(C) 2011 Bastian T. Reijnen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An overview of the octocoral and hydrozoan host species of pygmy seahorses is provided based on literature records and recently collected field data for Hippocampus bargibanti, Hippocampus denise and Hippocampus pontohi. Seven new associations are recognized and an overview of the so far documented host species is given. A detailed re-examination of octocoral type material and a review of the taxonomic history of the alcyonacean genera Annella (Subergorgiidae) and Muricella (Acanthogorgiidae) are included as baseline for future revisions. The host specificity and colour morphs of pygmy seahorses are discussed, as well as the reliability of (previous) identifications and conservation issues.
Acanthogorgiidae, Alcyonacea, Annella, Anthozoa, Hippocampus, host specificity, Hydrozoa, Indo-Pacific, Muricella, new associations, Octocorallia, Subergorgiidae
Pygmy seahorses (Hippocampus spp.) (Pisces: Syngnathidae)
are diminutive tropical fish that live in close association with
octocorals, colonial hydrozoans, bryozoans, sea grass and algae (
The first discovered pygmy seahorse was described as Hippocampus bargibanti Whitley, 1970 (redescribed by
This study deals with the octocoral (Cnidaria: Anthozoa: Octocorallia) and hydrozoan (Cnidaria: Hydrozoa) hosts of the pygmy seahorses Hippocampus bargibanti, Hippocampus denise, and Hippocampus pontohi.The taxonomic problems in the octocoral host genera Muricella (Acanthogorgiidae) and Annella (Subergorgiidae)are addressed, and type material is re-examined and depicted. In addition, a literature review of all documented host species is provided, as well as accounts on newly recorded associations. The distribution records of pygmy seahorses are updated with four localities in Indonesia and Malaysia.
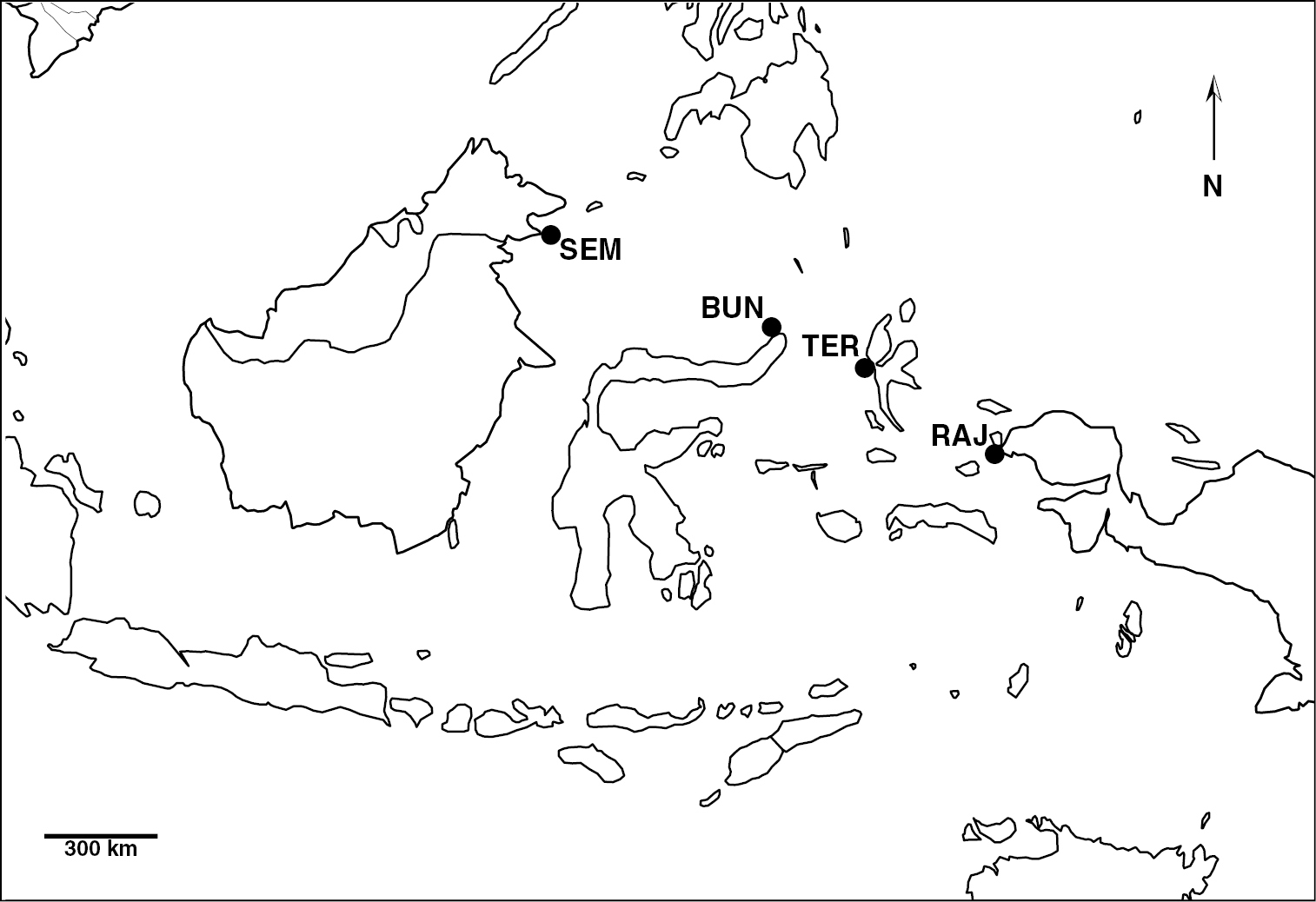
Material and methodsThe majority of the pygmy seahorse records in the present study was obtained during fieldwork in Raja Ampat, West Papua, Indonesia (2007). Additional observations were made in Bunaken National Marine Park, North Sulawesi (2008), Ternate and Halmahera, North Moluccas (2009) in Indonesia, and Semporna, eastern Sabah, in Malaysia (2010) (Fig. 1).
Map showing the fieldwork localities in Indonesia and Malaysia; BUN (Bunaken), RAJ (Raja Ampat), SEM (Semporna) and TER (Ternate).
Soft corals, gorgonians and hydrozoans were thoroughly searched for pygmy seahorses to a maximum depth of 40 m (using SCUBA), with the help of local dive guides where available (Raja Ampat, Bunaken). In situ photographs were taken of both the hosts and the associated seahorses (Fig. 2). The total number of seahorses per host colony was counted, the height of each host colony was estimated and a sample was taken for identification and as voucher material. All material is stored on 70% ethanol in the collections of NCB Naturalis, Leiden (catalogue numbers RMNH Coel.). Subsamples of the Ternate material are deposited in the collections of Museum Zoologicum Bogoriense (Java, Indonesia).
In-situ photographs A Hippocampus bargibanti on Muricella sp. 3 (RMNH Coel. 39866, see Fig. 7), Turtles Reef, Raja Ampat (photo F.R. Stokvis) B Hippocampus denise on Annella reticulata (RMNH Coel. 39880, see Fig. 10); W Mansuar, Raja Ampat (photo B.W. Hoeksema) C Hippocampus pontohi (host not collected) Timur I, Bunaken (photo S.E.T. van der Meij) D Hippocampus severnsi (host not collected) Siladen I, SE Siladen (photo B.T. Reijnen).
For the identification of the octocoral hosts, microscope slides and SEM photographs of the sclerites were made. These were obtained by dissolving the octocoral tissue in 10% sodium hypochlorite, after which they were rinsed five times with tap water and five times with double-distilled water. The sclerites were subsequently dried on glass microscope slides on a hot plate. After drying, the sclerites were brushed on a SEM stub and coated with platinum. A JEOL JSM6480LV electron microscope operated at 10 kV was used for the SEM photography. The hydrozoans were identified using a dissecting microscope.
ResultsIn the literature eight pygmy seahorse species have been recorded as associates of hydroids and octocorals (Table 1). During the fieldwork a total of 10 observations of Hippocampus bargibanti, 10 of Hippocampus denise, four of Hippocampus pontohi and one of Hippocampus severnsi was made. The total number of encountered pygmy seahorse individuals is 52 (Table 2), in which 10 host species were involved. For Hippocampus bargibanti and Hippocampus denise there is overlap in host species with the previous records. The present host records of Hippocampus pontohi do not correspond with previous ones (Table 1). On one occasion Hippocampus severnsi was observed, but the host organism was not sampled.
Distribution ranges of pygmy seahorses and their host species associations as obtained from literature.
| Species | Confirmed distribution | Host species | Reference |
|---|---|---|---|
| Hippocampus bargibanti | Australia, New Caledonia, Indonesia, Japan, Papua New Guinea, Philippines | Muricella paraplectana Grasshoff, 1999 |
|
| Muricella plectana Grasshoff, 1999 | |||
| Muricella sp. | |||
| Hippocampus colemani | Australia (Lord Howe Isl.) | Halophila sp. |
|
| Zostera sp. | |||
| Hippocampus denise | Indonesia, Malaysia, Micronesia, Palau, Papua New Guinea, Philippines, Solomon Isl., Vanuatu | Annella mollis (Nutting, 1910) |
|
| Annella reticulata (Ellis & Solander, 1786) | |||
| Muricella sp. | |||
| ?Acanthogorgia spp. | |||
| ?Echinogorgia sp. | |||
| ?Subergorgia sp. | |||
| Hippocampus pontohi | Indonesia (widespread) | Aglaophenia cupressina Lamouroux, 1812 |
|
| Halimeda sp. | |||
| Hippocampus satomiae | Indonesia (E Kalimantan, N Sulawesi), Malaysia (N Borneo) | ?Carijoa sp. |
|
| Nephthea sp. | |||
| Hippocampus severnsi | Indonesia, Japan, Papua New Guinea, Solomon Isl., Fiji | Antennellopsis integerrima Jäderholm, 1919 |
|
| Catenicella sp. | |||
| Halicordyle disticha [Halocordyle disticha (Goldfuss, 1820) = Pennaria disticha (Goldfuss, 1820)] | |||
| Halimeda sp. | |||
| Lytocarpus phoeniceus (Busk, 1852) | |||
| Muricella sp. | |||
| ?Menella sp. | |||
| Hippocampus waleananus | Indonesia (Walea Isl., Togian Isl.) | Nephthea sp. |
|
| Hippocampus sp. A | Japan (Hachijo Isl., Izu Isl.) | unknown |
|
Host-species associations as recorded in this study, no. = the number of observed pygmy seahorses per colony. Observations recorded in Raja Ampat, unless otherwise stated.
| Species | No. | Host species | RMNH Coel. | Colony height | Depth | Locality | Lat. | Long. |
|---|---|---|---|---|---|---|---|---|
| Hippocampus bargibanti | 1 | Muricella sp. 1 | 39868 | 30 cm | 16 m | S Friwin Isl. | 0°28’54.54”S; 130°41’54.06”E | |
| 2 | Muricella sp. 1 | 39871 | 100 cm | 21 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | ||
| 6 | Muricella sp. 1 | 39874 | 100 cm | 22 m | Maitara NW (Ternate) | 0°44’19.21”N; 127°20’59.99”E | ||
| 1 | Muricella sp. 2 | 39869 | 70 cm | 21 m | S Friwin Isl. | 0°28’54.54”S; 130°41’54.06”E | ||
| 1 | Muricella sp. 3 | 39864 | 30 cm | 18 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | ||
| 4 | Muricella sp. 3 | 39865 | 60 cm | 14 m | Sorido wall, E Kri | 0°33’13.20”S; 130°41’16.91”E | ||
| 1 | Muricella sp. 3 | 39866 | 70 cm | 12 m | Turtles Reef | 0°32’35.16”S; 130°41’51.06”E | ||
| 1 | Muricella sp. 3 | 39867 | 25 cm | 18 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | ||
| 5 | Muricella sp. 3 | 39870 | 70 cm | 23 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | ||
| 1 | Muricella sp. 3 | 39872 | 80 cm | 23 m | NW Batanta | 0°47’45.78”S; 130°30’21.24”E | ||
| Hippocampus denise | 1 | Annella mollis | 39875 | 50 cm | 20 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | |
| 1 | Annella mollis | 39876 | 80 cm | 20 m | Sleeping barracuda | 0°32’43.14”S; 130°42’01.62”E | ||
| 1 | Annella cf. mollis | 39877 | 100 cm | 22 m | S Kri, Kri Isl. | 0°33’32.26”S; 130°41’15.48”E | ||
| 1 | Annella cf. mollis | 39881 | 50 cm | 22 m | Mike’s Point, SE Gam Kerupiar Isl. | 0°30’57.06”S; 130°40’22.14”E | ||
| 4 | Annella reticulata | 39878 | 60 cm | 24 m | W Mansuar | 0°30’41.76”S; 130°33’35.34”E | ||
| 3 | Annella reticulata | 39879 | 40 cm | 22 m | W Mansuar | 0°30’41.76”S; 130°33’35.34”E | ||
| 4 | Annella reticulata | 39880 | 30 cm | 24 m | W Mansuar | 0°30’41.76”S; 130°33’35.34”E | ||
| 4 | Annella reticulata | 39882 | 30 cm | 22 m | Yeffam Isl., NW Pulau Keruo | 0°35’15.36”S; 130°17’42.66”E | ||
| 1 | Annella reticulata | 39952 | 60 cm | 22 m | Timba Timba Isl. (Semporna) | 4°33’37.70”N; 118°55’30.40”E | ||
| 1 | Muricella sp. 2 | 39873 | 100 cm | 20-25 m | Yeffam Isl., NW Pulau Keruo | 0°35’15.36”S; 130°17’42.66”E | ||
| Hippocampus pontohi | 2 | not collected | – | – | – | Timur I (Bunaken) | 1°36’38.46”N; 124°46’58.74”E | |
| 1 | Thyroscyphus fruticosus (Esper, 1793) | 39883 | 20 cm | 8 m | Nikson, SE Mansuar | 0°34’51.42”S; 130°38’31.62”E | ||
| 1 | Thyroscyphus fruticosus (Esper, 1793) / Lytocarpia phyteuma (Kirchenpauer, 1876) | 39884 / 39886 | – | 26 m | S Kri, Kri Isl. | 0°33’32.26”S; 130°41’15.48”E | ||
| 1 | Clytia cf. gravieri (Billard, 1904) | 39885 | – | 20 m | Mioskon Isl. | 0°29’48.48”S; 130°43’37.38”E | ||
| Hippocampus severnsi | 3 | not collected | – | – | – | Siladen I, SE Siladen (Bunaken) | 1°37’30.66”N; 124°47’53.88”E |
Family Acanthogorgiidae Gray, 1859
Genus Muricella Verrill, 1869
Muricella Verrill, 1869: p. 450
Muricella Bayer, 1981: p. 920, 945
Muricella Grasshoff, 1999: p. 33
In the remarks of the species descriptions of Muricella plectana Grasshoff, 1999, and Muricella paraplectana Grasshoff, 1999, from New Caledonia,
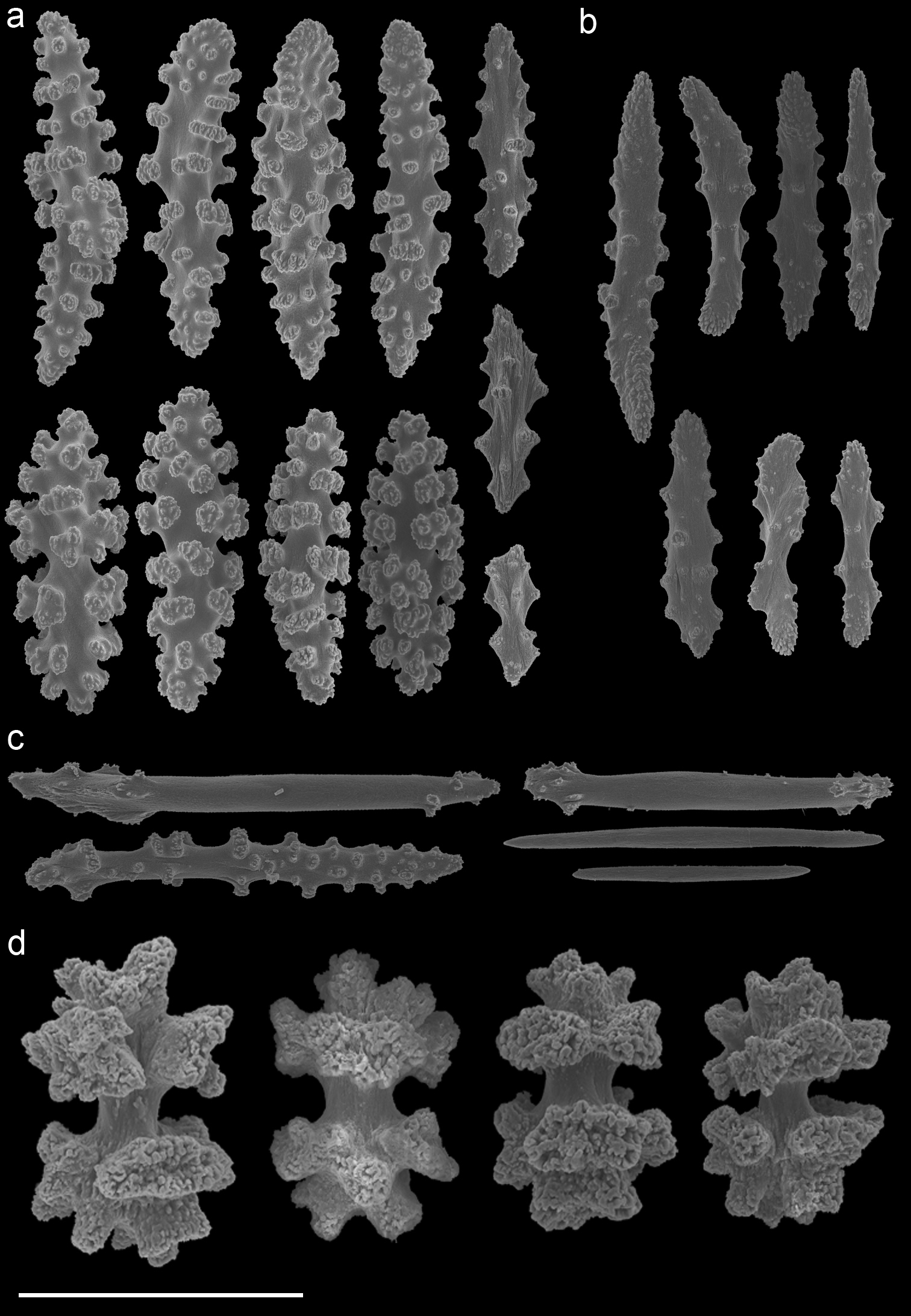
Firstly, Muricella sp. 1 (RMNH Coel. 39868, 39871, 39874) is characterized by wide, plump, capstans from the adaxial layer, up to 0.12 mm long (Fig. 5). Secondly, Muricella sp. 2 (RMNH Coel. 39869, 39873) is characterized by small, slender adaxial capstans, up to 0.05 mm long (Fig. 6). Thirdly, Muricella sp. 3 (RMNH Coel. 39864-67, 39870, 39872), is characterized by adaxial capstans intermediate in shape between the first two, up to 0.10 mm long, and big spindles with rounded ends (Fig. 7). The latter are lacking in the first two species. Muricella plectana has similar plump spindles in the coenenchyme but differs from the present material by lacking the bent spindles from the polyp (Fig. 3). Muricella paraplectana differs from all other material by having spindles with pointed ends (Fig. 4).
Family Subergorgiidae Gray, 1859
Genus Annella Gray, 1858
Annella Gray, 1858: p. 287
Suberogorgia Stiasny, 1937: p. 83
Subergorgia Bayer, 1981: p. 910
Annella Grasshoff, 1999: p. 16
According to
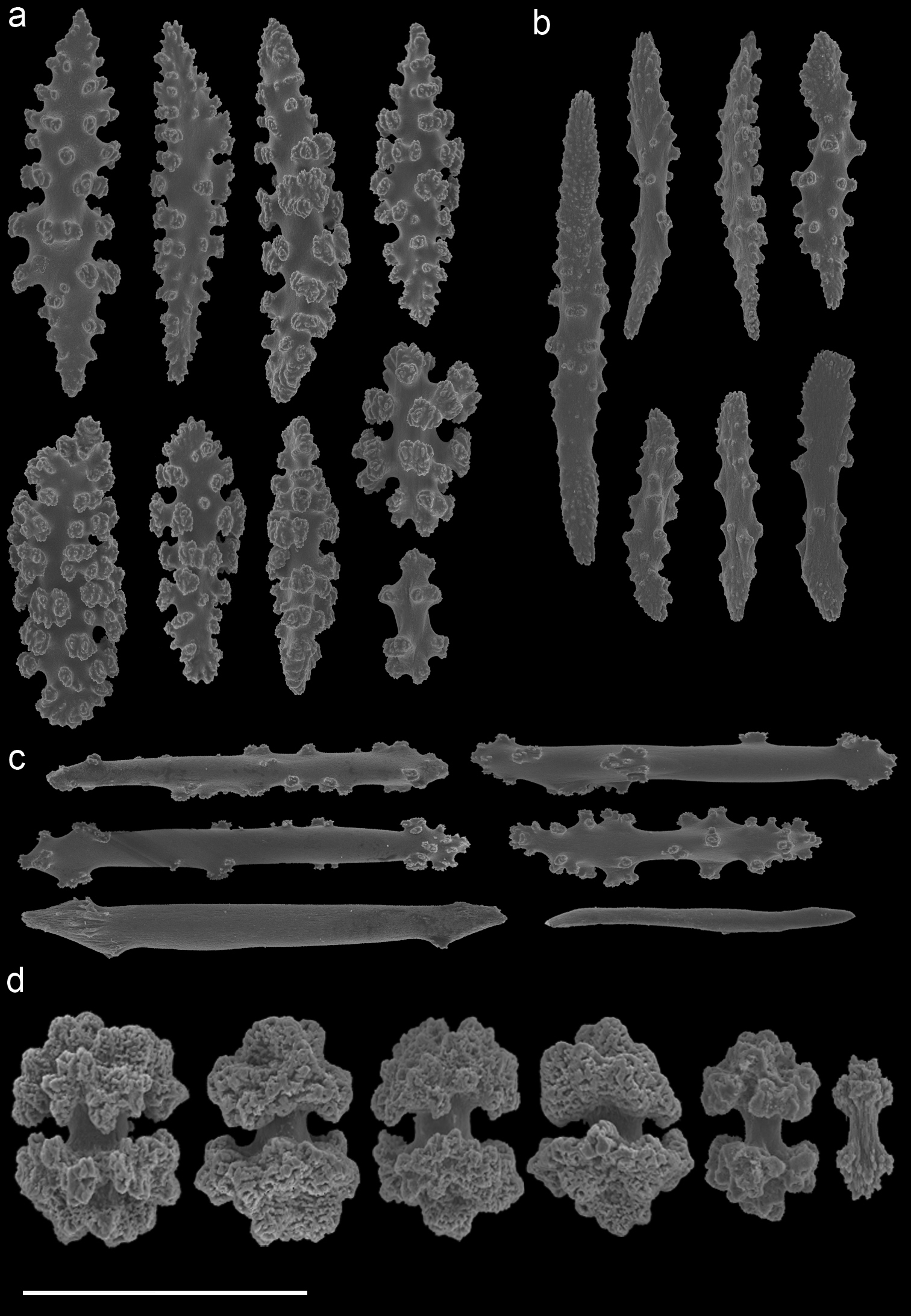
The taxonomic history of the genus Annella is puzzling. Ellis and Solander (1786) described Gorgonia reticulata and added a drawing of the habitus without further description or indication of its type locality. The type specimen of Gorgonia reticulata is presumably lost. Subsequently, Gray (1857[1858]) described the genus Annella, with Annella reticulata as type species, but it is unknown whether he associated this species with Gorgonia reticulata.Later, Nutting (1910) described Euplexaura reticulata (Fig. 8), probably without considering a possible homonymy involving Annella reticulata and Gorgonia reticulata.
Nutting (1910) described a different species as Euplexaura mollis (type locality Moluccas).
A taxonomic revision of Annella has not yet been made, but
The sclerites of the holotype of Euplexaura mollis from the Moluccas (= Annella mollis sensu
On four occasions specimens of Hippocampus pontohi were observed and three of their hosts were collected. Two records of Hippocampus pontohi individuals are from a colony of Thyroscyphus fruticosus (Esper, 1793) (RMNH Coel. 39883-4), a common littoral species on coral reefs with a distribution range throughout Indonesia (Prof. W. Vervoort, pers. comm.). A single individual from Kri Island (Raja Ampat) was found on a specimen of Thyroscyphus fruticosus intertwined with a specimen of the hydroid Lytocarpia phyteuma (Kirchenpauer, 1876) (RMNH Coel. 39886), therefore both co-host species are listed in Table 2. Lytocarpia phyteuma is an uncommon hydrozoan, which can be found at 0–50 m depth, especially in eastern Indonesia (Prof. W. Vervoort, pers. comm.). The Hippocampus pontohi individualfrom Mioskon Island was found on specimens of Clytia cf. gravieri (Billard, 1904) (RMNH Coel. 39885), a common hydrozoan on coral reefs with a wide (sub-) tropical distribution range. Due to the small amount of collected material, a positive identification is not possible. This hydroid species was also recorded during previous expeditions in Indonesia, such as the Snellius II expedition (1983–84) (unpublished data Prof. W. Vervoort). The host of Hippocampus severnsi was unfortunately not sampled and therefore its identity remains unknown.
DiscussionMany sessile marine organisms contribute to the high
marine biodiversity in the so-called Coral Triangle by acting as host
for many associated organisms (
Pygmy seahorses were observed to remain on a single
gorgonian for periods of at least 3–40 weeks. Information on the pygmy
seahorse whereabouts after this period is lacking, and movement between
different hosts was not directly observed (
In the case of Hippocampus bargibanti two host species have been recorded in the literature, Muricella plectana and Muricella paraplectana. Three additional Muricella species from the Indo-Pacific, different from Muricella plectana or Muricella paraplectana, were found during the present study. Hippocampus bargibanti is therefore associated with at least five different Muricella spp.Unfortunately, the genus Muricella is in need of a revision (
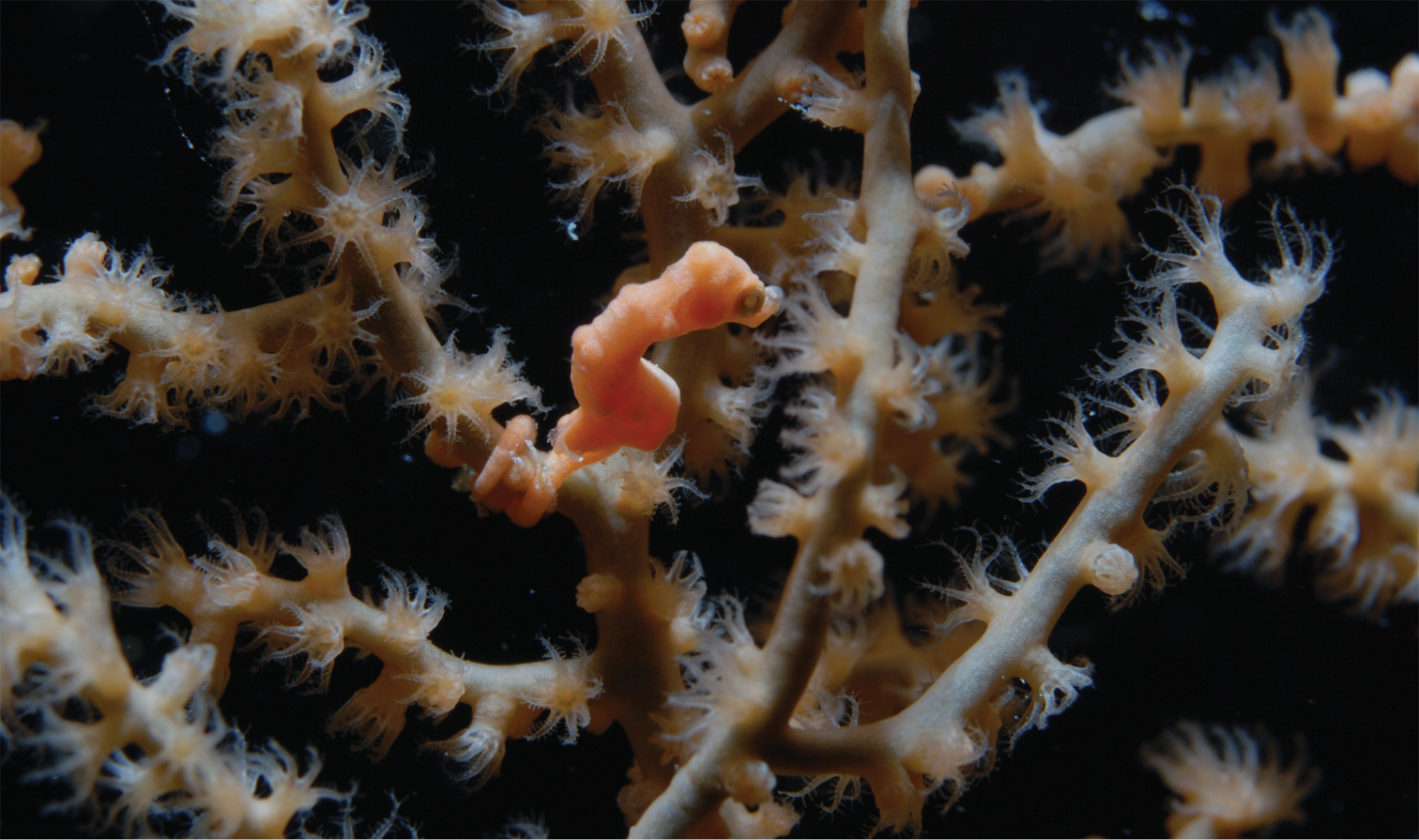
Individuals of Hippocampus denise primarily occur on colonies of Annella spp., which they strongly resemble in colour pattern, resulting in an optimal camouflage. Based on our results Hippocampus denise lives in association with at least three different Annella species: Annella reticulata, Annella mollis and Annella cf. mollis. No other Annella species are currently recognized, and a revision of the genus Annella is needed. This will most likely show that additional Annella species await description. One individualof Hippocampus denise was found on Muricella sp. 2 (Fig. 13). This association was already known (Table 1), but appears quite unusual. Additional host genera for Hippocampus denise are expected, based on published photographs (
Different colour morphs are recorded for the gorgonian-associated species Hippocampus bargibanti, and Hippocampus denise (Lourie et al.2004,
For other pygmy seahorse species new colour morphs may be encountered, since pygmy seahorses are enigmatic species that are popular objects for divers and underwater photographers. As a result they often appear in dive magazines and field guides. Occasionally these pictures show new colour morphs or maybe even new species. For future research such observations and sightings can contribute to the general knowledge and ecology of pygmy seahorses, especially when the host organisms are collected for taxonomic studies.
Reliability of identificationsPrevious identifications of the hosts of Hippocampus bargibanti may well be in error since they were made by non-gorgonian specialists, except for the identifications in
For many organisms molecular methods can be of help
to identify species, but so far barcoding of Octocorallia for the COI
gene has not been successful. Even when sequences are obtained,
information on species level is very limited. Most research is currently
limited to three genes, which are still unsatisfactory to identify
species (
The distribution ranges of the pygmy seahorses are largely situated within the Coral Triangle (
This paper shows that pygmy seahorses are associated with more gorgonian and hydrozoan hosts than previously assumed, resulting in new associations; Hippocampus bargibanti is associated with five species of the genus Muricella, Hippocampus denise is associated with three Annella species, and Hippocampus pontohi with four hydrozoan and one algae species. No new records are available for Hippocampus severnsi. The presumed association of colour morphs of Hippocampus bargibanti with certain Muricella species cannot be confirmed based on our present results. Future work on pygmy seahorses should preferably include more attention for their hosts, including taking tissue samples for identification by an octocoral taxonomist.
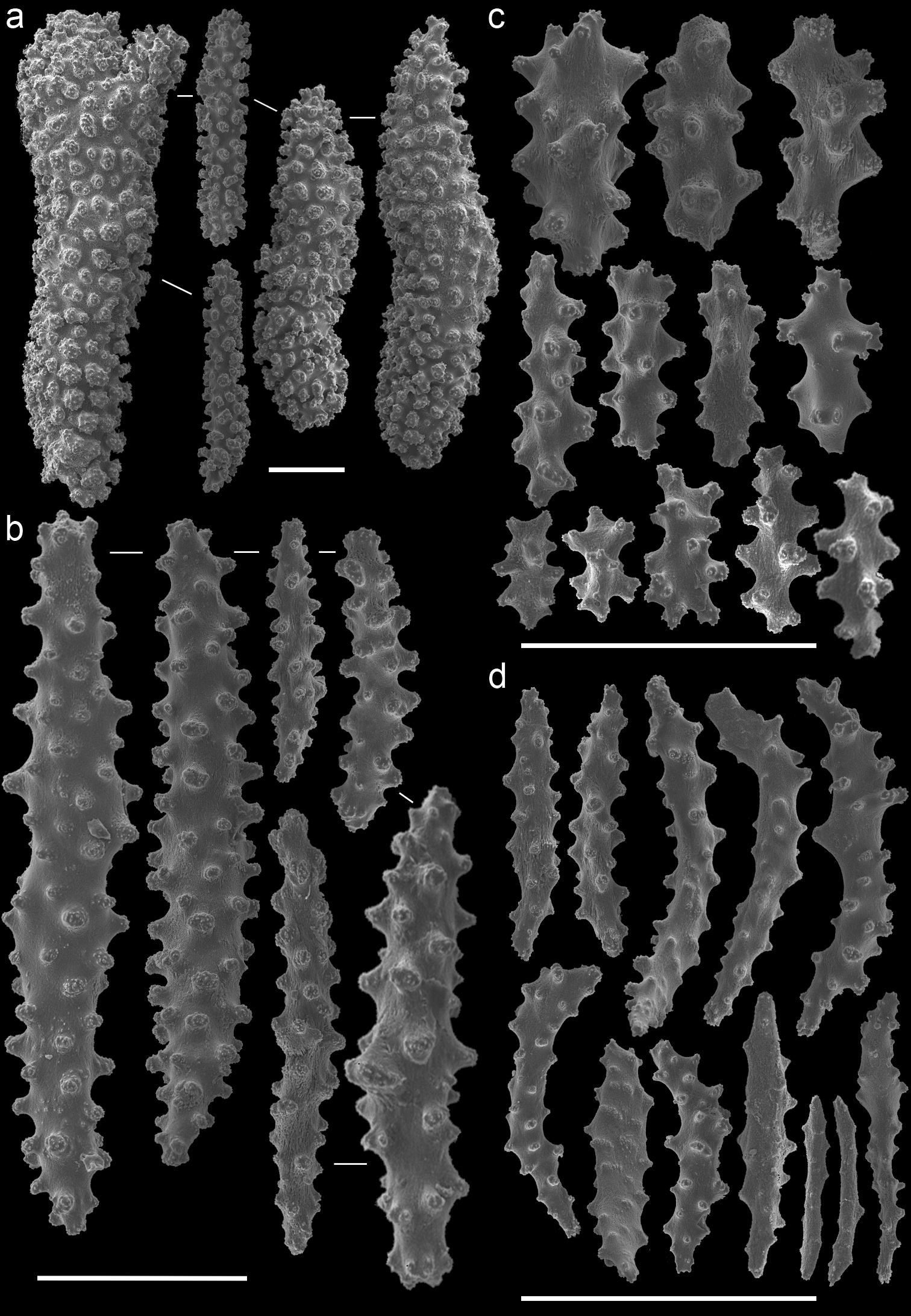
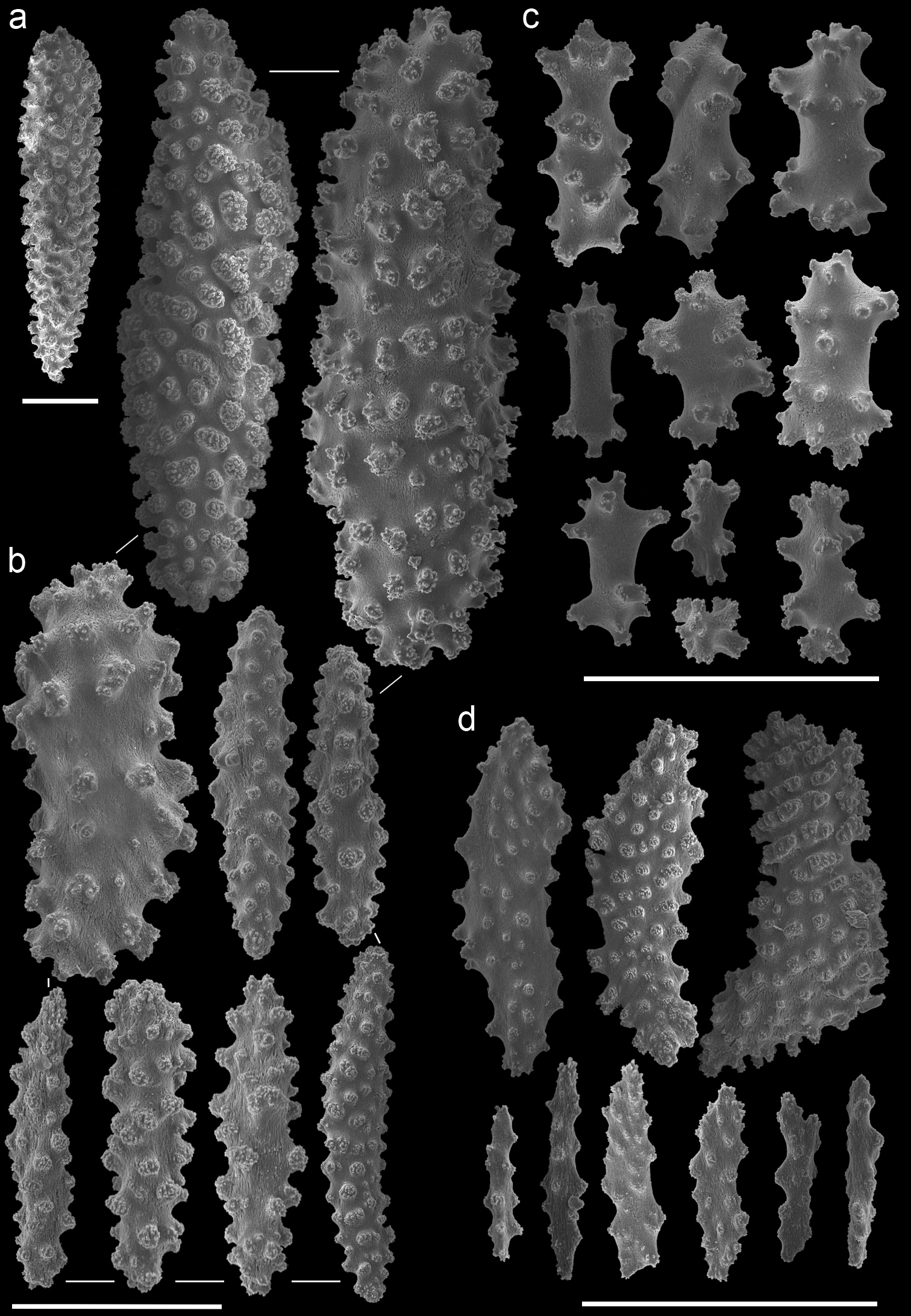
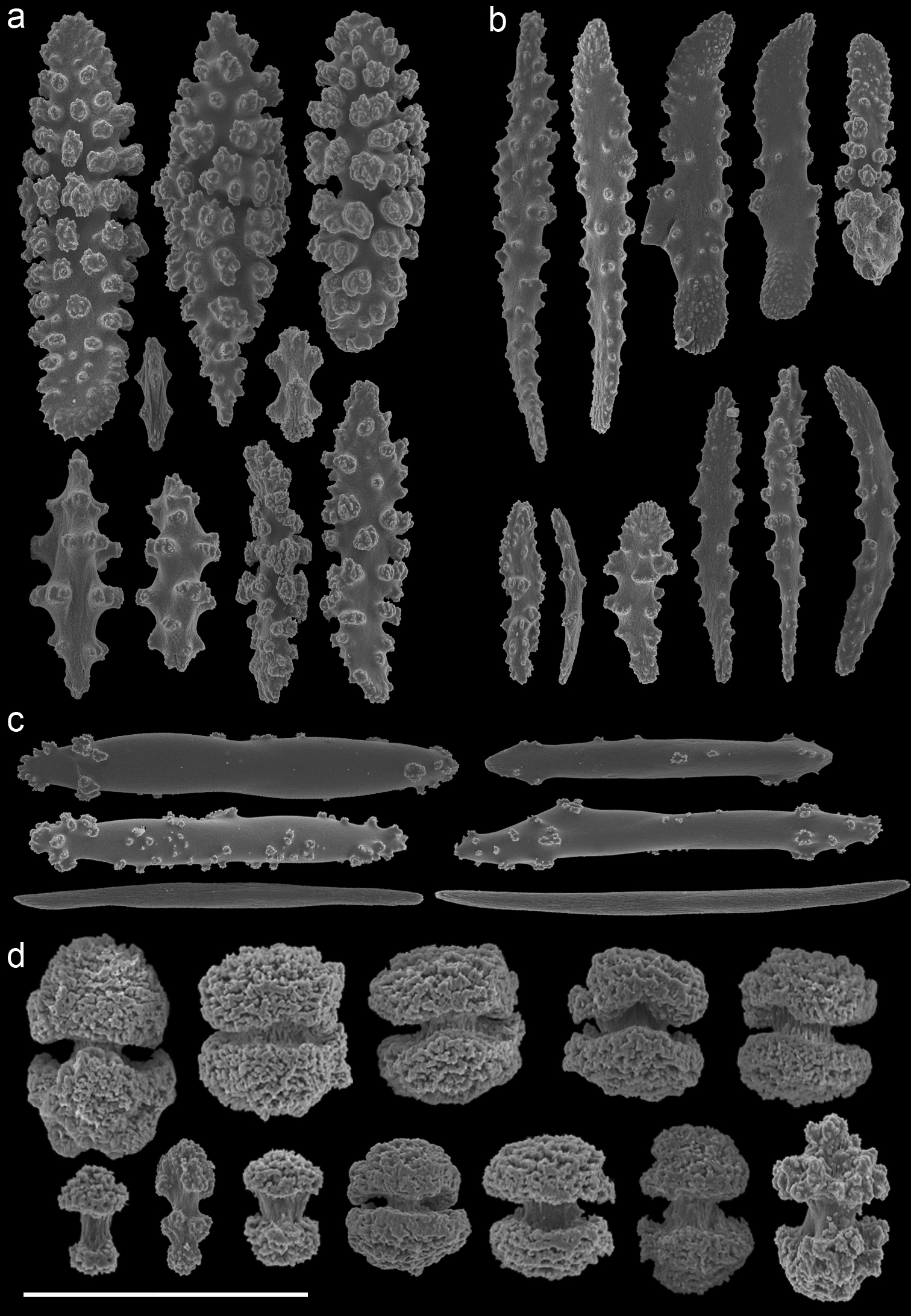
Muricella plectana (MNHN, HG-100 - holotype)A spindles from coenenchyme and polyp B smaller spindles from coenenchyme and polyp C capstans from adaxial layer D rods from tentacle. Scale bars represent 0.1 mm.
Muricella paraplectana (MNHN, HG-121 - holotype)A spindles from coenenchyme and polyp B smaller spindles from coenenchyme and polyp C capstans from adaxial layer D rods from tentacle. Scale bars represent 0.1 mm.
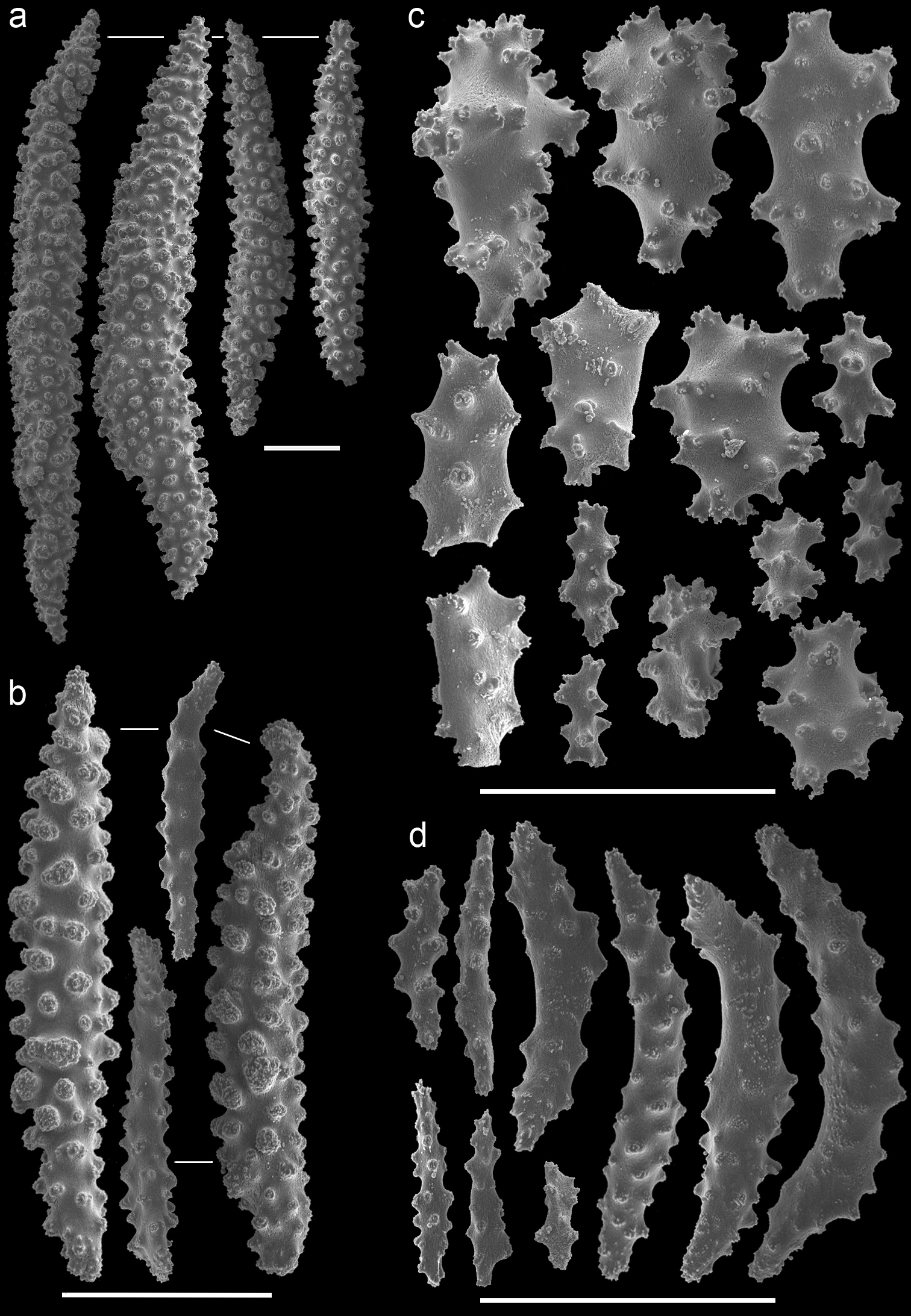
Muricella sp. 1 (RMNH Coel. 39871)A spindles from coenenchyme and polyp B smaller spindles from coenenchyme and polyp C capstans from adaxial layer D rods from tentacle. Scale bars represent 0.1 mm.
Muricella sp. 2 (RMNH Coel. 39873) A spindle from coenenchyme B smaller spindles from coenenchyme and polyp C capstans from adaxial layer D rods from tentacle. Scale bars represent 0.1 mm.
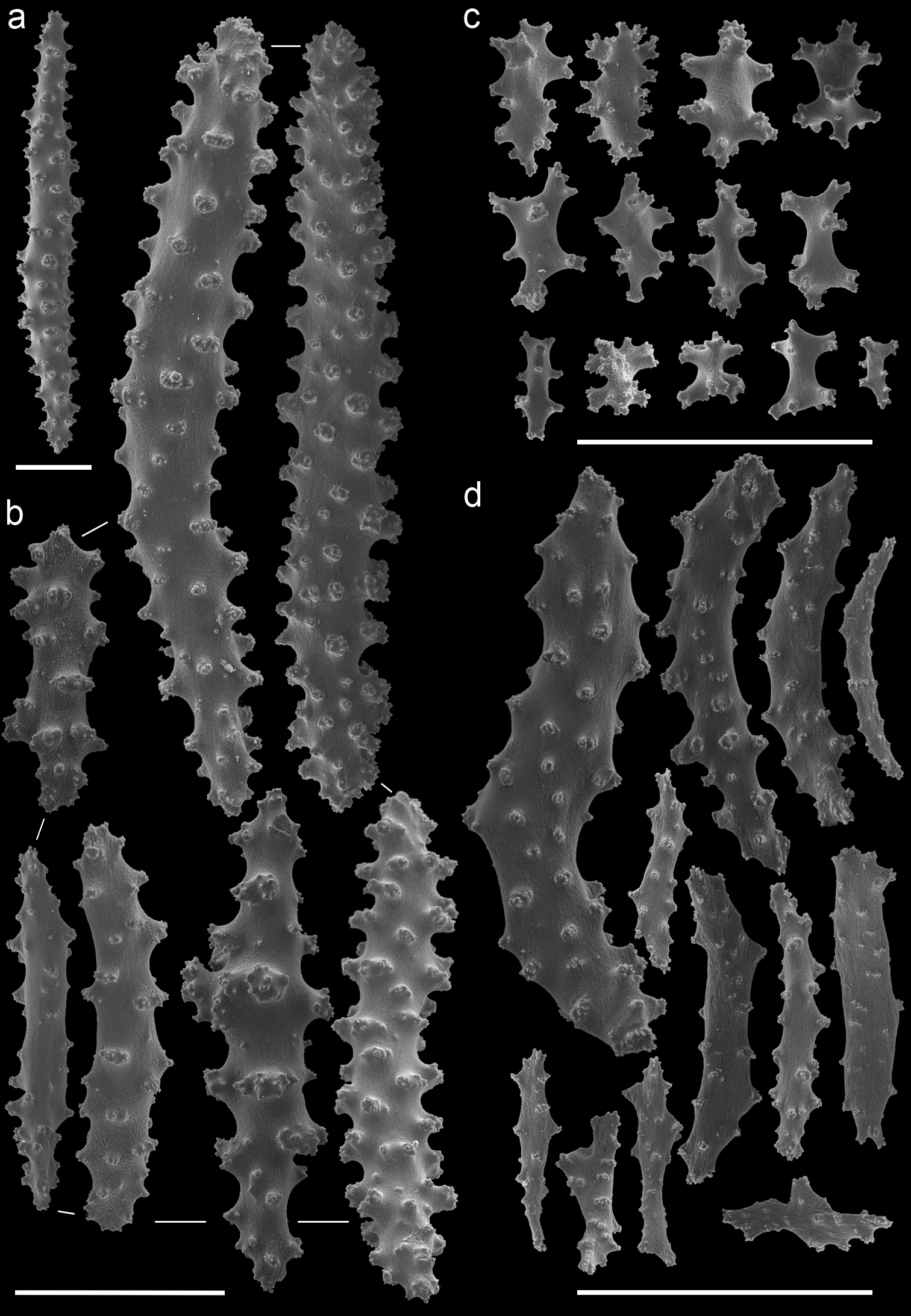
Muricella sp. 3 (RMNH Coel. 39865) A spindle from coenenchyme B smaller spindles from coenenchyme and polyp C capstans from adaxial layer D rods from tentacle. Scale bars represent 0.1 mm.
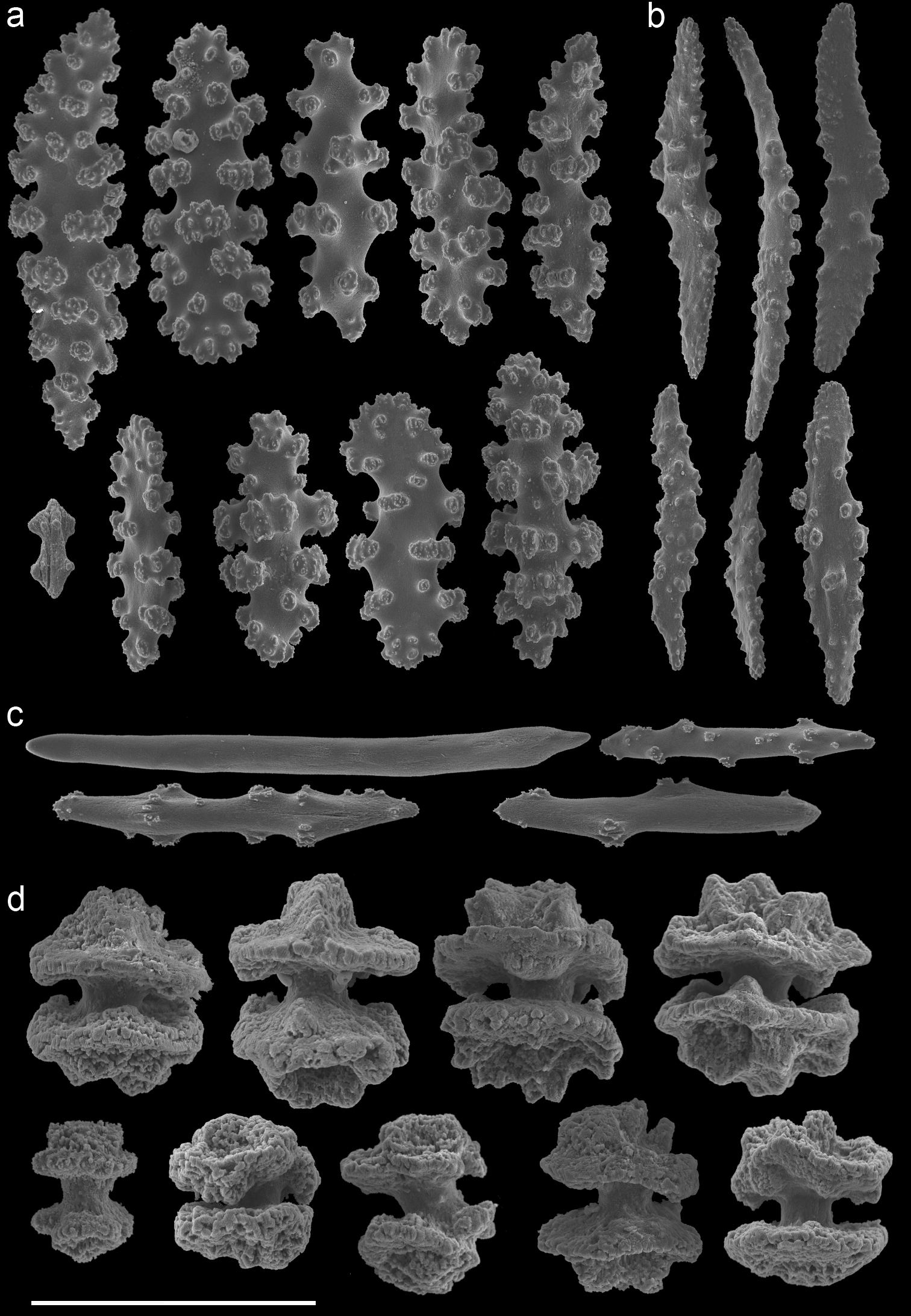
Euplexaura reticulata (ZMA Coel. 3504 - holotype) A spindles from the coenenchyme B tentacle rods C medulla spindles from the axis D double heads from the surface layer. Scale bar represents 0.1 mm, except for D which is 0.05 mm.
Euplexaura mollis (ZMA Coel. 3498 - holotype) A spindles from the coenenchyme B tentacle rods C medulla spindles from the axis D double heads from the surface layer. Scale bar represents 0.1 mm, except for D which is 0.05 mm.
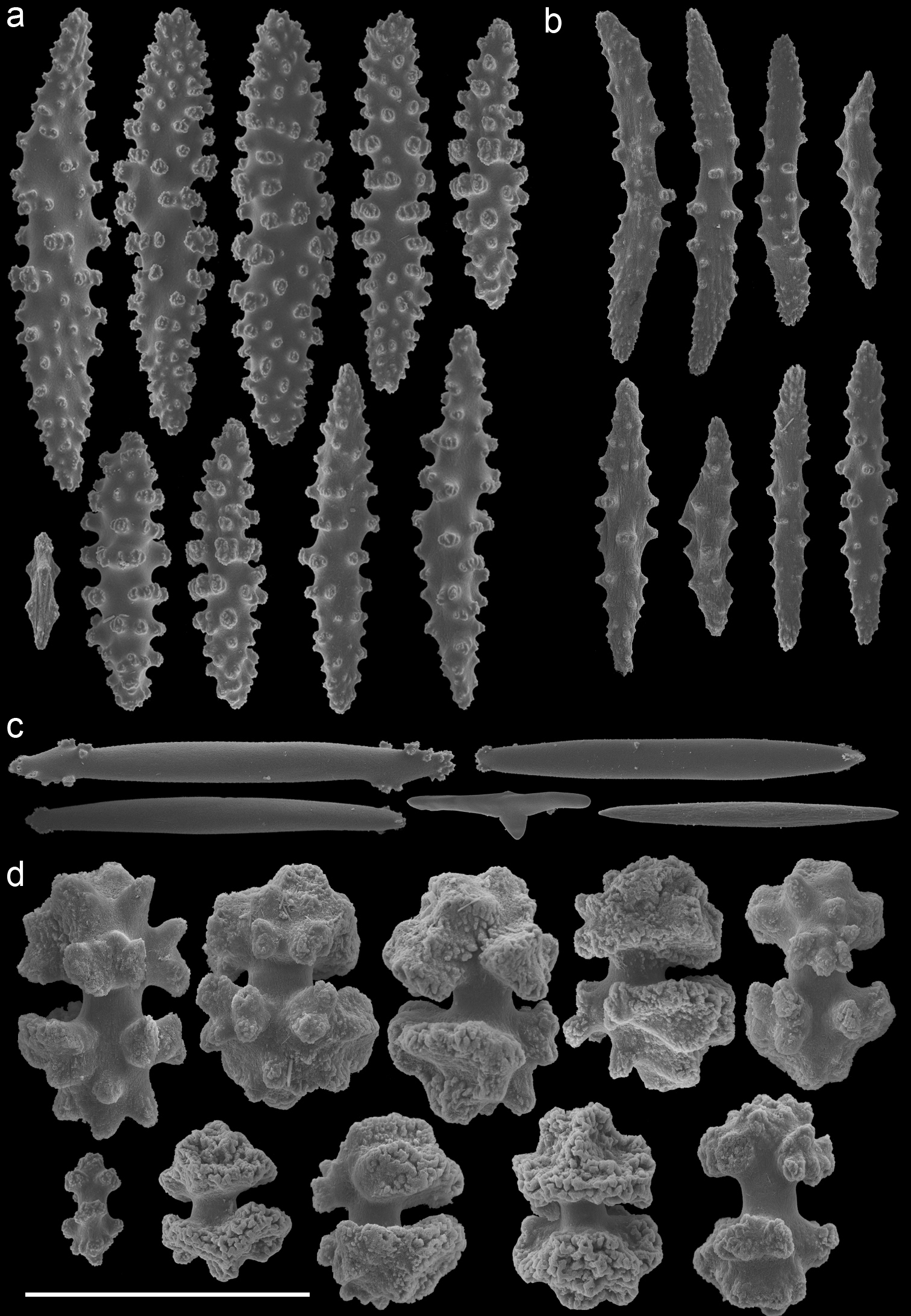
Annella reticulata (RMNH Coel. 39882) A spindles from the coenenchyme B tentacle rods C medulla spindles from the axis (d) double heads from the surface layer. Scale bar represents 0.1 mm, except for D which is 0.05 mm.
Annella mollis (RMNH Coel. 39875) A spindles from the coenenchyme B tentacle rods C medulla spindles from the axis D double heads from the surface layer. Scale bar represents 0.1 mm, except for D which is 0.05 mm.
Annella cf. mollis (RMNH Coel. 39877) A spindles from the coenenchyme B tentacle rods C medulla spindles from the axis D double heads from the surface layer. Scale bar represents 0.1 mm, except for D which is 0.05 mm.
A rare occurrence, Hippocampus denise on Muricella sp. 2 (RMNH Coel. 39873) at Raja Ampat (photo F.R. Stokvis).
Dr Bert W. Hoeksema (NCB Naturalis) and Mrs. Yosephine Tuti (RCO-LIPI) jointly organized the Raja Ampat (2007) and Ternate (2009) expeditions. Fieldwork in Bunaken (2008) was conducted by the first and second author. LIPI (in 2007 and 2008) and RISTEK (in 2009) granted the research permits for the work in Indonesia. The research was accommodated by Papua Diving, Bunaken Village and the LIPI-Ternate field station. The Semporna Marine Ecological Expedition (SMEE2010) was jointly organized by WWF-Malaysia, Universiti Malaysia Sabah’s Borneo Marine Research Institute, NCB Naturalis and Universiti Malaya’s Institute of Biological Sciences. Research permission for SMEE2010 was granted by Economic Planning Unit, Prime Minister’s Department, Economic Planning Unit Sabah, Sabah Parks and Department of Fisheries Sabah. The MV Celebes Explorer accommodated the research. Frank R. Stokvis photographed many of the pygmy seahorses and collected coral hosts in Raja Ampat. Additional pygmy seahorses were recorded by Dr Bert W. Hoeksema, Dr Willem Renema, and Eva M. van der Veer. Prof. Dr Wim Vervoort (NCB Naturalis) identified the hydrozoan hosts of Hippocampus pontohi. Dr James D. Reimer (University of the Ryukyus) helped with information on zooanthids, and Dr Sara Lourie (McGill University) checked our pygmy seahorse identifications, and provided extra background information. Dr Rob W.M. van Soest and Ms. Elly Begliner (NCB Naturalis, section ZMA) provided us with the holotypes of Euplexaura mollis and Euplexaura reticulata and Dr A. Andouche and Dr N. Ameziane (Muséum nationale d’Histoire naturelle) provided the holotypes of Muricella plectana and Muricella paraplectana. Funding for the various expeditions was provided by the Van Tienhoven Foundation for International Nature Protection, Schure-Beijerinck-Popping Fund (KNAW), a National Geographic Young Explorers Grant, Stichting Fonds Dr C. van Tussenbroek (Nell Ongerboerfonds), Alida M. Buitendijkfonds, Stichting L.B. Holthuisfonds, Jan-Joost ter Pelkwijkfonds (all NCB Naturalis), and Leiden University Funds. Singapore Airlines and SilkAir provided logistical support. Dr Yehuda Benayahu and an anonymous reviewer provided valuable comments on the manuscript.