(C) 2012 Camila Timm Wood. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The goal of this study was to compare the feeding rates of Balloniscus sellowii on leaves of different decomposition stages according to their phenolic and flavonoid content. Leaves from the visually most abundant plants were offered to isopods collected from the same source site. Schinus terebinthifolius, the plant species consumed at the highest rate, was used to verify feeding rates at different decomposition stages. Green leaves were left to decompose for one, two, or three months, and then were offered to isopods. The total phenolic and flavonoid contents were determined for all decomposition stages. Consumption and egestion rates increased throughout decomposition, were highest for two-month-old leaves, and decreased again in the third month. The assimilation rate was highest for green leaves. The mode time of passage through the gut was two hours for all treatments. Ingestion of leaves occurred after two or three days for green leaves, and on the same day for one-, two- and three-month-old leaves. The speed of passage of leaves with different decomposition stages through the gut does not differ significantly when animals are fed continuously. However, it is possible that the amount retained in the gut during starvation differs depending on food quality. The digestibility value was corrected using a second food source to empty the gut of previously ingested food, so that all of the food from the experiment was egested. The digestibility value was highest for green leaves, whereas it was approximately 20% for all other stages. This was expected given that digestibility declines during decomposition as the metabolite content of the leaves decreases. The phenolic content was highest in the green leaves and lowest in three-month-old leaves. The flavonoid content was highest in green leaves and lowest after two months of decomposition. Animals ingested more phenolics when consumption was highest. The estimated amount of ingested flavonoids followed the same trend as assimilation rate. Flavonoids accounted for a large portion of total phenolics, and the estimated amount of flavonoids consumed was similar for one-, two- and three-month-old leaves. Our results suggest that the high phenolic and flavonoid concentrations in green leaves are feeding deterrents. Isopods may discriminate among concentrations of flavonoids and modify their consumption rates to maintain their intake of flavonoids when ingesting leaves with lower flavonoid content.
Woodlice, digestibility, total phenolics, flavonoid concentration, consumption rate, assimilation rate
Litter dynamics are of great importance in ecosystem functioning and are influenced by many different organisms. Isopods, earthworms, lumbricids, diplopods, dipteran larvae, and termites are detritivores of organic soil litter that play a major role in the cycling of nutrients, which is an important ecosystem service. Detritivores have low assimilation efficiency (Szláveczs and Pobozny 1995), and thus contribute to leaf litter decomposition indirectly by returning large amounts of consumed litter as feces (
Detritivores exhibit feeding preferences that may be related to leaf senescence (
Frequent switching between different types of food and regulating the intake of specific defensive chemicals are behavioral mechanisms used to accommodate for chemically defended foods (
Phenolics are thought to play a fundamental role in the chemical defense of plants against herbivores and pathogens (
The ability to digest phenolic polymers such as tannins and lignin is essential in the use of litter (
Most studies examining detritivore feeding and phenolics have explored the relationship with total phenolics (concentrations at which animals avoid feeding), lignin content related to toughness (not included in the total phenolic determination since it is a non-soluble phenolic) (
The goal of this study was to observe how two interconnected food parameters (phenolic content and decomposition stage) affect feeding rates (consumption, egestion, and assimilation rates, as well as digestibility efficiency) of detritivores using the neotropical terrestrial isopod Balloniscus sellowii (Brandt, 1833) as a model. We relate the total phenolic content to feeding rates and examine the flavonoid contents, which constitute a specific group of phenolics that are known to deter insects. Finally, we test a new method of calculating digestibility (assimilation efficiency) that takes into consideration food retention in the gut.
Material and methods Species and source siteThe species Balloniscus sellowii (Brandt, 1833)is common in Southern Brazil, Uruguay, and the region surrounding Buenos Aires in Argentina (
The source-site consisted of an area where animals were abundant and there were trees characteristic of pioneer vegetation colonization. The three most abundant plant species in the site were Lithraea brasiliensis Marchand (Anacardiaceae), Ricinus communis Linnaeus (Euphorbiaceae), and Schinus terebinthifolius Raddi (Anacardiaceae).
Feeding preferenceLeaves from the visually most abundant local plants were offered to isopods to verify feeding preference based on the highest consumption. Green leaves from three different plant species were removed from branches and placed into litter bags (10 x 15 cm) fastened to the soil for decomposition in loco for 14 days. Leaves were then transported to the laboratory and circles of 18 mm in diameter were cut and oven dried at 60ºC for 48 hours. The discs were weighed (Gibertini E425-B) and remoistened with distilled water before being offered to animals for one week in individual units consisting of 8 cm diameter plastic containers with moist plaster of Paris covered with a net to minimize coprophagy. The treatments consisted of 10 units with one leaf disc of each plant species with one isopod, and five animal free control units. Animals were kept without food for two days prior to and after the experiment to empty gut contents. After the experiment, the remaining plant material and feces were oven dried and reweighed, and consumption rates were calculated. The control group consisted of units containing leaves and no animals, such that the mean percentage of leaf weight lost due to autogenic changes (weight lost independent of the action of consumers) was subtracted from the amount of plant consumed. The plant species that was consumed at the highest rate was used to verify feeding rates on leaves at different stages of decomposition, as well as the phenolic and flavonoid contents of the leaves.
Feeding rates on leaves in different stages of decompositionGreen leaves were collected from branches at the same site and placed into 20 litter bags. The litter bags were collected after one, two, and three months of decomposition. These leaves were then taken to laboratory along with additional green leaves that had been collected from the branches when litter bags were placed in the soil, and offered to animals. Oven dried leaves from each decomposition stage were stored under refrigeration for phenolic and flavonoid content analysis.
For each unit, two or three discs of 18 mm (approximated amount for the third month of decomposition, at which point the leaves were very fragmented) were oven dried, weighed, remoistened with distilled water, and offered to individual animals for 10 days. The remaining leaves and feces were collected from the units, oven dried, and weighed after the experiment to calculate feeding rates. We performed 20 repetitions with one animal per unit and 20 control repetitions for each stage of decomposition.
Consumption rates were calculated as the total mg of ingested leaves (subtracted mean percentage of autogenic losses) in dry weight (DW) per g of body weight (FW) per day. The egestion rate was calculated as the total mg of produced feces (DW) per g of body weight (DW), per day. The assimilation rate was calculated as the total mg of ingested leaves (DW) minus the total mg of produced feces (DW) per g of body weight (FW) per day (DW = dry weight; FW = fresh weight) (
We also measured the amount of time that the food was retained in the gut. Animals were kept in individual units containing carrot as a food source (generates fecal pellets that differ in color) for a week. Then, 10 animals were exposed to one disc of leaf litter for each decomposition stage, and monitored every two hours for 80 hours. We recorded the timing of the first sign of leaf consumption (i.e., evidence of nibbling on the leaf disc) and that of the first non-carrot feces appearance.
Digestibility was calculated using animals fed carrots for one week and placed into units containing one leaf disc and monitored daily until total consumption. Following full disc consumption, animals were fed carrots to maintain egestion of the leaf material from the gut. Feces from leaf feedings were collected, oven dried, and reweighted. Five units from each decomposition stage were used. The digestibility was calculated as a percentage based on the total mg of ingested leaf minus the total mg of feces produced per mg of ingested leaf. Given the variability in duration with this method, other feeding rates were not calculated.
Phenolic and flavonoid contentThe total phenolic content was determined using the Folin-Ciocalteau method (
Five samples of each decomposition stage were used to determine the flavonoid content. For each sample, five discs of dry leaves (or an approximate amount) were ground up and left for two days in 5 mL of ethanol 80% for flavonoid extraction. Flavonoid content was determined using the method reported by
The mean concentrations of phenolics and flavonoids were multiplied by the consumption rates to estimate the total ingested amount of each group of substances.
Statistical analysisAll data were tested for normality using the Kolmogorov-Smirnov test. The consumption, egestion, and assimilation rates were compared using a one-way analysis of variance (ANOVA) followed by Tukey’s test. Pearson correlations were used to verify the association between consumption and egestion rates among treatments. All statistical analyses were performed using InStat 3.01 software.
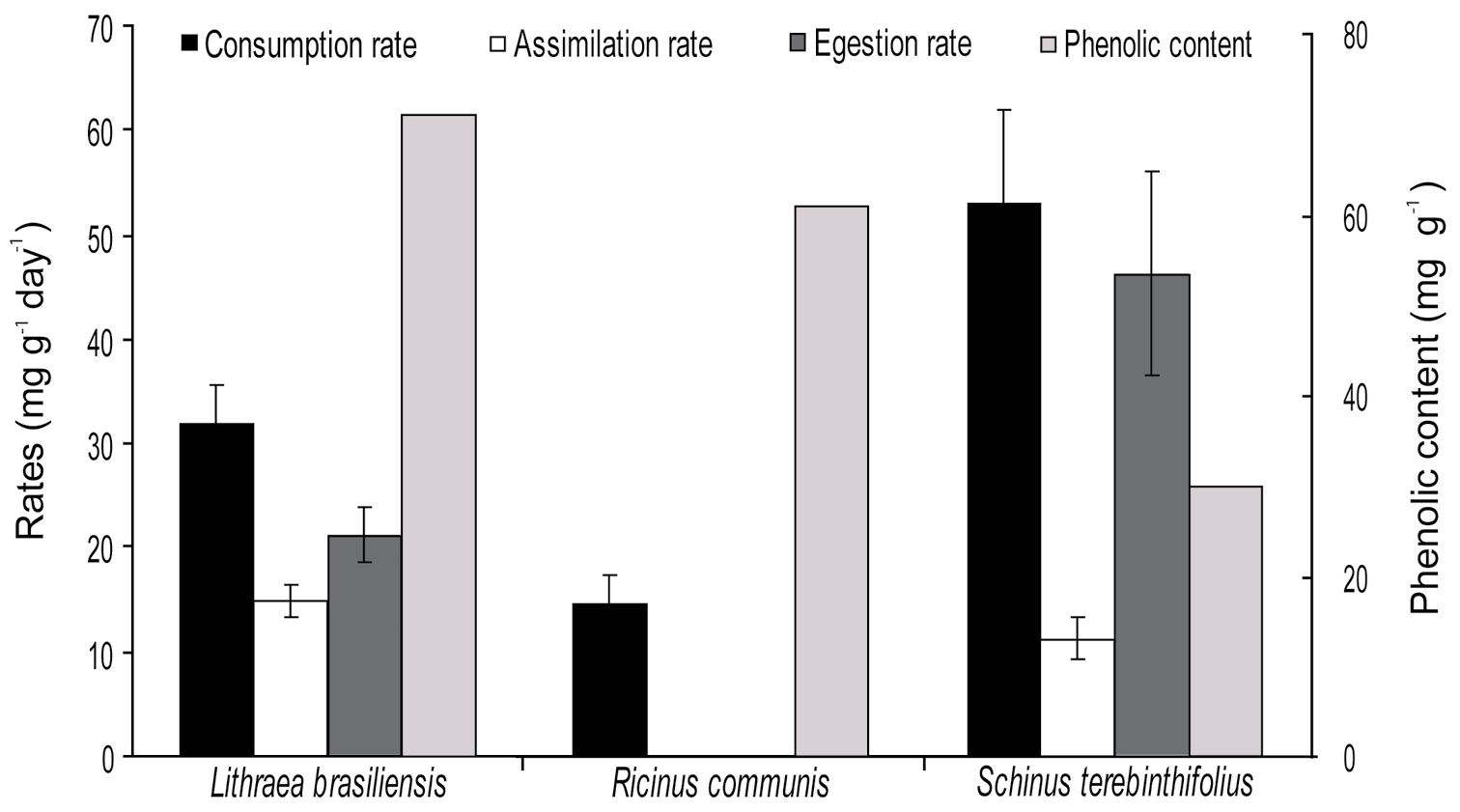
Results Feeding preferenceThe consumption rate was significantly higher when animals fed on Schinus terebinthifolius (52.9 ± 9.0 mg g-1 day-1, n = 10; mean ± SE) (F2, 26 = 9.395; p < 0.001), and no significant difference was recorded when animals fed on Lithraea brasiliensis (31.8 ± 4.0 mg g-1 day-1, n = 10) and Ricinus communis (15.5 ± 2.9 mg g-1 day-1, n = 9). The egestion and assimilation rates were 46.3 ± 9.8 (mg g-1 day-1) and 11.2 ± 2.0 (mg g-1 day-1) for Schinus terebinthifolius, and 21.1 ± 2.6 and 14.8 ± 1.62 (mg g-1 day-1) for Lithraea brasiliensis, respectively. Egestion and assimilation rates could not be calculated for Ricinus communis due to a low amount of fecal pellets. The phenolic content was highest in Lithraea brasiliensis (71.1 mg of tannic acid equivalent per g of dry leaf), followed by Ricinus communis (60.9), and was lowest in Schinus terebinthifolius (30.0) (Fig. 1). Standard error could not be calculated due to insufficient leaf material for additional replicates. The mass loss of leaves in control units was 0.08% for Schinus terebinthifolius, 0.09% for Lithraea brasiliensis, and 0.034% for Ricinus communis. Mortality was 20% or lower in all treatments.
Isopod feeding rates on leaves of Lithraea brasiliensis (n = 10), Ricinus communis (n = 9), and Schinus terebinthifolius (n = 10) with 14 days of decomposition and respective phenolic content (standard error was not calculated due to the low amount of leaf remains for chemical analysis). Egestion and assimilation rate could not be calculated for Ricinus communis (low amount of fecal pellets). The values are mean and SE. Superscript letters indicate significant difference among treatments (p < 0.05).
Schinus terebinthifolius was used to examine the feeding rates at different decomposition stages. The consumption rate was significantly higher on two month-old leaves (F3, 58 = 8.96; p < 0.0001), and there were no significant differences between green, one-month-old, and three month-old leaves. The egestion rate was significantly higher for two-month-old leaves (F3, 58 = 14.17; p < 0.0001) and there was no significant difference between green and one-month-old leaves, or between one-month-old and three-month-old leaves. The assimilation rate of green leaves was significantly higher than that of two- and three-month-old leaves and exhibited no significant difference between one-, two-, and three-month-old leaves (F3, 58 = 5.275; p = 0.0028) (Table 1). The mean reduction in leaf mass in the control units was 0.16% (green), 0.13% (one-month-old), 0.12 (two-month-old), and 0.06% (three-month-old). Mortality was 0.35% for green, 0.15% for one-month-old, and 0.20% for two- and three-month-old leaves.
There were significant correlations between consumption and egestion rates for all decomposition stages. The correlation was stronger for one-month-old leaves (r2 = 0.928; p < 0.0001) followed by those for three-month-old leaves (r2 = 0.9258; p < 0.0001), two-month-old leaves (r2 = 0.8342; p < 0.0001), and green leaves (r2 = 0.7240; p < 0.0002).
Feeding rates of Balloniscus sellowii on Schinus terebinthifolius for different stages of decomposition. Data are expressed as mean value and SE of mg of food source (DW), per g of animal (FW), per day. N differs among decomposition stages due to different mortality in treatments. Different letters indicate significant differences of each rate among treatments (p < 0.05).
| Decomposition stage | Consumption rate | Egestion rate | Assimilation rate |
|---|---|---|---|
| Green leaves (n=13) | 41.5 ± 5.1 a | 10.7 ± 3.2 a | 30.7 ± 2.9 a |
| 1 month-old leaves (n=17) | 52.2 ± 4.6 a | 29.1 ± 5.7 a, b | 23.0 ± 1.7 a, b |
| 2 months-old leaves (n=16) | 80.1 ± 6.2 b | 61.3 ± 6.4 c | 18.9 ± 2.6 b |
| 3 months-old leaves (n=16) | 53.4 ± 5.5 a | 33.4 ± 5.1 b | 20.0 ± 1.5 b |
The time required for passage through the gut did not differ among stages of decomposition. For all stages, the mode time for the appearance of leaf-based feces was two hours (one observation interval). However, leaf ingestion was initiated immediately for most units for the one-, two-, and three-month-old leaves, whereas the ingestion of the green leaves was initiated two days after the onset of the experiment. For 3 out of the 10 units containing green leaves, no apparent consumption had occurred after 80 h of observation.
The digestibility, calculated based on the total consumption of a leaf disc with a known mass and a second food source to push leaf material out of the gut, was 43.1 ± 3.8% for green leaves (n = 6), 19.7 ± 2.4% for one-month-old leaves (n = 4), 20.3 ± 1.6 for two-month-old leaves (n = 5) and 19.5 ± 2.8% for three-month-old leaves (n = 2).
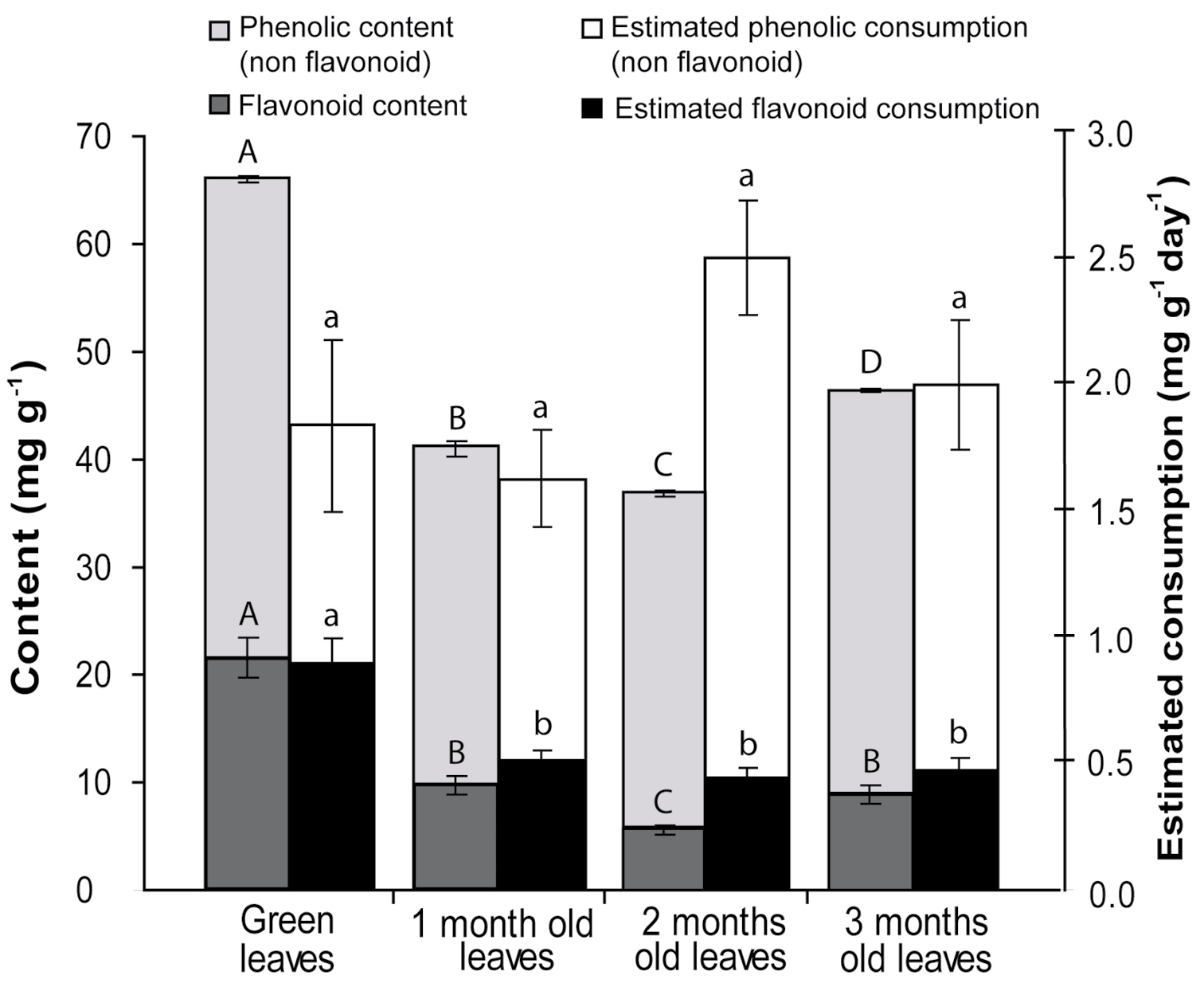
Phenolic and flavonoid contentThe phenolic content was significantly different across all decomposition stages (F3, 12 = 602.61; p < 0.0001), and was highest in green leaves (66.0 ± 0.3 mg of tannic acid equivalent per g of dry leaf) and lowest in two-month-old leaves (36.9 ± 0.4 mg g-1). The flavonoid content was significantly highest in green leaves (21.6±1.9 mg g-1), but did not differ significantly among the other stages (F3, 16 = 37.10; p < 0.0001). The estimated phenolic amount ingested by animals was not significantly different (F3, 58 = 2.065; p = 0.115), ranging from 2.141 ± 0.190 mg of phenolic per g of isopod per day (one-month-old) to 2.954 ± 0.229 (two-month-old). The estimated flavonoid amount ingested by the animals followed the same trend as the assimilation rate among decomposition stages, being significantly higher in green leaves, but not differing between those undergoing one, two, and three months of decomposition (F3, 58 = 10.783; p < 0.0001) (Fig. 2).
Given that the flavonoid content was not tested for every experimental unit, the average content of each leaf age was correlated with the average assimilation rate for each stage of decomposition, resulting in a high correlation (r2 = 0.9688; p = 0.0157, n = 4).
Total phenolic and flavonoid content and estimated amount of total phenolics and flavonoids ingested by Balloniscus sellowii on leaves of Schinus terebinthifolius for different stages of decomposition. The values are mg of equivalent of quercetin(flavonoid) or tannic acid (phenolic) per mg of dry leaf ± SE. Superscript letters indicatesignificant differences among treatments (p < 0.05).
Numerous studies have analyzed the effects of secondary metabolites in herbivores, whereas few studies have been conducted to understand the role of these compounds in detritivore and decomposer organisms. For example, an understanding of the presence of unpalatable or indigestible compounds and their rates of consumption related to leaf senescence is lacking. Our source site harbored plant species characteristic of a successional stage that do not exhibit mechanical structures to deter herbivores other than lignin, suggesting that chemical defenses are key for plant protection. Tropical plants inhabiting resource-poor environments invest heavily in chemical defenses such as phenolics (
Schinus terebinthifolius accounted for the highest consumption rate and it is known as a source of terpenoids, simple phenolic derivatives, and flavonols. Furthermore, the anti-oxidant activity of the extract derived from its aerial parts has been described in the literature (
When given a choice, animals avoid green leaves that provide high amounts of secondary compounds (
In general, detritivores show low digestibility, although differences in the digestibilities of high and low quality foods remain debated. In our feeding rates experiment, green leaves presented very high digestibility values (~80%, data not shown) and there was no difference in the time required for leaf-based feces to appear, contrary to the hypotheses of other researchers (Souza et al. 1998,
Although the consumption rate did not differ significantly between green and one-month-old leaves, the onset of feeding on green leaves was not immediate, whereas it was for the other stages, suggesting that the high phenolic and flavonoid concentrations of green leaves are feeding deterrents and therefore reduce leaf palatability. These phenolic substances are probably lost in the beginning of the decomposition process due to leaching. If substances that cause feeding deterrence and inhibit feeding are lost early in the leaf decomposition process, the leaves will be consumed more quickly by detritivores, and thus be returned to the soil to provide nutrients back to the trees. Therefore, this process may serve as an adaptive advantage to the plants. Indeed,
During leaf senescence, the total secondary metabolite content tends to diminish due to leaching (
Flavonoids represented a considerable portion of the total phenolics in Schinus terebinthifolius leaves. We observed a correlation between the flavonoid content and the assimilation rate, whereas no correlation was detected with the total phenolic content. Although the flavonoid content of the leaves differed among decomposition stages, as did the consumption rates, the estimated amounts of flavonoids consumed by the animals were almost the same for leaves after one, two, and three months of decomposition. Thus, given that a decrease in the consumption of high flavonoid leaves is not supported due to the significantly higher ingestion of flavonoids in green leaves, it appears that the animals increased their consumption of low flavonoid leaves, therefore suggesting that they might use these flavonoids as a food parameter.
Existing reports attribute the presence of phenolics and flavonoids in plants to defense against pathogens and herbivores, while only a few studies suggest possible benefits for organisms ingesting these substances. For example, when examining herbivores,
The authors wish to thank to CNPq for granting of the scholarship to CTW and research fellowship to PBA, to CAPES for granting of the scholarship to CCDS, and to EMAE for financial help on the ISTIB 2011. We are also grateful to Priscila Bugs and Carina Appel for help in the field, and to José Cláudio Fonseca Moreira, Milton de Souza Mendonça Junior and two anonymous reviewers for comments on the manuscript.