(C) 2010 Clifford D. Ferris. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Based on genitalic studies, the new genus Pionenta is established for two taxa formerly placed under Antepione. The taxa hewesata and ochreata (and previously associated synonyms) are now synonomized as Pionenta ochreata. Three species of Antepione are now recognized: Antepione thisoaria, Antepione imitata, Antepione tiselaaria with the taxa comstocki, constans, and indiscretata synonomized under Antepione imitata. No new species are described. Adults and genitalia are illustrated, including type specimens.
Antepione , Arizona, Colorado, Costa Rica, Ennominae, Geometridae, Guatemala, Lepidoptera, Mexico, New Mexico, nomenclature, North America, Pionenta , taxonomy, Texas
A genitalic study of the eight species recognized by
AMNH American Museum of Natural History, New York, NY, USA.
ANSP Academy of Natural Sciences, Philadelphia, PA, USA.
BMNH The Natural History Museum (formerly British Museum [Natural History]), London, UK.
CDF Personal collection of Clifford D. Ferris, Laramie, WY, USA.
CMNH Carnegie Museum of Natural History, Pittsburgh, PA, USA.
CNC Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada.
EME Essig Museum of Entomology, University of California, Berkeley, CA, USA.
FMNH Field Museum of Natural History, Chicago, IL, USA.
MCZ Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA.
MNHN Museum National d’Histoire Naturelle, Paris, France.
SEM Snow Entomological Museum Collection, University of Kansas, Lawrence, KS, USA.
USNM National Museum of Natural History [formerly United States National Museum], Washington, District of Columbia, USA.
Methods and general terminology Terms for genital structures and wing markings follow
AML Antemedial line.
DFW Dorsal forewing.
DHW Dorsal hindwing.
FWL Forewing length, measured along costa from base to apex.
MB medial band = area between DFW AML and PML.
PML Postmedial line.
TL Type locality.
Key to genera(based on DFW pattern and genitalia)
| 1 | DFW triangular costal dark patch present; male genitalia lack furca; female genitalia lack colliculum and signum | Antepione |
| – | DFW triangular costal dark patch absent; male genitalia with robust stubby furca; female genitalia with colliculum and signum | Pionenta ochreata |
(based on genitalia)
| 1 | Male genitalia: apical region of valva lacks spines. Female genitalia: corpus bursa oblong and initially swollen with membranous anterior sac | Antepione thisoaria |
| – | Not as above | 2 |
| 2 | Male genitalia: valva rounded at apex with 3 long robust spines and additional fine setae. Female genitalia: corpus bursae long and cylindrical with membranous anterior sac | Antepione imitata |
| – | Not as above | 3 |
| 3 | Male genitalia: valva rounded at apex with multiple short slender translucent spines over most of surface excepting toward base. Female genitalia unknown to author | Antepione tiselaaria |
Epione depontanata Grote, 1864. Location of type unknown; originally placed in ANSP. Described from Maryland, USA.
Mimogonodes constricta Warren, 1895 [BMNH].
Adults. Medium sized (FWL 13–21 mm) basically ochreous-colored moths with variable markings on DFW. DFW outer margin angulate at vein M3. Separation from similar genera is by the combination of characters: filiform antennae; male genitalia with stout tapered decurved uncus, valvae with even outer margins lacking projections, absence of furca; female genitalia without colliculum and signum.
Adults. Sexually dimorphic and sexes polyphenic; FWL 13–21 mm. Antenna simple, more slender in females. Head – Dark ochreous speckled with darker scales, concolorous collar; labial palpi broad, barely extending beyond frons, ochreous speckled with darker scales. Thorax, abdomen, legs – Ochreous or pale tan as in wings with widely scattered small brown scales. Wings – FW outer margin arcuate at vein M3 and HW; DFW apex acute to falcate. Usually obscure narrow dark DFW submarginal band; small dark discal spots both wings. Males. Dorsal color varies from gray, medium ochreous to medium brown. DFW AML and PML variable from pronounced and dark to broken and indistinct; medial band concolorous with remainder of wing, or paler and yellowish; a dark triangular patch with blunted or acute apex, with or without pale oblong spot, located along costa distad of PML. DHW with dark narrow medial band varying individually from dark to indistinct. Ventrally paler with dorsal maculation repeated, usually with less intensity. Females. Dorsal color varies from yellow through pale ochreous to medium ochreous and gray. Crosslines usually indistinct. DFW triangular patch as in males, PML above inner margin expanded into two large oblong brown spots. Ventrally paler with dorsal maculation repeated, usually with less intensity. Male genitalia – Uncus stout, slightly decurved, tapering to a rounded tip; medial gnathos with a few small teeth; valva rounded at apex; anellus with small spines; aedeagus truncate with one large oblong cornutus near base of vesica. Female genitalia – Apophyses long, slender; posterior apophyses ca. 1.8 × anterior apophyses; colliculum absent; ductus bursae ridged, short, partially sclerotized at posterior end; corpus bursae without signum, oblong with membranous anterior sac; ductus seminalis originates at top of ductus bursae.
Figs 1, 11–19, 59
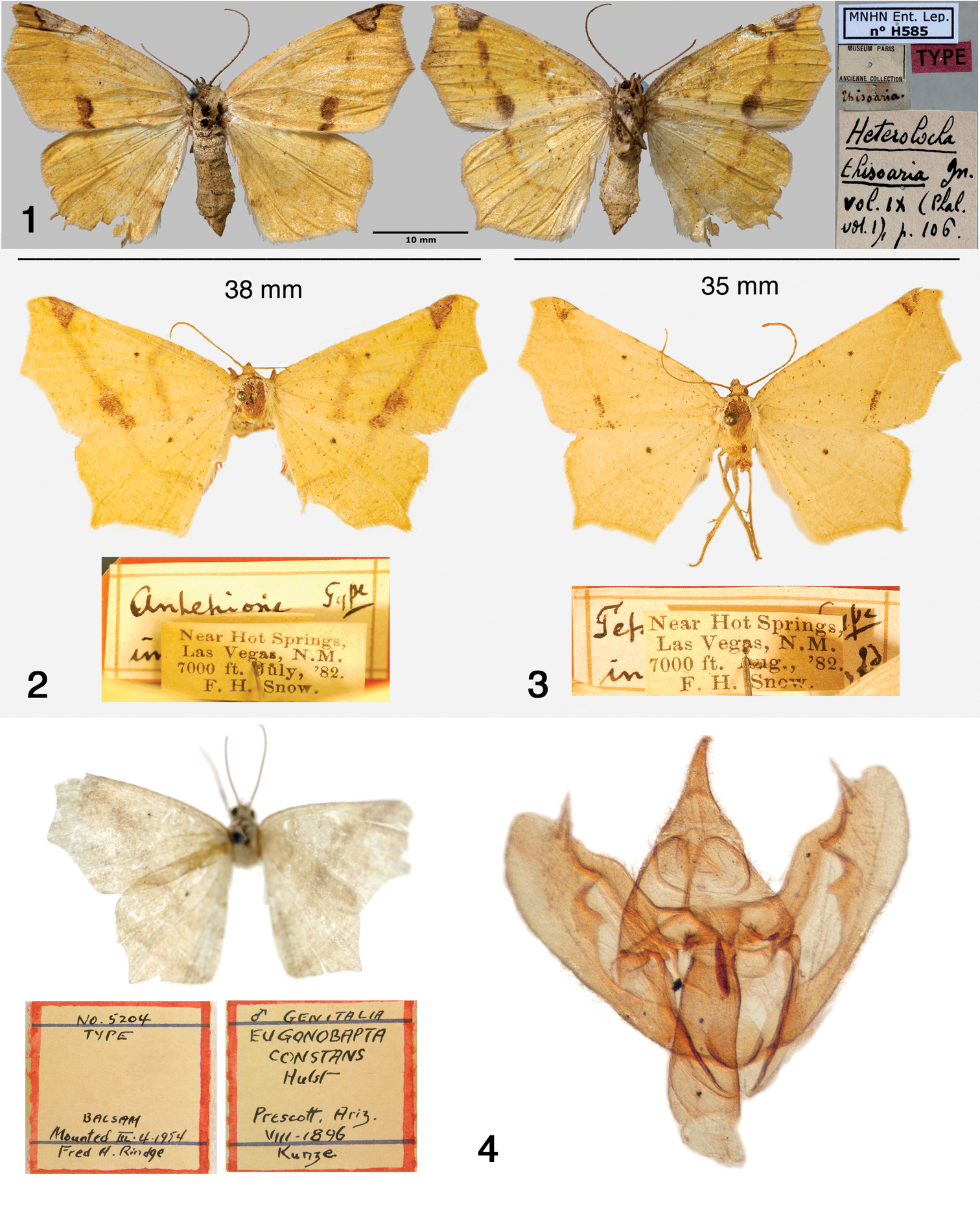
Female HT (Fig. 1), country of origin not stated [MNHN].
The Central American taxa were not recognized and described until 1892 (azonax) and 1912 (rhomboidaria). On this basis, I infer that specimens from this region were not available to Guenée in 1857 when he described thisoaria, and that the holotype was collected in eastern North America. In habitus, the HT matches exactly female specimens of the sulphuraria/sulphurata
form. The HT was most probably collected in the Middle Atlantic region.
I hereby fix the type locality as eastern North America. Based on my
research, it appears that
84 specimens (a few by photograph) from Alabama, Indiana, Kentucky, Mexico, Michigan, New Jersey, New York, Nova Scotia, Pennsylvania, Quebec, Tennessee, Virginia. Additional distribution records were obtained from individuals and several museums, including 439 from the Carnegie Museum of Natural History, Pittsburgh, PA.
Antepione species. 1 Antepione thisoaria HT (dorsal and ventral) with pin labels (MNHN photo) 2 Antepione imitata HT with pin labels (SEMC photo) 3 Antepione (Tetracis) indiscretata HT with pin labels (SEMC photo) 4 Antepione (Eugonobapta) constans HT, adult, pin labels (AMNH photo) and male genitalia. The balsam embedding medium has fogged with age producing the apparent lack of focus in the genitalia photo.
Antepione thisoaria is most easily separated from Antepione imitata based on geography. It does not occur west of the 95th parallel, while Antepione imitata extends eastward only to west Texas, and is not recorded from Central America. In the male genitalia, the apical region of the valva lacks spines, which are present in the valva of imitata. In the female genitalia, the corpus bursae is initially swollen while not so in Antepione imitata.
Adults. As described above for the genus. Genitalia. Figs 17–19. Two dissections (male and female) by author; illustrations in McGuffin (1987, Figs 242g, 245e); Pitkin (2002, Figs 202, 460). Uncus stout, slightly decurved, tapering to a rounded tip; gnathos with unjoined slender arms, medial gnathos with a few small teeth; valva rounded at apex without spinesMale genitalia –, produced ventral ridge forming two short projections; anellus with two sclerotized spinose lobes; aedeagus truncate with one large narrow elliptical cornutus near base of vesica. Female genitalia – Apophyses long, slender; posterior apophyses ca. 1.8 × anterior apophyses; ductus bursae ridged, moderately short, partially sclerotized at posterior; corpus bursae without signum, corpus bursae without signum, oblong and initially swollen with membranous anterior sac; ductus seminalis originates at top of ductus bursae.
(Fig. 59). McGuffin (1987: 88–89) described the early stages and cited three specific larval hosts: Alnus rugosa (Du Roi) Spreng; Physocarpus opulifolius (L.) Maxim; Prunus serotina
Ehrh. Various additional larval hosts are reported in the literature in
the families Aceraceae, Anacardiaceae, Betulaceae, Ebenaceae, and
Rosaceae. The last instar larva was illustrated by Wagner et al. (2001, p. 155) and Wagner (2005, p. 195).
Adults fly April–May with an occasional mid-March and mid-June record,
July–August with occasional September to mid-October records. There is
one generation in Canada, and at least two southward. The distribution
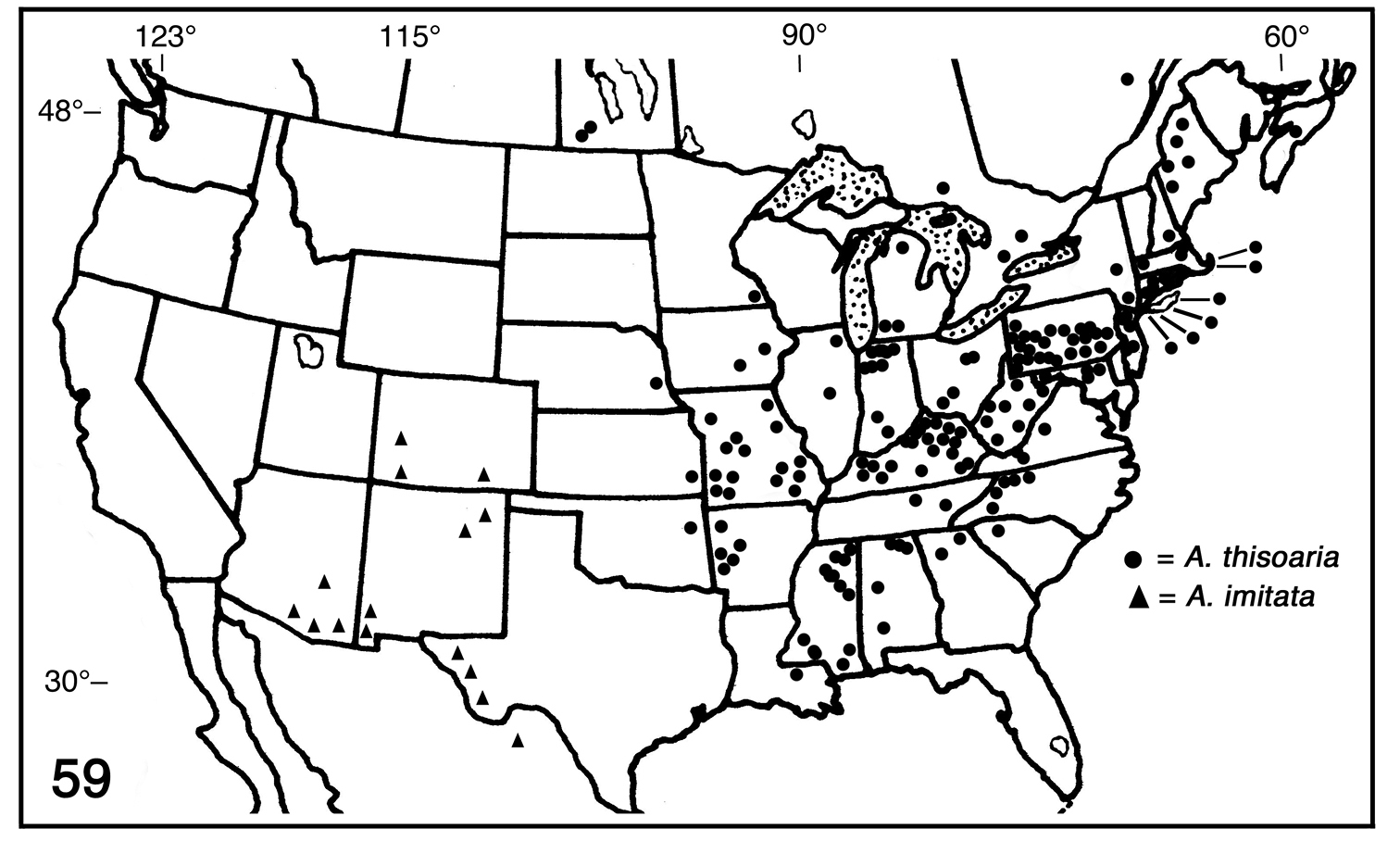
map (Fig. 59)
represents the data that I was able to locate. The heavy distribution
in Pennsylvania reflects intensive collecting in that state by CMNH
personnel and volunteers. Undoubtedly similar efforts in neighboring
areas should produce additional records. The overall range of this
species is: in CANADA from Nova Scotia to Manitoba; in the UNITED STATES (county records in parentheses) then south and west to the Gulf states to the 95th parallel, including Alabama (Bibb, DeKalb, Jackson, Madison, Monroe), Arkansas (Logan, Montgomery, Polk, Scott, Washington), Connecticut (Fairfield, Hartford, New Haven, New London, Tolland, Windham), Georgia (Cherokee, Rabun), Illinois (Cook, Macon), Indiana (Elkhart, Jackson, Jasper, Lagrange, Laporte, Monroe, Newton, Perry, Pulaski, St. Joseph), Iowa (Johnson, Monroe), Kansas (Crawford), Kentucky
(Bell, Boone, Bracken, Bulitt, Calloway, Carter, Fayette,
Graves, Harlan, Jefferson, Madison, McCracken, Meade, Menifee,
Metcalfe, Morgan, Muhlenberg, Oldham, Owsley, Powell, Rowan,
Russell, see Covell 1999), Louisiana (Feliciana Parish), Maine (Aroostook, Franklin, Oxford, Penobscot, Piscataquis), Maryland (Allegheny, Anne Arundel, Baltimore, Cecil, Garrett, Harford, Howard, Washington, Worcester), Massachusetts (Berkshire, Dukes, Essex, Middlesex, Nantucket), Michigan (Berrien, Cass, Otsego), Minnesota (Houston), Mississippi
(Franklin, George, Grenada, Harrison, Kemper, Lee, Marshall,
Oktibbeha, Pike, Pontotoc, Tishomingo, Union, Warren, Webster,
Winston), Missouri (Barry, Benton, Camden, Cape Girardeau,
Carter, Greene, Jasper, Lafayette, Lewis, Madison, Morgan,
Newton, Stoddard, Warren, Wayne), Nebraska (Cass), New Jersey (Burlington, Essex, Gloucester, Morris, Passaic, Sussex, Union, Warren), New Hampshire (Rockingham), New York (Albany, Kings, Queens, Nassau, Suffolk, Westchester), North Carolina (Allegheny, Ashe, Avery, Stokes, Swain, Transylvania), Ohio (Adams, Ashland, Ross, Wayne), Oklahoma (Cherokee, see
The gray spring form of the moth (Figs 11, 15) was described by Packard as the species furciferata. The male (Fig. 14) represents the summer form arcasaria, and the female (Fig. 16) represents the summer form sulphuraria = sulphurata.
Figs 2–5, 20–33, 59
Female HT (Fig. 2), New Mexico, [San Miguel Co.], Las Vegas, July, 1882. [SEMC].
Antepione comstocki male HT (Fig. 5), Arizona, [Pima Co.], Baboquivari Mts., 26 April, 1938 [CNC]. Tetracis indiscretata female HT (Fig. 3), New Mexico, [San Miguel Co.], Las Vegas, August, 1882 [SEMC]. Eugonobapta constans male HT (Fig. 4), Arizona, [Yavapai Co.], Prescott, August, 1896 [AMNH].
145 specimens in [CDF] from Arizona, Colorado and New Mexico; additional material (some by photographs) from Arizona (including a reared series), Colorado, New Mexico, Texas, Mexico.
Antepione species. 5 Antepione comstocki HT with pin labels (CNC photo) 6–10 Antepione tiselaaria. 6 HT with pin labels (USNM photo) 7–8 adult males 9 male genitalic capsule, aedeagus removed 10 aedeagus with vesica everted.
Antepione imitata is most easily separated from Antepione thisoaria based on geography. It does not occur east of west Texas and is not recorded from Central America, while Antepione thisoaria extends west only to the 95th parallel. In the male genitalia, the apical region of the valva exhibits 3 long robust spines and additional fine setae, which are not present in the valva of thisoaria. In the female genitalia, the corpus bursae is not initially swollen as in Antepione thisoaria.
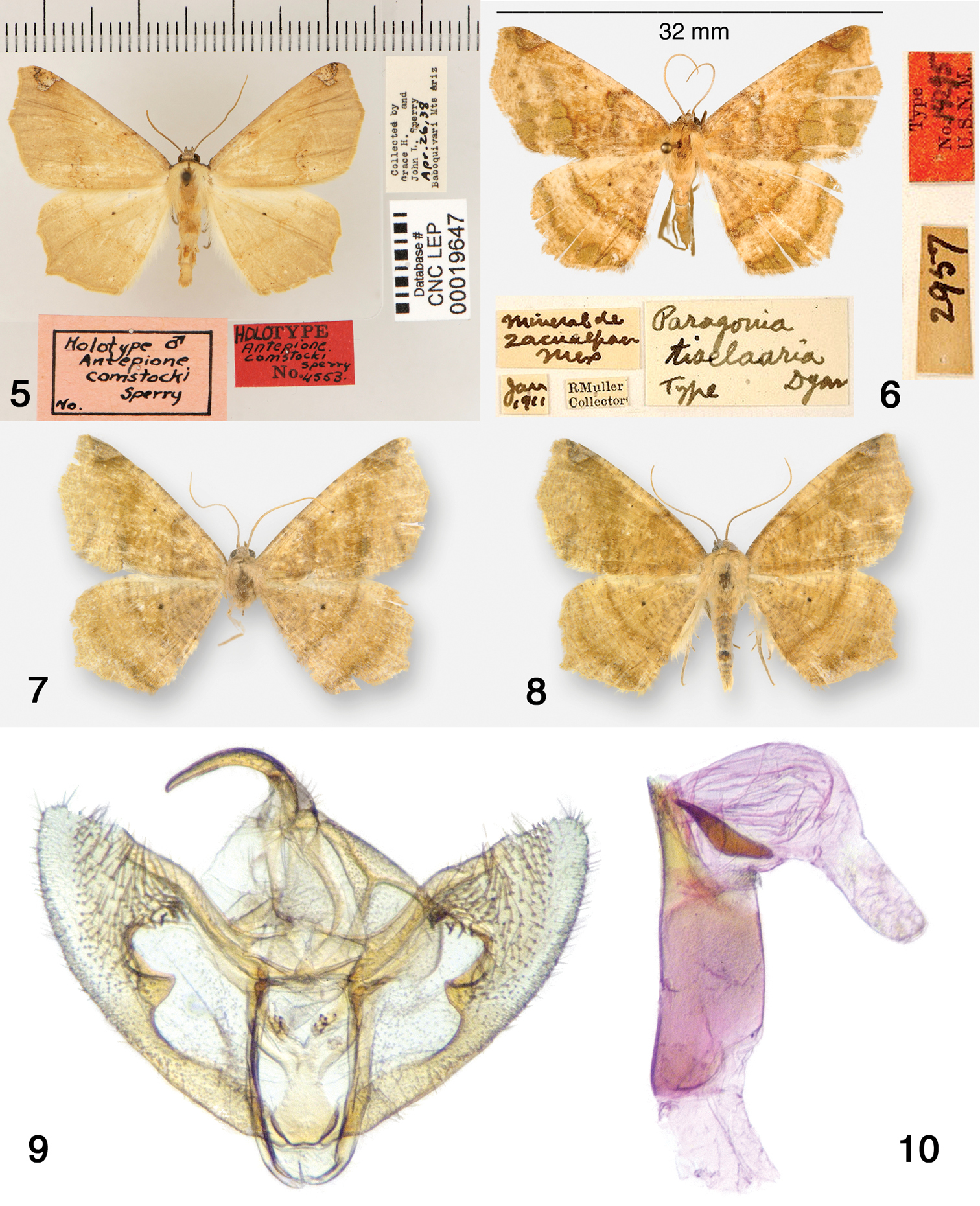
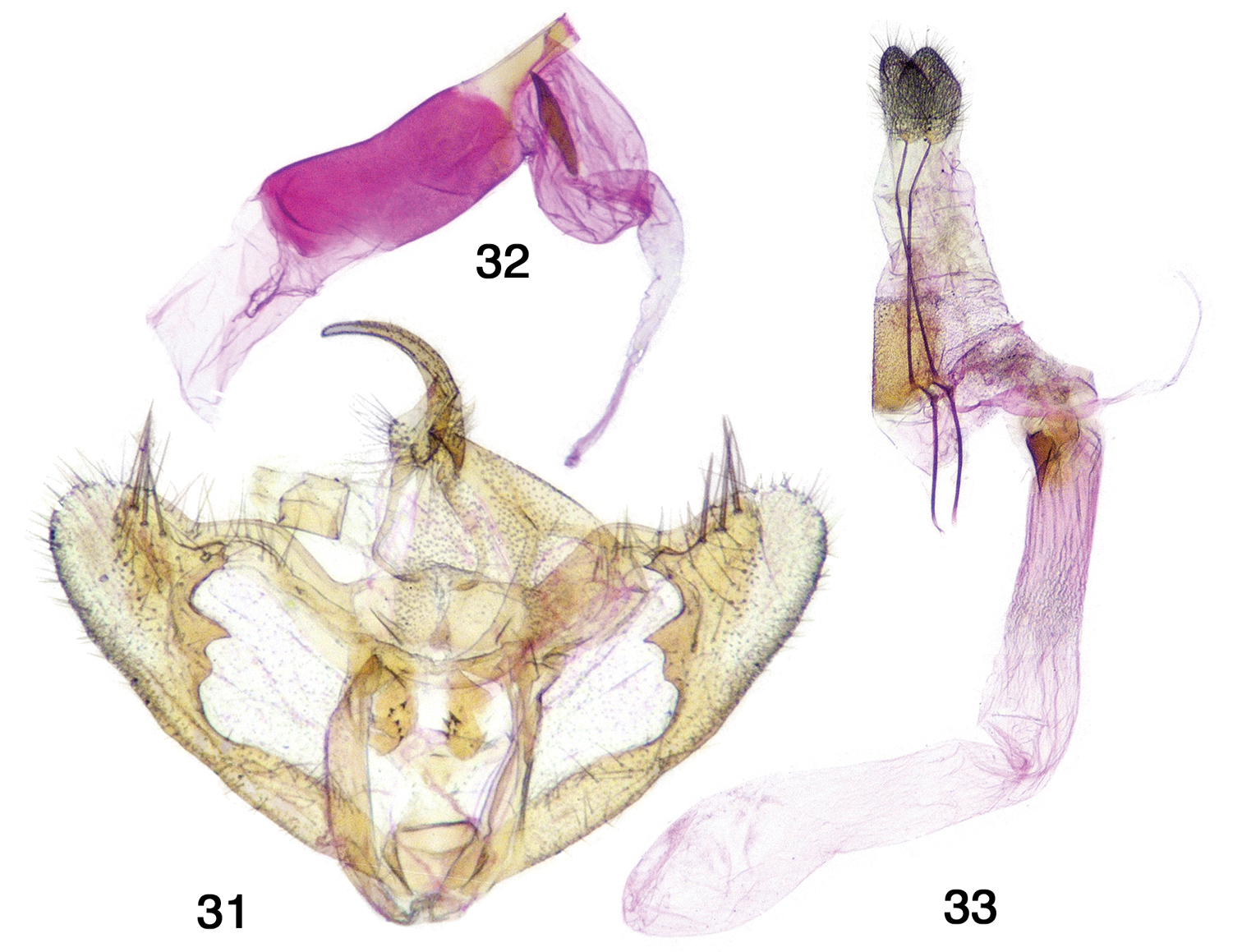
Adults. As described above for the genus. Genitalia. Figs 4, 31–33. Dissections 8m, 2f comprising full range of phenotypes). Male genitalia – Uncus stout, slightly decurved, tapering to a rounded tip; gnathos with unjoined slender arms, medial gnathos with a few small teeth; valva rounded at apex with 3 long robust spines and additional fine setae, produced ventral ridge forming two short projections; anellus with two sclerotized spinose lobes; aedeagus truncate with one large oblong cornutus near base of vesica. Female genitalia – Apophyses long, slender; posterior apophyses ca. 1.8 × anterior apophyses; ductus bursae ridged, short, partially sclerotized at posterior; corpus bursae without signum, long and cylindrical with membranous anterior sac; ductus seminalis originates at top of ductus bursae.
Antepione thisoaria. 11–14 adult males 15–16 adult females 17 male genitalic capsule, aedeagus removed 18 aedeagus with vesica everted 19 female genitalia.
Antepione imitata adults. 20–25 males 26–30 females.
Antepione imitata genitalia. 31 male genitalic capsule, aedeagus removed 32 aedeagus with vesica everted 33 female genitalia.
Remarks. One male specimen (Fig. 25) of the comstocki phenotype examined from Las Animas Co., Colorado lacks the characteristic DFW costal triangular patch, causing it to resemble superficially the ligata form of Pionenta ochreata. The male genitalia, however, are typical of Antepione imitata.
(Fig. 59). Noel McFarland (Hereford, AZ) reared the species on Ribes aureum Push. from ova from an adult female of the nearly uniformly brownish-ochreous April–May generation; adults emerged June–July. The resulting adults are of the form with yellow females and males in which the DFW medial band has a yellow flush. Based on my field studies over many years in southeastern Arizona and southwestern New Mexico and McFarland’s reared material, there appear to be three generations in southeastern Arizona and Southwestern New Mexico. There is a strong early flight starting in April and early May, with a weaker flight in late June into July, and another strong flight beginning in mid-August after the monsoonal rains with a few individuals into early October. This species ranges from west Texas (Brewster, Culberson, Jeff Davis), Colorado (Delta, La Plata, Las Animas), New Mexico (Grant, Harding, Hidalgo, San Miguel), to southern Arizona (Cochise, Gila, Pima, Santa Cruz). A typical male specimen was examined [CMNH] with the collection data: Mexico: Coahuila, Sierra La Madera, upper Canada Desiderio, 15–17 March 1985, 27–08N, 102–31W, 1810m, J. Rawlins, S. Thompson. This locality is essentially due south of the western Texas records, and one might anticipate that with further collecting Antepione imitata will prove to be widespread in northern Mexico. It is generally associated with riparian canyons up to 6000’ (1830m).
As is also the case with Antepione thisoaria, most spring individuals of Antepione imitata are rather drab in appearance with lightly maculated brownish males (the comstocki phenotype) and pale creamy colored or ochreous females. The strongly maculated males and yellow females appear in the later generations in company with the rather drab early-season phenotypes. In his original descriptions of imitata and indiscretata, Edwards provided no insight as to why he assigned imitata to Antepione and indiscretata to Tetracis. Both taxa are described on the same page with the description of imitata preceding that of indiscretata. He characterized the color of imitata as similar to the yellow sulphurata phenotype of thisoaria, and indiscretata as “Ochraceus drab.” Over the years the type specimens have faded to some extent so that they now appear nearly identical in color, the only difference being the extent of the dark maculation. The name constans appears to have been applied to the heavily maculated male phenotype, as best can be determined from the poor condition of the HT.
Figs 6–10
Male HT (Fig. 6), Mexico, Minerale de Zacualpan, January, 1911 [USNM]. Comment: Dyar (1912: 87) stated the type locality only as “Zacualpan” and not “Minerale de Zacualpan” as shown on the specimen label. I interpret the label to mean the Zacualpan mining region located in the state of Morelos south of Mexico City, today still an active silver mining district.
(Figs 7–10). MEXICO. Puebla, 2 mi. SW Tehuacan, 5300’, 4.x.1975, Powell (1m, dissected); same, 5.x.1975, J. Powell (1m) [EME].
Females not known to the author. Mexican specimens of Antepione tiselaaria males are most easily separated from Antepione imitata based on geography, since the latter species does not penetrate south to central Mexico. In Costa Rica, where Antepione thisoaria is also reported, Antepione tiselaaria manifests a more orange-brown overall color than the drab ochreous-gray form of thisoaria. In the male genitalia, the apical region of the valva is covered with multiple short slender translucent spines over most of the surface except toward the base; spines are absent in the valva of Antepione thisoaria, and 3 long robust spines occur in Antepione imitata.
Adults. Only males were available for examination. As described above for the genus, other than the wings. FWL 17–18 mm. Wings – FW outer margin arcuate (roundly produced about) vein M3 and HW; DFW apex sightly acute, not falcate. Dorsal color pale orange-brown-ochreous with darker maculation. AML a narrow band centrally with a few paler scales, PML an interrupted band with irregular edges and centrally paler, widening substantially approaching inner margin; MB not clearly defined with splotchy brown maculation over paler ground color; a dark triangular patch with blunted or acute apex, with or without pale oblong spot, located along costa distad of PML; small dark discal spots FW and HW. Ventrally paler with dorsal maculation repeated with slightly less intensity. Male genitalia. Figs 9–10. Dissection 1m. Uncus stout, slightly decurved, tapering to a rounded tip; gnathos with unjoined slender arms, medial gnathos with a few very small dark teeth; valva rounded at apex with multiple short slender translucent spines over most of the surface excepting toward the base, produced ventral ridge forming one large and one short projection; anellus with two sclerotized spinose lobes; aedeagus truncate with one large (equal to diameter of aedeagus shaft) oblong triangular cornutus near base of vesica; fully everted vesica initially spherical becoming a tapered tube.
Early stages unknown. Current distribution records are for the Mexican states of Morelos and Puebla, and Costa Rica.
urn:lsid:zoobank.org:act:95BF80CA-F55F-40D9-B877-A25252635770
Antepione ochreata Hulst, 1898.
Pionenta is a masculine anagram of Antepione.
Pionenta ochreata lacks the DFW triangular costal patch found in all species of Antepione. The well-developed DFW AML and PML form a wedge-shaped medial band absent in Antepione. The robust centered furca in the male genitalia and large stellate signum in the female genitalia of Pionenta are absent in Antepione.
Adults. Sexually dimorphic and both sexes are polyphenic; FWL 14–19 mm. Antenna simple. Head – Uniformly ochreous, collar concolorous; labial palpi relatively narrow, slightly upcurved, ochreous, barely extending beyond frons. Thorax, abdomen, legs – Uniformly colored as in ground color of wings with a few widely scattered small brown scales on legs. Wings – Outer margin arcuate FW (about M3) and HW; DFW apex normally sightly falcate. Wing color variable from pale creamy white to ochreous tan. AML and PML narrow and brown (occasionally reddish-brown), PML continues on DHW as medial line; AML with narrow pale shading basad, PML with narrow pale shading distad. MB trapezoidal tapering inward from costa to inner margin. Small dark discal spots present FW and HW. Scattered dark patches may be present basally and submarginally on DFW, and submarginally on DWH. Ventrally paler with dorsal maculation only weakly repeated. Male genitalia (7 dissections by author, additional museum slides examined) – Uncus stout, slightly decurved, tapering at apex to a rounded tip; gnathos v-shaped with well-sclerotized edges, medially a sharp upcurved tip with numerous very small teeth; valva rounded at apex, but with blunt triangular projection at end of sclerotized costa; anellus membranous without spines or setae, with central robust cylindrical furca covered by numerous short spines on rounded apex; aedeagus truncate with two long sclerotized pointed extensions from apical margin and a variable patch of apparently deciduous dark setae near base of otherwise membranous short cylindrical vesica. Female genitalia (6 dissections) – Posterior apophyses short, anterior apophyses much reduced ca. 0.4 × posterior apophyses; sterigma well sclerotized; posterior margin of lamella antevaginalis rounded at ends with central depression; well-defined colliculum; partially ridged short ductus bursae opens into ovoid membranous corpus bursae; one large centrally located oval stellate signum; ductus seminalis originates immediately below colliculum.
Figs 34–58, 60
Male HT (Fig. 34), Arizona, Senator [probably Senator Mine, Yavapai Co.] [AMNH].
Antepione hewesata female HT (Fig. 35), Arizona [Coconino Co.], Oak Creek Canyon, Todd’s Lodge, [AMNH]. Sabulodes arizonata male HT (Fig. 38), Arizona [Cochise Co.], Huachuca Mts. [USNM]. Sabulodes dyari male HT (Fig. 39), Arizona [Cochise Co.], Huachuca Mts. [USNM]. Sabulodes ligata male HT (Figs 36–37), Arizona [Cochise Co.], Huachuca Mts. [USNM].
135 specimens in [CDF] from Arizona and New Mexico; 61 additional specimens (some by photographs) from Arizona.
As for genus.
General description as for genus.
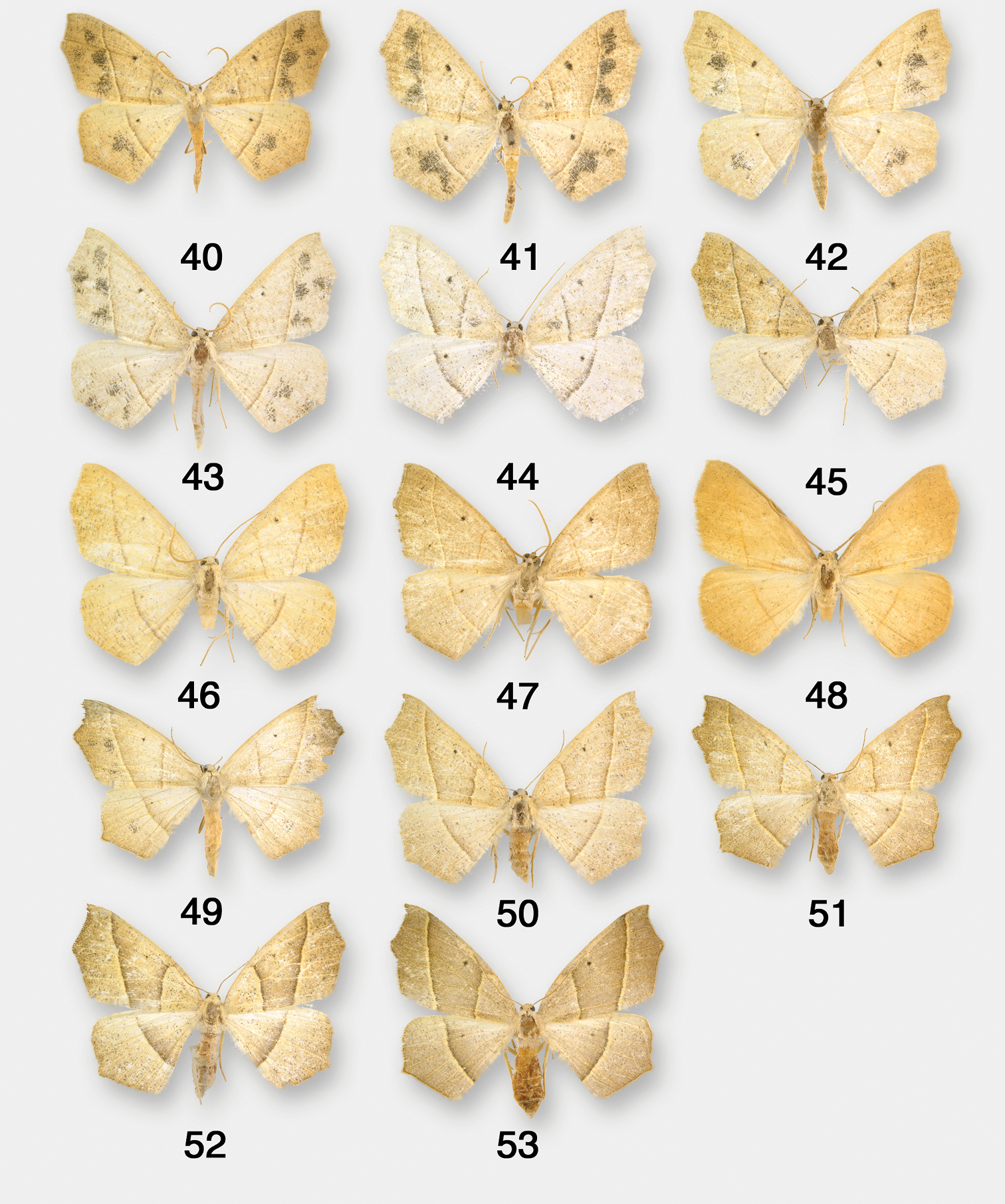
Pionenta ochreata. 34 Pionenta ochreata HT with pin labels (AMNH photo) 35 Pionenta (Antepione) hewesata HT with pin labels (AMNH photo) 36–37 Pionenta (Sabulodes) ligata 36 HT with pin labels 37 genitalia 38 Pionenta (Sabulodes) arizonata HT with pin labels 39 Pionenta (Sabulodes) dyari HT with pin labels. (36–39 USNM photos).
Pionenta ochreata adults. 40–48 males 49–53 females.
Pionenta ochreata genitalia. 54–57 male genitalic capsules, adeagii removed and adeagii with vesicas everted (arrows point to deciduous setae) 58 female genitalia.
Antepione ochreata has two distinct phenotypes. The male form with pale ochreous wing color and varying numbers of multiple dark patches (Figs 34, 40–43) was described as ochreata. The female described as hewesata (Figs 35, 44) is intermediate between typical ochreata and the brownish-tan phenotype without dark patches described as ligata (Figs 36, 46, 50–53) which is the usual female form based on my field experience and examination of museum material. Regarding the taxa arizonata and dyari, apparently some years ago an accident occurred with a drawer containing type specimens and they were badly damaged. Figs 38 and 39 illustrate what remains of these two specimens. Their associated genitalia slides were not damaged and the preparations agree with Figs 37, 54–57.
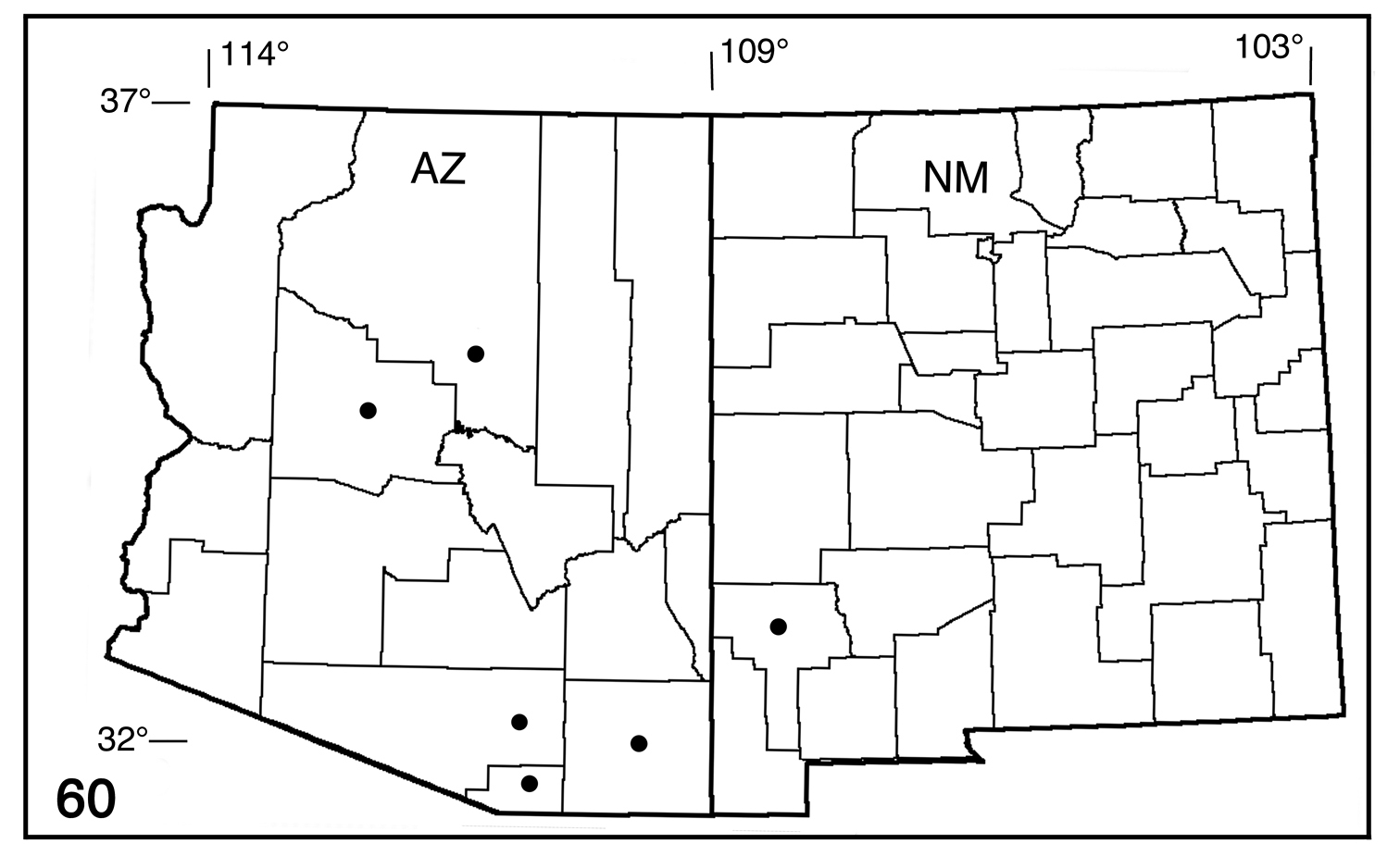
(Fig. 60). Early stages unknown. Adults from mid-May to August in riparian canyons and dry coniferous forest to 8400’ (2560m); probably more than one generation. Collection records include Arizona (Cochise, Coconino, Pima, Santa. Cruz, Yavapai), New Mexico (Grant).
Partial distribution map for Antepione thisoaria and Antepione imitata.
Distribution map for Pionenta ochreata (counties only).
Based on the male and female genitalia, Pionenta is closely related to Tetracis. The male genitalia of both genera possess a well defined central furca. The female genitalia of both genera possess a well defined colliculum and prominent single signum. The gnathos in Pionenta does not have a quadrate dorso-caudal margin with two to four (occasionally five) widely separated, dorsally projecting teeth as found in Tetracis (Ferris & Schmidt, 2010). Once barcoding of the North America geometrid genera has been completed, the relationship of Pionenta can be established.
Special thanks to my friends for permitting me to camp and run light traps on their properties: in Cochise Co., Arizona, Karen Coreg, Noel and Dienie McFarland, Todd Hoyer and Hayley Smith, Ralph and Rosemary Snapp, Gwen B. Wright; Nate Gibson in Patagonia, Arizona; the Coles in Grant Co., NM. Photographs, locality data, and loan material were kindly provided by: James Adams, Calhoun, GA; Susan Borkin, Milwaukee, WI; Jerome Barbut (MNHN), Paris, France; Richard L. Brown, Mississippi State, MS; Charles V. Covell, Jr., Gainesville, FL; Diane M. Debinski, Ames, IA; Michael S. Engle and Jennifer C. Thomas, (SEMC), Lawrence, KS; Irving Finkelstein, Atlanta, GA; Patricia Gentili-Poole, (USNM), Washington, DC; Suzanne Rab Green (AMNH), New York, NY; Howard Grisham, Huntsville, AL; John Gruber, Wynnewood, PA; Ed Knudson, Houston, TX; Timothy McCabe, Albany, NY: Hugh McGuiness, East Hampton, NY; J.S. Nordin, Laramie, WY; Paul Opler, Ft. Collins, CO; Bob Patterson, Bowie, MD; Jerry Powell (EME), Berkeley, CA; John E. Rawlins and Vanessa Verdecia (CMNH), Pittsburgh, PA; J. A. Snyder, Greenville, SC; J. B. Sullivan, Beaufort, NC; Jim Vargo, Mishawaka, IN; David Wagner, Storrs, CT; J. B. Walsh. Tucson, AZ; Jocelyn Gill, Don Lafontaine, Chris Schmidt, (CNC), Ottawa, Ontario, Canada. Two anonymous reviewers provided useful suggestions.
Antepione Packard, 1876
Antepione imitata H. Edwards, 1884, New Mexico, Las Vegas [HT female, SEMC]
Antepione comstocki Sperry, 1939, syn. n., Arizona, Baboquivari Mts. [HT male, CNC]
Antepione constans (Hulst, 1898), syn. n., Arizona, Prescott [HT male, AMNH]
Antepione costinotata Taylor, 1906, Colorado, Durango [HT female, USNM] Note 1
Antepione indiscretata (H. Edwards), 1884, syn. n., New Mexico, Las Vegas [HT female, SEMC]
Antepione vanusaria (Strecker, 1899), syn. rev., New Mexico [HT male, FMNH]
Antepione thisoaria Guenée, 1857 [1858], fixed herein as eastern North America [HT female, MNHN]
Antepione arcasaria (Walker, 1860), [HT female, BMNH]
Antepione azonax (Druce, 1892) Guatemala, San Geronimo; Costa Rica, Volcan de Irazu [ST female, BMNH] Note 2
Antepione constricta (Warren, 1895), ? South America [HT male, BMNH] Note 2
Antepione depontanata (Grote, 1864), Maryland [HT male] Note 3
Antepione fuciferata (Packard, 1876), New York [HT male, MCZ]
Antepione rhomboidaria (Oberthür, 1912), Costa Rica, San Jose [STs, BMNH] Note 2
Antepione rivulata (Warren, 1897), Costa Rica [HT female, BMNH] Note 2
Antepione sulphuraria (Packard, 1873), New York, West Farms; Middle States [HT female, MCZ]
Antepione sulphurata (Packard, 1876) Note 4
Antepione tiselaaria (Dyar. 1912), Mexico, Minerale de Zacualpan [HT male, USNM]
Pionenta Ferris, 2010, gen. n.
Pionenta ochreata (Hulst, 1898), comb. n., Arizona, Senator [HT male, AMNH]
Pionenta arizonata (Taylor, 1905), syn. rev., Arizona, Cochise Co., Huachuca Mts. [HT male, USNM]
Pionenta dyari (Grossbeck, 1908), syn. rev., Arizona, Huachuca Mts. [HT male, USNM]
Pionenta hewesata (Sperry, 1948), syn. n., Arizona, Oak Creek Canyon, Todd’s Lodge [HT female, AMNH]
Pionenta ligata (Grossbeck, 1908), syn. rev., Arizona, Huachuca Mts. [HT male, USNM]
Notes:
1. Taylor stated in his original description that the HT was type number 9800 in the USNM. A recent attempt to locate the type failed, and it is presumed misplaced or lost. The TL was incorrectly stated as Prescott, Arizona in Parsons et al. (1999).
2. Barcoding of these taxa may ultimately indicate species distinct from thisoaria.
3. The type was originally placed in the collection of the Entomological Society of Philadelphia and subsequently in ANSP. In the 1960s, the bulk of the ANSP Lepidoptera collection went to CMNH. The type cannot be located in either ANSP or CMNH and is presumed lost.
4. Packard (1876) redescribed Heterolocha sulphuraria Packard, 1873 as Antepione sulphurata.