(C) 2010 Chao-Chun Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The millipede genus Aponedyopus is endemic to Taiwan and contains three species. All previously described nominal species are considered to represent one species: Aponedyopus montanus Verhoeff, 1939 (the type species), including Aponedyopus reesi (Wang, 1957) and Aponedyopus maculatus Takakuwa, 1942, syn. n. Two further species are described as new: Aponedyopus similis sp. n. and Aponedyopus latilobatus sp. n. The genus is re-diagnosed, all of its three species are keyed, and their distributions mapped.
Millipede, Aponedyopus, taxonomy, new species, distribution, key, Taiwan
The genus Aponedyopus Verhoeff, 1939 was first proposed to incorporate the single species Aponedyopus montanus
Verhoeff, 1939 said to be from the foot of Mt Fuji in Japan. The
generic diagnosis was very poor, relying on a highly superficial
resemblance in the gonopod conformation of Aponedyopus to the genus Nedyopus Attems, 1914 (
The present study reviews the millipede genus Aponedyopus, based on abundant fresh material, including some near-topotypes of one of included species, covering various parts of Taiwan. Thus, the previously described species could be re-assessed, two new species added, and anew synonym established.
Material and methodsNew extensive collections of millipedes covering most parts of Taiwan were made between 1989 and 2009, using hand-sorting of the soil and litter. Specimens were preserved in 70% ethanol. External structures were examined and the drawings prepared with a LEICA MZ 16 stereomicroscope, as well as with a HITACHI S2400 scanning electron microscope. Coloration of the specimens is described from alcohol material. This material has been shared between the collections of Department of Life Science, National Chung Hsing University (NCHUL), Taiwan; Department of Biological Sciences, National Sun Yat-Sen University (NSYSUB), Taiwan; Department of Life Sciences, National Taiwan Normal University (NTNUL), Taiwan; National Museum of Natural Science (NMNS), Taiwan; Taiwan Forestry Research Institute (TFRI), Taiwan; and Zoological Museum of the State University of Moscow (ZMUM), Russia.
Systematic AccountMedium- to large-sized Paradoxosomatidae (15–55 mm long, 2.0–5.0 mm wide) with 20 segments. Pore formula normal. Paraterga poorly developed, evident only on segment 2. An evident sternal lobe between ♂ coxae 4; ♂ segment 7 with or without a pair of prominent sternal cones (= spiracles) flanking gonopod aperture. ♂ tarsal brushes present.
Gonopod coxae long, subcylindrical, setose distodorsally, cannula as usual. Telopodites rather long, their distal parts crossingmedially in situ. Femorite long, moderately to evidently broadened parabasally on dorsal side, apically separated from postfemoral region by a clear oblique sulcus on lateral side; postfemoral part enlarged at base, tapering thereafter, demarcated from solenophore by a sulcus on mesal side; solenophoreshorter than to as long as femorite, curved first ventrad and then dorsad on mesal face, distally holding subparallel to broadened part of femorite; base of solenophore with a small to obvious, apically deeply bifid lobe; seminal groove first running fully on mesal face of femorite, then turning dorsad near postfemoral part and continuing onto solenomere at base of solenophore on dorsal face; solenomereflagelliform, long, at most only slightly longer than, and nearly completely supported/sheathed by, solenophore, with only tip of solenomere sometimes exposed.
Figs 1–23, 40–43, 50–53
1 ♀ (NSYSUB-DI 60), Taiwan, Taipei City, BeiTou area (北投區), 101 Jiia county road (101甲縣道), ca 860 m a.s.l., 4 May 2002, leg. S. Y. Wu. 1 ♂ (NCHUL), Taipei County (台北縣), Gongliao Township (貢寮鄉), upstream of Yuanwangkeng Stream (遠望坑溪上游), 6 June, 1998, leg. S. H. Wu. 1 ♂ (NSYSUB-DI 67), Taipei County (台北縣), ULai Township (烏來鄉), TaManShan (塔曼山), in decaying wood, 2, 100 m a.s.l., 23 August 2002, same collector. 1 ♀ (NSYSUB-DI 59), same township, WuLai (烏來), 1, 000–1, 200 m a.s.l., March 2002, leg. C. C. Chen & C. S. Iang. 1 ♂, 2 ♀ (TFRI), same township, FuShan Botanical garden (福山植物園), ca 730 m a.s.l., 18–25 May 2001, leg. W. B. Huang. 1 ♂, 3 ♀ (NSYSUB-DI 61–64), Taiwan, Taoyuan County (桃園縣), FuSiing Township (復興鄉), HuaLeng Village (華稜村), Northern Cross-Island Highway (北部橫貫公路)/Provincial # 7 Highway (台七線), 53 km, ca 1, 030 m a.s.l., 22 April 2003, same collector. 1 ♀ (NSYSUB-DI 73), same locality, 58 km, ca 1, 110 m a.s.l., 29 May 2003, same collector. 1 ♀ (NSYSUB), same locality, 56 km, ca 1, 030 m a.s.l., 23 June 2006, same collector. 3 ♂ (NSYSUB-DI 444–446), same township, Baling (巴陵), ca 600 m a.s.l., 3 April 2004, leg. H. D. Zhu. 1 ♀ (NTNUL-My 15), Hsinchu County (新竹縣), Wufeng Township (五峰鄉), GuanU (觀霧), ca 2, 000 m a.s.l., 28 June 1993, leg. S. H. Chen. 1 ♂ 1 ♀ (NSYSUB), same township, ShihLu old path (石鹿古道), ca 1, 600 m a.s.l., 22 September 2005, leg. H. D. Zhu. 1 ♀ (NSYSUB), same township, Syueba farm (雪壩農場), DaLu forest path (大鹿林道), ca 1, 890 m a.s.l., 1 October 2006, leg. S. Y. Wu. 1 ♂, 1 ♀ (ZMUM), Yilan County (宜蘭縣), Yuanshan Township (員山鄉), Shuanglian Pond (雙連埤), ca 500 m a.s.l., 11 May 2007, same collector. 1 ♀ (NSYSUB), Datong Township (大同鄉), Cueifong Lake (翠峰湖), ca 1, 900 m a.s.l., 29 July 2004, same collector. 1 ♂ (NSYSUB), same township, Northern Cross-Island Highway (北部橫貫公路)/Provincial # 7 Highway (台七線), MingChih (明池), ca 1, 200 m a.s.l., 13 April 2006, same collector. 1 ♂ (NSYSUB), same township, Mt Taiping (太平山), ca 1, 930 m a.s.l., 26 February 2007, 24°28'46"N, 121°31'03"E, leg. M. H. Hsu. 1 ♀ (NSYSUB), same township, forest path # 100 (100號林道), 21 km, ca 1, 600 m a.s.l., 9 September 2009, leg. C. J. Jheng. 1 ♂ (NSYSUB-DI 65.), Taichung County (台中縣), HePing Township (和平鄉), AnMaShan forest amusement zone (鞍馬山森林遊樂園), ca 2, 000 m a.s.l., 7 May 2003, leg. S. Y. Wu. 1 ♂ (NCHUL), Nantou County (南投縣), LuGu (鹿谷鄉), SiTou (溪頭), ca 1, 140 m a.s.l., 31 October 1997, leg. S. H. Wu. 1 ♀ (NCHUL), same locality, ca 1, 160–1, 400 m a.s.l., 31 October 1997, leg. S. H. Chen. 1 ♀ (NSYSUB-DI 58), same locality, ShenMu walking path (神木步道), under stones, ca 1, 200 m a.s.l., 15 November 2002, leg. J. D. Lee. 1 ♀ (JDLee20021114008, deposited at NSYSUB), same locality, TuDiGongLun walking path (土地公崙步道), ca 1, 160–1, 400 m a.s.l., same date and collector. 2 juveniles (JDLee20021114004, deposited at NSYSUB), same locality, SiTou walking path (溪頭步道), same date and collector. 3 ♀ (NTNUL-My 6–9), same county, ShinYi Township (信義鄉), Zjhong (自忠), ca 2, 340 m a.s.l., 1 July 1989, leg. S. H. Chen. 3 ♂ (NTNUL-My 25–28), same locality, date and collector. 1 ♀ (NSYSUB), same county, Zhushan Township (竹山鎮), ShanLinSi amusement park (杉林溪遊樂園), ca 1, 600 m a.s.l., 7 October 2004, leg. S. Y. Wu. 1 ♂ (NSYSUB), Taiwan, Hualien County (花蓮縣), Xiulin Township (秀林鄉), Mt. JiaLiWan (加禮宛山), ca 1, 290 m a.s.l., 29 July 2005, leg. F. S. Jhou. 1 ♀ (NSYSUB), same township, Toroko (太魯閣), Lianhua Pond walking path (蓮花池步道), ca 1, 060 m a.s.l., 24°13'10"N, 121°28'49"E, 28 February 2007, leg. M. H. Hsu. 1 ♀ (NSYSUB), same county, FengBin Township (豐濱鄉), Ruigang Highway (瑞港公路), ca 130 m a.s.l., 23°28'50"N, 121°27'31"E, 7 May 2009, leg. M. H. Hsu. 2 ♂ (NSYSUB-DI 69–70), same county, JhuoSii (卓溪鄉), WaLaMi (瓦拉米), YuShan National Park (玉山國家公園), ca 1, 080 m a.s.l., 24 February 2003, leg. H. D. Zhu. 1 ♂, 3 juveniles (NTNUL-My 29–32), Chia-I County (嘉義縣), ALiShan Township (阿里山鄉), ALiShan (阿里山), ca 2, 260 m a.s.l., 11 March 1989, leg. S. H. Chen. 1 ♂, 2 ♀ (NTNUL-My 49–51), 2, 250 m a.s.l., 3 July 1989, same locality and collector. 1 ♀ (NSYSUB-DI 74), same locality, ALiShan amusement park (阿里山遊樂園), under stones on soil, ca 2, 280 m a.s.l., 24 June 2003, leg. Y. H. Lin. 1 ♀ (NSYSUB-DI 66), Kaohsiung County (高雄縣), TaoYuan (桃源鄉), TengJhih (藤枝), ShihShan forest path (石山林道), 6 km, ca 1, 600 m a.s.l., 21 August 1998, collector unknown. 1 ♂ (NSYSUB-DI 66), same locality, 1 August 2001, leg. C. R. Wu. 1 ♀ (NSYSUB-DI 72), same locality, ca 1, 450 m a.s.l., 14 April 2003, leg. S. Y. Wu. 1 ♂ (NSYSUB), same township, NanSi forest path (楠溪林道), ca 2, 000 m a.s.l., 24 September 2002, leg. M. J. Hong & M. J. Wu. 1 ♀ (NSYSUB), same township, Southern Cross-Island Highway (南部橫貫公路), DaGuanShan (大關山), YaKou forest path (啞口林道), ca 2, 720 m a.s.l., 13 May 2007, leg. Y. C. Chang. 1 ♀ (NSYSUB-DI 71), at boundary between MaoLin County (茂林鄉) of Kaohsiung and UTai County (霧臺鄉) of PingTung, YuGuTing (雨谷亭), under stone, ca 2, 150 m a.s.l., 28 March 2003, leg. H. W. Chang. 1 ♂ (NSYSUB), Taitung County (台東縣), JinFeng Township (金峰鄉), Yima forest path (依麻林道), ca 1, 110 m a.s.l., 2 July 2009, leg. M. H. Hsu. 1 ♀ (NSYSUB), PingTung County (屏東縣), ChunRih Township (春日鄉), DaHan forest path (大漢林道), 20 km, under stone, ca 250 m a.s.l., 9 July 2004, leg. W. J. Lee. 2 ♂ (NSYSUB), same county, Taiwu Township (泰武鄉), entrance to North DaWu Mountain (北大武山登山口), ca 1, 400 m a.s.l., 23 January 2004, leg. H. D. Zhu.
Aponedyopus montanus Verhoeff, 1939, showing different colour patterns, ♂♂ from Mt Taiping (太平山) 1 Zjhong (自忠) 2 Yima forest path (依麻林道)3 NanSi forest path (楠溪林道) 4 dorsal view. Scale bars: 5.0 mm.
Aponedyopus montanus Verhoeff, 1939, showing different colour patterns, ♂♂ from upstream of Yuanwangkeng Stream (遠望坑溪上游) 5 ShanLinSi amusement park (杉林溪遊樂園) 6 ShihLu old path (石鹿古道) 7 dorsal view. Scale bars: 5.0 mm.
Aponedyopus montanus Verhoeff, 1939, showing different colour patterns, ♂♂ from Mt Taiping (太平山) 8 Zjhong (自忠) 9 Yima forest path (依麻林道) 10 NanSi forest path (楠溪林道) 11 lateral view. Scale bars: 5.0 mm.
Aponedyopus montanus Verhoeff, 1939, showing different colour patterns, ♂♂ from upstream of Yuanwangkeng Stream (遠望坑溪上游) 12 ShanLinSi amusement park (杉林溪遊樂園) 13 ShihLu old path (石鹿古道) 14 dorsal view. Scale bars: 5 mm.
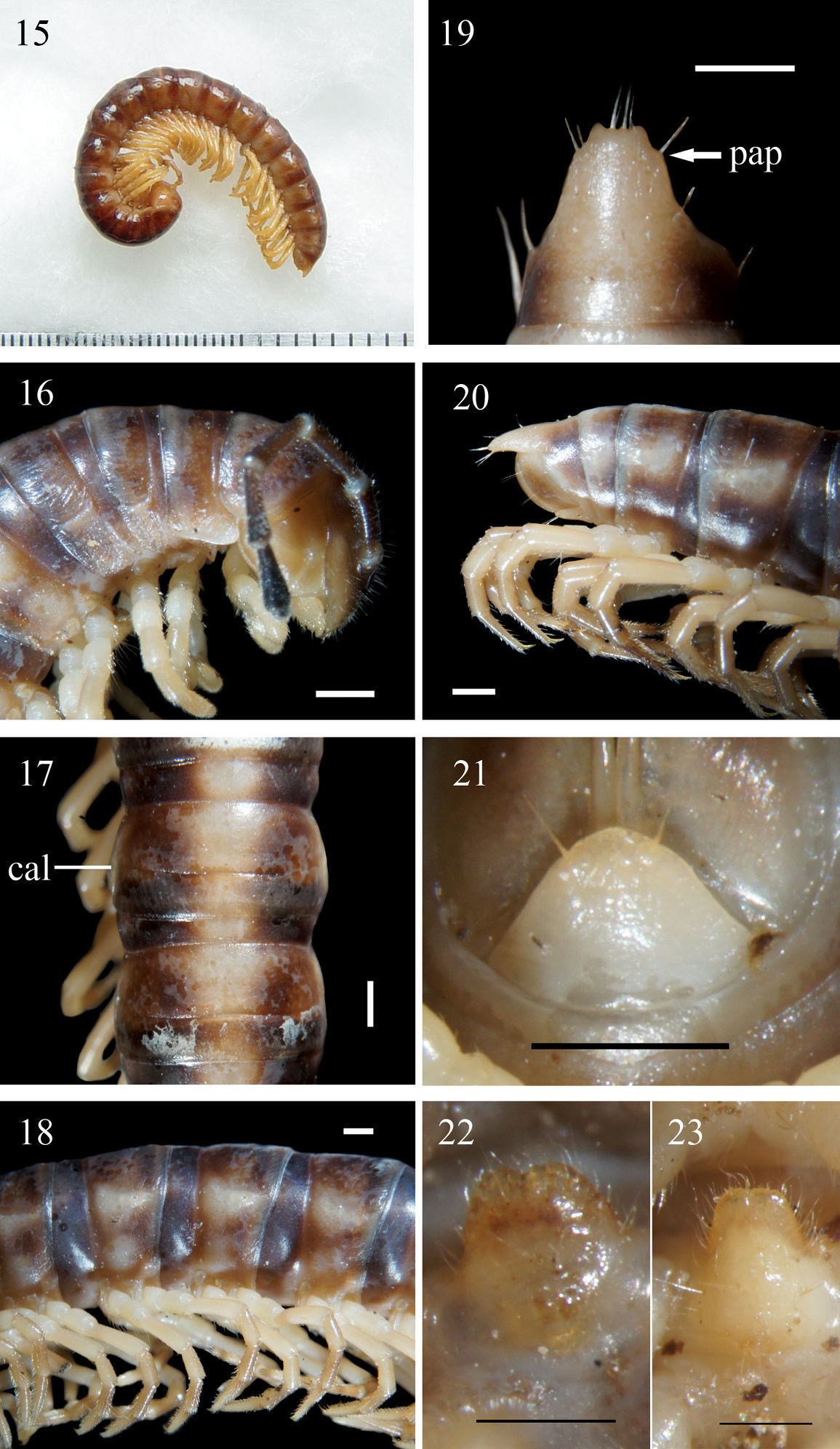
Aponedyopus montanus Verhoeff, 1939, ♂ from Mt JiaLiWan (加禮宛山). 15 Entire body, lateral view 16 Anterior body portion, lateral view. 17, 18 Midbody segments, dorsal and lateral views, respectively 19–20 Epiproct (epi), dorsal and lateral views, respectively 21 Hypoproct (hyp), ventral view 22, 23 Sternal lobe between ♂ coxae 4, subventral views. Scale bars: 1.0 mm for 15–21, 0.5 mm for 22, 23. al: axial line; cal: calluses; col: collum; meta: metazona; o: ozopore; pap: pre-apical papillae; par: paraterga; ple: pleurosternal region; pro: prozona; rug: rugulose; str: stricture; sul: transverse sulcus; tar: tarsal brushes.
Differs from the other Aponedyopus species in often containing specimens considerably more than 40 mm long, in the considerably longer ♂ legs (usually about twice as long as midbody height), a dentiform process b at the base of the gonopod prefemoral part and, above all, the slender terminal branches (x and y) of the solenophore (Figs 40, 42 & 43).
Length 40–55 (♂, n=11) or 47–58 mm (♀, n = 13); width of midbody metazona 10 ca 3.5–5.0 (♂) or 5.0–6.0 mm (♀).
Coloration in alcohol entirely light yellow to dark brown (Figs 1–14). Antennae light yellow to dark brown, increasingly blackish distally, but tip pallid; head to anterior half of epiproct (epi) (Fig. 19), pleurosternal region (ple) (Fig. 18) light yellow to dark brown, prozona (pro) always darker than metazona (meta) (Fig. 18), anterior and hind edges of metazona evidently to slightly lighter brown; posterior half of epiproct, sterna and legs light yellow to orange-brown in ♂.
Head densely setose in clypeolabral region, vertex nearly bare, epicranial suture distinct. Postcollum constriction faint; in width, segments 2 = 3 = 4 < head = segment 5 = 6 < collum (col) (Fig. 16) = segments 7–17 in ♂, or segments 2 = 3 = 4 < head < collum = segments 5–16 in ♀; thereafter body gradually and gently tapering both in width and height towards telson. Antennae medium-sized to long, stout, reaching behind middle of metatergite 3 to middle of metatergite 4 dorsally (♂) (Fig. 16), or midway to end of segment 3 (♀). Surface generally shining and rather smooth, only metaterga rugulose (rug) (Fig. 16) (post-sulcus halves (Fig. 17) usually slightly more so); surface below paraterga (par) (Fig. 18) visibly and densely granular on anterior segments, increasingly sparsely granular towards telson in both sexes, sometimes densely granular until segment 19 in ♀. Paraterga (par) (Fig. 18) poorly developed, especially evident as low ridges drawn considerably forward into a rounded lobe on segment 2 in both sexes, nearly to totally wanting on segments 16–19 (sometimes only a dorsal sulcus above ozopore (o) (Fig. 18) still present); calluses (cal) (Fig. 17) always delimited by a sulcus dorsally, calluses thinner on poreless segments, broader on pore-bearing ones, but a ventral sulcus mostly observed in caudal 1/3 only until segment 15; paraterga even more strongly reduced in ♀. Axial line (al) usually absent to traceable in places on collum and following metaterga, sometimes evident on metaterga in both sexes (Fig. 17). A medially sinuate transverse sulcus (sul) (Fig. 17) evident on segments 5–17, traceable on segments 4 and 18(19) in both sexes, narrow, shallow, very faintly beaded to smooth at bottom, not reaching bases of paraterga. Limbus (= region between two arrows, Fig. 16) thin, caudal margin entire. Stricture (str) (Fig. 17) between pro- and metazona shallow, narrow, faintly ribbed at bottom in both sexes. Pleurosternal carinae (arrow) (Fig. 16) nearly wanting, present as slight flaps only on segment 2, barely traceable on segment 3 (Fig. 16). Tergal setae almost fully abraded, pattern traceable mostly as 1+1 or 2+2 insertion points at anterior edge of collum in both sexes, as well as 2+2 in anterior (pre-sulcus) and 2+2 in posterior (post-sulcus) row on following metaterga. Ozopores (o) (Fig. 18) lateral, lying on callus ca 1/3 metatergal length in front of caudal edge (Figs 17 & 18). Epiproct (epi) (Figs 19 & 20) moderately long, conical, only slightly curved in lateral view, ratio of epiproct length to pre-epiproct length of telson 1.3:1 in ♂, tip emarginated in both sexes in dorsal view (Fig. 19); pre-apical papillae (pap) (Fig. 19) evident, close to apex. Hypoproct (hyp) (Fig. 21) usually subtrapeziform (♂, ♀), more rarely subtriangular to semi-circular (♀), 1+1 setae at caudal corners situated on well-separated knobs, sides straight (♂) or slightly convex (♀).
Sterna sparsely setose, each cross-impression with neither a transverse sulcus nor an axial groove; a slightly to very slightly notched, setose, ventrally bulging lamina only between ♂ coxae 4 (Figs 22 & 23). Ridges/cones (= spiracles) flanking gonopod aperture present or absent. Legs long, ca twice as long as midbody height, shorter and slenderer in ♀; legs 1 to posterior legs of segment 15 with obvious tarsal brushes (tar) (Fig. 18) only in ♂, ♀ without tarsal brushes; ♂ coxa 2 with a small apical process carrying a gonopore.
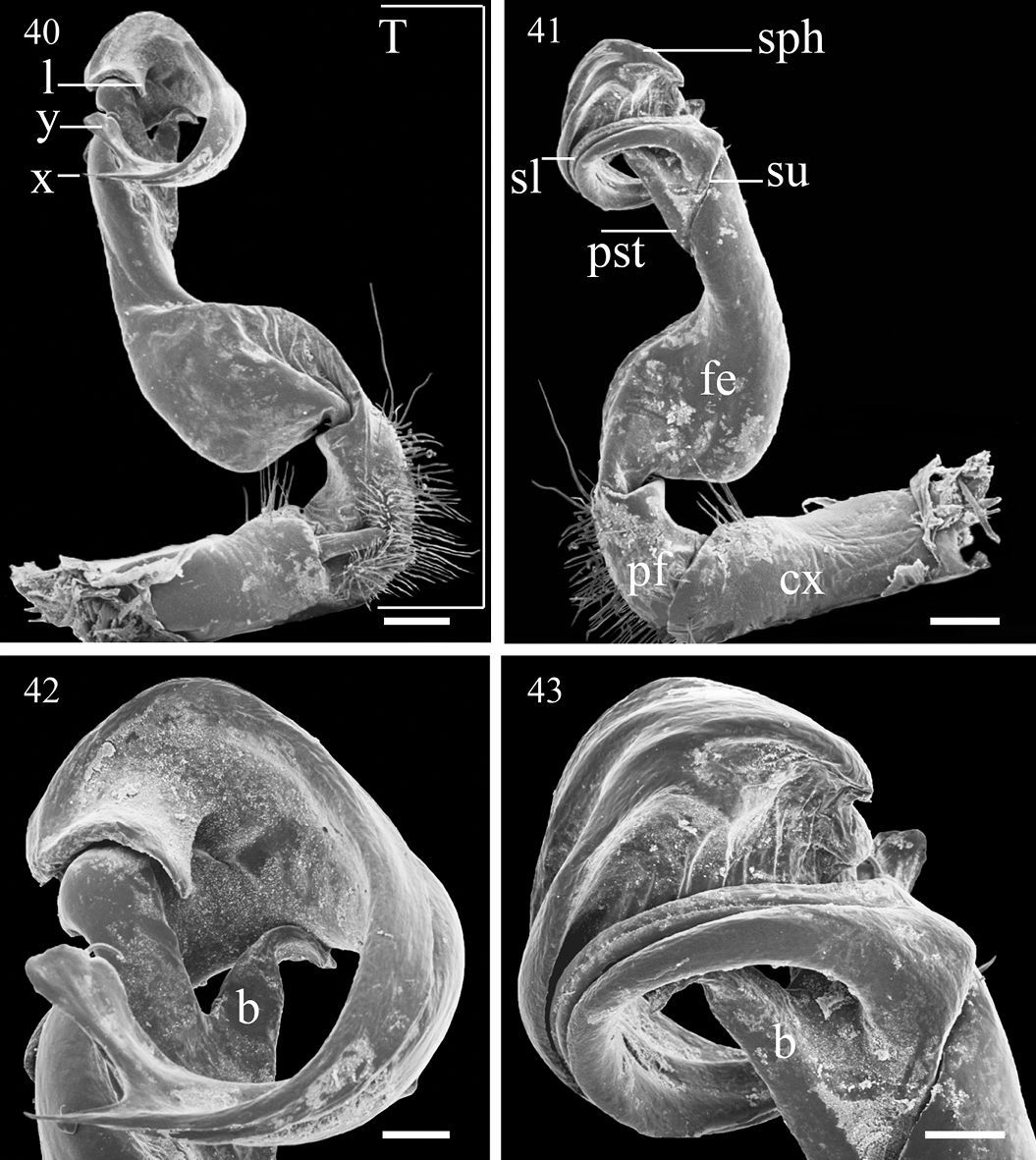
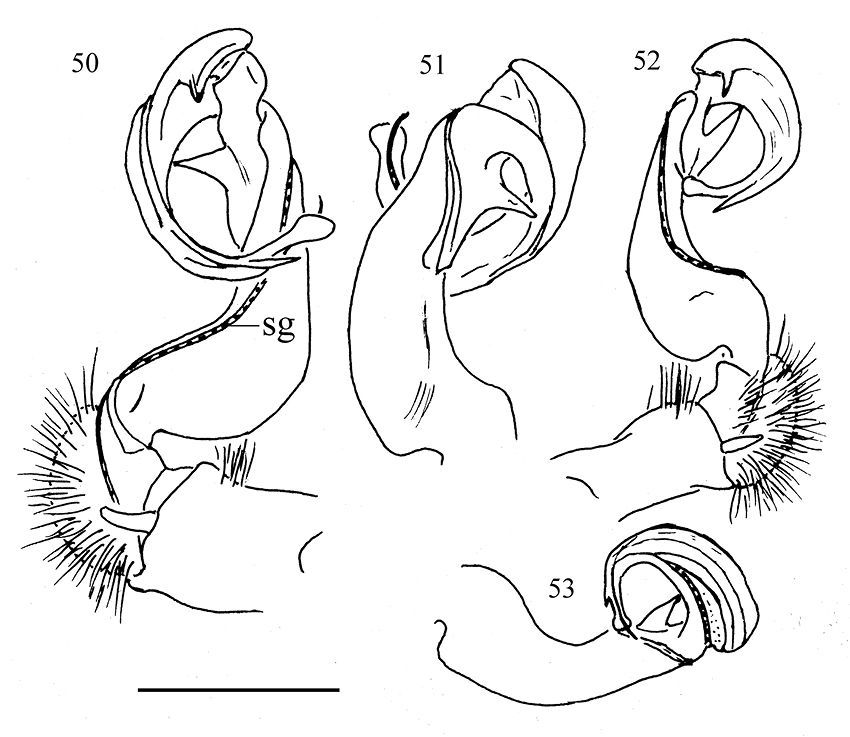
Gonopods (Figs 40–43, 50–53) simple. Coxite (cx) (Fig. 41) elongate, subcylindrical, setose distodorsally; cannula normal. Telopodites (T) (Fig. 40) curved distally, longer than coxite. Prefemoral part (pf) (Fig. 41) short and stout, almost 1/3 femur length, as usual densely setose. Femorite (fe) (Fig. 41) evidently broadened near base on dorsal side, with a clear demarcation sulcus (su) (Fig. 41) on lateral side separating a postfemoral part (pst) (Fig. 41); the latter showing an obvious, spiniform, (nearly) pointed branch (b) (Figs 42 & 43) parabasally on lateral side; solenophore (sph) (Fig. 41) with another demarcation sulcus separating it from pst on medial side, long, only slightly shorter than to as long as femorite, twisted and curved first ventrad and then dorsad on medial side in ventral view, distally holding subparallel to broadened part of femorite; base of sph with an obvious, subspiniform lobe (l) (Fig. 40), either well separated from or holding quite adjacent to sph base; terminal part of sph divided into two slender, separated branches: one wide, flattened dorsoventrally, with a rounded membranous end (y), the other spiniform (x) (Fig. 40). Seminal groove (sg) (Fig. 50) first running fully on mesal face of fe, then turning dorsad near pst to continue onto solenomere (sl) (Fig. 41) at base of sph on dorsal face; sl flagelliform, long, only slightly longer than sph and nearly completely supported/sheathed by sph, only tip of sl exposed.
Type material has not been revised, presumably in the collection of the Zoologische Staatssammlung in Munich, Germany.
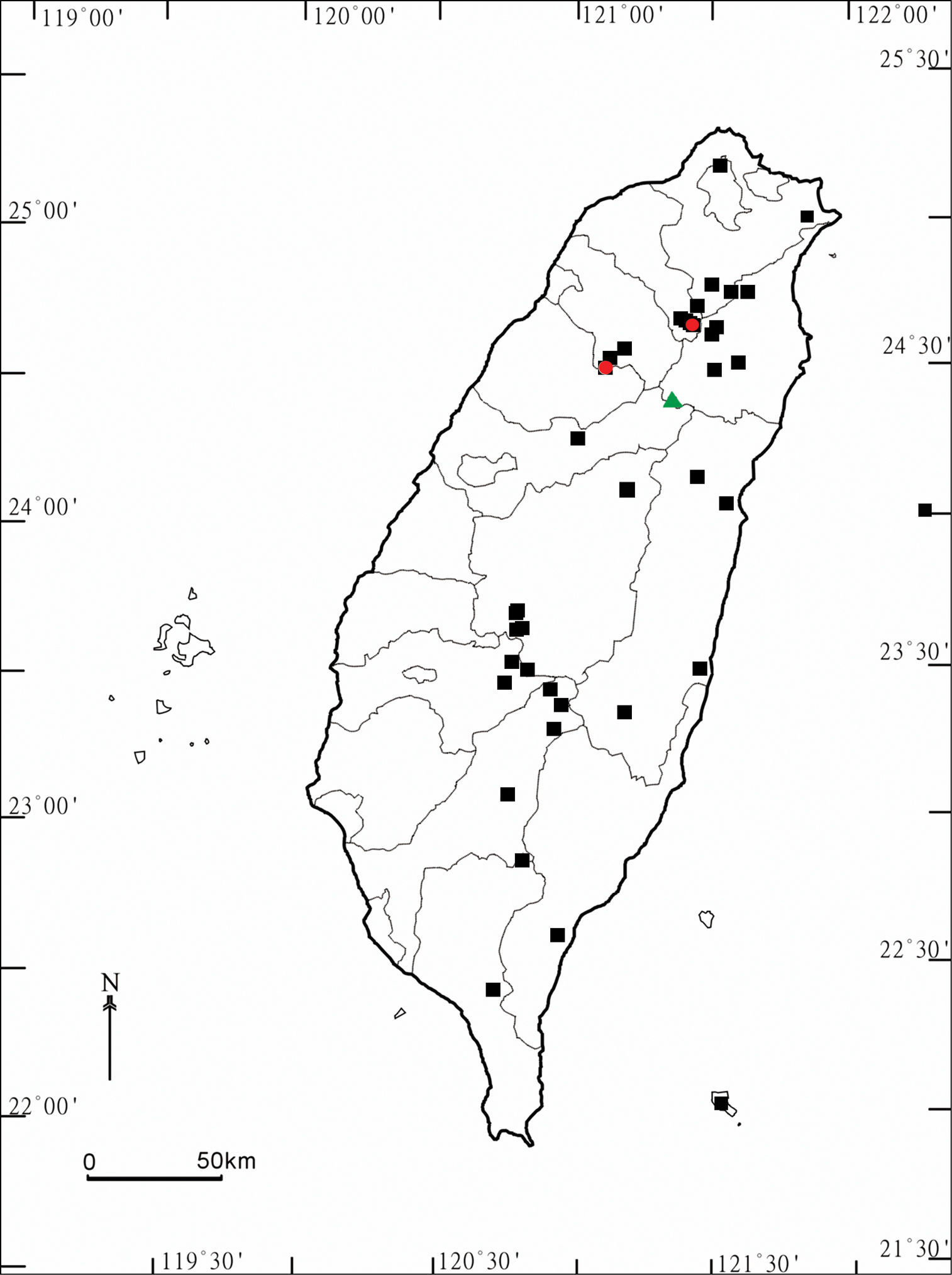
This species is highly variable in size and coloration, and is the most widespread amongst Aponedyopus species in Taiwan. Its distribution covers much of the island and vertically ranges from 175 to over 2, 720 m a.s.l. (Map).
urn:lsid:zoobank.org:act:80CFF96F-2331-45EB-B4B5-DF5814A57660
Figs 24–31, 44, 45, 54 & 55Holotype ♂ (TFRI), Taiwan (R. O. C.), Taichung County (台中縣), HePing (和平鄉), Shengguang (勝光), ca 2, 200 m a.s.l., 26 March – 25 April, 2003, leg. W. C. Yeh.
Paratype ♂ (NSYSUB-DI 75), Taiwan (R. O. C.), Hsinchu County (新竹縣), Wufeng Township (五峰鄉), GuanU (觀霧), 24.5 km from entrance to national park, ca 2, 000 m a.s.l., 13 August 2002, leg. C. C. Chen, Y. H. Lin & J. N. Huang.
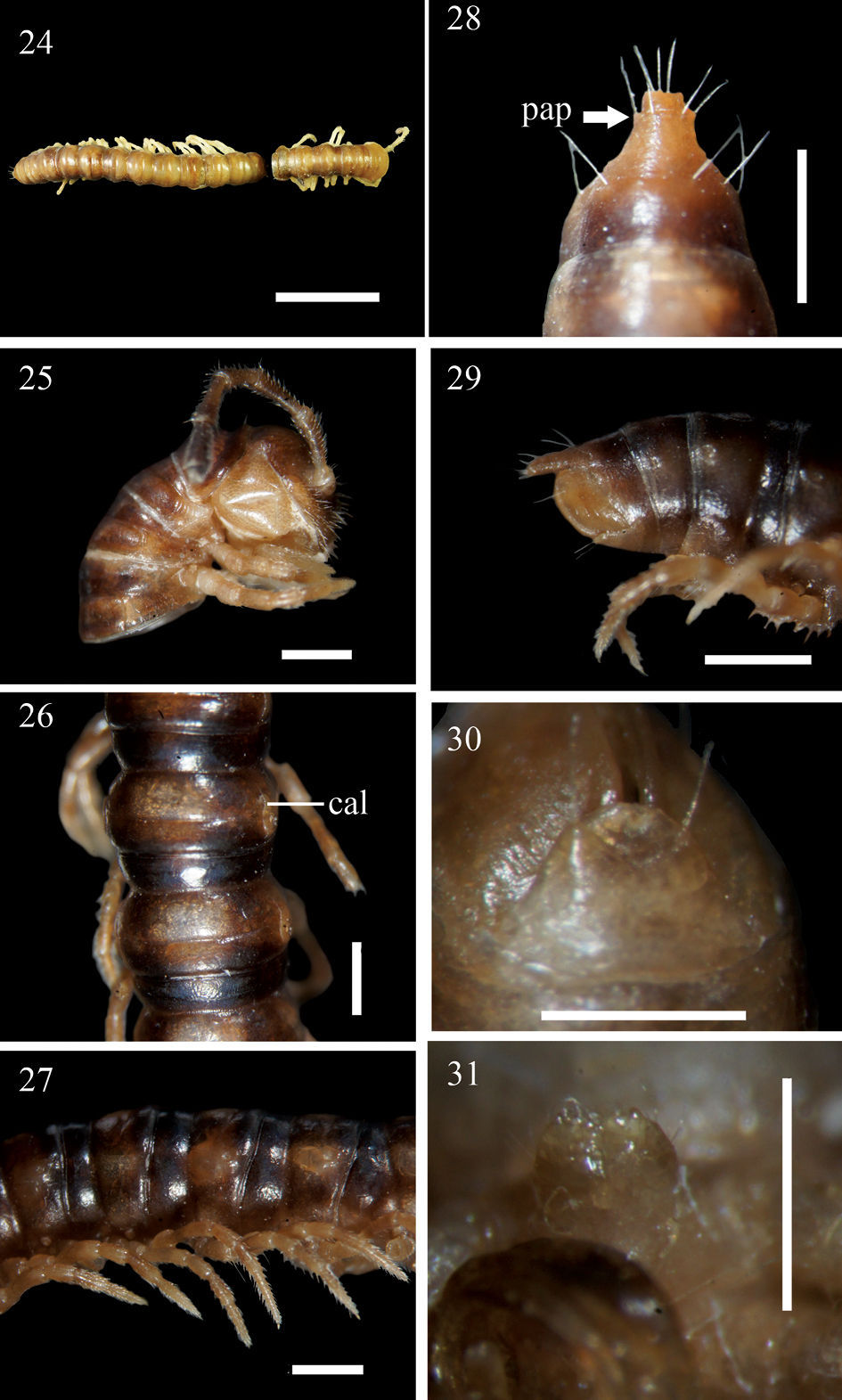
Aponedyopus similis sp. n., ♂ holotype (24) ♂ paratype (25–31). 24 Entire body, dorsal view 25 Anterior body portion, lateral view 26–27 Midbody segments, dorsal and lateral views, respectively 28–29 Epiproct, dorsal and lateral views, respectively 30 Hypoproct, ventral view 31 Sternal cones near gonopod aperture. Scale bars: 1.0 mm for 24–29, 0.5 mm for 30, 31. cal: calluses; pap: pre-apical papillae.
To emphasize the close resemblance to the next new species.
Being apparently the most similar to Aponedyopus latilobatus sp. n., based both on several peripheral characters (shorter legs, mostly a smaller body size etc.) and gonopod conformation, it is distinguished by the gonopod lobe b being membranous and lobiform, the terminal branches of the solenophore differing in length and crossing each other, with branch x carrying an inconspicuous lobe (see also Key below).
Length ca 22 mm (♂, n=2); width of pro- and metazona 10 ca 1.8 and 2.0 mm, respectively.
General coloration in alcohol brown to dark brown (Figs 24–27), with a clear pattern of a lighter brown to yellow brown axial stripe consisting of narrower subtriangular spots on proterga and twice as wide central spots on metaterga, these spots growing slightly infuscate, to blackish both towards stricture and posterior half of metaterga; prozona slightly darker than metazona, thus providing a vague cingulate pattern as well; paraterga, legs and venter slightly lighter than background, light grey-brown; head marbled brown, especially well so in vertigial region, genae contrastingly yellowish, a square median spot above antennal sockets contrastingly dark brown; antennae increasingly infuscate, up to blackish distad, distinctly darker at margins, marbled and lighter centrally, only tip contrastingly pallid; both collum and segment 2 with a very faint, yellow-brown, axial line; epiproct uniformly light brown, only very slightly infuscate near base.
Postcollum constriction evident; in width, segment 2 = 3 < 4 < collum < head = segments 5–15; thereafter body gradually and gently tapering towards telson both in width and height. Antennae (Fig. 25) medium-sized, slender, reaching behind stricture of tergite 3. Paraterga (Figs 26 & 27) very poorly developed, very evident and low only on segment 2, calluses (cal) (nearly) completely delimited by a sulcus dorsally, in caudal 1/3 also ventrally only on pore-bearing segments. Transverse sulcus (Figs 26, 27) developed on segments 5–17, traceable on segment 18, wanting on 19th, narrow, shallow, neither beaded at bottom nor reaching bases of paraterga. Surface smooth throughout, slightly granulated only below paraterga 2–4. Limbus thin, caudal margin entire. Stricture dividing pro- and metazona shallow, narrow, not beaded at bottom (Figs 26 & 27). Pleurosternal carinae present only on segments 2 and 3 (Fig. 25). Tergal setae almost fully abraded, 2+1 retained only at anterior edge of collum; pattern untraceable. Ozopores lateral, lying on calluses ca 1/2 metatergal length in front of caudal edge (Figs 26 & 27). Epiproct long (Figs 28 & 29), flattened dorsoventrally, straight, not curved caudoventrad in lateral view, ratio of epiproct length to pre-epiproct length of telson 1: 1.3, tip of epiproct slightly concave; pre-apical papillae (pap) evident, close to apex. Hypoproct (Fig. 30) rounded, subtrapeziform, 1+1 setae at caudal corners situated on well-separated knobs, sides slightly concave.
Sterna sparsely setose; lamina between coxae 4 setose and emarginate (Fig. 31); segment 7 with a pair of prominent sternal cones (= spiracles) flanking gonopod aperture; each cross-impression with a transverse sulcus, but without axial groove. Legs (Fig. 27) moderately long and slender, legs 1 to anterior legs of segment 17 with tarsal brushes, thereafter legs broken off in both available ♂♂, each midbody leg ca 1.2 times as long as body height, coxa 2 with a small apical process supporting a gonopore.
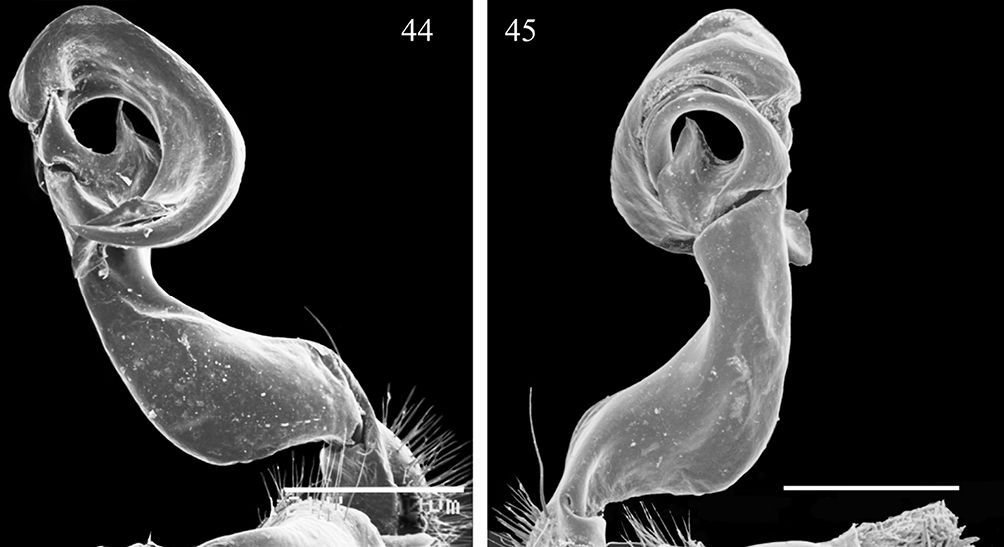
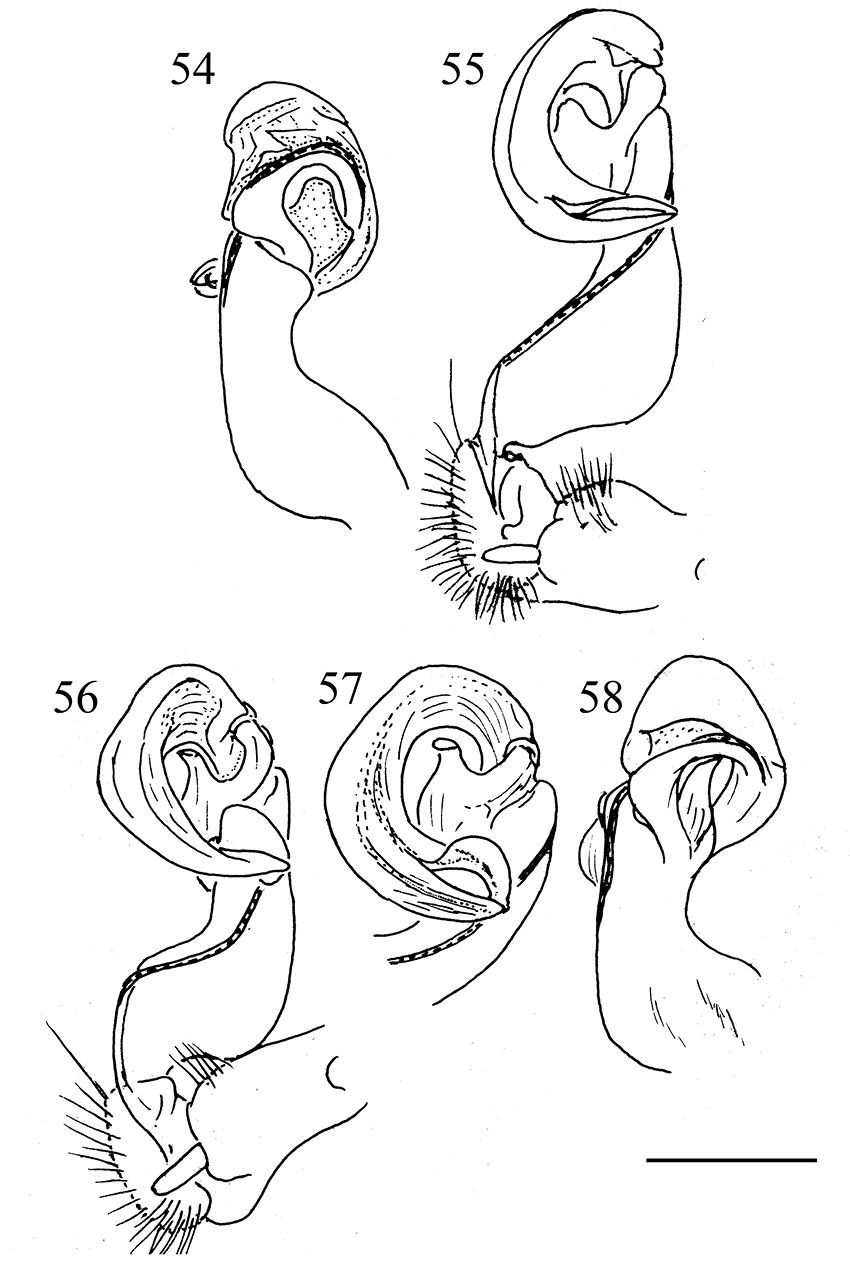
Gonopods (Figs 44, 45, 54 & 55) with process b at base of postfemoral part lobe-shaped, membranous, not like a distinct process; l at base of solenophore rather vague; distal part of gonopod deeply bifid, divided into a longer solenomere (sl), more complex at end and bearing a low terminal lobe, and a slightly shorter, simple, nearly pointed solenophore branch (sph); ends of both branches crossing.
This species seems to be local, occurring only rather high (2, 000–2, 200 m a.s.l.) in the mountains of northern Taiwan (Map).
urn:lsid:zoobank.org:act:9365404E-6E43-4CBA-ABA9-4C0466D27E42
Figs 32–39, 46–49, 56–58Holotype ♂ (NSYSUB-DI 76), Taiwan (R. O. C.), Taichung County (台中縣), HePing (和平鄉), Sihyuanyakou (思源啞口), forest path no. 710, 1.5 km from entrance to path, ca 2, 050–2, 100 m a.s.l., 21 August 2002, leg. C. C. Chen & Y. H. Lin.
Paratypes: 3 ♀ (NSYSUB-DI 77–79), same locality, date, and collectors, together with holotype.
Aponedyopus latilobatus sp. n., ♂ holotype. 32 Entire body, dorsal view 33 Anterior body portion, lateral view 34–35 Midbody segments, dorsal and lateral views, respectively 36–37 Epiproct, dorsal and lateral views, respectively 38 Hypoproct, ventral view 39 Sternal lobe between coxae 4. Scale bars: 0.5 mm for 36, 39, 1.0 mm for others.
Aponedyopus montanus Verhoeff, 1939, ♂ fromFuShan Botanical Garden (福山植物園), left gonopod, (40, 41) mesal and lateral views, respectively 42–43 telopodite tip, mesal and lateral views, respectively. Scale bar = 0.5 mm for 40, 41, 0.2 mm for 42, 43. cx: coxite, b: spiniform pointed branch, fe: femorite, l: lobe, pf: prefemoral part, pst: postfemoral part, sl: solenomere, sph: solenophore, su: sulcus, T: telopodite.
Aponedyopus similis sp. n., ♂ paratype, left gonopod 44, 45, submesal and sublateral views, respectively. Scale bar = 0.5 mm.
Aponedyopus latilobatus sp. n., ♂ holotype, left gonopod 46, 47, submesal and dorsal views, respectively 48, 49 telopodite tip, submesal and lateral views, respectively. Scale bar = 0.5 mm for 46, 47, 0.25 mm for 48, 49.
Aponedyopus montanus Verhoeff, 1939, ♂♂. 50, 51 right gonopod, mesal and lateral view, respectively. 52–53 left gonopod, mesal and lateral view respectively. Scale bar = 1 mm. sg: seminal groove.
Aponedyopus similis sp. n., ♂ paratype, right gonopod, lateral and mesal views, respectively 54, 55. Aponedyopus latilobatus sp. n., ♂ holotype, right gonopod and telopodite tip, mesal view, respectively 56, 57, right gonopod, lateral view 58. Scale bar = 0.5 mm.
Apparently being the most similar to Aponedyopus similis sp. n., it differs in the texture of the tegument (mostly rugulose in Aponedyopus latilobatus sp. n.) and, especially, in certain details of gonopod structure: lobe l is neither so wide nor membranous, the terminal branches are subequal in length, and the solenomere is supplied with a far more evident terminal lobe (see also Key below).
Length 15 mm (♂, n=1) and 18 mm (♀, n =3); width of pro- and metazona 10 ca 1.8 and 2.0 (♂) or 1.9–2.0 and 2.0–2.2 mm (♀), respectively.
Coloration in alcohol entirely light brown to brown (Figs 32–35); antennae light brown, growing increasingly blackish distally, but tip pallid; pattern much clearer in ♀, much like in Aponedyopus similis sp. n.: a light brown, wide, axial stripe from anterior edge of collum to end of epiproct; paraterga and sternites contrastingly lighter brown; legs pallid to yellow; axial line wanting.
Postcollum constriction clear (♂) or faint (♀), segment 4 < 3 < 2 < collum = segments 5–16 < head (♂), or collum = segments 2–4 < head = segments 5–18 (♀), thereafter body gradually and gently tapering both in width and height towards telson. Antennae (Fig. 33) medium-sized (♂) to short (♀), slender, reaching behind stricture of tergite 3 dorsally (♂), or end of collum to posterior edge of segment 2 (♀). Paraterga (Figs 34 & 35) as in Aponedyopus similis sp. n., but sometimes not or nearly not delimited by a ventral sulcus (♀). Surface transversely rugulose on metaterga 2 close to paraterga, sparsely longitudinally rugulose in places on post-sulcus halves of metaterga. Pleurosternal carinae (Fig. 33) present only on segments 2 and 3. Tergal setae almost fully abraded, 3+3 retained only at anterrior edge of collum; pattern untraceable. Epiproct (Figs 36 & 37) same as in Aponedyopus similis sp. n., but tip either slightly concave or subtruncate.
Sterna sparsely setose; lamina (Fig. 39) between ♂ coxae 4 evidently emarginate and setose; ♂ segment 7 with a pair of prominent, ventral, sternal cones (= spiracles) flanking gonopod aperture. Legs short and slender, shorter than to almost as long as midbody height; tarsal brushes present from legpair 1 to anterior legs of segment 10; coxa 2 with a small apical process supporting a gonopore.
Gonopod (Figs 46–49, 56 & 57) with b more like in Aponedyopus montanus, but l especially indistinct, and distal part of solenophore (sph), albeit also deeply bifid, having both terminal branches of subequal length, as well as a far more evident terminal lobe on solenomere (sl) not crossing a simple end of sph.
This seems to be a very local high-montane species in central Taiwan (Map).
| 1 | Midbody legs about twice as long as body height. Gonopod with terminal part of solenophore divided into two slender branches (x and y, Figs 40–43, 50–53) | Aponedyopus montanus |
| – | Midbody legs only up to 1.2 times as long as body height. Gonopod with terminal part of solenophore divided into wide branches | 2 |
| 2 | Terminal branches of solenophore differing in length and crossing each other, branch y with a rather inconspicuous terminal lobe (Figs 44, 45, 54 & 55) | Aponedyopus similis sp. n. |
| – | Terminal branches of solenophore subequal in length and not crossing each other, branch y with a highly inconspicuous terminal lobe (Figs 46–49, 56–58) | Aponedyopus latilobatus sp. n. |
Aponedyopus
seems to be a small genus endemic to Taiwan. Based on available
information, among its three constituent species two are pretty local
in distribution, Aponedyopus latilobatus sp. n. and Aponedyopus similis sp. n., each restricted to the northern or central, mostly montane parts of the island, respectively (Map). In contrast, Aponedyopus montanus
appears to be extremely widespread, living at various elevations in
all parts of Taiwan, including the small islet of Lanyu off the
southeastern coast of Taiwan. Whether this species could have been
introduced to, and originally described from, Japan, remains open to
question. There is only a single example of a basically Taiwanese
paradoxosomatid to have become successfully established at least in
southern Japan: Chamberlinius hualienensis Wang, 1956 in Kyushu Island and the Ryukyus (
Distribution of Aponedyopus species in Taiwan. Aponedyopus montanus Verhoeff, 1939: filled black squares; Aponedyopus similis sp. n.: filled red circles; Aponedyopus latilobatus sp. n.: filled green triangle.
The distribution of Aponedyopus
species in Taiwan shows allopatry. Syntopic occurrences are nearly
missing, a feature already reported, e.g., for the paradoxosomatid
genus Anoplodesmus (
Concerning the tribal position of Aponedyopus, it has long been placed in the tribe Tonkinosomatini (