(C) 2011 Carmelo Andújar. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the genus Parazuphium (Coleoptera, Carabidae, Zuphiini), Parazuphium aguilerai sp. n., is described from the Tingitan peninsula in North Morocco. The only known specimen was found under a large deeply buried boulder, and belongs to an anophthalmous, depigmented and flattened species. This is the second species of blind Parazuphium known so far, the other being Parazuphium feloi Machado 1998 from a lava tube in the Canary Islands. Molecular data of the only known Parazuphium aguilerai sp. n. specimen are provided, and a reconstructed phylogeny based on these molecular data confirms its inclusion inside Zuphiini within Harpalinae. Identification keys to the Mediterranean and Macaronesian species of Parazuphium are provided.
Taxonomy, new species, Parazuphium, identification key, endogean fauna, molecular phylogeny
The genus Parazuphium Latreille (Coleoptera, Dryptinae, Zuphiini)
is characterized by the presence in the aedeagus of a strong ventral
constriction between the basal and the distal part of the median lobe, a
unique structure within the Carabidae (
The species of the genus seem to be associated with deep soil or the soil crevices near rivers or temporary flooded areas (
During an entomological expedition to North Morocco we found the single specimen of a new species of Parazuphium,
anophthalmous and with strong modifications apparently related to its
endogean habitat. Despite an attempt to collect additional material the
following year no other specimen was found, possibly due to the
endogean habits of this species. We describe the species here, and
provide some molecular data to characterize it and to postulate its
phylogenetic position among the Zuphiini for which genetic data are available (
The unique specimen was killed and stored in absolute ethanol in the field, and total DNA was extracted using the QIAGEN Dneasy tissue kit (Qiagen, Hilden, Germany), without destroying the external cuticle. The extracted specimen was mounted in DMHF (Dimethyl Hydantoin-Formaldehyde) on a transparent acetate label. For the morphological study and photographs we used a Zeiss Stemi 2000C Trinocular Zoom Stereomicroscope with Spot Insight Firewire digital camera and software.
Molecular methodsTotal genomic DNA for the single specimen of Parazuphium aguilerai sp. n. was extracted using QIAGEN Dneasy tissue kit (Qiagen, Hilden, Germany). To characterize the new species we amplified fragments of six genes, four mitochondrial and two nuclear: 3' end of cytochrome c oxidase subunit (cox1); a single fragment including the 3' end of the large ribosomal unit (rrnL), the whole tRNA-Leu gene (trnL) and the 5' end of the NADH dehydrogenase 1 (nad1); 5' end of the small ribosomal unit, 18S rRNA (SSU); and an internal fragment of the large ribosomal unit, 28S rRNA (LSU). Primers used are given in Table 1. Additionally, we extracted DNA from one specimen of Parazuphium cf. baeticum (K. and J. Daniel 1898), Zuphium olens Rossi 1790, Ildobates neboti Español 1966 and several other outgroups among Carabidae (Table 2), which were amplified for the same molecular gene fragments. PCR reactions were made using PuReTaq Ready-To-Go PCR beads (GE Healthcare, UK) and standard conditions [39 cycles using 48–50°C as annealing temperature]. New sequences have been deposited in GenBank (NCBI) with Acc. Nos JF778779-JF778845. Each individual gene matrix was aligned in MAFFT with the Q-ins-i option and default parameters. The four genes fragments were concatenated to get a final dataset of 20 taxa and 3376 bp that was employed in phylogenetic analyses. Table 2 shows taxa information, source and accession number for each DNA sequence.
Primers used in the study. F, forward; R, reverse. Length refers to the aligned matrix.
| Type DNA | Gene | Length | Primer | S | Primer sequence (5'- 3') | Described in: |
|---|---|---|---|---|---|---|
| Mitochondrial protein coding | cox1 | 755 | Jerry (M202) | F | CAACATTTATTT-TGATTTTTTGG | ( |
| Pat (M70) | R | TCCA(A)TGCACTA-ATCTGCCATATTA | ( |
|||
| Mitochondrial ribosomal | rrnL | 744 | 16SaR (M14) | F | CGCCTGTTTA-WCAAAAACAT | ( |
| 16s-ND1a (M223) | R | GGTCCCTTACGAA-TTTGAATATATCCT | ( |
|||
| Nuclear ribosomal | LSU | 1240 | LS58F (D1) | F | GGGAGGAAA-AGAAACTAAC | ( |
| LS998R (D3) | R | GCATAGTTC-ACCATCTTTC | ( |
|||
| Nuclear ribosomal | SSU | 625 | 5'b5.0 | F | GACAACCTGGTT-GATCCTGCCAGT | ( |
| R | TAACCGCAA-CAACTTTAAT | ( |
Species, locality of collection, voucher reference and accession numbers for each sequence.
| Especie | Locality | Voucher | cox1 | rrnL | LSU | SSU |
|---|---|---|---|---|---|---|
| Laemostenus terricola | Alicante, Spain | 1583BG | JF778779 | JF778796 | JF778812 | JF778829 |
| Leistus spinibarbis | Albacete, Spain | 1581BG | JF778780 | JF778797 | JF778813 | JF778830 |
| Calosoma sycophanta | Albacete, Spain | 1590BG | JF778781 | JF778798 | JF778814 | JF778831 |
| Carabus (Eucarabus) deyrollei | Lugo, Spain | 1553BG | JF778782 | JF778799 | JF778815 | JF778832 |
| Carabus (Limnocarabaus) clathratus | Susuz, Turkey | 1600BG | JF778783 | JF778800 | JF778816 | JF778833 |
| Dixus capito | Albacete, Spain | 1578BG | JF778784 | N/A | JF778817 | JF778834 |
| Pseudotrechus mutilatus | Cádiz, Spain | 36_EN | JF778785 | JF778801 | JF778818 | JF778835 |
| Licinus punctatulus | Alicante, Spain | 1582BG | JF778786 | JF778802 | JF778819 | JF778836 |
| Elaphropus (Tachyura) parvulus | Pays Zaer Zaine, Morocco | 64_EN | JF778787 | JF778803 | JF778820 | JF778837 |
| Bembidion (Peryphus) hispanicum | Pays Zaer Zaine, Morocco | 62_EN | N/A | JF778804 | JF778821 | JF778838 |
| Bembidion (Emphanes) latiplaga | Pays Zaer Zaine, Morocco | 65_EN | JF778788 | N/A | JF778822 | JF778839 |
| Perileptus aerolatus | Agadir, Morocco | MNHN-AF113 | GQ293688 | FR729593 | GQ293625 | GQ293503 |
| Trechus quadristriatus | Huesca, Spain | MNHN-AF96 | FR733908 | GQ293743 | GQ293619 | GQ293534 |
| Typhloreicheia laurentii | Sardinia, Italy | 56_EN | JF778789 | JF778805 | JF778823 | JF778840 |
| Dyschiriodes sp. | Pays Zaer Zaine, Morocco | 63_EN | JF778790 | JF778806 | JF778824 | JF778841 |
| Nebria salina | Albacete, Spain | 1579BG | JF778791 | JF778807 | JF778825 | JF778842 |
| Ildobates neboti | Castellón, Spain | MNCN-6409 | JF778792 | JF778808 | AM051084 | DQ130051 |
| Drypta dentata | Ciudad Real, Spain | 98_EN | N/A | JF778809 | N/A | N/A |
| Zuphium olens | Murcia, Spain | 97_EN | JF778793 | N/A | JF778826 | JF778843 |
| Parazuphium cf. baeticum | Castellón, Spain | 87_EN | JF778794 | JF778810 | JF778827 | JF778844 |
| Parazuphium aguilerai | Tanger, Morocco | 31_EN | JF778795 | JF778811 | JF778828 | JF778845 |
Bayesian phylogenetic analyses (BA) were performed with MrBayes v.3.1. (
urn:lsid:zoobank.org:act:B4718866-DA9E-4096-9291-A38E90FD7A0A
http://species-id.net/wiki/Parazuphium_aguilerai
Figs 1–3Souk-Khemis-des-Anjra, Tetuan, Morocco (Fig. 4).
Holotype: 1♂, “MOROCCO 28-III-2008 / Souk-Khemis-des-Anjra, Tetuan / 123m N35°43'18" W5°31'23" / Andújar, Hernando, Ribera & Aguilera leg."; voucher number label “31_EN"; plus red holotype label. Type specimen mounted in DMHF in a transparent acetate label, genitalia dissected and mounted in DMHF in a separate label pinned with the specimen. Deposited in the Museu de Ciències Naturals de Barcelona (MCNB), DNA aliquots deposited in the IBE (CSIC) and Univ. Murcia (ZAFUMU col.).
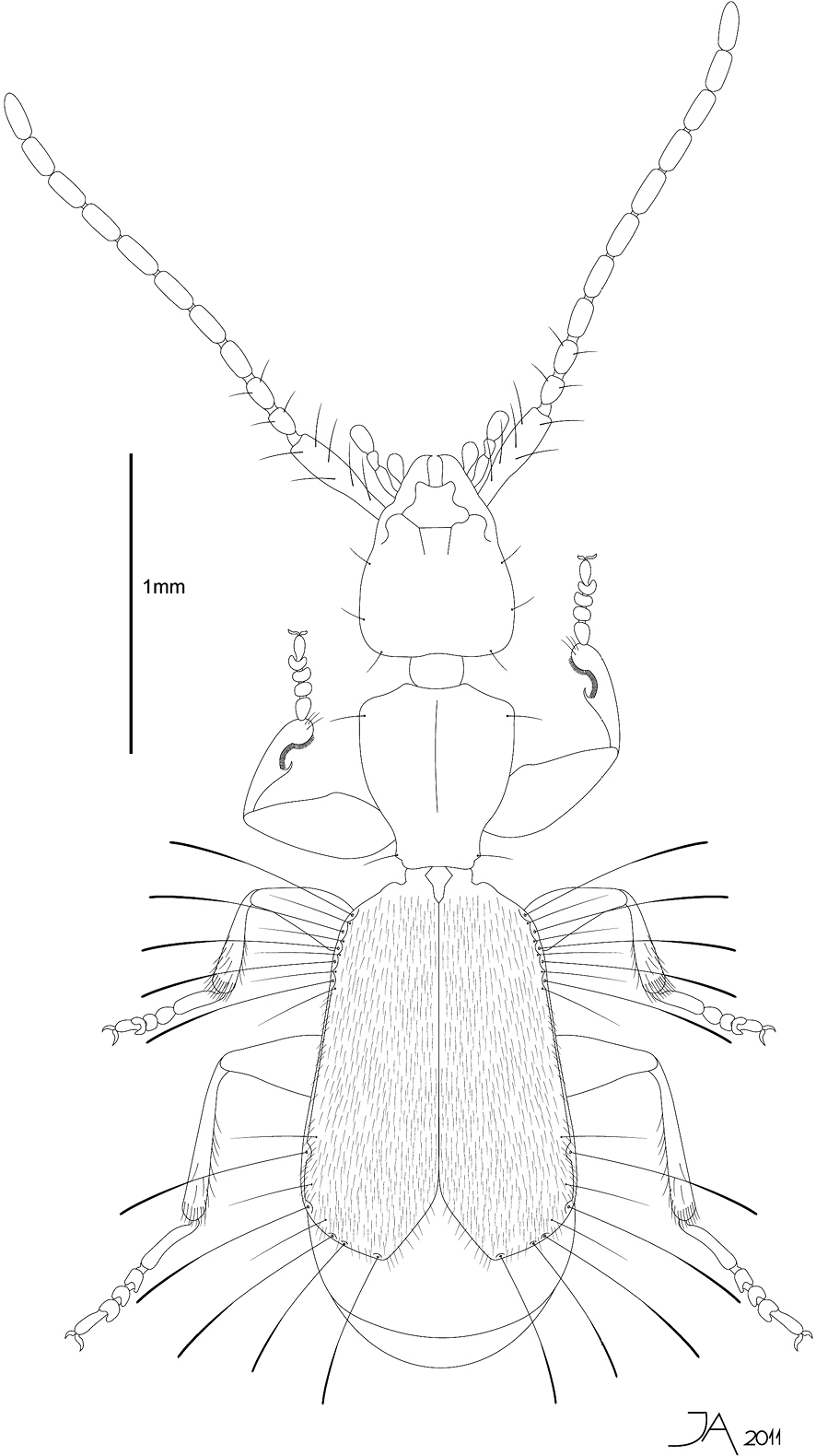
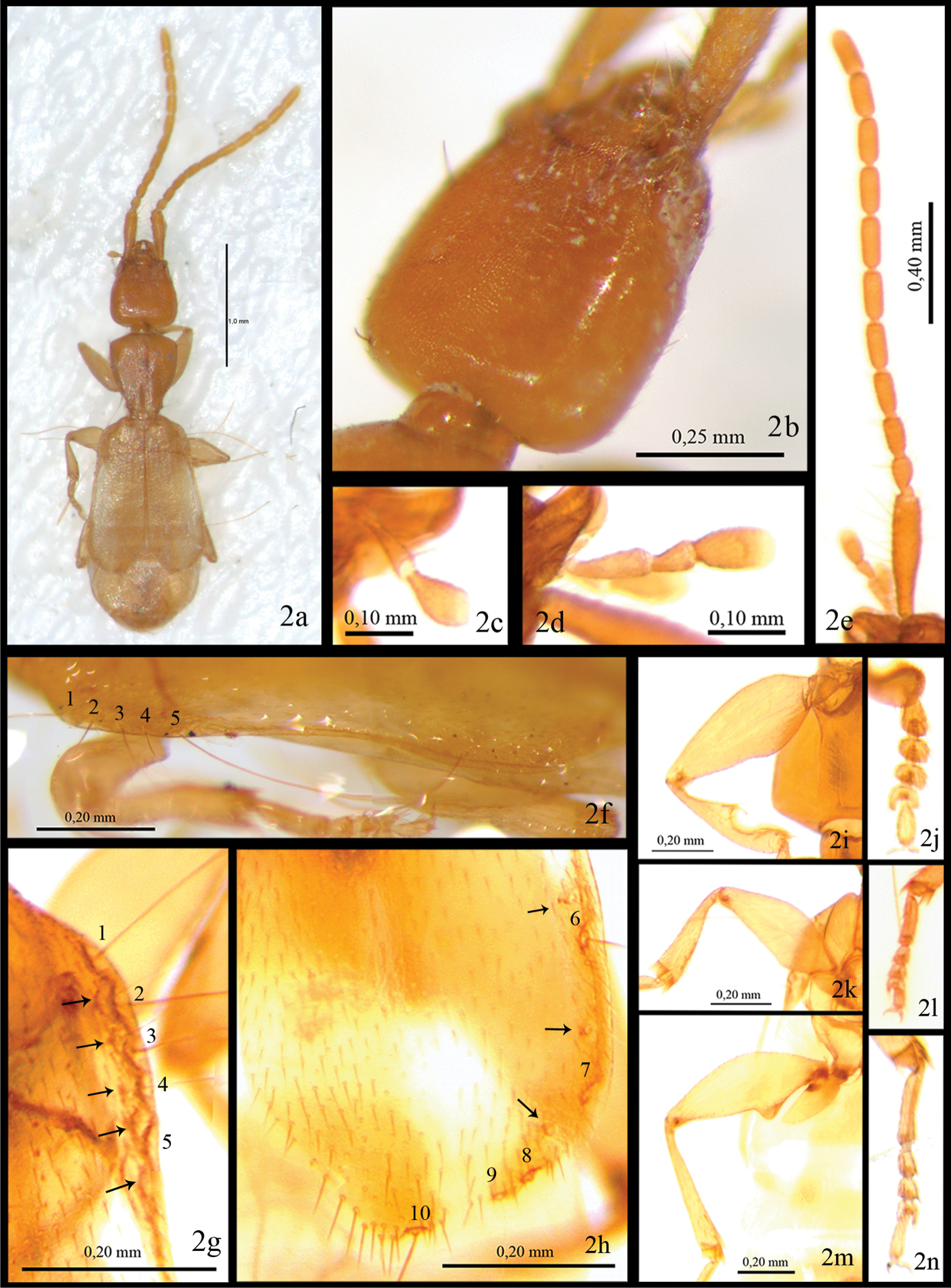
Total Length 2.7 mm (from apex of mandible to apex of elytra). Body depressed, flattened, light brown (Fig. 1). Eyes absent (Fig. 2b). First antennomere (0.41mm) as long as antennomeres 2–4 combined (0.37 mm) (Fig. 2e). Pronotum cordiform (Fig. 1). Elytra flat, not fully covering abdomen (Figs 1, 2a). Umbilicate lateral series of elytra with 5+5 spatuliform setae (Figs 2f-h). Apex of elytra divergent (Figs 1, 2a). Metafemora with an acute tooth on interior margin (Fig. 2m).
Line drawing ofhabitus of Parazuphium aguilerai sp. n. Total length 2.7 mm.
Length of holotype: 2.7 mm. Body depressed, flattened and depigmented, light brown. Surface microreticulate, with mesh pattern regular polygonal (observed on the dried specimen) and scattered short setae.
Head (Fig. 1) with trapezoidal shape. No trace of eyes or ocular scars (Fig. 2b). Length of head (from apex of mandible to base) 0.63 mm; maximum width close to base (0.51 mm). Surface microreticulate, microlines deeper on sides. Neck pedunculate. With three long setae, two lateral and one basal. Appendages: antennae (Fig. 2e) with first antennomere (0.41mm) as long as total length of antennomeres 2–3-4 together (0.37 mm); second antennomere pedunculate (0.1 mm), slightly shorter than third (0.13 mm) and fourth (0.14 mm); from fifth to tenth with same length (0.16–0.17 mm); last antennomere longer (0.23mm). Antennomeres from 3° to 11° cylindrical. Labial and maxillary palpi as in Figs 2c, d.
Photographic images ofParazuphium aguilerai sp. n. A whole specimen B head in dorso-lateral view C labial palpus; (d), maxillary palpus C antenna F margin of left elytron in lateral view G margin of right elytron, detail for anterior umbilicate setae, numbers 1 to 5 H margin of right elytron, detail of posterior umbilicate setae, numbers 6 to10, arrows over them point other smaller setae I–N details of anterior, median and posterior legs respectively.
Pronotum cordiform (Fig. 1), longer (0.60 mm) than wide (0.51–0.27 mm), maximum width (0.51 mm) close to anterior angles, almost double minimum width (0.27 mm), at the posterior angles. Anterior angles obtuse, rounded. Anterior margin regularly convex. Median line apparent, marked with two depressions. Two lateral setae at anterior and posterior angles. Lateral margin sinuate before posterior angles.
Elytra (Figs 1, 2a) flattened, short, not totally covering abdomen, wider apically (maximum width, 0.90mm, close to apex); width at humeral angle 0.65mm. Punctuation forming longitudinal series, more evident at basal third, disappearing towards apex. Entire surface with short pubescence. Anterior umbilicate series with 5 spatuliform setae (Figs 2f-g, numbers 1–5), deeply inserted in small marginal indentations, with some other minor setae over them (Fig. 2g, arrows). Posterior umbilicate series with 5 spatuliform setae, the last one just before apex (Fig. 2h, numbers 6–10), with three smaller setae over them (Fig. 2h, arrows). Margin of elytra from 5° umbilical anterior to 2° umbilical posterior seta with a marginal carina (Fig. 2f). Apices divergent (Figs 1, 2a).
Legs. Pro- and meso-femora dilated proximally, forming an obtuse interior angle (Figs 2i, k). Metafemora with a strong acute tooth on the interior margin (Fig. 2m). Front tibia with antennal cleaner (toilette organ), as reported in other species of the genus (Fig. 2i). Meta-tibia long and straight, with an internal spine at apex. Meso and meta tibiae with a circle of seta round the apex. Pro-tarsomeres 1–4 dilated (Fig. 2j). First meso- and meta-tarsomeres as long as 2° to 4° combined (Figs 2l, n). Fourth tarsomere cordiform. Trochanters without tooth or any other special structure.
Aedeagus. Median lobe as in Fig. 3, short and robust with a ventral constriction between the basal and the distal part as described for the genus. Basal margin arcuate, bisinuate, with the apex rounded. Internal sac with two small sclerites. Parameres asymmetric, as in other species of the genus.
Photographic image of median lobe of Parazuphium aguilerai sp. n. Left lateral aspect.
The single known specimen of Parazuphium aguilerai sp. n. was found under a large, deeply buried boulder, in the humid soil on a hillside with herbaceous vegetation (Chamaerops humilis, Nerium oleander and Pistacia lentiscus, Fig. 4). The same sample included some endogean ants (Leptanilla sp, Amblyopone sp.) and remains of anendogean weevil, Torneuma sp. (Curculionidae, Cryptorhynchinae).
Habitat of Parazuphium aguilerai sp. n.
The specific epithet is a Latinized eponym, genitive case, based on the name of our late friend Pedro Aguilera, who collected the specimen with us during his last trip to Morocco.
Parazuphium aguilerai
sp. n. can be clearly distinguished from any other species of the genus
through the combinations of the following characters: lack of eyes,
reduced size (2.7 mm), length and proportions of 2°, 3° and 4°
antennomeres (0.1, 0.13 and 0.14mm respectively) and the presence of a
tooth on metafemora. Parazuphium feloi from the Canary Islands is also anophthalmous, but it is larger than Parazuphium aguilerai sp. n. and without a tooth on the hind femora (
Key to adults of the West Mediterranean and Macaronesian Parazuphium species, modified from
| 1 | Eyeless | 2 |
| – | With eyes | 3 |
| 2 | Third antennal segment only slightly longer than 2nd and slightly shorter than 4th, anterior margin of pronotum trapezoidal, presence of a tooth on metafemora. Length 2.7mm. North Morocco | Parazuphium aguilerai sp. n. |
| – | Third antennal segment more than twice longer than 2nd and similar to 4th. Anterior margin of pronotum bisinuate, without tooth on metafemora. Length 4.9–5.1mm. Canary Islands | Parazuphium feloi Machado |
| 3 | Third antennal segment not twice as long as 2nd and distinctly shorter than 4th, legs short and robust, metaibiae curved, strongly so in male. North Africa, Middle East, Iberian Peninsula | Parazuphium damascenum (Fairmaire) |
| – | Third antennal segment at least twice as long as second and similar to 4th, metatibiae straight | 4 |
| 4 | Third antennal segment three times longer than 2nd. Length 7 mm. Algeria, Morocco | Parazuphium punicum (K. & J. Daniel) |
| – | Third antennal segment at most twice longer than 2nd. Length 2.8–6mm. | 5 |
| 5 | Eyes convex, as long as temporae, pronotum as long as wide. Length 5–5.5mm. Morocco, Tunisia | Parazuphium vaucheri (Vauloger) |
| – | Eyes flattened | 6 |
| 6 | Head darker than pronotum and elytra | 7 |
| – | Head concolorous with pronotum and elytra, body entirely yellowish brown | 8 |
| 7 | Eyes well developed, distance between hind margin of head and hind margin of eyes at most 2 times longer than diameter of eyes. Apical part of aedeagus short and robust, with slightly curved ventral margin. Length 4.5–6mm. Central and southern Europe, Turkmenistan | Parazuphium chevrolati (Castelnau) |
| – | Eyes reduced, distance between hind margin of head and hind margin of eyes at least 2.5 times longer than diameter of eyes. Apical part of aedeagus long and narrow. Morocco | Parazuphium angusticullum Hürka |
| 8 | Apical part of aedeagus straight, long and narrow. Length 4–5mm. Spain | Parazuphium ramirezi J. and E.Vives |
| – | Apical part of aedeagus sinuate, curved, robust and hooked. Length 3.8–5.4mm. North Africa, Italy, Spain | Parazuphium baeticum (K. & J. Daniel) |
The cox1 gene fragment was aligned with no gaps, and its correct translation to amino acids confirmed. Alignment of the three ribosomal markers resulted in several gaps, which were included in the analyses as obtained from MAFFT without further modifications. Bayesian analysis reached a convergence value of 0.0005 after 20 million generations. The initial 10% saved trees were removed as a burning value and the half consensus tree was built with the “sumt" option in MrBayes v.3.1. Figure 5 represents the obtained phylogeny, were most of nodes showed very high Bayesian posterior probabilities, which are interpreted as Bayesian support.
Phylogenetic tree obtained with MrBayes for the combined dataset (cox1, rrnL, LSU, SSU). Numbers in nodes correspond to Bayesian posterior probabilities. Zuphiini and Dryptinae (sensu
We recovered a monophyletic Zuphiini, with the two studied species of Parazuphium as sisters, and sister to Zuphium (Fig. 5). Zuphiini was sister to Drypta, in a monophyletic Dryptinae (sensu
The genus Parazuphium is currently included in subtribe Zuphiina (tribe Zuphiini), together with Ildobates, Zuphium and Polistichus
among the Palaearctic fauna (Baehr 2003). Although the scarcity of data
does not allow a comprehensive study, our molecular results support
this taxonomic position, with both studied Parazuphium species clustered together as a sister group of Zuphium (Fig. 5). Zuphium and Parazuphium species are recovered as related to Ildobates neboti, which was found as belonging to the Zuphiini by
Parazuphium has traditionally been divided in three subgenera, Neozuphium, with only one valid species, Parazuphium damascenum (
The subgenus Neozuphium was described by
Parazuphium aguilerai
sp. n. differs from all other known species of the genus in its clear
adaptations to an endogean way of life. Other species are regularly
found in soil crevices, specially among the cracks of the dried
substratum of areas which are regularly inundated (
This research was supported in part by the project CGL2007-61665 of the Spanish Ministry of Science and Innovation and the project S/REF.08724/PI/08 of the Fundacion Seneca. CA had a FPU predoctoral grant of the same Ministry. Thanks are due to José Serrano and José Luis Lencina for specimens of Parazuphium damascenum and Parazuphium ramirezi used in comparisons and for the help in the identification of some species. Joaquin Mateu, Arnaud Faille and two anonymous Referees helped with their comments. Jesús Arribas did the drawing of habitus. Phylogenetic analyses were carried out on the freely available Bioportal (www.bioportal.uio.no)