(C) 2012 Sara Novak. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Nanoparticles of titanium dioxide are one of most widely used nanomaterials in different products in everyday use and in industry, but very little is known about their effects on non- target cells and tissues. Terrestrial isopods were exposed to food dosed with nano-TiO2 to give final nominal concentration 1000 and 2000 µg TiO2/g dry weight of food. The effects of ingested nano-TiO2 on the model invertebrate Porcellio scaber (Isopoda, Crustacea) after short-term (3 and 7 days) and prolonged (14 and 28 days) dietary exposure was assessed by conventional toxicity measures such as feeding rate, weight change and mortality. Cell membrane destabilization was also investigated. No severe toxicity effects were observed after 3, 7, 14 or 28 days of dietary exposure to nano-TiO2, but some animals, particularly those exposed to lower concentrations of nanoparticles, had severely destabilized digestive cell membranes. It was concluded that strong destabilization of the cell membrane was sporadic, and neither concentration- nor time-related. Further research is needed to confirm this sporadic toxic effect of nanoparticles.
Isopods, Porcellio scaber, TiO2 nanoparticles , prolonged feeding, toxic effects
During the last decade the presence of nanomaterials has increased extraordinarily, and information on their toxicity is urgently needed. Nanomaterials have unique physical and chemical properties as a result of their small particle size, shape, conductivity and surface characteristics. TiO2 nanoparticles are most commonly encountered nanoparticles and as a consequence they could become a substantial environmental pollutant. Nanoparticles of TiO2 have been shown to have different types of effects in vivo (
Many reports using terrestrial isopods as toxicity test organisms for chemicals and particles in laboratory single-species tests can be found in the literature, and Porcellio scaber (Isopoda, Crustacea) is among themost frequently used species in such studies. The species was found to be suitable in tests of the effects of elevated concentrations of metals (
Conventional measures of toxicity such as investigations of growth, reproduction, and life-cycle are not the most suitable when terrestrial isopods are the test organism. Rates of growth in terrestrial isopods over several weeks are variable even for a single individual (
In toxicity tests with isopods however, feeding parameters have proved to be an integrated organism-level response, appropriate evidence of the effects of chemicals. Feeding rate changes are relatively fast and have been observed in relation to added metals or organic chemicals. Reduced feeding rate in comparison to controls was recorded after exposure of isopods to metals and biocides (
Recently, studies on the effects of nanoparticles were performed with Porcellio scaber. When added to food, TiO2 particles had no adverse effect on the feeding rate of Porcellio scaber after 3 or 14 days dietary exposure (
In the present study, the effects of nano-TiO2 on the model invertebrate Porcellio scaber (Isopoda, Crustacea) after brief (3 and 7 days) and prolonged (14 and 28 days) dietary exposure are examined. We discuss the toxic effect of ingested TiO2 nanoparticles on this terrestrial isopod. The feeding rate was used as evidence of a toxic effect and the cell membrane destabilization as a measure of a cytotoxic effect. We have found that after 14 days of exposure to nano-TiO2, the feeding rate of Porcellio scaber was not significaly affected, but cell membranes were destabilized in more than 40% of the population. If cell membrane destabilization leads to cytotoxicity, prolonged exposure of Porcellio scaber to nano-TiO2 will result in toxic effects which can be assessed by conventional toxicity measures. If there is a reduced feeding rate after prolonged exposure, this will confirm the time- and dose-dependency of the effects of nano-TiO2 which has been seen with other materials.
Materials and methods ChemicalsAcridine orange (AO), ethidium bromide (EB) and titanium dioxide nanoparticles (nano-TiO2) were purchased from Sigma-Aldrich. The nano-TiO2 was the same as was used in our earlier experiments (
Terrestrial isopods (Porcellio scaber, Isopoda, Crustacea) were collected in July and August 2010 at location (46°4'20"N, 14°26'51"E) near Ljubljana, Slovenia. The animals were kept in a terrarium filled with a layer of moistened soil and a thick layer of partly decomposed hazelnut tree leaves (Corylus avellana), at a temperature of 20 ± 2°C and a 16:8-h light:dark photoperiod. Adult animals of both sexes, weighing more than 30 mg, were used in the experiments. If moulting or the presence of marsupia were observed at any time, the animals were removed from the experiment in order to keep the investigated population as physiologically homogenous as possible.
Characterization of nanoparticlesNanoparticles were inspected with transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET) analysis, dynamic light scattering (DLS) and X-ray powder diffraction techniques. TEM micrographs were published in a previous report by
In the DLS analysis, the dispersions of nanoparticles (100 µg nano-TiO2/ml distilled water) were examined with a 3D DLS-SLS spectrometer (LS Instruments) which allows the assessment of the hydrodynamic radii of particles in extremely turbid suspensions by a 3D cross-correlation technique that eliminates light scattering. The light source used was a HeNe laser operating at a wavelength of 632.8 nm and scattering was measured at an angle of 90°. At higher concentrations of nanoparticles (1000, 2000 µg nano-TiO2/ml distilled water), measurements were not possible, due to the low transparency of the samples (
Hazelnut leaves were dried at room temperature, and cut into pieces weighing ~100 mg. The TiO2 nanoparticles were suspended in distilled water to obtain different final concentrations (1000 and 2000 µg/ml). In a control group, the leaves were treated with pure distilled water. A suspension of particles was brushed onto the abaxial leaf surface and the leaf was allowed to dry, giving final nominal concentrations of nanoparticles on the leaves of 1000 and 2000 µg nano-TiO2 per gram (dry wt) of leaf.
A single hazelnut leaf treated with either distilled water or nano-TiO2 suspension was placed in a Petri dish with one animal in each Petri dish. The leaf was the only food source for animal. The Petri dishes were kept in a large glass container under controlled conditions in terms of humidity (≥80%), temperature (21±1°C) and light regime (16:8-h light:dark photoperiod). In experiments 1-4, animals were exposed for 3 days, 7 days, 14 and 28 days, respectively. During the 14 and 28 days exposure feces were removed every 7 days to eliminate the possibility of coprophagy. After the exposure the animals were weighed, and anasthetized and decapitated. The digestive glands were isolated and used for assessment of digestive gland cell membrane stability as described below.
Feeding parameters, weight change and mortalityAfter 3, 7, 14 or 28 days of exposure of animals to treated leaves, fecal pellets and leaves were removed from the Petri dishes and the leaves were dried at room temperature for 24 h. The dried leaves and fresh animals were weighed and the feeding rate of the isopods was calculated as the mass of consumed leaf per animal’s wet weight per day. The animal’s weight-change in each case was defined as the change in animal wet weight from the beginning to the end of the experiment.
Digestive gland cell membrane stabilityThe AO/EB assay is based on the assumption that changes in cell membrane integrity result in differences in permeability of cells to AO and EB dyes. Different permeability to the two dyes results in differentially stained nuclei. Acridine orange is taken up by cells with membranes that are intact or destabilized, and in the cell, emits green fluorescence, as a result of its intercalation into double-stranded nucleic acids. Ethidium bromide on the other hand, is taken up only by cells with destabilized cell membranes, and it emits orange fluorescence, after intercalation into DNA (
Cell membrane stability was tested with a modified method described by
The differences in the medians of measured parameters in exposed and unexposed groups were tested with the non-parametric Mann-Whitney U test. All calculations were done using Statgraphics Plus 4.0 statistics software. Statistical differences between exposed and control animals were categorized into three groups to which different numbers of asterisks were assigned (* p < 0.05, ** p < 0.01, ***p < 0.001).
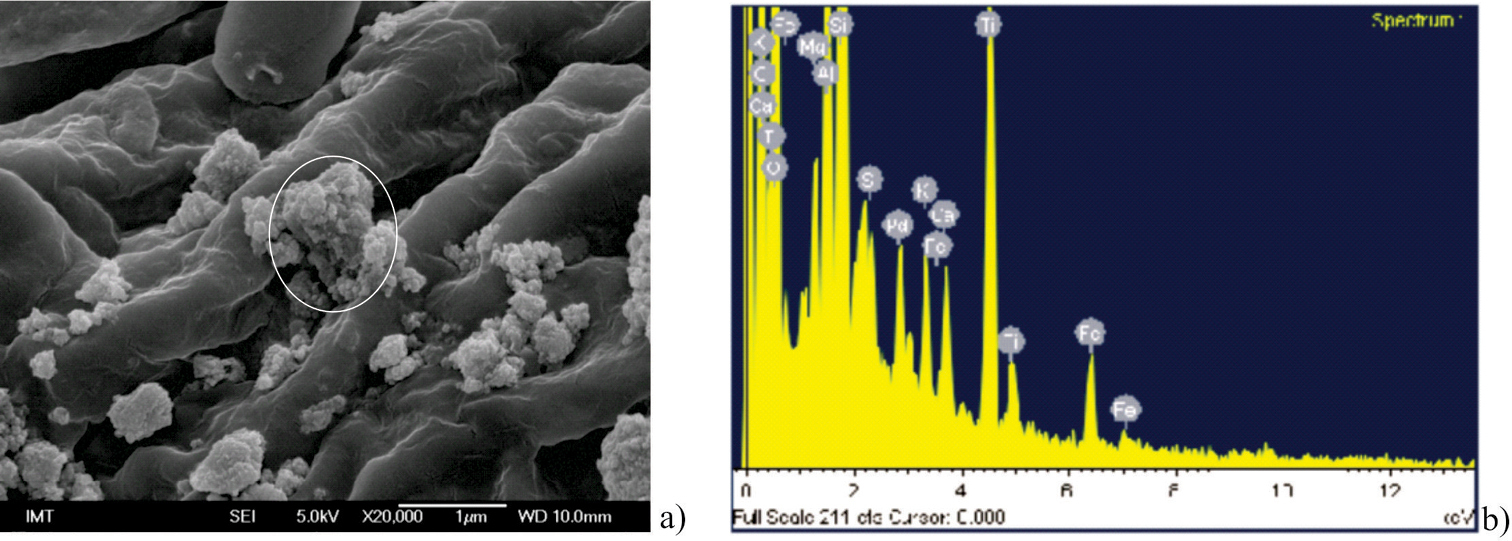
Results Characterization of nanoparticlesScanning electron microscopy revealed the distribution of TiO2 particles applied on the lower leaf surface (Fig. 1a) and EDX confirmed their composition (Fig. 1b). The same particles were tested in other studies which provide a more detailed description of their characteristics (
TiO2 nanoparticles dispersed over the abaxial leaf surface to give final concentration of 1000 µg/g dry wt of leaf a EDX spectrum of area encircled on Figure 1a, where presence of Ti is confirmed b.
The number of exposed animals at the beginning of the exposure and that at the end of the exposure failed to correspond because some animals molted during the course of experiment and consequently were excluded from further analysis. Based on the amount of consumed food it was estimated that when animals were fed on 1000 µg nano-TiO2/g of leaf they consumed approximately 0.01 ± 0.01 µg TiO2 per day in 3 days, 0.05 ± 0.03 µg TiO2 per day in 7 days, 0.07 ± 0.02 µg TiO2 per day in 14 days and 0.05 ± 0.02 µg TiO2 per day in 28 days. When fed on 2000 µg nano-TiO2/g of leaf they consumed approximately 0.08 ± 0.04 µg TiO2 per day in 3 days, 0.09 ± 0.03 µg TiO2 per day in 7 days, 0.09 ± 0, 03 µg TiO2 per day in 14 days and 0.1 ± 0.05 µg TiO2 per day in 28 days. No significant effect of ingested nano-TiO2 on survival and weight change was observed in animals fed with TiO2 nanoparticles when compared to control animals fed with untreated food.
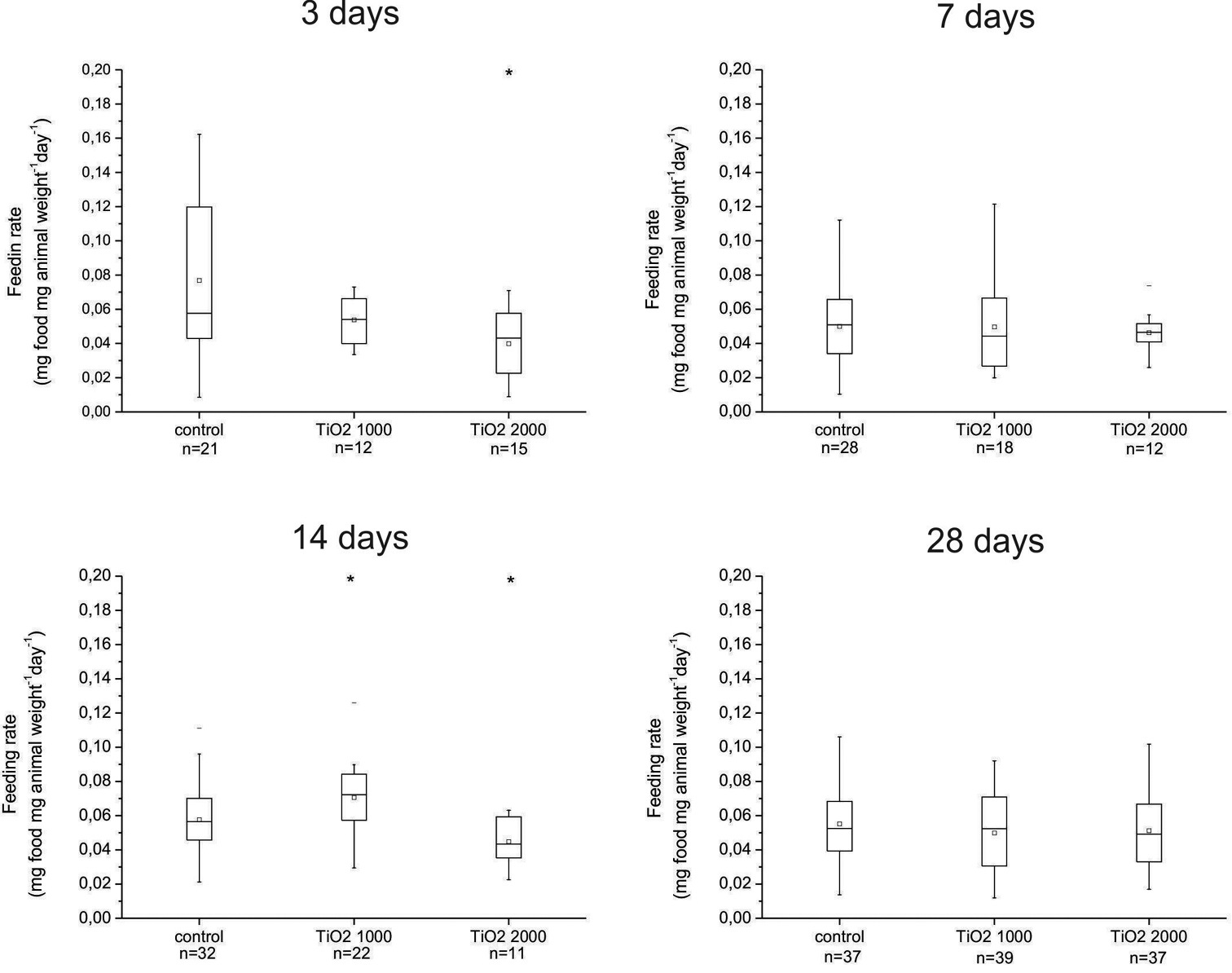
There was a statistically significant decrease in feeding rate in animals exposed for 3 days or 14 days on food dosed with 2000 µg/g nano-TiO2 when compared with controls (Fig. 2). However, an increase, also statistically significant, occurred in the feeding rate of animals exposed for 14 days to food dosed with 1000 µg/g nano-TiO2 when compared with control (p = 0.03). These data indicate a dynamic response of feeding behavior to presence of particles in the food, which was not consistent over time (Fig. 2). In more or less all exposed groups the average feeding rate was similar, indicating reproducibility of the feeding parameters in different experiments.
Daily feeding rate (mg of consumed leaves/animal weight) of animals fed on control (untreated) leaves and leaves dosed with 1000 or 2000 µg/g nano-TiO2 for 3, 7, 14 or 28 days. On x scale also number of animals in each group is represented (n). There are statistically significant differences between animals exposed to food dosed with 1000 µg/g nano-TiO2 for 3 and 14 days compare to control of the corresponding group and between control and 2000 µg/g nano-TiO2 in animals exposed for 14 days (* p < 0.05). Symbols on the box plot represent minimum and maximum data values (whiskers), mean value (□), 75th percentile (upper edge of box), 25th percentile (lower edge of box), median (line in box) and max and min value ( - ).
Our previously published data demonstrate that in animals from a stock culture and in good physiological condition, the digestive gland cell membrane stability classification was higher than 2 in only 5% of animals, and this was considered to be a benchmark (
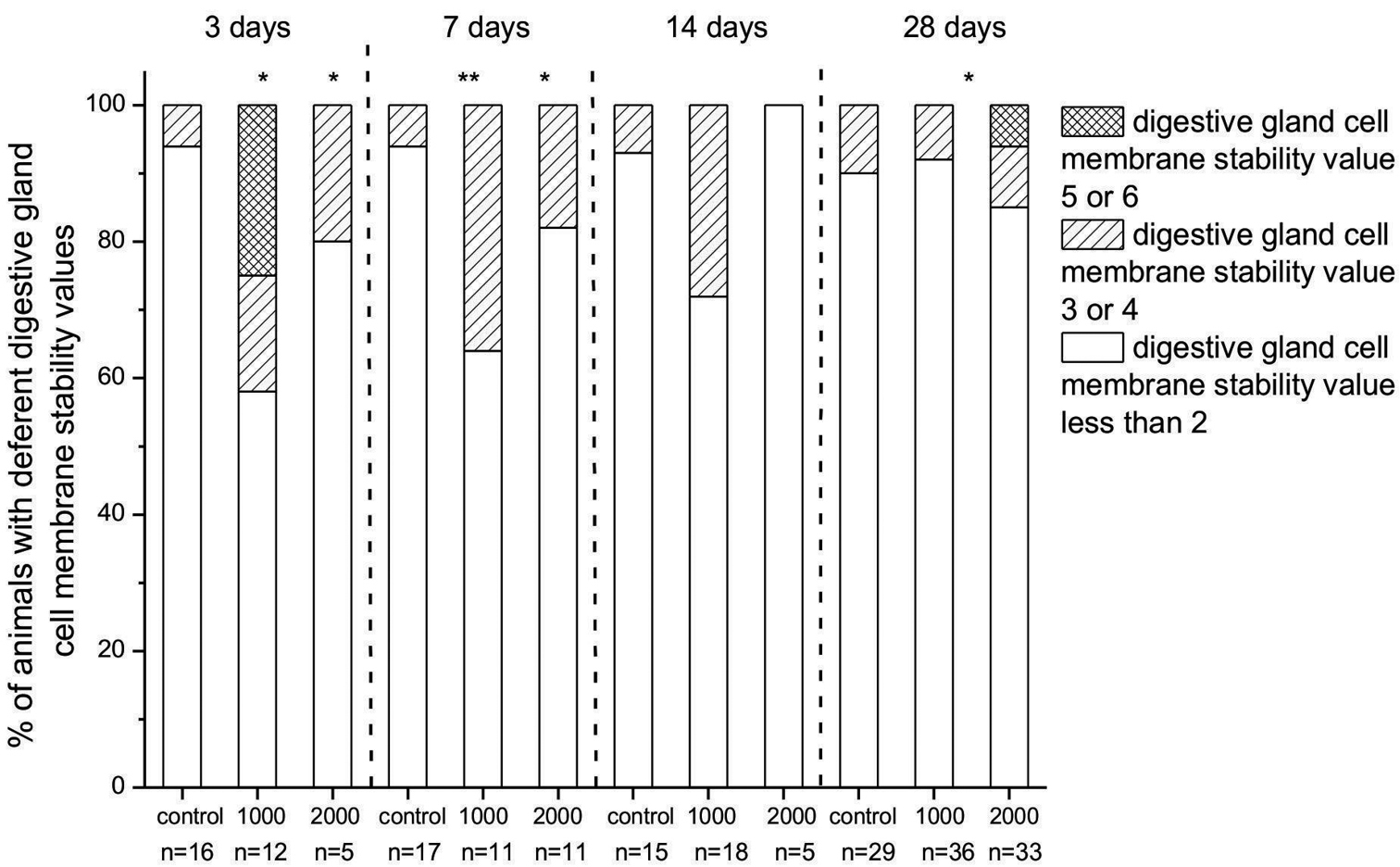
Our data show that among the control animals fed with uncontaminated food, the digestive cell membranes were affected in up to 10% of exposed animals. We consider this to be a response to suboptimal experimental conditions in terms of isolation of animals, inappropriate shelter during the experiment, and poor food. We consider 10% of animals with affected cell membrane to be normal (benchmark).
The most significantly affected groups were those exposed to 1000 µg/g nano-TiO2 for 3 days. In this group, 25% of animals had a cell membrane destabilization value of 5 or greater. After 7 and 14 days of exposure to food dosed with 1000 µg/g nano-TiO2, digestive cell membrane destabilization was detected in 36% and 28%, respectively, of the exposed animals, but after 28 days of feeding on food dosed with 1000 µg/g nano-TiO2 there was almost no effect (8%) on digestive gland cell stability. The highest concentration of TiO2 particles in food (food dosed with 2000 µg/g nano-TiO2) investigated was generally less harmful to digestive cell membranes, although in some (6%) of the animals exposed for 28 days, serious destabilization of the digestive cell membranes was observed (Fig. 3). These severe biological effects (after 3 and 28 days of exposure) were neither dose- nor duration-related and this cell membrane damage, which was never seen in control animals, could be interpreted as a sporadic effect.
Percentage of animals in fed on food dosed with 1000 or 2000 µg/g nano-TiO2 for 3, 7, 14 or 28 days with different degrees of destabilization of cell membranes, assessed visually and classified from 0 to 6 according to the scale defined in Materials and Methods, above. On x scale also number of animals in each group is represented (n). Digestive gland cell membrane stability values ≤ 2 represent animals which had no destabilized cell membrane and digestive gland cell membrane stability values 3 or 4 animals with destabilized cell membranes. Those with value 5 or 6 had the most destabilized cell membranes. Statistical differences between exposed and control animals (within one exposure duration) are marked with an asterisk (* p < 0.05 and ** p < 0.01).
In our study no toxic effects could be confirmed by conventional toxicity parameters such as weight change or mortality in short-term (3 and 7 days) and prolonged (14 and 28 days) exposure. The changed feeding rate of exposed animals compared to controls is also convenient evidence of the effects of chemicals on isopods. We hypothesize that the adverse effect of chemicals is manifested in a reduced feeding rate. In cases where the feeding rate significantly increases, it is thought to be a hormetic like response (
In this study, the feeding rate of the animals increased, decreased or was not affected at all. These observations coincide with our previous results in which nanosized TiO2 enhanced the feeding rate of Porcellio scaber (
Different studies report changed feeding rates after feeding animals on chemically dosed food for different periods of time. For example, the feeding rate in Porcellio scaber was assessed after 3 days (
Feeding rate changes appear to be a suitable measure of effects of ingested chemicals. Whether this is a convenient measure of effects of nanoparticles is needed to be confirmed in future research. Data obtained with nanoparticles suggested that feeding rate changes are neither dose nor time dependent. Feeding rates of exposed animals either increased or decreased when compared to controls. Such result may indicate that: (a) exposure duration was not long enough to provoke effect; (b) exposure concentration was too low to exert effect or (c) nanoparticles have stochastic type of effects which occur by chance and are not time nor dose dependant. To confirm this is a change for future research.
In the study presented here feeding rates were not severely affected even at exposures of up to 28 days, but an effect was seen at shorter exposure duration. Consequently, a concentration of nano-TiO2 of 2000 µg/g in the food may not be assumed to be a “no observed effect concentration” (NOEC).
In contrast to the not so significant effect on standard toxicity parameters in our study the cell membranes of digestive glands in almost half of exposed animals (42%) fed on 1000 µg/g nano-TiO2 were destabilized after as little as 3 days of exposure. After 7 days of exposure to food containing 1000 µg/g nano-TiO2, digestive cell membrane destabilization was detected in 36% of the exposed animals. Animals exposed for longer periods, 14 or 28 days, did not exhibit such intensive membrane damages as was expected, but in animals exposed for 28 days to highest exposure concentration, the digestive gland membrane was severely damaged, a result that was never observed in controls. We conclude that the severe damage of membrane was neither dose- nor exposure duration dependent but occurs sporadically. Here again, we observed different type of response to nanoparticles when compared to non-nanoparticulate chemicals. Performed with soluble chemicals, the AO/EB assay reveals a dose response effect (
A moderate effect was found to be more common in animals exposed to lower concentrations and for shorter times. In the light of currently available knowledge we speculate that in such cases, the cell membrane is destabilized, but the organism has a mechanism to restore its normal activity. The ability of cells to alter their lipid composition and thus their rigidity after exposure to CuO nanoparticles has been demonstrated by
We speculate that in our study, TiO2 nanoparticles interact first with the cell membrane, and this interaction is diagnosed as cell membrane destabilization. Subsequently, the cells respond to this destabilization of the cell membrane by repairing its stability. This is indicated by the failure to observe intensification of cell membrane destabilization after prolonged exposure durations, such as 28 days. Not with standing this, the cell membrane in some animals was severely affected. Future research is needed to learn if this severe damage could lead to toxic effects or if it can be reversed.
ConclusionsWe found nano-TiO2 to manifest no severe toxicity after 3, 7, 14 or 28 days of dietary exposure to 1000 µg/g or 2000 µg/g of nano-TiO2when measured by conventional toxicity measures such as feeding rate, weight change, and mortality.
Severe cell membrane destabilization was sporadic, and was independent of dose and duration of exposure.
The highest tested concentration with 28 days of exposure is not the NOEC because the membrane destabilization effects were observed at shorter duration periods.
The toxic effect of nanoparticles has to be interpreted differently from that of soluble chemicals. It appears more a stochastic-like effect which is not dose responsive.
Work of PhD student Sara Novak was supported by the Slovenian Research Agency (No. J1–4109). We thank G.W.A. Milne for editorial assistance and Matej Hočevar from IMT Institute, Ljubljana for SEM micrographs.