(C) 2010 Jan Bosselaers. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Heser Tuneva, 2005 (Gnaphosidae) is described from the south of India. A key is provided to the species of Heser and the importance of Gnaphosidae for the study of world spider biodiversity is briefly discussed.
Dionycha, Karnataka, Hampi, Zelotinae, key, vijayanagara
The genus Heser Tuneva, 2005 belongs in the gnaphosid subfamily Zelotinae by the presence of metatarsal preening combs on legs III and IV, and was delimited by
The specimens were observed, photographed and drawn
under Euromex MIC 465 and Olympus SZC-X9 binocular microscopes equipped
with an eyepiece grid and a Praktica DC42 digital camera.. The vulva
(cleared in methyl salicylate) was observed and drawn using a Wild M12
compound microscope. All measurements are in mm, unless otherwise
stated. The format for leg spination follows
Abbreviations:
AER anterior eye row; ALE anterior lateral eyes; ALS anterior lateral spinnerets; AME anterior median eyes; fe femur; MOQ median ocular quadrangle; mt metatarsus; pa patella; PER posterior eye row; PLE posterior lateral eyes; PLS posterior lateral spinnerets; PMS posterior median spinnerets; ti tibia.
Abbreviation of institutional collection (curator in parentheses):
RBINS Royal Belgian Institute of Natural Sciences, Brussels (L. Baert)
TaxonomyHeser Tuneva, 2005
urn:lsid:zoobank.org:act:4CD46D69-8560-49BD-B58F-825F96510C14
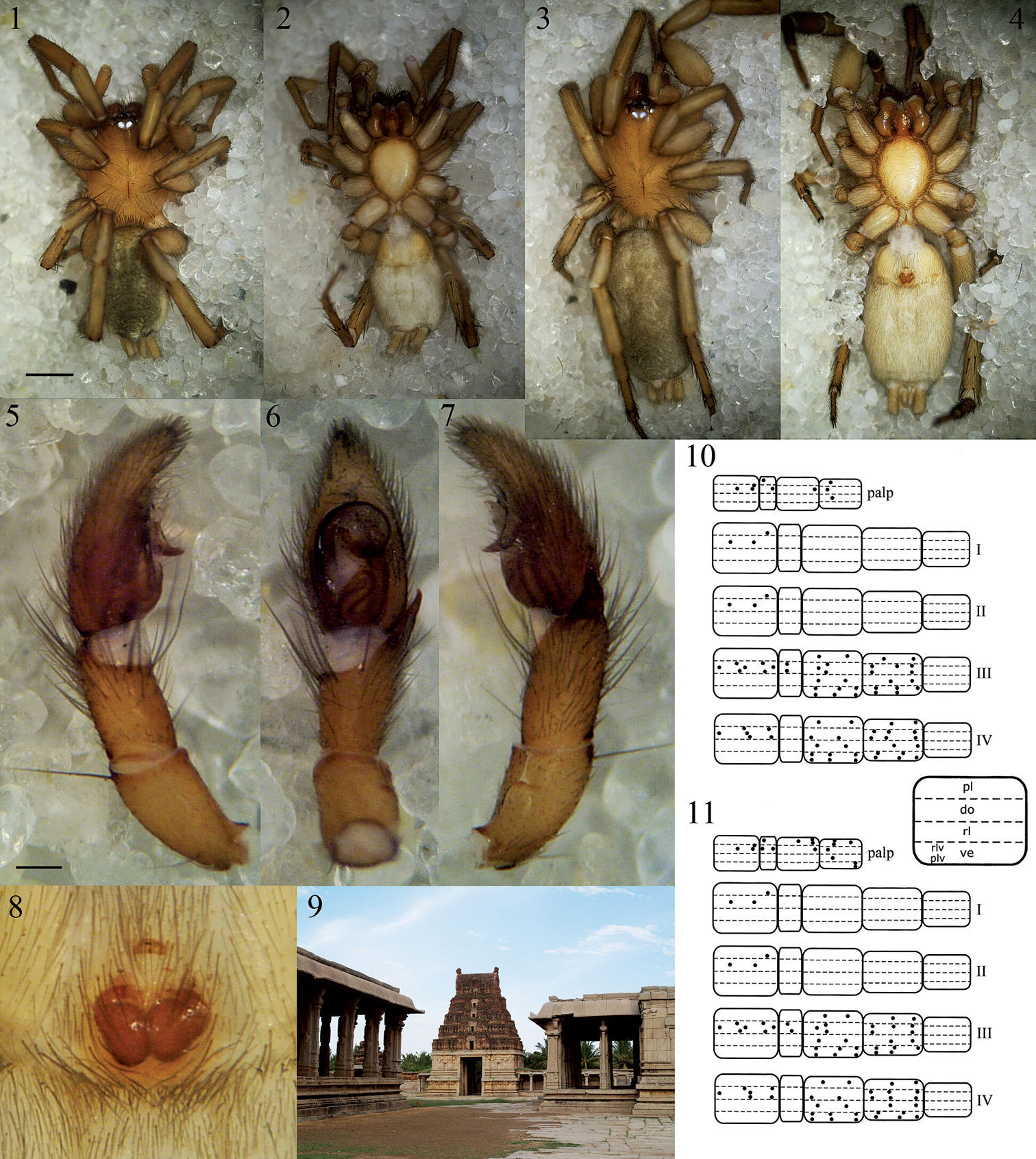
Figs 1–8, 10–11, 12–19Holotype male:India, Karnataka, Hospet, Hampi World Heritage Site, 15°18'27.7"N, 76°28'32.8"E, alt. 455 m, under stone close to archaeological office, 25 November 2006, J. Bosselaers leg. (RBINS). Allotype female, same data (RBINS).
The species can be distinguished from the other three species of Heser by the abdominal dorsal scutum in males, the pronounced S-shaped curve of the sperm duct, the small, transversally oriented median apophysis and the hook-shaped embolus tip circling the broad membranous conductor in the male palp, as well as the narrow anterior epigynal hood, the large spermathecae and the circular insemination ducts in females.
The species epithet is a noun in apposition and refers to the imperial city of Vijayanagara (Hampi, Karnataka, India) among whose ruins the type specimens of the new species were found (Fig. 9).
Male (holotype). Total length: 5.00. Carapace
length: 2.24; width: 1.63. Carapace orange brown, unicolorous, with a
deep and narrow fovea in the posterior half (Fig. 1).
Eight eyes in two rows, ringed with black, AER width 0.47, straight
from above, slightly procurved from front, PER width 0.56, procurved
from above, strongly procurved from front. MOQ depth 0.35, anterior
width 0.29, posterior width 0.32. Eyes of AER subequal, AME grey and
circular, separated by half their diameter, ALE oval, pearly white,
touching AME. PME oval to subtriangular, pearly white, touching,
larger than AME (Fig. 1).
PLE subquadratic, pearly white, slightly smaller than ALE, separated
from PME by half the PLE diameter. Clypeus vertical, slightly larger
than diameter of AME. Chilum small, sclerotised, subtriangular and
single, orange-brown. Chelicerae brown, with a few scattered thin
setae on anterior face, anterior cheliceral rim with two very small
teeth close to fang base and three larger teeth further from fang base,
posterior cheliceral rim with one very small tooth close to fang base
end two medium-sized teeth further from fang base. Sternum smooth,
orange-brown, shield-shaped with a thin border, length 1.32; width
1.08. No precoxal triangles (
Heser vijayanagara sp. n. 1 Male holotype, dorsal 2 Male holotype, ventral 3 Female allotype, dorsal 4 Female allotype, ventral 5 Male palp, prolateral 6 Male palp, ventral 7 Male palp, retrolateral 8 Epigyne, ventral 9 Pattabhirama temple in close vicinity of the locus typicus, giving a good impression of the type of terrain where the type specimens were found 10 Male leg spination diagram, legend below right 11 Female leg spination diagram. Scale bars: 1–4: 1.0; 5–8: 0.25.
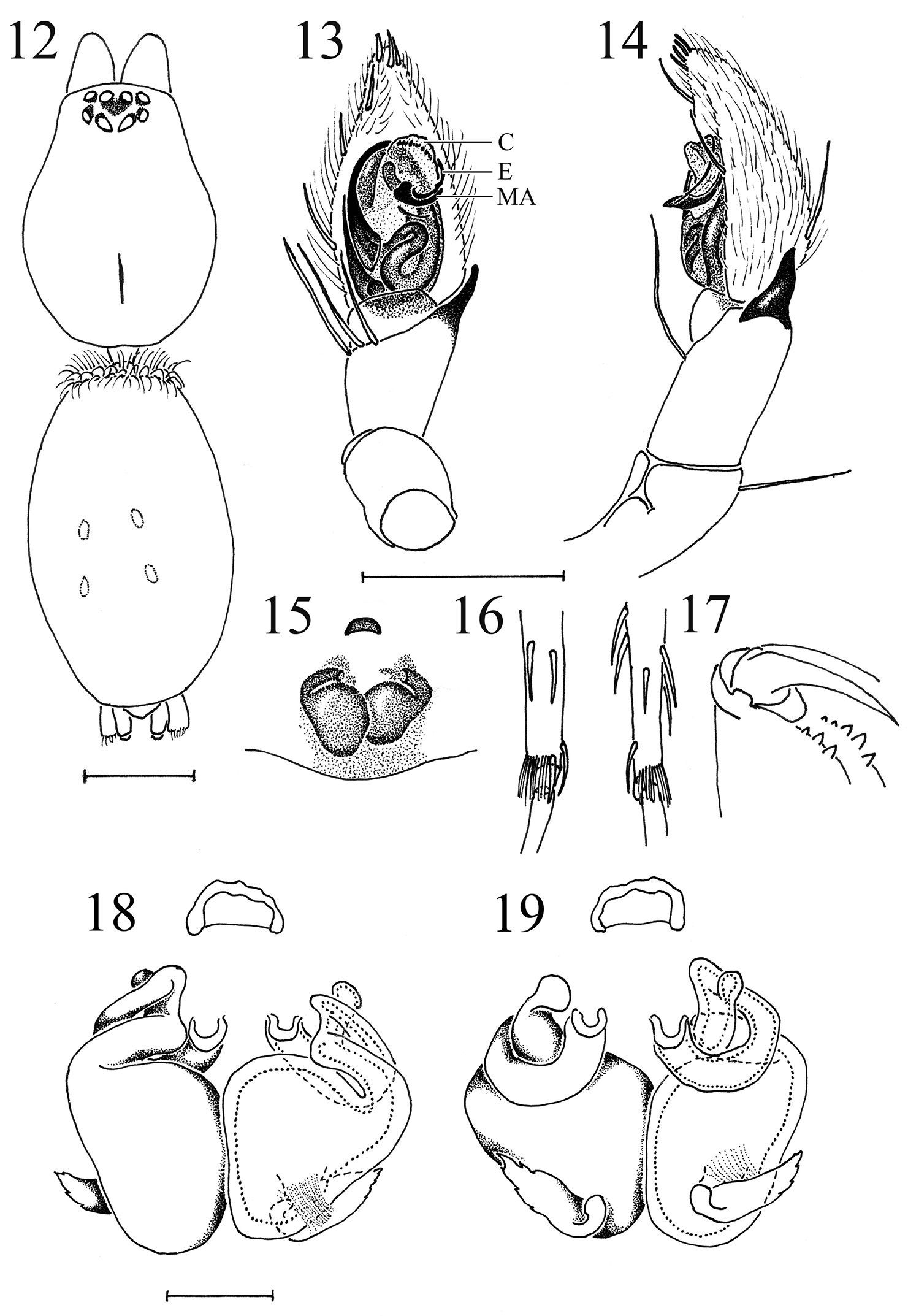
Heser vijayanagara sp. n. 12 Female allotype, dorsal view of body 13 Male palp, ventral, with conductor (C), embolus (E), and median apophysis (MA) indicated 14 Male palp, retrolateral 15 Epigyne, ventral 16 Female metatarsi III (left) and IV, with ventral terminal preening comb 17 Female cheliceral teeth 18 Vulva, ventral 19 Vulva, dorsal. Scale bars: 12: 1.0; 13–17: 0.5; 18–19: 0.1.
| fe | pa | ti | mt | ta | Total | |
|---|---|---|---|---|---|---|
| I | 1.66 | 0.97 | 1.45 | 1.21 | 0.92 | 6.21 |
| II | 1.34 | 0.74 | 1.08 | 1.08 | 0.87 | 5.10 |
| III | 1.16 | 0.63 | 0.89 | 1.03 | 0.74 | 4.44 |
| IV | 1.71 | 0.87 | 1.42 | 1.66 | 1.03 | 6.68 |
Male palp with a slender, basally-prolaterally inserted embolus circling more than half of the tegulum, having a hook-shaped tip pointing in prolateral direction, which is curling around the broad, membranous conductor. Median apophysis small and subtriangular, oriented transversally. Sperm duct with a pronounced, S-shaped curve in basal half of tegulum. Retrolateral tibial apophysis pointed, subtriangular (Figs 5–7, 13–14).
Female (allotype). Total length: 6.31. Carapace length: 2.37; width: 1.79. Carapace as in male (Fig. 3). Eyes as in male, AER width 0.53, PER width 0.58, MOQ depth 0.39, anterior width 0.30, posterior width 0.31. PME subtriangular, somewhat smaller than in male, almost touching (Figs 3, 12). Clypeus and chilum as in male. Cheliceral teeth as in male (Fig. 17). Sternum smooth, yellow-brown with a darker margin (Fig. 4), shield-shaped with a thin border, length 1.45; width 1.13. No precoxal triangles or intercoxal sclerites, pleural bars as in male. Labium and endites as in male (Fig. 4). Abdomen pale grey dorsally, with a frontal row of curved hairs and a number of paler chevrons in posterior half, no do scutum (Fig. 3). Ventral side of abdomen pale white (Fig. 4). Legs yellow-brown, unicolorous (Figs 3, 4). No trochanter notch, no retrocoxal hymen, patellar indentation as in male. Metatarsi III and IV with ventral terminal preening comb composed of stiff, black setae (Fig. 16). Tarsi with two pectinate claws, no claw tufts. Leg formula 4123. Leg spination (Fig. 11): fe: palp do 0–1-2; I pl 0–0-1 do 1–1-0;II pl 0–0-1 do 1–1-0;III do 1–3-3; IV do 1–3-2; pa: palp pl 1–0 do 1–1; III do 0–2-0; ti: palp pl 0–1-2 do 0–0-1; III pl 1–0-1 do 3–0-0 rl 1–0-1 ve 2–2-2; IV pl 1–0-1 do 1–1-0 rl 1–0-1 ve 2–2-2; mt: III pl 0–1-1 do 2–1-2 rl 0–1-1 ve 2–2-1; IV pl 0–1-1 do 2–2-2 rl 0–1-1 ve 2–2-1; ta: palp pl 0–2-1 do 1–0-0 rl 0–1-0 ve 0–0-2.
| fe | pa | ti | mt | ta | Total | |
|---|---|---|---|---|---|---|
| I | 1.63 | 0.92 | 1.34 | 1.05 | 0.84 | 5.79 |
| II | 1.37 | 0.79 | 1.00 | 0.87 | 0.74 | 4.76 |
| III | 1.24 | 0.55 | 0.82 | 0.92 | 0.71 | 4.23 |
| IV | 1.71 | 0.84 | 1.34 | 1.58 | 0.92 | 6.39 |
Epigyne simple and poorly sclerotised, with a narrow anterior hood and showing large, oval spermathecae, as well as the connected, stout, inward directed part of the looped anterior insemination ducts. Copulatory openings small and medially situated (Figs 8, 15, 18, 19). Vulva (Figs 18, 19) with two large, thick-walled, oval, posterior spermathecae, connected to the anterior, medially situated copulatory openings by an insemination duct looped over 360°. The first, hemicircular stretch of the looped insemination duct passes dorsally behind the anterior part of the spermathecae, while the second, straight, longitudinally directed stretch carries an accessory gland (Fig. 19), and the third, stout, outward directed and ventrally situated stretch connects to the large spermathecae (Fig. 18).
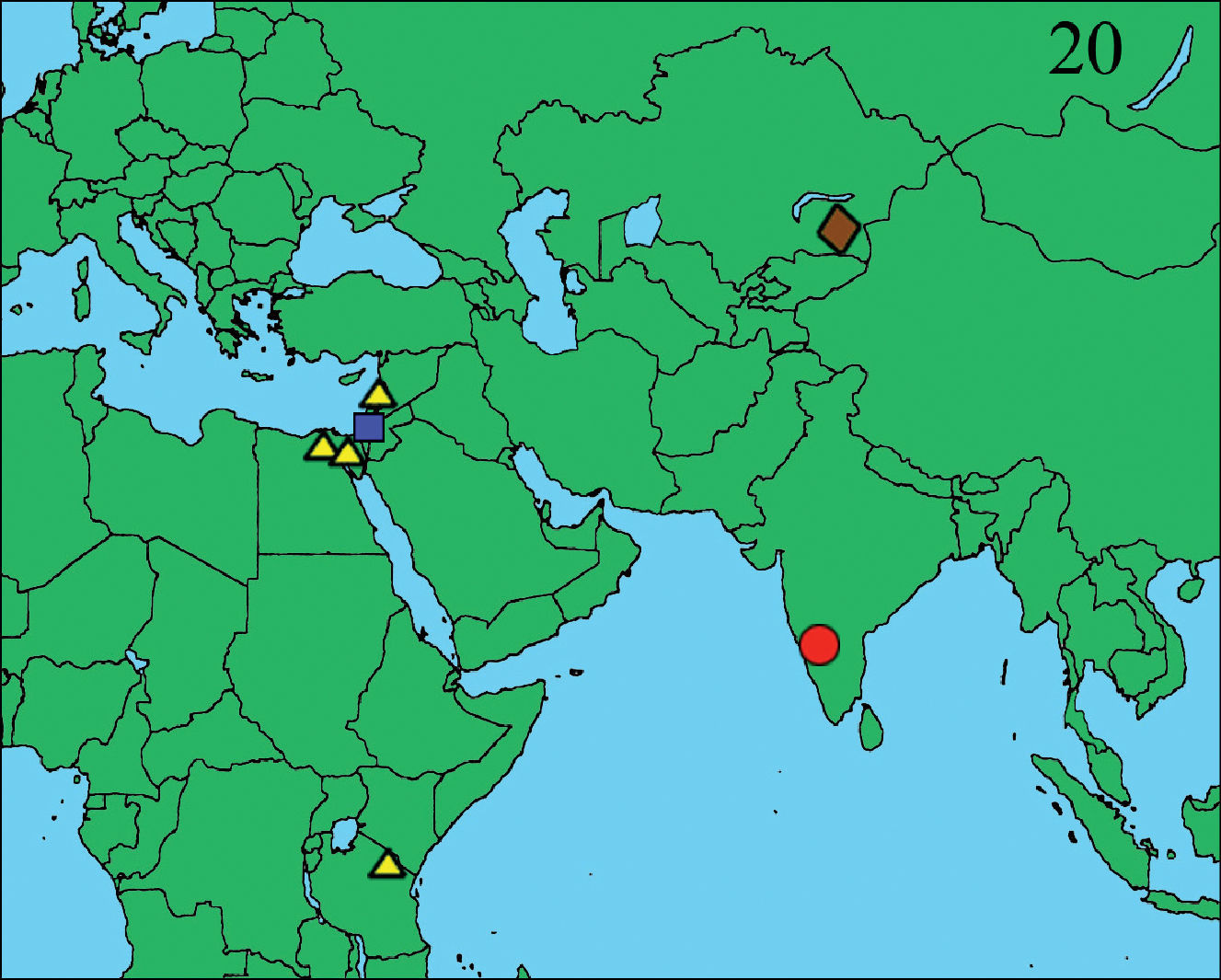
Distribution map of the four species of the genus Heser. Heser aradensis: blue square; Heser infumatus: yellow triangle; Heser malefactor: brown lozenge; Heser vijayanagara sp. n.; red circle.
| 1 | Males | 2 |
| – | Females | 5 |
| 2 | Total length less than 4 mm. Cymbium blunt-tipped, male palpal ti longer than wide, retrolateral tibial apophysis short and blunt (Levy 1998, fig. 116) | Heser aradensis |
| – | Total length more than 4 mm. Cymbium pointed, male palpal ti at least as long as wide, retrolateral tibial apophysis triangular and pointed | 3 |
| 3 | Membranous conductor of the male palp subtriangular and more or less pointed (Levy 1998, fig. 112) | Heser infumatus |
| – | Membranous conductor broad and blunt | 4 |
| 4 | Embolus tip pointing towards bulbus base, median apophysis large, with a long basally oriented tip bent outwards ( |
Heser malefactor |
| – | Embolus tip curled around conductor, pointing in prolateral direction. Median apophysis small, triangular, directed transversally (Fig. 13). Small anterior dorsal abdominal scutum. | Heser vijayanagara sp. n. |
| 5 | Total length 4 mm or less | 6 |
| – | Total length 5 mm or more | 7 |
| 6 | No anterior epigynal hood present, copulatory openings relatively small, their long axis directed transversally (Levy 1998, fig. 118) | Heser aradensis |
| – | Broad anterior epigynal hood present, copulatory openings large, their long axis oriented longitudinally ( |
Heser malefactor |
| 7 | Anterior epigynal hood broad, copulatory openings a transversal slit, spermatheca diameter smaller than half the longitudinal dimension of the epigyne (Levy 1998, fig. 114) | Heser infumatus |
| – | Anterior epigynal hood narrow, copulatory openings a small oval, their long axis longitudinally oriented, spermatheca diameter about half the longitudinal dimension of the epigyne (Figs 8, 15, 18, 19) | Heser vijayanagara sp. n. |
The family Gnaphosidae is one of the largest spider families. In
The Gnaphosidae of the Indian subcontinent and its surroundings have been studied by
The recent progress made in the taxonomic study of ground spiders demonstrates that, for a long time to come, Gnaphosidae, and Gnaphosoidea in general, will remain a prime target for biodiversity studies within Araneae.
The author is grateful to Dr. S.V.P. Halakatti and to Dr. C.S. Seshadri (Archaeological Survey of India) for their expert guidance through the ruins of Hampi as well as for taking care of many practical aspects of the field visit, and to Mr. Jayant Nadiger (Flanders Investment and Trade, Bangalore) for skilfully organising many of the logistic aspects of the author’s stay in India. Thanks are also due to Yuri Marusik, Mykola Kovblyuk and an anonymous referee for skilled comments that helped improve the manuscript.