarmed; basis with 1 transverse spinule row on surface, a spinule row inserted on inner margin; exopod 1-segmented, well-defined at base, with surface spinule row, bearing 3 lateral and 2 apical setae; free endopod 2-segmented; both segments robust, of about the same length; segment 1 with inner spinule row; segment 2 with two inner spinule rows; armature consisting of 2 inner spines and 1 seta, 1 unipinnate apical spine, 4 geniculate setae, 1 apical slender seta and 1 subapical slender seta; a row of spinules at outer corner.
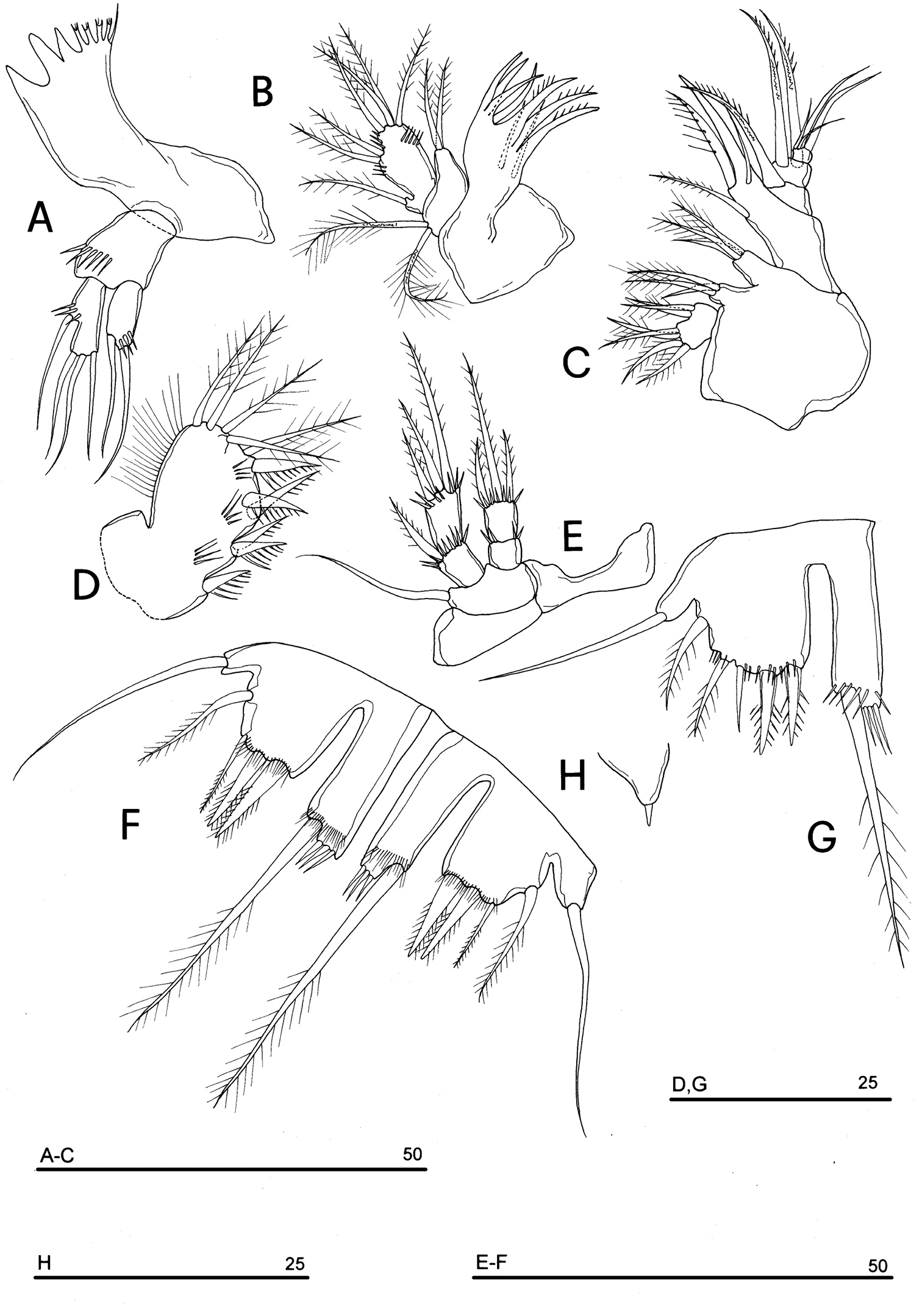
Phyllognathopus viguieri (Maupas, 1892) (♀). A antennule B antenna C mandible D maxillule E maxilla F maxilliped (scale bars in μm).
Labrum (Fig. 1D): trapezoidal, with two spinule rows on free distal margin. Paired rows of hair-like elements on medioventral surface.
Mandible (Fig. 4C): coxal gnathobase elongate, cutting edge with 2 large and coarse teeth, three smaller teeth and row of fringed teeth; naked seta at dorsal corner. Mandibular palp biramous, basis with inner spinule row, exopod with 1 apical and 1 inner bipinnate setae; endopod with 1 inner, 1 subapical and 2 apical bipinnate setae.
Maxillule (Fig. 4D): well developed arthrite incorporated into praecoxa, with 7 strong curved spines inserted on free distal margin and 1 short seta inserted on a sort of surface peduncle and 2 anterior surface setae. Coxal epipodite represented by 2 setae; coxo-endite with 2 plumose setae. Exopod and endopod incorporated into basis, with a total of 7 plumose setae.
Maxilla (Fig. 4E): syncoxa with 3 endites. Proximal endite with 6 setae; medial and distal endites, each with 3 plumose setae. Allobasis drawn out into a strong claw, distally spinulose, accompanied by 2 robust and 1 thin setae; endopod 3-segmented; segment 1 with 1 robust curved seta; segment 2 with 2 robust curved setae; segment 3 with 2 robust curved and 2 slender setae.
Maxilliped (Fig. 4F): phyllopodial, lamelliform, and 1-segmented. Trace of ancestral 2-segmented condition marked by the presence of outer incision, representing original segmentation boundary between former segments 1 and 2. Armature consisting of 10 elements, of which 5 bipinnate setae in apical position, 1 unipinnate seta inserted along inner margin together with 4 strong unipinnate stout spines. No trace of incision along inner margin.
P1-P3 with 3-segmented exopods and endopods. P4 with 3-segmented exopod and 2-segmented endopod. Intercoxal sclerites: boundary between intercoxa and basis not well defined at posterior surface of P2-P4 (Fig. 5B–D). P1-P3 praecoxa well developed, with outer spinule row. P4 praecoxa absent.
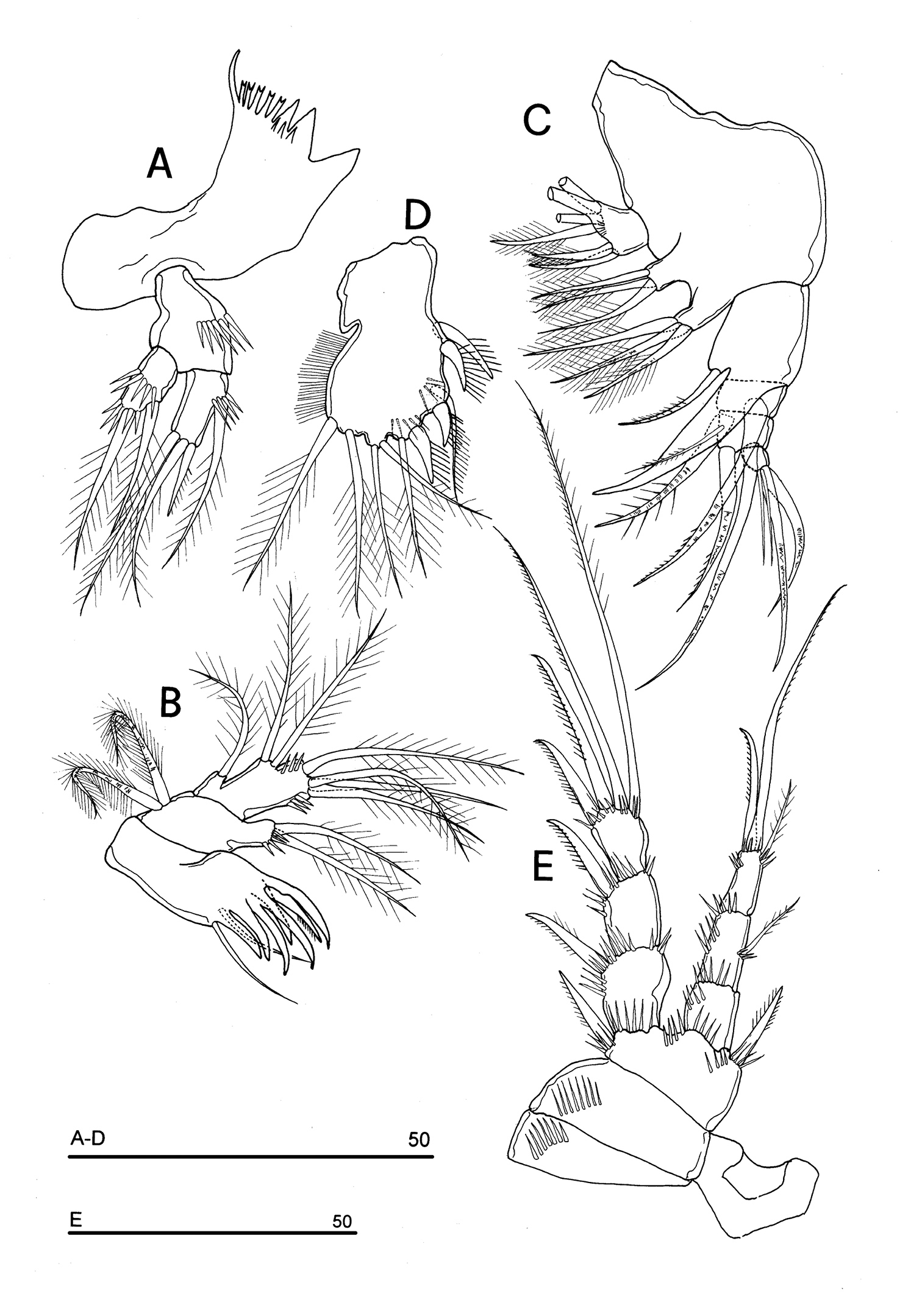
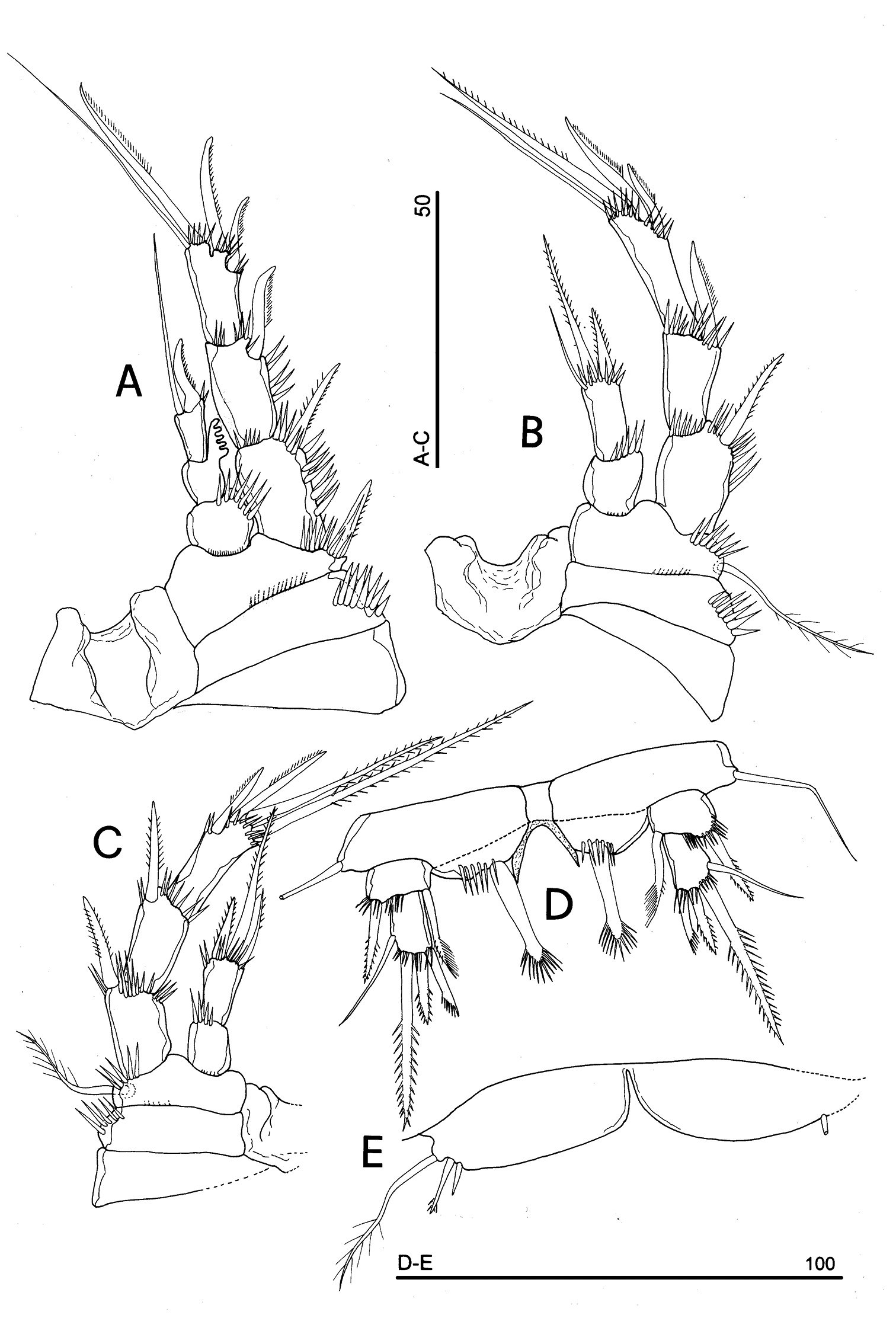
P1 (Fig. 5A): praecoxa and coxa with outer spinule row on anterior surface; one posterior row of thin spinules inserted close to coxo-basis boundary. Basis with 1 outer spiniform seta and 1 inner spine, with spinule rows along outer margin, between exopod and endopod and at the insertion of inner spine, respectively. Exopod about as long as endopod; exp-1 and -2 with 1 outer unipinnate spine; exp-3 with 2 unipinnate spines in apical position, and 1 apical and 1 subapical inner setae. Endopod: enp-1 unarmed, about as long as enp-2 and enp-3, wider than enp-2 and enp-3. Enp-2 cylindrical, with short inner seta inserted at the middle of segment. Enp-3 with 1 inner spine, 1 apical seta and 1 curved apical spine. Ornamentation as in Fig. 5A.
P2 (Fig. 5B): ornamentation of praecoxa and coxa as in P1. Basis with 1 outer spine, with spinule rows along outer margin, and between exopod and endopod. Exopod slightly longer than endopod; exopodal segments of about the same length; exp-1 and -2 with 1 outer unipinnate spine; exp-3 with 2 outer unipinnate spines, 1 apical unipinnate seta and 1 subapical long inner seta. Endopod: enp-1 unarmed; enp-2 with 1 naked inner seta; enp-3 with 1 spine and 1 long bipinnate seta in apical position, and 1 short bipinnate subapical seta. Ornamentation as in Fig. 5B.
P3 (Fig. 5C): ornamentation of praecoxa and coxa as in P2. Basis with short outer seta and spinule rows along outer margin and at the insertion of the endopod. Exopod distinctly longer than endopod. Exp-1 and -2 with 1 unipinnate outer spine; exp-3 with 2 unipinnate outer spines, 1 bipinnate apical seta and 1 long bipinnate subapical seta. Endopod: enp-1 and -2 unarmed; enp-3 with 1 spine and 2 bipinnate setae in apical position. Ornamentation as in Fig. 5C.
P4 (Fig. 5D): reduced in size, praecoxa absent, coxa and basis without ornamentation; basis with long outer naked seta; exopod and endopod about as long as half of remaining legs; the exopod only slightly longer than endopod. Exp-1 with 1 unipinnate outer spine; exp-2 unarmed; exp-3 with 1 bipinnate outer spine and 2 apical setae of different length. Endopod: enp-1 unarmed; enp-2 with 3 apical setae. Ornamentation as in Fig. 5D.
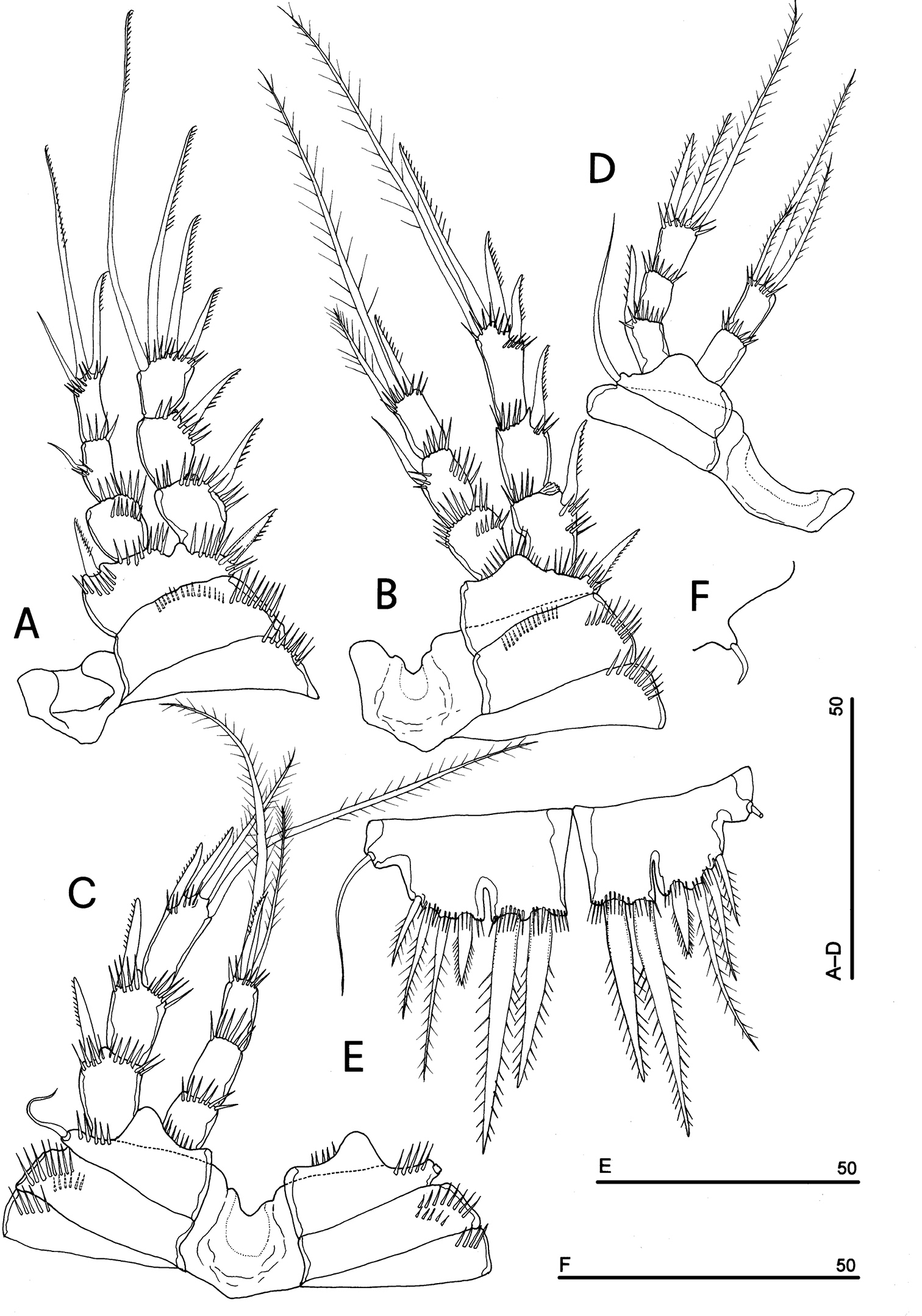
Phyllognathopus viguieri (Maupas, 1892) (♀). A P1 B P2 C P3 D P4 E P5 F P6 (scale bars in μm).
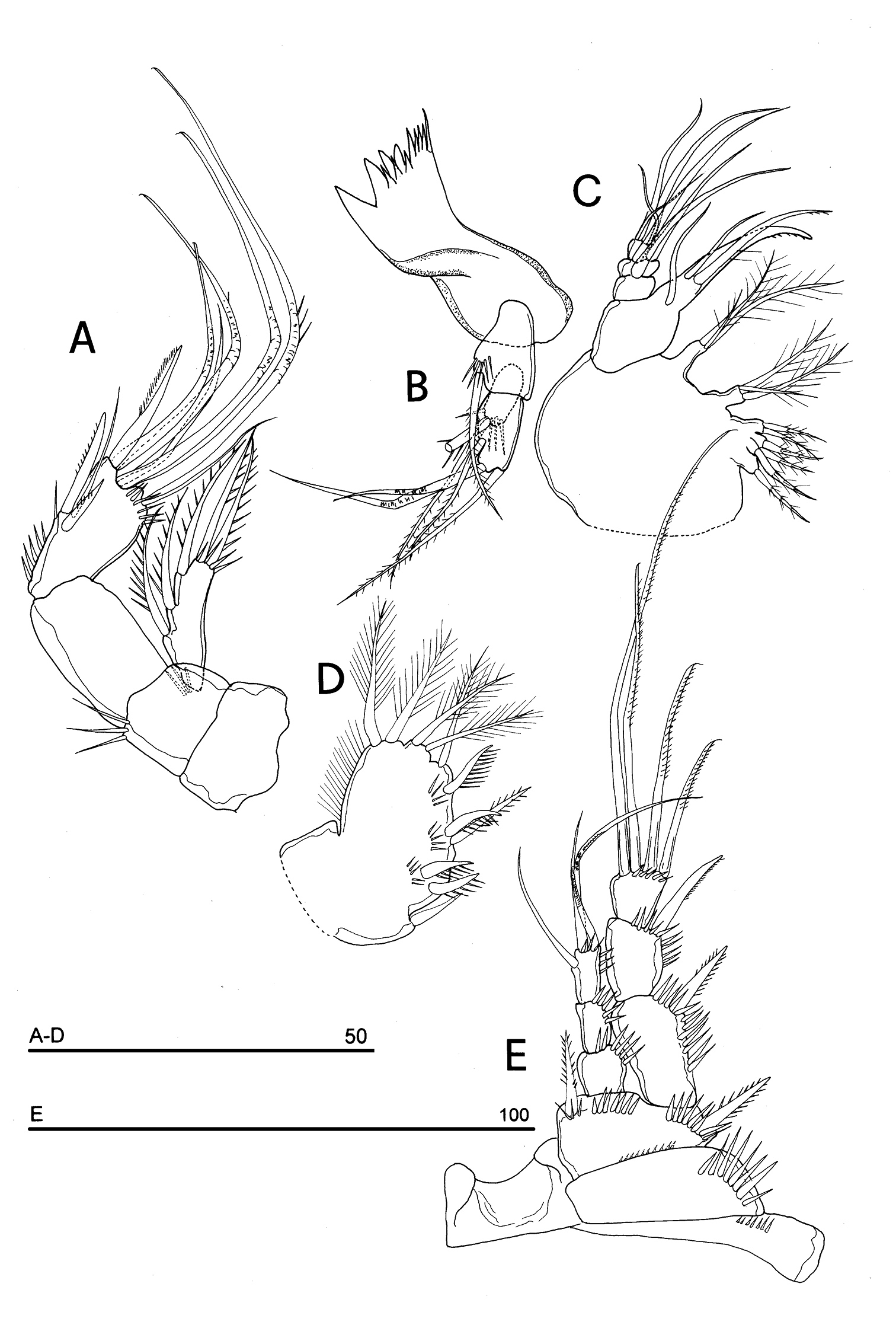
P5 (Figs 3A, 5E): free, with clear articulation to P5-bearing somite; right and left legs distinct; baseoendopod and exopod coalescent, incision marked original segmentation between them still present; basipodal outer seta present, exopodal armature consisting of 3 bipinnate setae and 1 stout spine: all elements in apical position; baseoendopod armed with 2 robust bipinnate setae, the outermost the longest.
P6 (Figs 3B, 5F): rudimentary, consisting of paired small chitinous lamellar plates not coalescent along medial margin, partially covering seminal receptacles. Armature consisting of 1 short naked spine with rounded tip on each leg.
Body length, measured from tip of rostrum to posterior margin of caudal rami, from 370 to 541 µm, with mean of 424 µm based on 8 individuals. Sexual dimorphism in antennule, abdominal segmentation, P5, P6 and caudal setae morphology. Habitus, cephalosome (Figs 6A, 7A–B), sensilla and pore patterns as in female. Integument with surface pits. Urosome as in Figs 6B, 7C. Caudal rami with 6 setae (Fig. 6B). Anterolateral seta (II) as in female, posterolateral seta (III) setiform, not transformed (length seta/length caudal ramus: about 2) and bipinnate. Outer terminal seta (IV) as in female, inner terminal seta (V) not transformed, plumose and long, not articulated at base; terminal accessory seta (VI) and dorsal seta (VII) as in female (Fig. 6). Ornamentation and pore patterns as in female. Anal operculum as in female.
Antennule (Figs 6C, 7D–F): elongate, basically 10-segmented, last segment still showing a surface suture line only on anterior surface, indicating an incipient 11-segmented condition. Segment 1 with 1 ventral spinule row and 1 tube-pore (Fig. 8A). Segment 2 with tube-pore. Segment 4 represented by small U-shaped sclerite. Segment 6 the largest, sclerotized. Segment 8 elongate and transformed, moderately sclerotized, segment 9 distinct, not incorporated into segment 8, segment 10 derived by incomplete fusion between former segments 10 and 11 (Fig. 7D–F). Armature formula: 1-[1], 2-[9], 3-[8], 4-[2], 5-[7+(1 + ae)], 6-[2], 7-[2], 8-[0], 9-[1], 10-[10 + (1 + ae)]. Aesthetasc on segment 5 very large. Segment 8 with medial pointed protrusions as in Fig. 6C.
Phyllognathopus viguieri (Maupas, 1892) (♂). A habitus, dorsal view B abdomen, ventral view (first urosomite omitted) C antennule D P5 E P6 (scale bars in μm).
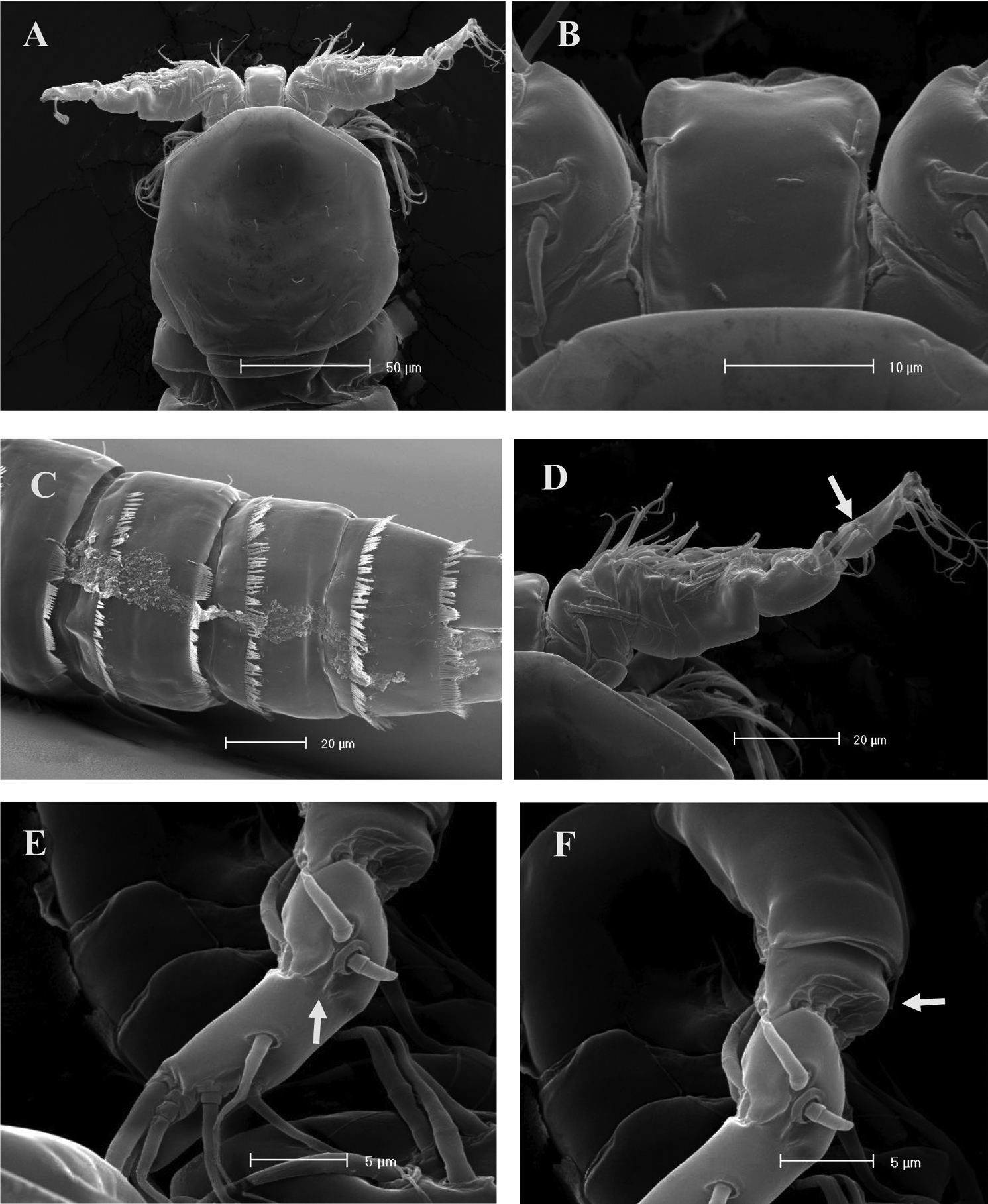
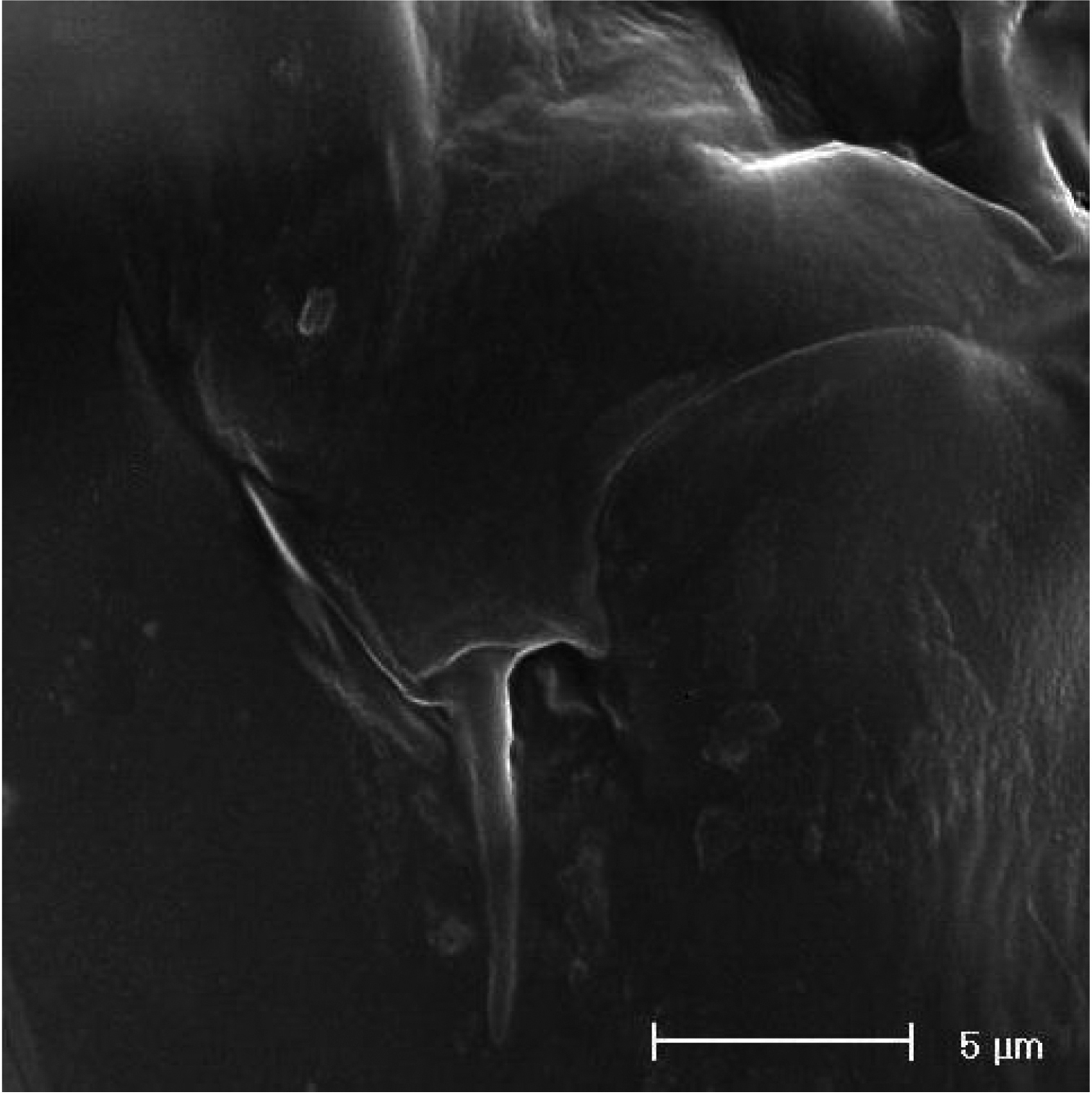
SEM micrographs of Phyllognathopus viguieri (Maupas, 1892) (♂). A cephalosome, rostrum and antennule B rostrum (detail) C ornamentation of third to six urosomites, ventral surface D antennule, segments 10 and 11 discrete on posterior surface (boundary line arrowed) E antennule, detail of segments 10 and 11 fused on anterior surface (suture line arrowed) F antennule, segment 9 arrowed.
P1-P4 as in female; for morphological details of P1-P4 see Fig. 8B. P5 (Figs 6D, 9A): free, with clear articulation to P5-bearing somite; right and left legs coalescent; exopod clearly discernible but incorporated to basis: no trace of articulation between them observable, bearing 2 inner, 2 apical and 2 outer bipinnate setae; endopod discrete, distinctly 1-segmented, bearing 1 large leaf-like transformed seta and a spinule row along its free outer margin. Basipodal outer seta slender and naked, one pore near its insertion.
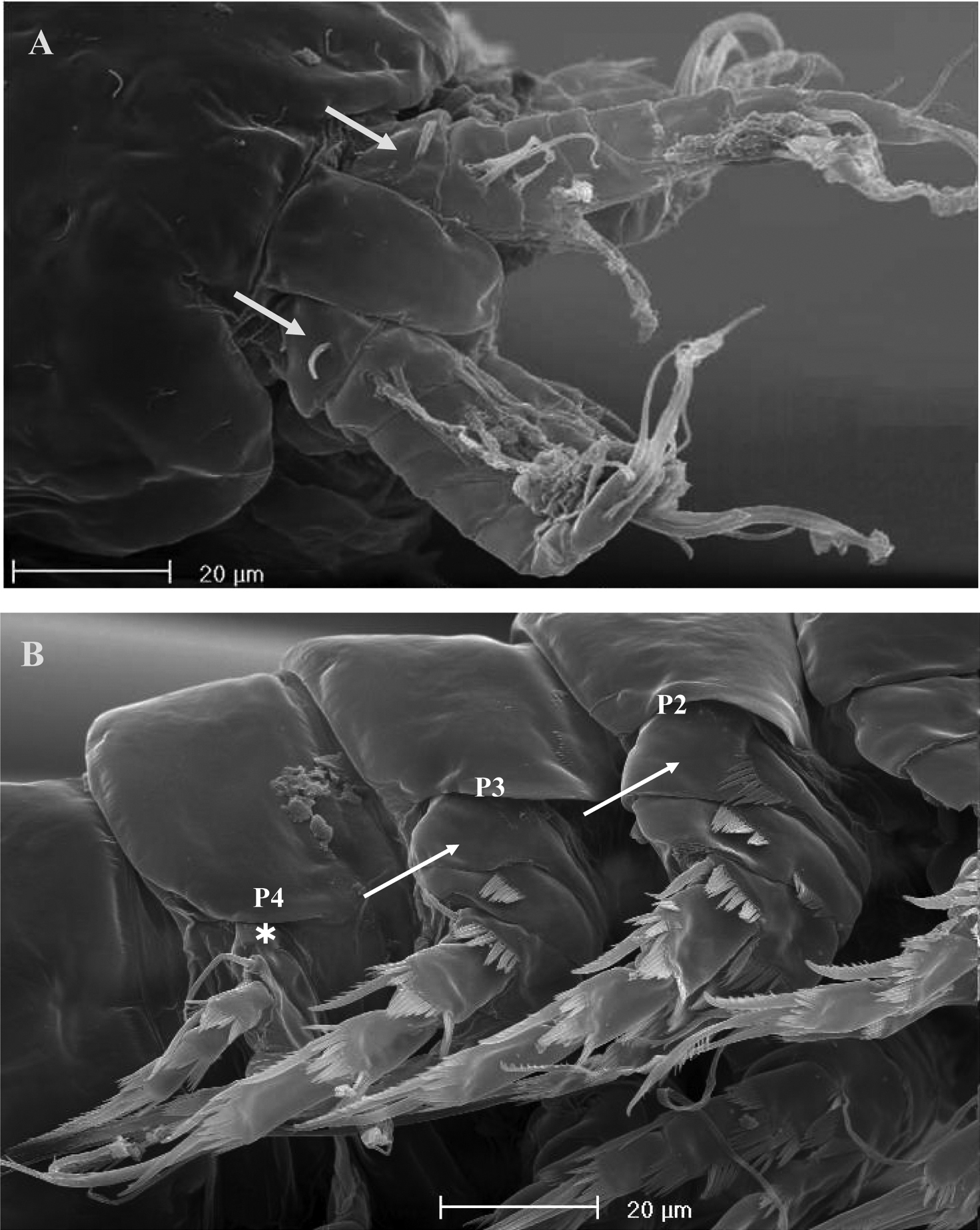
SEM micrographs of Phyllognathopus viguieri (Maupas, 1892) (♂). A antennule, tube-pores on the first antennulary segment arrowed B P2-P4 (P2-P3 praecoxa arrowed; asterisk indicates the P4 praecoxa missing).
P6 (Figs 6E, 9B): right and left legs coalescent, forming a single linear lamellar plate, with no trace of incision between right and left P6; armature consisting of 2 spines and 1 outer seta.
SEM micrographs of Phyllognathopus viguieri (Maupas, 1892) (♂). A P5 B P6 (arrowed).
urn:lsid:zoobank.org:act:0BCBB7D6-0796-49AE-8856-BE37771B0F66
http://species-id.net/wiki/Phyllognathopus_inexspectatus
Figs 10–13♀ holotype completely dissected and mounted in glycerine, deposited at the Natural History Museum, London (UK); January 2003, D. Cipriani coll.; 3 ♀♀ paratypes completely dissected and mounted in lactophenol; May 2003; January 2004. D. Cipriani coll..
Mazzoccolo karstic spring (Latium, central Italy), coordinates: 41°15'17"N, 13°27'08"E; Western Aurunci Mountains; 20 m a.s.l.; water temperature 13.5 ± 0.3 °C; pH 7.5 ± 0.1; O2 9.1 ± 0.9 mg/L (n = 11).
FEMALE. Total body length, measured from tip of rostrum to posterior margin of caudal rami, 474 µm (holotype), 468 µm (paratypes mean value; n = 3). Body depigmented and eyeless. Habitus slender, no clear demarcation between prosome and urosome. Integument with surface pits, moderately sclerotized. Cephalosome subquadrate, with a dorsal rounded protuberance, hardly observable, referable to the dorsal integumental window (Fig. 10A). Couples of setule rows located on surface of cephalic shield. Rostrum elongate, subrectangular in shape, clearly articulated to the cephalosome; two dorsal sensilla laterally inserted on distal third, and one pore apically. Cephalosome and both thoracic and abdominal somites with cuticular ornamentation represented by reduced number of paired sensilla (Fig. 10A). First pedigerous somite free. Hyaline frills of cephalosome, somites bearing P1-P4 and urosome dorsally smooth. P5-bearing somite with large paired pores laterodorsally. Genital double-somite with three lateral spinule rows and three pairs of setule rows inserted dorsally. Female genital field located at the middle of genital double-somite. Genital apparatus simplified, copulatory pore located at half of the genital double-somite. Seminal receptacles laterally located and condensed close to the lamellar sixth legs.
Phyllognathopus inexspectatus sp. n. (♀). A habitus, dorsal view B abdomen, ventral view C caudal ramus, ventral view (scale bars in μm).
Urosomites with smooth hyaline frill ventrally, except third urosomite (Fig. 10B). Last two urosomites with spinular fringe on proximal third; anal somite with distal continuous spinule row.
Anal somite with paired sensilla on dorsal surface (Fig. 11A), and two short spinule rows close to the anal operculum. Anal operculum rounded, protruding beyond insertion line of caudal rami and armed with strong spinules on free distal margin (Fig. 11A). Caudal rami rectangular with strongly expanded inner margin, slightly divergent, distinctly longer than wide (length/width ratio: about 1.5), with incomplete setal pattern (6 setae) (Figs 10C, 11A); anterolateral accessory seta (I) absent, anterolateral seta (II) smooth, inserted at proximal third of caudal ramus; posterolateral seta (III) inserted on distal third of ramus, transformed in a short and stout spiniform seta, with tuft of spinules apically. Outer terminal seta (IV) very short, thin, and naked, without articulation at base (Fig. 10C), distinctly shorter than caudal ramus; inner terminal seta (V) not transformed, very long, without articulation at base; terminal accessory seta (VI) as long as outer terminal seta, thin and naked; dorsal seta (VII) inserted at half of caudal ramus, about as long as caudal ramus or slightly shorter. A continuous spinule row along inner margin of caudal ramus and three spinule rows inserted close to the anterolateral seta (Figs 10B, 11A), at the basis of the posterolateral seta and at distal margin of ramus ventrally, respectively. Two pores are located dorsally on each caudal ramus, and one pore ventrally.
Phyllognathopus inexspectatus sp. n. (♀) A urosome, dorsal view B antennule C antenna (scale bars in μm).
Antennule (Fig. 11B): short, 8-segmented. Segment 1 with ventral spinule row. Both segments 1 and 2 bearing long and flaccid tube-pores. Armature formula: 1-[1], 2-[8], 3-[5], 4-[1 + (1 + ae)], 5-[1], 6-[3], 7-[4], 8-[6 +(1+ ae)]. Aesthetasc on segment 4 very large, reaching about the last antennulary segment.
Antenna (Fig. 11C): coxa unarmed; basis with 1 transverse spinule row on surface; exopod 1-segmented, well-defined at base, with spinule row on surface, bearing 3 lateral unipinnate and 2 apical bipinnate setae; free endopod 2-segmented; both segments robust, of about the same length; segment 1 with inner spinule row; segment 2 with one inner and one surface spinule rows; armature consisting of 2 inner spines and 1 thin seta, 1 apical unipinnate spine, 4 geniculate setae, and 1 apical and 1 surface slender setae; two rows of spinules at outer corner and in subapical position on free distal margin, respectively.
Mandible (Fig. 12A): coxal gnathobase elongate, cutting edge with 3 large and coarse teeth, 5 smaller fringed teeth; naked seta at dorsal corner. Mandibular palp biramous, basis with inner strong spinule row, exopod with 1 apical and 1 inner bipinnate setae; endopod with 1 inner bipinnate, and 1 spiniform and 2 bipinnate apical setae. Ornamentation as in Fig. 12A.
Phyllognathopus inexspectatus sp. n. (♀). A mandible B maxillule C maxilla D maxilliped E P1 (scale bars in μm).
Maxillule (Fig. 12B): well developed arthrite incorporated into praecoxa, with 7 strong curved spines inserted on free distal margin, and 2 anterior surface setae. Proximal surface bipinnate seta inserted on tubercle absent (vs. present in Phyllognathopus viguieri, see Fig. 4D). Coxal epipodite represented by 2 setae; coxo-endite with 2 plumose setae. Exopod and endopod incorporated into basis, bearing 7 plumose setae.
Maxilla (Fig. 12C): syncoxa with 3 endites. Proximal endite free, with 6 setae; medial and distal endites incorporated to syncoxa, each with 3 plumose setae, inserted as in Fig. 12C. Allobasis drawn out into a strong claw apparently smooth, accompanied by 1 robust and 2 thin setae; endopod 3-segmented; segment 1 with 1 robust curved seta; segment 2 with 2 robust curved setae; segment 3 with 2 robust curved and 2 slender setae.
Maxilliped (Fig. 12D): phyllopodial, lamelliform, 1-segmented, and slender than in Phyllognathopus viguieri. Trace of ancestral 2-segmented condition marked by the presence of outer incision, probably representing original segmentation boundary between segments 1 and 2. Armature consisting of 10 elements, of which 5 bipinnate setae in apical position, two of which with independent insertion, 1 unipinnate seta inserted along inner margin together with 4 strong unipinnate spines. No trace of incision along inner margin.
P1-P3 with 3-segmented exopods and endopods. P4 with 2-segmented exopod and endopod. Intercoxal sclerites: boundary between intercoxa and basis not well defined at posterior surface in P2-P4. P1-P3 praecoxa well developed, with 1 outer spinule row. P4 praecoxa absent.
P1 (Fig. 12E): praecoxa and coxa with outer spinule row on anterior surface. Basis with 1 outer spiniform seta and 1 inner spine; with spinule rows along outer margin, between exopod and endopod and at the insertion of inner spine, respectively. Exopod slightly longer than endopod: exp-1 and -2 with 1 outer unipinnate spine; exp-3 with 2 outer unipinnate spines and 2 setae, respectively inserted apically and subapically. Endopod: enp-1 unarmed, about as long as enp-2 and enp-3. Enp-2 cylindrical, with 1 inner short seta. Enp-3 with 1 inner bipinnate seta, 1 long unipinnate curved seta and 1 spiniform curved seta in apical position. Ornamentation as in Fig. 12E.
P2 (Fig. 13A): praecoxa and coxa as in P1; basis with 1 outer spiniform seta, with spinule rows along outer margin, and between exopod and endopod. Exopod distinctly longer than endopod; exopodal segments of about the same length; exp-1 and -2 with 1 outer bipinnate spine; exp-3 with 2 outer unipinnate spines, 1 apical unipinnate seta and 1 subapical long bipinnate seta. Endopod: enp-1 and-2 unarmed; enp-3 with 1 spine and 1 long bipinnate seta in apical position, 1 subapical short bipinnate seta. Ornamentation as in Fig. 13A.
Phyllognathopus inexspectatus sp. n. (♀). A P2 B P3 C P4 D P5 E P6 (scale bars in μm).
P3 (Fig. 13B): ornamentation of praecoxa and coxa as in P1-P2. Basis with long outer seta and spinule rows along outer margin and at the insertion of the endopod. Exopod distinctly longer than endopod. Exp-1 and -2 with 1 outer bipinnate spine; exp-3 with 2 outer unipinnate spines, and 2 apical long bipinnate setae. Endopod: enp-1 and -2 unarmed; enp-3 with 1 unipinnate spine and 1 long bipinnate seta in apical position, 1 subapical bipinnate seta. Ornamentation as in Fig. 13B.
P4 (Fig. 13C): small sized, if compared to P1-P3; praecoxa absent, coxa and basis without ornamentation; basis with outer long and naked seta; exopod as long as endopod. Exp-1 with 1 outer long unipinnate spine; exp-2 with 1 outer long spine and 2 apical setae. Endopod: enp-1 unarmed; enp-2 with 2 apical plumose setae. Ornamentation as in Fig. 13C.
P5 (Fig. 13D): free, with clear articulation to P5-bearing somite; right and left legs distinct; baseoendopod and exopod coalescent, incision marked original segmentation still present; basipodal outer seta present, exopodal armature consisting of 1 outer spine, 2 apical short setae, of about the same length, and 1 apical spine; baseoendopod armed with 2 robust bipinnate setae, the outer the longest.
P6 (Fig. 13E): rudimentary, consisting of small paired chitinous lamellar plates not coalescent along medial margin, partially covering the genital field. Armature consisting of 1 long and slender bipinnate seta on each side.
Male unknown.
The specific name derives from the Latin adjective inexspectatus which means “unexpected”, alluding to the surprising geographical location of the species, being the taxonomically related Phyllognathopus distributed in the Southern Hemisphere, and to the ecological finding of this species, which was collected from a large karstic aquifer in Central Italy, whereas all the other members of the genus are epigean.
At present knowledge the species is to be
considered a stygobiotic species, collected from a karstic aquifer of
the Western Aurunci Mountains (Latium) (
http://species-id.net/wiki/Parbatocamptus_jochenmartensi
Figs 14–16♂ holotype, completely dissected and mounted on slide labeled SMF 14657, deposited at the Senckenberg Museum (Germany).
Phyllognathopodidae. Body flattened. First pedigerous somite free. Hyaline frills of abdominal somites ventrally smooth. Anal operculum prominent, subdistally crenulated. Caudal ramus subquadrate in ventral view, tapering on free distal margin in dorsal view, with 6 setae; anterolateral accessory seta (I) absent; dorsal seta inserted on spinulose protuberance closely located to free distal margin. Male antennule 10-segmented, with geniculation between segments 7 and 8; segment 9 distinct, as usual in the family; tube-pores on segments 1 and 2; aesthetascs on segments 5 and 10. Antenna: exopod 1-segmented, well-defined at base, bearing 3 lateral and 2 apical unipinnate setae; free endopod 2-segmented; both segments robust, of about the same length. Mandibular palp biramous; basis with inner long bipinnate seta; exopod with 2 setae; endopod with 2 apical geniculate setae; 1 inner and 1 subapical bipinnate setae. Maxillary syncoxa with 3 endites. Proximal endite quadrilobate, with 6 apical setae; medial and distal endites, each with 3 setae. Allobasis drawn out into a strong claw, accompanied by 1 curved and 2 normal setae; endopod 3-segmented; segment 1 with 1 robust curved seta; segment 2 with 2 robust curved setae; segment 3 with 2 robust curved and 2 slender setae. Maxilliped phyllopodial, lamelliform, 1-segmented. Clear trace of ancestral 2-segmented condition well discernible and marked by outer and inner incisions; armature consisting of 11 elements: 1 strong spine inserted on inner corner of former proximal segment; 4 spines and 6 setae along free distal margin of former second segment, two of which inserted on independent little knob. P1-P2 with 3-segmented exopods and endopods. P3-P4 with 3-segmented exopods and 2-segmented endopods. P1-P4 praecoxa present. P1 exp-1 long, about as long as exp-2 and -3 together. P1-P4 endopods distinctly shorter than exopods, not overreaching distal margin of exp-2. P2 enp-2 transformed: outer margin produced into a comb-like structure; P2 enp-3 with 1 apical strong curved spine and 1 apical seta. P5 with 2-segmented exopod; endopod incorporated to basis, forming a baseoendopod; suture line still observable on posterior surface; rudimentary intercoxal sclerite still discernible. P6 symmetrical, consisting of a well developed, deeply incised lamella, marking original division between left and right legs. Armature consisting of 1 outer seta and 2 inner short spines of different length.
Female unknown.
The description deals with major morphological details, omitted or overlooked in the original description, and with improvements of observational errors.
Integumental pitting not detectable on the dissected holotype; integument well sclerotized.
Urosomites 3–5 with smooth hyaline frills ventrally (Fig. 14A); third and fourth urosomites with spinular fringe closely located to hyaline frill; fifth urosomite with surface spinule rows ventrally, anal somite with distal continuous spinule row. Other ornamentation as in Fig. 14A. Urosomites 3–5 with crenulated hyaline frills dorsally. Indented surface rows are observable on third, fourth and fifth urosomites dorsally (Fig. 14B). Anal somite with row of fine spinules on proximal third; spinule row at the insertion of each caudal ramus; paired dorsal sensilla. Anal operculum slightly protruding the insertion line of caudal rami, subdistally crenulated (Fig. 14B).
Parbatocamptus jochenmartensi Dumont and Maas, 1988 (♂). A abdomen, ventral view B abdomen, dorsal view C antennule D antennule, detail of segments 5-7 (scale bars in μm).
Caudal rami ventrally subquadrate, parallel, longer than wide (length/width ratio: about 1.4), with incomplete setal pattern (6 setae) (Fig. 14A–B). Anterolateral accessory seta (I) absent, anterolateral seta (II) unipinnate, inserted at second third of caudal ramus; posterolateral seta (III) inserted in subdistal position, transformed in spiniform bifid seta. Outer terminal seta (IV) well developed, unipinnate, with articulation at base; inner terminal seta (V) bipinnate and long; terminal accessory seta (VI) thin and naked, about as long as posterolateral seta; dorsal seta (VII) inserted on a spinulose knob, close to free distal margin of caudal ramus, distinctly longer than caudal ramus. A spinule row on proximal third of each caudal ramus dorsally; three spinule rows inserted ventrolaterally and one spinule row at distal margin of caudal ramus, ventrally.
Antennule (Fig. 14C): 10-segmented. Segment 1 with spinule row and tube-pore. Segment 2 with tube-pore. Segment 4 represented by small U-shaped sclerite. Segment 5 large, sclerotized (Fig. 14D). Segment 8 very large and transformed, moderately sclerotized, segment 9 short, discrete, segment 10 derived by complete fusion of former segments 10 and 11. Armature formula: 1-[1], 2-[9], 3-[8], 4-[2], 5-[5?+(1 + ae)], 6-[2], 7-[2], 8-[0], 9-[1], 10-[10 + (1 + ae)].
Antenna (Fig. 15A): coxa unarmed; basis with 1 transverse spinule row on surface, a spinule row inserted on apical inner margin; exopod 1-segmented, well-defined at base, bearing 3 lateral and 2 apical unipinnate setae; free endopod 2-segmented; both segments robust, of about the same length; segment 1 naked; segment 2 with one inner spinule row, armature consisting of 2 inner spines and 1 seta, 1 apical unipinnate spine, 4 geniculate setae, 1 apical slender seta and 1 subapical tiny seta; a row of spinules at outer corner.
Parbatocamptus jochenmartensi Dumont and Maas, 1988 (♂). A antenna B mandible C maxilla D maxilliped E P1 (scale bars in μm).
Mandible (Fig. 15B): coxal gnathobase elongate, cutting edge with 2 large and coarse teeth, three smaller teeth and row of tiny teeth; naked seta at dorsal corner. Mandibular palp biramous, basis with inner long bipinnate seta and spinule row, exopod with 2 bipinnate setae; endopod with 2 apical geniculate setae, 1 inner and 1 subapical bipinnate setae.
Maxillule not observable.
Maxilla (Fig. 15C): syncoxa with 3 endites fully incorporated to syncoxa. Proximal endite quadrilobate, with 6 setae; the first two distal lobes each bearing 2 setae; the proximal ones, each with 1 plumose seta; medial and distal endites, each with 3 bipinnate setae. Allobasis drawn out into a strong claw, distally spinulose, accompanied by 1 robust and curved seta and 2 naked setae, respectively; endopod 3-segmented; segment 1 with 1 robust curved seta; segment 2 with 2 robust curved setae; segment 3 with 2 curved and 2 slender setae.
Maxilliped (Fig. 15D): phyllopodial, lamelliform, 1-segmented. Clear trace of ancestral 2-segmented condition marked by the presence of both outer and inner incisions. Armature consisting of 11 elements: 1 strong spine inserted at inner corner of former segment 1; 4 strong unipinnate spines along inner margin, 1 bipinnate seta inserted along inner margin and 5 bipinnate setae in apical position.
P1-P2 with 3-segmented exopods and endopods. P3-P4 with 3-segmented exopods and 2-segmented endopods. P1-P4 praecoxa well developed. P1 (Fig. 15E): praecoxa and coxa with outer spinule row on anterior surface; one posterior row of thin spinules inserted on coxo-basis boundary. Basis with 1 outer spiniform seta and 1 inner spine, with spinule rows along outer margin, between exopod and endopod and at the insertion of inner spine, respectively. Endopod distinctly shorter than exopod, reaching about distal third of exp-2; exp-1 long, about as long as exp-2 and -3 together; exp-1 and -2 with 1 outer spine; exp-3 with 2 outer curved, unipinnate spines, and 1 apical and 1 subapical geniculate setae. Endopod: enp-1 unarmed, about as long as enp-2 and enp-3, wider than enp-2 and enp-3. Enp-2 cylindrical, unarmed. Enp-3 with 1 inner long seta, and 2 apical geniculate setae of different length. Ornamentation as in Fig. 15E.
P2 (Fig. 16A): praecoxa unornamented, coxa as in P1 and P2. Basis with 1 outer spiniform seta, with spinule rows along outer margin. Exopod distinctly longer than endopod; endopod reaching about the proximal half of exopodal segment 2; exopodal segments of about the same length; exp-1 and -2 with 1 outer spine; exp-3 with 2 outer unipinnate spines, 1 apical fringed seta and 1 subapical long and slender seta. Endopod: enp-1 unarmed; enp 2 transformed, with outer strong comb-like process; enp-3 with 1 transformed spine and 1 long slender and naked seta in apical position. Ornamentation as in Fig. 16A.
Parbatocamptus jochenmartensi Dumont and Maas, 1988 (♂). A P2 B P3 C P4 D P5 E P6 (scale bars in μm).
P3 (Fig. 16B): praecoxa unornamented; ornamentation of coxa as in P1 and P2. Basis with long outer plumose seta and spinule rows along outer margin and at the insertion of the endopod. Exopod distinctly longer than endopod; endopod reaching about half of exp-2. Exp-1 and -2 with 1 outer spine; exp-3 with 2 outer fringed spines, 1 apical unipinnate seta and 1 subapical long and naked seta. Endopod: enp-1 unarmed; enp-2 with 2 apical spinulose setae and 1 subapical thin and naked seta. Ornamentation as in Fig. 16B.
P4 (Fig. 16C): slightly smaller than the other swimming legs, praecoxa present, unornamented; coxa with outer spinule row, one posterior row of thin spinules inserted on coxo-basis boundary; basis with outer plumose seta, and spinule rows along outer margin and at the insertion of endopod; exopod distinctly longer than endopod. Exp-1 and -2 with 1 outer bipinnate spine; exp-3 with 2 outer unipinnate spines and 2 apical bipinnate setae of different length. Endopod: enp-1 unarmed; enp-2 with 1 spine and 1 seta in apical position, and 1 subapical short unipinnate seta. Ornamentation as in Fig. 16C.
P5 (Fig. 16D): free, with clear articulation to P5-bearing somite; right and left legs distinct, trace of intercoxal sclerite present but hardly observable, with coxo-basis protrusions; exopod discrete, 2-segmented, segment 1 with 1 outer spine and 1 inner seta distally fringed; segment 2 with 4 elements: 1 outer slender and naked seta, 1 apical long spine, 1 medial short spine and 1 short seta distally crested; endopod incorporated to basis forming a baseoendopod, trace of original segmentation still recognizable on posterior surface (Fig. 16D); rudimentary endopod 1-segmented, bearing 1 strong spiniform element, crested on its distal margin. Basipodal outer seta slender and naked.
P6 (Fig. 16E): well developed, symmetrical, right and left legs distinct, deep medial incision marking boundary between legs; armature consisting of 1 outer long, bipinnate seta and two inner short spines, the innermost the shortest.
urn:lsid:zoobank.org:act:D06A6D6C-129B-4142-8C3C-B220A4E7CFAD
Phyllognathopodidae. Habitus slightly dorsoventrally flattened with no clear demarcation between prosome and urosome. Integumental dorsal window on cephalosome not confirmed. Integument without surface pits, moderately sclerotized. Cephalosome rounded; rostrum elongate, clearly articulated to cephalosome. Cephalosome and both thoracic and abdominal somites with cuticular ornamentation represented by dorsal sensilla. First pedigerous somite free. Hyaline frills of cephalosome, somites bearing P1-P4 plain both dorsally and ventrally. P5-bearing somite with large paired pores laterodorsally. Sexual dimorphism in antennule, P5, P6, urosomal segmentation and ornamentation, and morphology of anal operculum. Female first and second abdominal somites fused forming the genital double-somite. Female urosomal segments with plain hyaline frills ventrally. Female genital apparatus simplified; copulatory pore located at the end of the proximal third of the genital double-somite. Seminal receptacles laterally located and condensed close to the lamellar sixth legs. Male urosome with different arrangement of hyaline frill ornamentations: urosome consisting of 6 segments, second urosomite with indented hyaline frill, third and fourth urosomites with deep ventral sockets; socket on third urosomite plicate, with smooth free distal margin, and 2 setules laterally inserted close to the socket opening; socket on fourth urosomite with free distal margin ornamented by strong and long spinules, covering the opening; fifth urosomite with indented hyaline frill. Anal somite with paired sensilla on dorsal side. Anal operculum protruding free distal margin of anal somite and extruded in strong spinular processes. Sexual dimorphism in the number of spinular processes of anal operculum (3 in females vs. 4 in males; and, in general, anal operculum in male more armed than in female). Caudal rami sub-quadrate, with incomplete setal pattern (6 setae). Dorsal seta inserted on distal third of caudal ramus. Antennule: 8-segmented in female, basically 9-segmented in male; geniculation between segments 7 and 8; penultimate and last segments, each with suture line marking original segmentation between former segments 8 and 9, and 10 and 11, respectively. Long tube-pores on segments 1 and 2 in both sexes. Antenna: armature of the second endopodal segment as in Phyllognathopus and Parbatocamptus, consisting of 10 elements. Exopod 1-segmented, with 3 lateral and 2 apical setae. Mandible: mandibular palp biramous, basis with inner spinule row, exopod with 1 apical and 1 inner setae; endopod with 1 inner, 1 subapical and 2 apical setae. Armature of maxillule and maxilla as in Phyllognathopus. Maxilliped: phyllopodial, lamelliform, 1-segmented. Clear trace of ancestral 2-segmented condition marked by the presence of outer and inner incisions as in Parbatocamptus. Armature consisting of 11 elements: 1 strong spine inserted at inner corner of former segment 1; 4 spines and 1 spiniform short seta inserted along inner margin, 5 bipinnate setae in apical position, armature topology basically referable to that of Parbatocamptus.
P1-P3 with 3-segmented exopods and endopods. P4 with 2-segmented exopod and endopod. P1-P3 praecoxa well developed. P1 exopod and endopod of about the same length; P2-P3 endopods shorter than exopods, reaching about tip of exp-2. P4 small - sized, praecoxa missing. Female P5: free, with clear articulation to P5-bearing somite; right and left legs distinct; baseoendopod and exopod coalescent, deep incision marking original segmentation between them; endopodal lobe well developed, elongate, longer than exopodal lobe, rectangular in shape, bearing 1 long pinnate seta, subdistally inserted, close to outer margin and a spinule row apically inserted. Exopodal lobe wide, fully incorporated into baseoendopod; exopodal armature consisting of 4 elements, the outermost bipinnate seta inserted in subdistal position, and three apical elements: 2 spinulose and 1 short setae; basipodal outer seta present. Female P6 rudimentary, each leg defined by a small cuticular lateral plate bearing a short, naked seta with rounded tip. Male P5: free, with clear articulation to P5-bearing somite; right and left legs separate, intercoxal sclerite rudimentary, but still discernible. Basis of each leg expanded, endopod strongly trasformed, consisting of a sclerotized and strong protrusion articulated to basis. Endopodal seta bipinnate, inserted on posterior surface of the endopod, close to its articulation to basis. Exopod distinct, clearly articulated to basis, wide and short, rectangular in shape, representing most part of the free distal margin of each leg; exopodal armature consisting of 6 elements, the innermost spiniform seta curved inward. Male P6: right and left legs distinct but closely adjacent to each other along their medial margin, and symmetrical; each leg consisting of a well developed lamellar plate, with spinule row on the anterior surface; armature consisting of 2 inner spines of different length and 1 outer seta.
Phyllognathopus bassoti Rouch, 1972 = Neophyllognathopus bassoti (Rouch, 1972), comb. n.
The genus name is derived from the type genus Phyllognathopus and the Latinised Greek prefix νέοσ which means “new”, referring to the new position of Phyllognathopus bassoti in the systematics of the family Phyllognathopodidae.
http://species-id.net/wiki/Neophyllognathopus_bassoti
Figs 17–25Female neotype completely dissected and
mounted in polyvinyl lactophenol, deposited at the Natural History
Museum, London (reg. No. NHM.2008. neotype). Other material: 5 ♀♀ and 3
♂♂ mounted on slides, 5 ♀♀ and 5 ♂♂ processed for SEM; India, 7
January 1999, Y. Ranga Reddy coll.; 1 ♀, slide code 66/49, 1 ♀,
slide code 66/53, 1 ♂, slide code 66/55, Santa Fe, Bantayan island,
Pooc, Philippines, V. Cottarelli coll. (see
Neophyllognathopus bassoti is proposed herein as new combination for Phyllognathopus bassoti assigned by
The specimens on which
India, Andhra Pradesh, town of Guntur,
Brindavan Gardens, domestic water reservoir filled by a freshwater bore
well; coordinates: approx. 16°18'N, 80°29'E (see
FEMALE NEOTYPE. Body length, measured from tip of rostrum to posterior margin of caudal rami, 348 µm. Habitus slightly dorsoventrally flattened (Fig. 17A), with no clear demarcation between prosome and urosome. Body depigmented and eyeless. Integumental dorsal window on cephalosome not confirmed. First pedigerous somite free. Integument without surface pits, moderately sclerotized. Cephalosome rounded; rostrum elongate, clearly articulated to cephalosome. Hyaline frills of cephalosome, somites bearing P1-P4 and urosome plain both dorsally and ventrally (Fig. 17 A, B). Cephalosome and both thoracic and abdominal somites (except fourth urosomite) with cuticular ornamentation represented by dorsal sensilla. P5-bearing somite with lateral paired and large pores (Fig. 18A). Female genital field located between first and second third of genital double-somite. Genital apparatus simplified; copulatory pore located at half of genital double-somite. Seminal receptacles laterally located and condensed close to the lamellar sixth legs. Three spinular processes on free distal margin of anal operculum (Figs 17A–C). Caudal rami sub-quadrate, with incomplete setal pattern (6 setae). Dorsal seta inserted close to free distal margin of caudal ramus (Fig. 17C).
Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♀). A habitus, dorsal view B abdomen, ventral view C anal somite and operculum D antennule E antenna (scale bars in μm).
SEM micrographs of Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂). A lateral large pore (arrowed) on P5-bearing somite B anal somite and operculum.
Antennule (Fig. 17D): consisting of 8 segments, segments 1 and 2 with long tube-pores. Armature formula: 1-[1], 2-[8], 3-[5], 4-[1 + (1 + ae)], 5-[1], 6-[3], 7-[4], 8-[6 + (1 + ae)]. Aesthetasc on segment 4 very large and long, well overreaching the last antennulary segment.
Antenna (Fig. 17E): exopod and armature of the second endopodal segment as in Phyllognathopus and Parbatocamptus. Exopod 1-segmented, with 3 lateral and 2 apical setae.
Mandible (Fig. 19A): mandibular palp biramous, basis with inner spinule row, exopod with 1 apical and 1 inner setae; endopod with 1 inner, 1 subapical and 2 apical setae. Armature of maxillule (Fig. 19B) and maxilla (Fig. 19C) as in Phyllognathopus.
Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♀) A mandible B maxillule C maxilla D maxilliped E P4 F P5 G P5 (anomaly) H P6 (scale bars in μm).
Maxilliped (Fig. 19D): phyllopodial, lamelliform, 1-segmented. Clear trace of ancestral 2-segmented condition marked by the presence of both outer and inner incisions. Armature consisting of 11 elements: 1 strong spine inserted at inner corner of former segment 1; 4 spines and 1 spiniform short seta inserted along inner margin, 5 bipinnate setae in apical position, armature topology basically referable to that of Parbatocamptus.
P1-P3 with 3-segmented exopods and endopods.
P4 with 2-segmented exopod and endopod. P1-P3 praecoxa well developed.
P4 praecoxa absent (Fig. 19E).
P1 exopod of about the same length of endopod. P2-P3 exopods longer
than endopods, endopod not overreaching exp-2, fitting the original
description (
P5 (Fig. 19F, G): free, with clear articulation to P5-bearing somite; right and left legs separate; baseoendopod and exopod coalescent, deep incision marking original segmentation between them; endopodal lobe well developed, elongate, rectangular in shape, longer than exopod, bearing 1 long pinnate seta, subdistally inserted, close to outer margin, and 2 spinule rows, the proximal one composed by tiny elements, the distal one of long spinules; exopodal lobe well discernible, with armature consisting of 4 (rarely 5 elements, observed in only one female) elements, the outermost seta inserted in subdistal outer position, the remaining ones in apical position; the outer apical seta slender and bipinnate, the remaining two spiniform. Basipodal outer seta present.
P6 (Figs 19H, 20): rudimentary, consisting of small paired chitinous lamellar plates not coalescent along medial margin, partially covering seminal receptacles. Armature consisting of 1 short smooth spine with rounded tip on each side.
SEM micrograph of Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♀): P6.
No marked sexual dimorphism in body size. Body length, measured from tip of rostrum to posterior margin of caudal rami, 335 µm. Rostrum and ornamentation of cephalosome as in female (Fig. 21A). Male urosome consisting of 6 segments (Fig. 21A), third and fourth urosomites with deep ventral sockets (Figs 21B, 22A); socket on third urosomite plicate, with smooth free distal margins, and 2 setules laterally inserted close to the socket opening (Fig. 22B); socket on fourth urosomite with ornamented anterior margin, armed by strong spinules covering the opening (Fig. 22C). Anal somite with paired sensilla on dorsal side. Anal operculum protruding free distal margin of anal somite and extruded in 4 strong spinular processes, rarely 5 (in general anal operculum in males more armed than in females) (Fig. 18B). Antennule (Fig. 21C): basically 9-segmented, geniculation between segments 7 and 8; penultimate and last segments, each with suture line marking original segmentation between former segments 8 and 9, and 10 and 11, respectively. Long tube-pores on segments 1 and 2. Armature formula: 1-[1], 2-[8], 3-[8], 4-[1], 5-[7+(1 + ae)], 6-[2], 7-[1], 8-[1], 9-[10 + (1 + ae)].
Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂). A habitus, dorsal view B abdomen, ventral view C antennule D P5 E P6 (scale bars in μm).
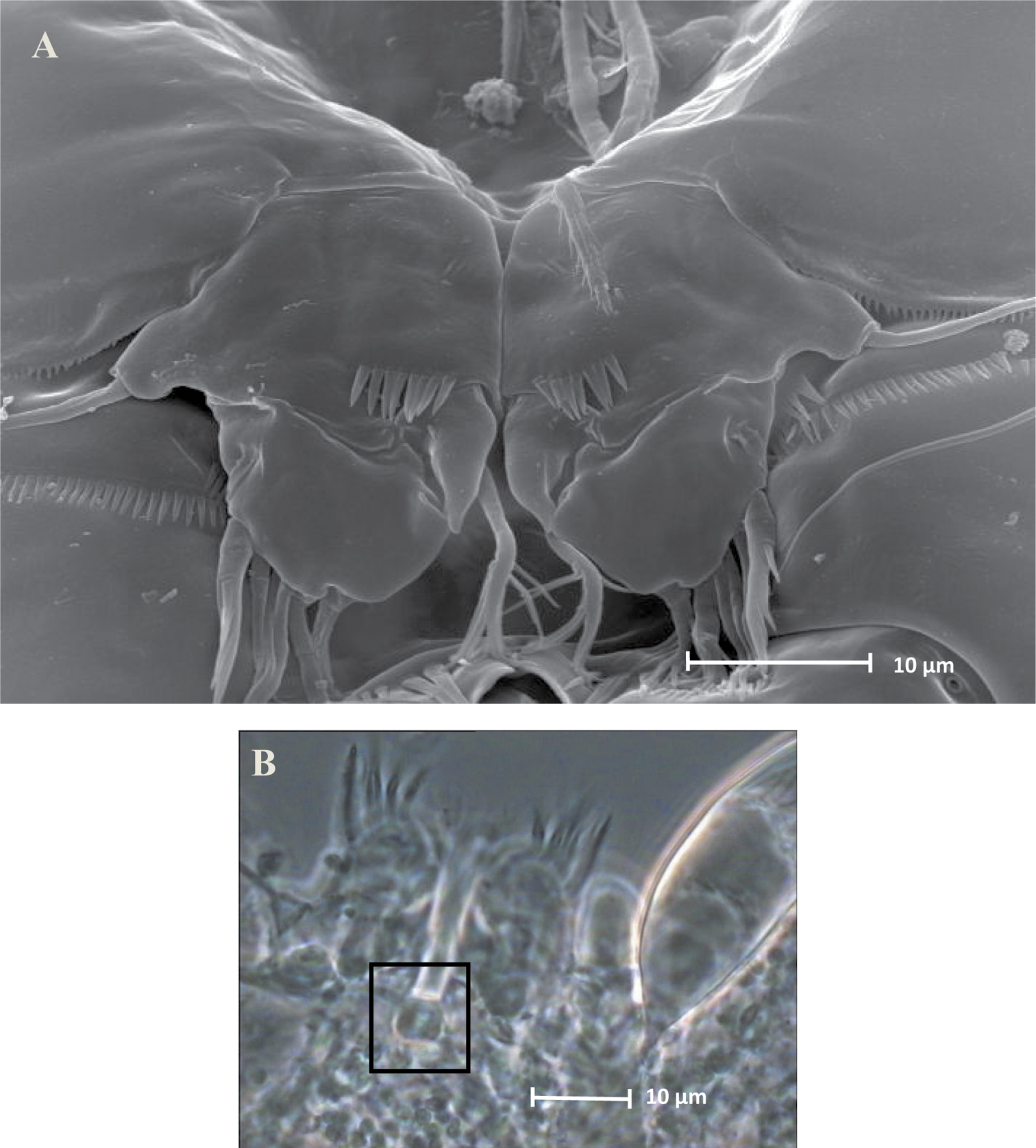
SEM micrographs of Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂). A general view of the urosomal sockets B plicate socket on third urosomite (paired lateral setules arrowed) C socket on fourth urosomite covered by strong spinules.
P5 (Figs 21D, 23A): free, with clear articulation to P5-bearing somite; right and left legs separate, intercoxal sclerite rudimentary but still discernible (Figs 21D, 23B). Basis of each leg well developed, representing most part of each leg; endopod rudimentary, consisting of a sclerotized and strong process articulated to basis. Row of surface spinules inserted near articulation between endopod and basis. Endopodal seta bipinnate, inserted on posterior surface of the endopod, close to its articulation to basis. Exopod distinct, clear articulated to the basis, wide and short, with unusual topology, being placed at the inner free distal margin of basis; exopodal armature consisting of 6 elements, all of which in apical position. Inner spinulose seta short and distinctly curved inward, the remaining setae of about the same length, 2 of which (the second and the fourth, beginning from the inner margin of the exopod) are respectively bipinnate and unipinnate; the remaining 3 smooth and slender, frequently closely adherent to each other and not easily discernible as distinct (Fig. 24). Male P6 (Figs 21E, 25A–B): right and left legs distinct, closely adherent along inner margin, and symmetrical, each leg consisting of a well developed lamellar plate, with some spinule rows on the anterior surface. A membranous lamella is observable between right and left P6 (rudimentary intercoxa?) (Fig. 25B); armature consisting of 2 inner spines of different length and 1 outer naked seta.
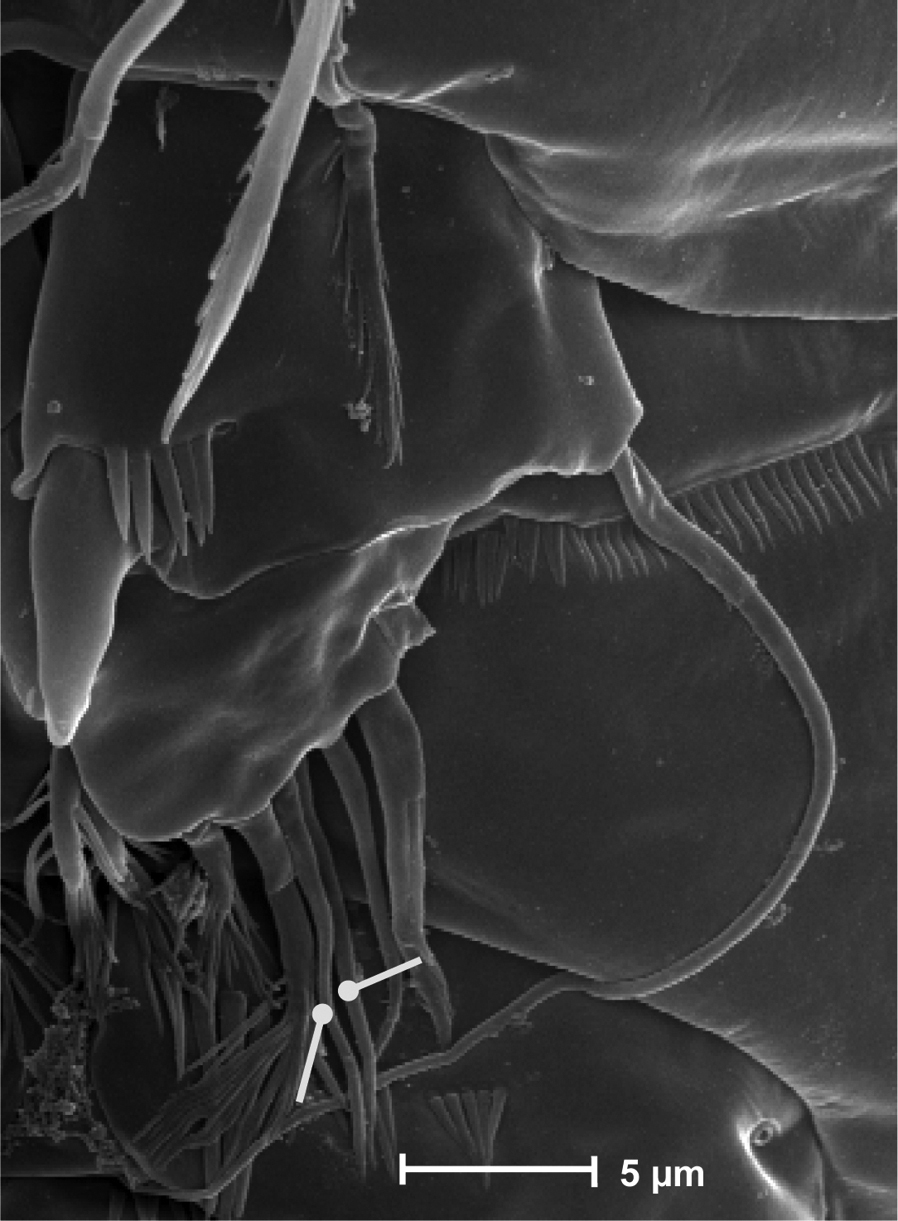
Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂). A SEM micrograph of P5 B contrast phase micrograph of P5 rudimentary intercoxa (framed).
SEM micrograph of Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂) P5, detail (white lines showing setae III and IV, which are closely adherent to each other and hardly discernible as distinct under contrast phase microscope).
SEM micrographs of Neophyllognathopus bassoti (Rouch, 1972), comb. n. (♂). A P5 and P6 B detail of P6 showing an interconnecting lamella (rudimentary intercoxa?) between right and left legs arrowed.
According to
Recently, cross-breeding experiments carried out by
A taxonomic dilemma was raised with the description of Phyllognathopus bassoti. The most striking morphological features of Phyllognathopus bassoti, as reported in the original description given by
It is therefore not unlikely that differences observed in
the relative development and armature of the P5 endopodal lobe are
related to different perception of morphological details, because of
intrinsic difficulties in observing the mutual position of the inner
protrusion (which is a modified endopod) and the relative seta, whose
surface insertion is hardly discernible under optical microscopy. Both
populations of Phyllognathopus bassoti recently described from India (
Review of morphological characters in the family Phyllognathopodidae.Comparisons were based almost exclusively on material examined directly and only sporadically on existing descriptions. This course of action was necessary because in most descriptions many morphological details are missing and the drawings are usually so deficient that any comparisons made may be potentially misleading.
Genera, species and subspecies presently recognized in the family Phyllognathopodidae.
| Genus Phyllognathopus Mrázek, 1893 | |
| Phyllognathopus viguieri (Maupas, 1892) | |
| Phyllognathopus viguieri menzeli (Chappuis, 1928) | |
| Phyllognathopus paludosus Mrázek. 1893 | |
| Phyllognathopus insularis Chappuis, 1940 | |
| Phyllognathopus chappuisi Delachaux, 1924 | |
| Phyllognathopus camptoides Božic, 1965 | |
| Phyllognathopus paracamptoides Božic, 1968 | |
| Phyllognathopus volcanicus Barclay, 1969 | |
| Phyllognathopus inexspectatus sp. n. | |
| Species and subspecies inquirendae | |
| Phyllognathopus coecus brevisetosus (Daday, 1901) | |
| Phyllognathopus fodinatus (Ziegelmayer, 1923) | |
| Phyllognathopus viguieri parvulus (Jakubisiak, 1929) | |
|
Phyllognathopus sp. (sensu |
|
|
Phyllognathopus sp. (sensu |
|
|
Phyllognathopus cf. camptoides (sensu |
|
| Genus Allophyllognathopus Kiefer, 1967 | |
| Allophyllognathopus brasiliensis Kiefer, 1967 (type-species by monotypy) | |
| Genus Parbatocamptus Dumont and Maas, 1988 | |
| Parbatocamptus jochenmartensi Dumont and Maas, 1988 (type-species by monotypy) | |
| Neophyllognathopus gen. n. | |
| Neophyllognathopus bassoti (Rouch, 1972), comb. n. (type-species by monotypy) | |
Male antennules. Male antennules are primarily
11-segmented, but, at present knowledge, this segmental pattern is
not showed by any member of the family, and the derived condition of 10
segments is the most widespread in the family. An 8-segmented male
antennule has been reported in Phyllognathopus viguieri (cf.
Our re-examination of the segmental patterns of the male antennule revealed that Phyllognathopus viguieri possesses a basically 10-segmented antennule, where the penultimate and last segments are still distinct only on posterior surface (giving an incipient 11-segmented antennule) and fused on frontal surface. Parbatocamptus jochenmartensi shows a 10-segmented antennule, whereas Neophyllognathopus bassoti comb. n. displays a 9-segmented antennule, because both segments 8 and 9, and 10 and 11 respectively, are not distinct, probable reflection of heterochrony.
Novel structures were discovered on the antennules in
both sexes; in particular, a truncated tubular extension, probably
ending in a distal opening, is discernible on the first and second
antennulary segments in both males and females. In many harpacticoid
families, these segments commonly possess (tube-) pores, as in, for
example, the Neobradyidae (
Antenna. The antenna of Phyllognathopus viguieri has been reported as 4-segmented, vs. 3 segmented in Phyllognathopus paludosus,
but this different segmentation pattern stems from failure to identify
the segment boundary between the basis and the proximal endopod
segment, and consequently the correct position of the exopod. In the
original description (
Mandible. The basic structure of the mandible and the setation of the mandibular palp in the type-genus Phyllognathopus appear to be identical in all observed populations, the only exception being Neophyllognathopus bassoti comb. n., which, according to
Re-examination of the male holotype of Parbatocamptus jochenmartensi
revealed the presence of a well developed pinnate seta on the basis of
the mandibular palp (not figured nor described in the original
description), accompanied also by the typical spinule row. This
observation suggests that the basis is armed in primitive
phyllognathopodids. Interestingly, Parbatocamptus jochenmartensi also shows two transformed, prehensile setae on the endopod of the mandibular palp, a character already reported by
Maxillule and maxilla.The structure of the maxilla and maxillule is almost identical in all specimens observed, although comparison of the setation patterns with previously described species is hampered by inconsistencies and deficiencies contained in most descriptions. As regards to the maxilla, number of setae and their topology are identical in all the species examined. In Parbatocamptus jochenmartensi the proximal endite is incorporated into the syncoxa and is composed by four lobes vs. the same endite is articulated to the syncoxa and the lobes are only hardly discernible in other members of the family. The maxillule is more conservative in both morphology and setation. The only exception is represented by the maxillule of Phyllognathopus viguieri, where the praecoxal arthrite bears 10 elements, whereas in all the other phyllognathopodids it bears 9 elements, being the proximal surface seta (inserted on a small knob) missing.
Maxilliped. The phyllopodial maxilliped is bilobed, with the basal part (syncoxa) fully incorporated in the compound distal part (basis and endopod fused). With regard to armature, a direct analysis of material among all the populations analysed, revealed two different setation patterns, accompanied by a different degree of fusion between the former syncoxa and the baseoendopod. In all the examined populations of Phyllognathopus the maxilliped bears 10 elements, and only a rudimentary incision between syncoxa and baseoendopod is still discernible. On the contrary, the primitive distinction between syncoxa and baseoendopod is still retained in Parbatocamptus jochenmartensi and Neophyllognathopus bassoti comb. n.with both inner and outer incisions marking original segmentation; moreover, an additional element (a robust stout spine) is inserted along inner side of the boundary syncoxa-baseoendopod, suggesting this condition as primitive within the family.
Integumental windows patterns.The dorsal integumental window (“nuchal organ”) on the cephalosome has been reported only for Neophyllognathopus bassoti comb. n. by
Swimming legs P1-P4.Female P1-P3 are relatively identical
in both morphology and armature in virtually all species and
populations of Phyllognathopus.
The only remarkable difference is observable in members of the family
characterized by a 3-segmented P4 exopod. In particular, in Phyllognathopus viguieri and related species, the P4 exp-2 lacks the outer spine in all the examined populations, whereas it is present in Parbatocamptus jochenmartensi and absent in Allophyllognathopus brasiliensis. Another exception refers to a population of Phyllognathopus viguieri collected from Lake Léman (Switzerland) by (
The major differences between members of the family are
found in the segmentation of the P4 exopod which can show three
different patterns: 1) 3-segmented; 2) distinctly 2-segmented; or 3)
1-segmented with (Phyllognathopus paracamptoides) or without (Phyllognathopus sp. sensu
Some variation is also expressed in the segmentation of
the P4 endopod (in general, it is 2-segmented, but it was described
and figured as 1-segmented in Phyllognathopus sp. by
By analysing different Phyllognathopus populations and species, as well as Neophyllognathopus bassoti
comb. n., the P4 praecoxa is always absent in both males and females,
a character never described or reported for the family, whereas all
the remaining swimming legs possess it. The absence of praecoxa is
always accompanied by a noticeable reduction in the size of P4,
irrespective of the segmentation pattern of this leg. Although the
absence of the P4 praecoxa may be a potential synapomorphy of the Phyllognathopus-Neophyllognathopus lineage, its presence/absence in Allophyllognathopus requires confirmation before any phylogenetic inference can be drawn. Parbatocamptus still possesses a P4 praecoxa. Interestingly the P4 is large, and the exp-2 still bears 1 outer spine, suggesting that Parbatocamptus jochenmartensi possesses the most primitive P4 in the family. Members of the genus Phyllognathopus
do not display sexual dimorphism in the segmentation of swimming legs.
However, some authors have documented transformations of particular
setae, especially on the male P2 endopod such as in Phyllognathopus viguieri by Gurney (1932: fig. 362) and in Phyllognathopus viguieri menzeli by
Fifth legs. The fifth pair of legs is sexually dimorphic in both structure and armature, and sometimes differs among species in the morphology of the exopod, in the degree of fusion between exopod and baseoendopod, in the number of exopodal and baseoendopodal setae, or alternatively, in their relative length. The female of Phyllognathopus viguieri shows an exopodal lobe with 4 apical elements, and a baseoendopod with 2 pinnate setae. The exopod is incorporated into the baseoendopod but the right and left legs are distinctly separate. Phyllognathopus inexspectatus sp. n. possesses a female P5 quite similar to that of Phyllognathopus viguieri, differing in the length of the exopodal setae, all shorter than in Phyllognathopus viguieri and, more importantly, in the topology of the outermost exopodal seta which is inserted in a clear outer subapical position. Only a few Phyllognathopus species show differences in the number of armature elements (e.g. Phyllognathopus camptoides). Neophyllognathopus bassoti comb. n.differs from any phyllognathopodid species in the unique morphology of female P5. The female P5 baseoendopod and exopod are coalescent, deep incision marking original segmentation between them; the endopodal lobe is well developed, elongate, rectangular in shape, longer than exopod, bearing 1 long pinnate seta, subdistally inserted, close to outer margin; exopodal lobe well discernible; exopodal armature consisting of 4 elements, the outermost seta inserted in subdistal outer position, the remaining ones in apical position.
The male P5 exhibits much more variation within the family, especially in the structure and armature of the baseoendopod. The most primitive condition is observed in Phyllognathopus viguieri, which exhibits a discrete 1-segmented endopod, on which the endopodal seta is terminally inserted. In some Phyllognathopus species, as well as in Allophyllognathopus brasiliensis and Neophyllognathopus bassoti comb. n., the endopod is secondarily transformed in a strong spinular process, sometimes described as incorporated into the basis, but more frequently articulating with it. In Parbatocamptus jochenmartensi the endopod is partially fused to basis forming a baseoendopod, although the suture line marking original segmentation is still discernible. In most phyllognathopodid species the endopod bears 1 element, but it was also reported without ornamentation. Despite its variation, no significance has been attributed to this character, in so far that populations displaying different setation patterns have been assigned to the same species.
The male P5 exopod shows different construction and
armature among members of the family. The most primitive condition is
showed by Parbatocamptus jochenmartensi, which retains a 2-segmented exopod; the exopod is distinctly 1-segmented in Neophyllognathopus bassoti comb. n., and in Allophyllognathopus brasiliensis, and appears as incorporated to the basis in Phyllognathopus (see also
Direct examination of the Philippine material assigned to Phyllognathopus bassoti by
Sixth legs. The sixth pair of legs has only sporadically been described and solely in males. It is bilaterally symmetrical and bears 3 elements on either side: a long outer seta, presumably representing the original outer basal seta, and 2 spinulose inner setae. The male sixth pair of legs shows three different morphologies: 1) in most species it appears as a hyaline linear and continuous lamella, lacking any trace of the primitive paired state showing distinct right and left legs (as in Phyllognathopus viguieri); 2) in a second species-group, the lamella appears medially incised as in some taxa currently under study, and in Parbatocamptus jochenmartensi, and 3) in Neophyllognathopus bassoti comb. n. the sixth legs are deeply incised forming a more complex structure, where also an interconnecting lamella has clearly been observed (rudimentary intercoxa?).
The P6 has never been described nor observed in females, frequently reported as absent (cf.
Ornamentation of urosome.The ornamentation of the urosome deserves special attention. By comparing several populations of Phyllognathopus viguieri sensu lato, several other Phyllognathopus species, Neophyllognathopus bassoti comb. n., and Parbatocamptus jochenmartensi,
clearly distinct ornamentation patterns could be distinguished, as
well as previously unnoticed enigmatic structures located on both dorsal
and ventral sides of the male urosome. The more complex structures were
observed in Neophyllognathopus bassoti using SEM (Fig. 22A–C) A similar ornamentation is presumably present in Phyllognathopus camptoides, as figured by
Both male and female urosome show large dorso-lateral
pores located on the P5-bearing somite. They are referable to the pores
observed in several Ameiridae (
Caudal rami.Caudal rami are frequently sexually dimorphic and generally considered polymorphic in the females of Phyllognathopus viguieri. Variation in caudal rami morphology and setation pattern has been reported for several harpacticoids (
The re-examination of type-material and/or topotypes of different phyllognathopodid species revealed a systematic scenario more complicate than supposed. The type species of the genus Phyllognathopus, Phyllognathopus viguieri, was redescribed in detail, analysing several populations coming from different localities world-wide. Some morphological characters within the genus Phyllognathopus revealed taxonomic significance, giving ground for the description of Phyllognathopus inexspectatus sp. n. from ground water in Italy. The discovery of new informative phylogenetic characters led also to the proposal of a new genus for Phyllognathopus bassoti, namely Neophyllognathopus bassoti comb. n.
Re-examination of different populations and species of the genus Phyllognathopus
led to the conclusion that the mouthparts show virtually no variation
in structure and setation, and the few differences observed are usually
autapomorphies of individual species (with the exception of the
structure and setation of the maxilliped and the absence of a seta on
mandibular basis which is a synapomorphy of a wider group of
species/genera). The most robust discriminating features between
species-groups are the segmentation of the P4 exopod, the general
morphology of legs 5 and 6 in both sexes, and the morphology and
ornamentation of male urosome. The different segmental patterns of P4
exopods observed among species of Phyllognathopus allow the identification of three morphological groups: 1) species with 3-segmented exopod (here defined Phyllognathopus viguieri-group, including Phyllognathopus viguieri, Phyllognathopus viguieri menzeli, Phyllognathopus viguieri parvulus, Phyllognathopus viguieri brevisetosus, Phyllognathopus paludosus, Phyllognathopus volcanicus); 2) species with 2-segmented exopod (here defined Phyllognathopus chappuisi-group, including Phyllognathopus chappuisi, Phyllognathopus insularis, Phyllognathopus camptoides, Phyllognathopus cf. camptoides sensu
Exopodal segmentation patterns of swimming legs are generally more conservative than those of endopods, and the explanatory power of derived states may be potentially higher in resolving phylogenetic issues. Within the Harpacticoida, members of the same genus very rarely exhibit different segmental patterns in the exopods of P2-P4. For example, in the canthocamptid genus Hypocamptus Chappuis, 1929, Hypocamptus brehmi (Van Douwe, 1922) shows P3 and P4 with 3-segmented exopods, whereas Hypocamptus paradoxus (Kreis, 1926) has 2-segmented P3 and P4. Similarly, the laophontid genus Laophontina Norman & T. Scott, 1905 contains species with 2-segmented (Laophontina acantha Noodt, 1955; Laophontina noodti Kunz, 1983) and 3-segmented (Laophontina dubia Norman & T. Scott, 1905; Laophontina posidoniae Fiers, 1986) P4 exopods. In another laophontid genus, Robustunguis Fiers, 1992, the type species Robustunguis ungulatus Fiers, 1992 possesses 3-segmented P2-P4 exopods but in its congener Robustunguis minor Fiers, 1992 these rami are only 2-segmented. Unfortunately, our analysis failed to reveal any congruence between exopodal segmentation patterns and other derived character states of phylogenetic significance, and consequently we were unable to delimit any “natural groups” within the genus Phyllognathopus. The Phyllognathopus chappuisi-“lineage” almost certainly evolved within the Phyllognathopus viguieri-group, although its phylogenetic position remains unresolved. Species belonging to this “lineage” all have the 2-segmented P4 exopod, which may have evolved independently and several times in the evolutionary history of the genus Phyllognathopus. However, since these species do not share any other derived character states, the monophyly of the chappuisi-“lineage” remains questionable as it may include the species of the paracamptoides-group and therefore be paraphyletic. On the other hand, if Neophyllognathopus bassoti comb. n. may theoretically enter into the “chappuisi-lineage” for the possession of a 2-segmented P4 exopod, this species is defined by a combination of unique apomorphies, most of which discovered in the present study: 1) P4 with 2-segmented exopods; 2) unique morphology of P5 in both sexes; 3) peculiar arrangement of the ornamentation of the hyaline frills on the male urosome ventrally; 6) anal operculum bearing 3–4 long and stout spinules, not articulated to the operculum. It also shows several plesiomorphic character states, such as the presence of an intercoxal sclerite between male fifth legs, the bilobed, separate male sixth legs (with rudimentaruy intercoxa?), the phyllopodial maxilliped with clear trace of articulation between syncoxa and basis-endopod, and an additional spiniform seta on the same maxilliped. Within the Phyllognathopodidae, the new genus Neophyllognathopus shows feeble relationships with the genus Parbatocamptus, which plausibly is the most primitive genus within the family, showing the most plesiomorphic state of male P5, with 2-segmented exopod, trace of endopod together with the presence of a rudimentary intercoxal sclerite; a deeply incised and well sclerotized male P6, the basis of the mandibular palp bearing one long bipinnate seta, the phyllopodial maxilliped with 11 elements and clear trace of the primitive articulation between syncoxa and basis-endopod, and the presence of P4 praecoxa and the outer spine on P4 exp-2, always absent in all members of the family showing a 3-segmented P4 exopod. Neophyllognathopus gen. n. shares with Parbatocamptus the identical construction and armature of the maxilliped, and the presence of a rudimentary intercoxa in male P5. Such similarities are however symplesiomorphic and more detailed information about Parbatocamptus is required (for instance, its female is unknown) before such a relationship can be corroborated. Pending the arrival of new data (e.g. molecular analysis, Glatzel, in litt.), it seems justifiable to maintain the Phyllognathopus chappuisi-group and the Phyllognathopus paracamptoides-group in the genus Phyllognathopus, considering the differences in P4 exopodal segmentation as intrageneric variation, and to assign generic rank to Phyllognathopus bassoti by creating the new genus Neophyllognathopus.
Among members of the chappuisi-group, all defined by 2-segmented exopods and endopods, Phyllognathopus inexspectatus
sp. n. is easily distinguishable by a P4 enp-2 with only 2 apical
elements (vs. 3 in all members of this group), a spinulose free distal
margin of anal operculum (vs. ciliate in Phyllognathopus insularis and armed with strong spinular processes in Phyllognathopus camptoides), caudal rami with posterolateral seta III transformed and subapical (vs. apical and not transformed in Phyllognathopus insularis). Phyllognathopus camptoides, as originally described by
Phyllognathopus inexspectatus sp. n. is defined by the combination of the following morphological characters: maxillule with syncoxal proximal surface seta absent (vs. present in Phyllognathopus viguieri); P2 enp-2 without inner seta (vs. present in Phyllognathopus viguieri); 2-segmented P4 exopod (vs. 3-segmented in Phyllognathopus viguieri); 2 apical elements on P4 enp-2 (vs. 3 elements in Phyllognathopus viguieri); female P5 exopod with 3 apical and one distinctly subapical outer seta (vs. 4 apical setae in Phyllognathopus viguieri); female P6 with long bipinnate and slender seta (vs. short, naked and with rounded tip in Phyllognathopus viguieri); anal operculum with spinules (vs. smooth in Phyllognathopus viguieri); inner terminal seta normally shaped (vs. short and with proximal part enlarged in Phyllognathopus viguieri).
Ecology and biogeography. Phyllognathopodidae
occur in both temperate and tropical areas, and at different
altitudes, with high preference for phytotelmata, leaf litter, moist
soils, pitcher plants, man-made and altered habitats. More rarely they
occur in mosses (
At present, it is difficult to speculate about the plesiotypic habitat of the family, but it is not unlikely that epigean, semi-terrestrial habitats represent the ancestral and still preferred environment for the family in temperate and, especially, in tropical areas. Circumstantial evidence supporting this hypothesis is provided by the high likelihood of discovering phyllognathopodids in these habitats world-wide, where they also appear to have their highest abundance and species diversity. How they can survive dehydration during low-water periods is unknown. Resting stages have never been found, and dormancy has not been documented until now.
From a biogeographical point of view, the cosmopolitanism of Phyllognathopus viguieri has unjustly been overemphasized as discussed also by
The new genus Neophyllognathopus shows a disjunct geographical distribution in the Oriental and Australasian regions and it is thus far predominantly restricted to ground water sensu lato (subsurface freshwater habitats: this is the case of Neophyllognathopus bassoti from Long Island (Papua, New Guinea) and from Philippines and India). Some doubts there are also for Allophyllognathopus brasiliensis, collected from the “caatingas” located in arid areas in Brazil. These areas are characterized by xeric vegetation and their hydrological regimes are regulated by intermittent rivers, which exist only during the rainy season.
The geographical distribution of the four defined groups
is not of great assistance in corroborating or refuting phylogenetic
affinity between or within groups. Members of the same group do not show
a common track of distribution. The Phyllognathopus viguieri-group
is cosmopolitan, and, at present, any speculation about the centre
of origin of the group is premature. Members of the chappuisi-group
are distributed in both Northern and Southern Hemispheres,
predominantly in tropical areas with the exception of the geographically
disjunct location of Phyllognathopus inexspectatus, which is recorded from temperate Europe. Two alternative hypotheses may be proposed: 1) the chappuisi-group was widespread in the past, and descended directly from a Phyllognathopus viguieri-like
ancestral stock, but disappeared from the plesiotypic surface habitats
in the Northern Hemisphere as a consequence of the drastic climatic
changes linked to the Quaternary glaciations, and survived as relict
species in refuge environments (e.g. ground water); 2) members of the chappuisi-group may have originated independently in different geographical areas from different surface ancestors closely related to the Phyllognathopus viguieri-group. In the first scenario, a common origin is hypothesized for the chappuisi-group, which could be considered monophyletic within the Phyllognathopus viguieri-group; in the second one, the “lineage” should be considered polyphyletic. The new genus Neophyllognathopus established for the Phyllognathopus bassoti-lineage
seems to be the only one for which a monophyletic origin may be
reasonably inferred. Its distribution is thus far limited to tropical
India, the Philippines and New Guinea, in both the Oriental and
Australasian Regions, a very problematic area from a biogeographical
point of view (
Males
| 1 | P5 with 1-segmented exopod and endopod, the latter cylindrical or transformed in a spiniform element | 3 |
| 2 | P5 with 2-segmented exopod; endopod incorporated to basis forming a baseoendopod; endopod boundary marked by rudimentary suture; rudimentary intercoxa present; P6 represented by a deeply incised hyaline lamella | Parbatocamptus |
| 3 | P3 endopod not transformed and with setae and/or spines normally conformed | Phyllognathopus |
| 4 | P3 endopod transformed, with aesthetasc-like elements on enp-3 | Allophyllognathopus |
| 5 | P5 with intercoxa, exopod discrete, very large and translated at the inner margin of the basis; right and left P6 distinctly separated; third and fourth urosomites with deep ventral sockets | Neophyllognathopus gen. n. |
Females*
| 1 | P5 baseoendopod and exopod coalescent; incision marking original articulation between basis and endopod little pronounced; baseoendopod bearing two elements | Phyllognathopus |
| 2 | P5 baseoendopod and exopod coalescent; deep incision marking original articulation between basis and endopod; endopod bearing one element | Neophyllognathopus gen. n. |
*female unknown for Parbatocamptus and Allophyllognathopus.
This study was supported by the PASCALIS project granted by the European Community (Contract n° EVK2-CT-2001-00121) and by PRIN (Italy) “Phylogenetic and biogeographical assessment of endemic patterns of distribution in the Apennine Province (Italy): new tools for biodiversity assessment and conservation strategies”. We are grateful to Janet W. Reid (Virginia Museum of Natural History, U.S.A.), Sophie Conroy-Dalton (NHM, London, U.K.) and Thomas Glatzel (University of Oldenburg, Germany) for suggestions, criticism, and bibliographical support. Rony Huys (NHM, London) critically revised the first draft of the manuscript, and supported one of us (D.G.) in suggesting the most appropriate way to solve several systematics problems. We are also much indebted to the Senckenberg Museum (Germany), the Natural History Museum (London), the Smithsonian Institution (Washington, D.C.) and the Museum National d’Histoire Naturelle (Paris) for the loan of type material and for having put at our disposal almost all the collections of Phyllognathopodidae species. A special thank is also due to Y. Ranga Reddy (Nararjuna University, India) for having provided us with consistent material of Phyllognathopus bassoti and of an undescribed species of Phyllognathopus from India, to Vezio Cottarelli (University of Viterbo, Italy) who put at our disposal Philippine material of Phyllognathopus bassoti and several specimens of Phyllognathopus viguieri, to Raffaella Berera for the SEM preparation of specimens, and to Thomas Glatzel for having provided Phyllognathopus specimens from Germany. Two anonymous reviewers made useful comments and improvements of the manuscript.