(C) 2010 Ralph W. Holzenthal. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new species of Notidobiella Schmid (Insecta: Trichoptera) are described from South America: Notidobiella amazoniana sp. n. (Brazil), Notidobiella brasiliana sp. n. (Brazil), and Notidobiella ecuadorensis sp. n. (Ecuador). In addition, the 3 previously described species in the genus, Notidobiella chacayana Schmid, Notidobiella inermis Flint, and Notidobiella parallelipipeda Schmid, all endemic to southern Chile, are redescribed and illustrated, including the females of each species for the first time, and a key to males of the species in the genus is provided. The occurrence of Notidobiella in Brazil and Ecuador represents a significant extension of the range of the genus beyond southern Chile where it previously was thought to be endemic. The biogeography of Sericostomatidae and other austral South American Trichoptera is reviewed. The presence of the family in South America may not be part of a “transantarctic” exchange, but instead may represent an earlier occurence in the region. The distribution of Notidobiella in tropical South America likely represents recent dispersal from southern South America to the north.
caddisfly, Neotropics, transantarctic, new species, biogeography, South America, taxonomy
The caddisfly family Sericostomatidae
occurs in all biogeographic regions, except the Australasian, but its
species diversity is very unevenly distributed across these regions (
In this paper, we describe 3 new species of Notidobiella,
1 from the Amazon basin, Brazil, 1 from southeastern Brazil, and 1
from Ecuador, thus extending the range of this genus well beyond its
Chilean representation. In addition, we provide illustrations and
diagnoses of males and females (the latter for the first time) of the 3
previously described species of Notidobiella, Notidobiella chacayana Schmid, Notidobiella inermis Flint, and Notidobiella parallelipipeda Schmid, and a key to males of species in the genus. The Neotropical species of Sericostomatidae, including those in the genus Notidobiella, appear to be members of a southern Gondwana fauna (
Techniques and procedures used in the preparation and examination of specimens are those outlined by
(modified from
Figs 1–5
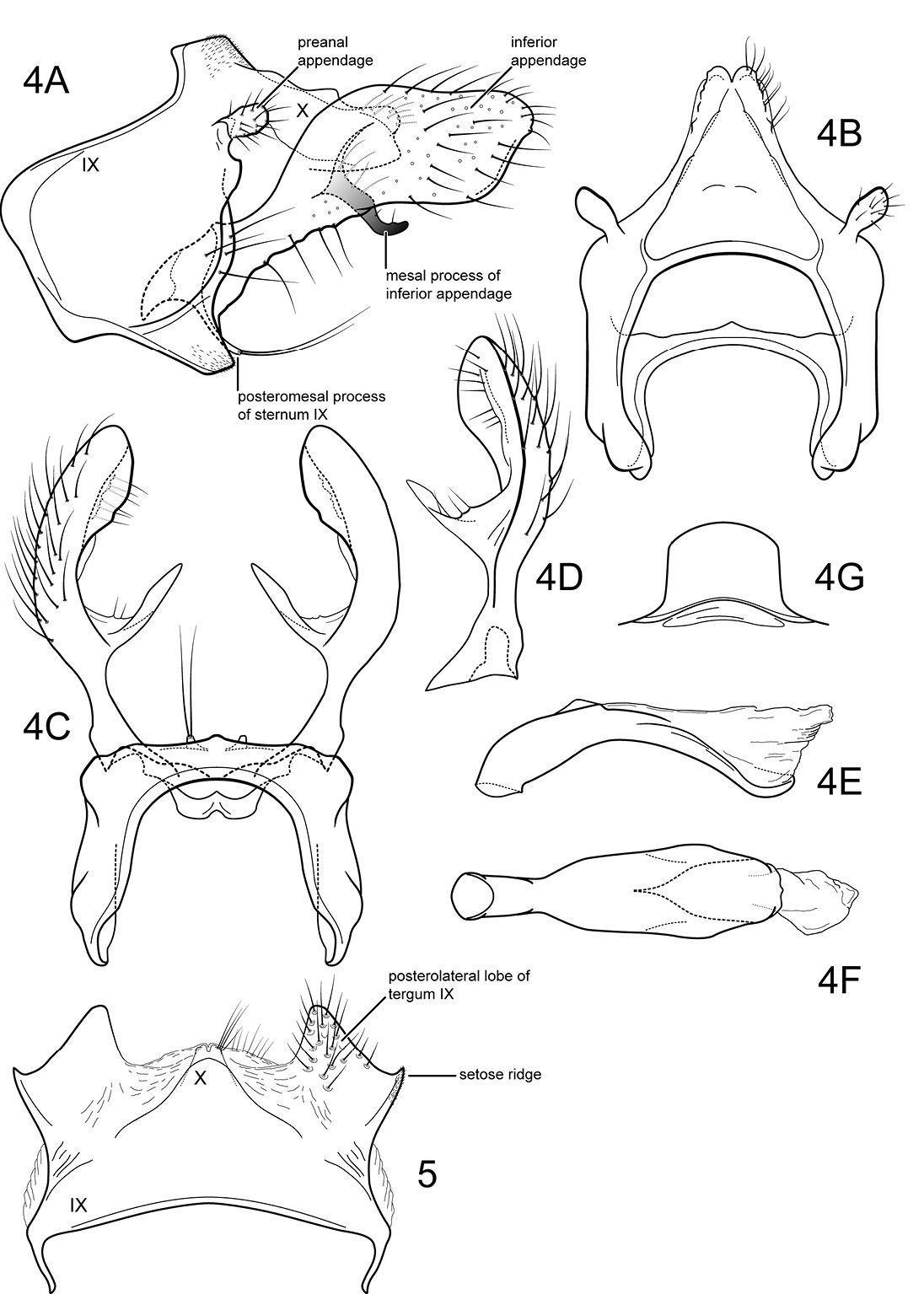
Of the species in the genus with broad, spatulate inferior appendages (all species except Notidobiella amazoniana), the type species is the most distinctive based on the parallel-sided inferior appendages with their prominent, mesally directed, mesal processes.
Adult. Forewing length 7.8–8.0 mm male (n=2); 8.8–9.0 mm female (n=2). Color light brown, palps and legs stramineous; forewings light brown, with scattered golden setae. Sternum VII of male with broad, fingernail-like, posteromesal process.
Male genitalia (Fig. 4). Segment IX with anterior margin broadly produced midlaterally; tergum IX narrow; sternum IX with pair of very short, posteromesal processes, bearing long apical setae. Tergum X simple, subquadrate in lateral view, with slight apicolateral elevation, with slight dorsomesal excavation, setose apically. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, spatulate, dorsal and ventral edges parallel, narrow basally, with prominent, elongate mesal process on mesal margin; strongly directed mesally in ventral view; apex exposed in lateral view. Phallic apparatus simple, tubular, slightly curved from base to apex; endophalic membranes prominent, but simple; phallotremal sclerite not apparent.
Female genitalia (Fig. 5). Tergum VIII quadrate; pleural membranes extensive, highly folded; sternum VIII broad, anterior margin with apodemal ridge, extending dorsolaterally; posterolateral corners rounded, heavily setose, especially posteriorly. Tergum IX with heavily setose, posterolateral lobes, rounded in lateral view, triangular in dorsal view; with lateral, microsetose, elevated ridge; sternum IX highly membranous, the membranes with parallel pleats or folds; tergum IX semimembranous dorsally. Tergum X with short setose projection. Internal vaginal sclerites complex (no discernable differences among the species); bursa copulatix subspherical, semisclerotized; pair of small, oval sclerites lying in membranes above vaginal sclerites (position variable).
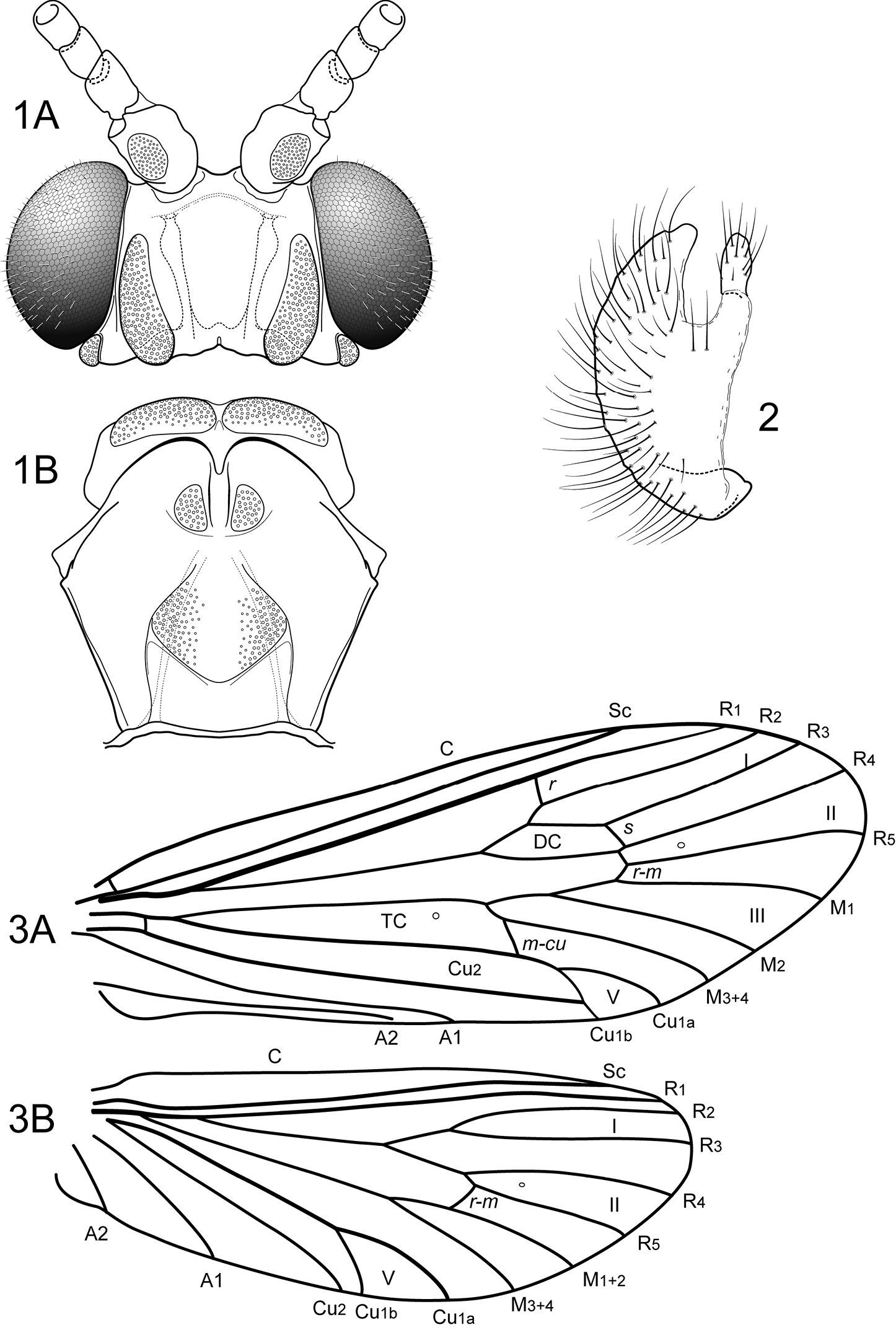
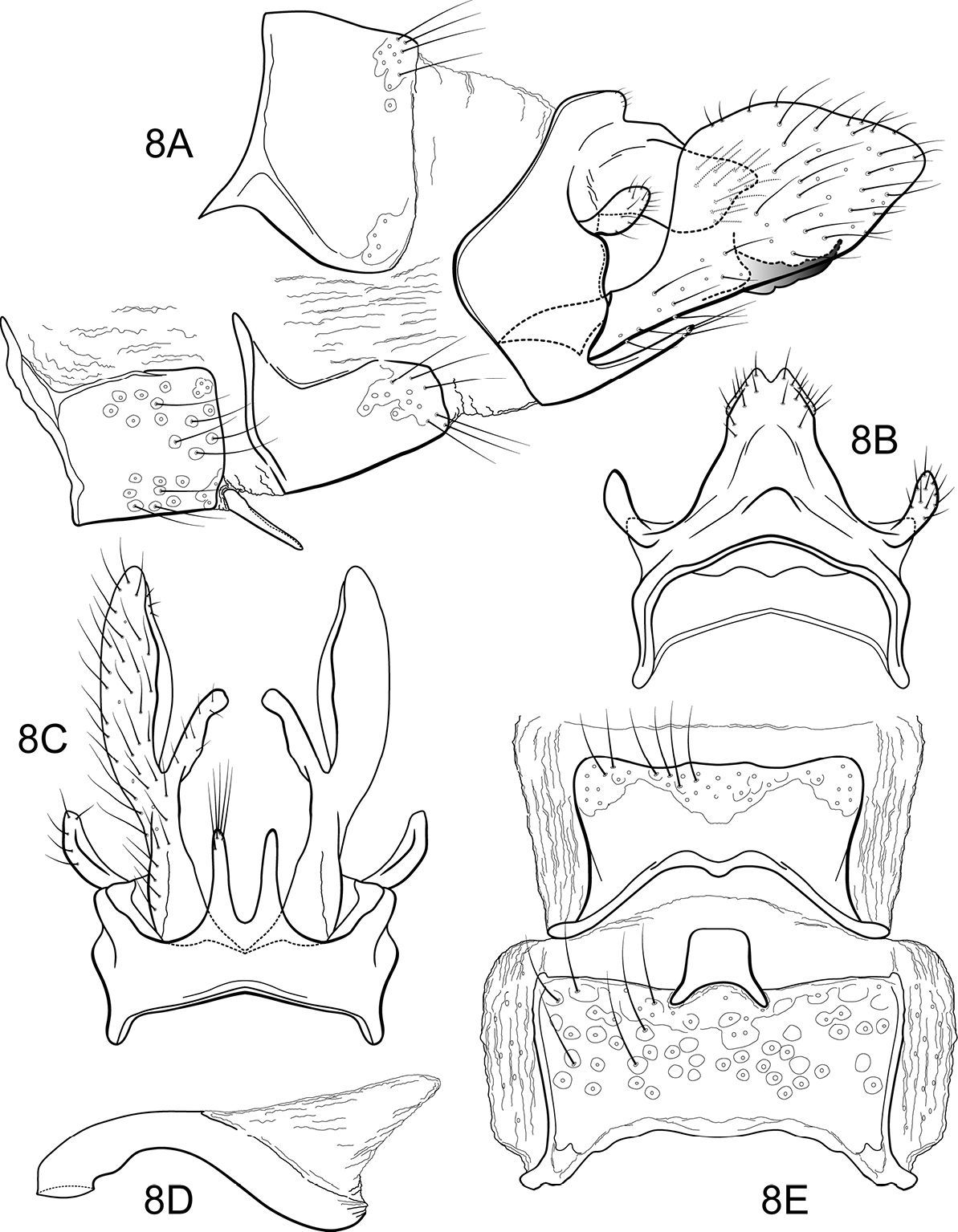
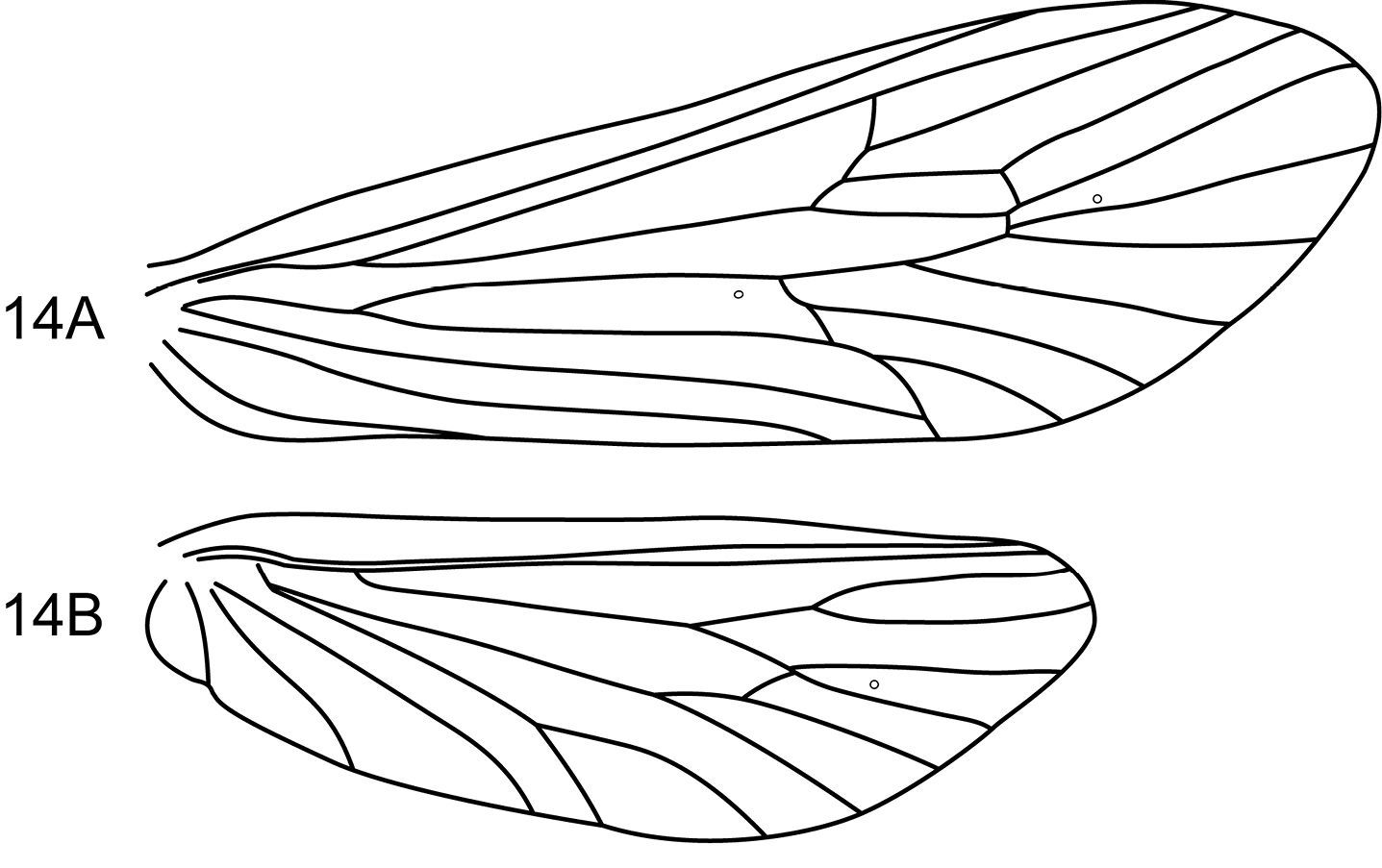
Notidobiella parallelipipeda Schmid. 1 Male head and thorax A head, dorsal B pro- and mesonota, dorsal. 2. Notidobiella parallelipipeda Schmid. Maxillary palp, male, frontal view. 3. Notidobiella parallelipipeda Schmid. Male wings A forewing B hind wing. Abbreviations: DC = discoidal cell, TC = thyridial cell.
Notidobiella parallelipipeda Schmid. 4 Male genitalia A segments IX, X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D inferior appendage, dorsal E phallus, lateral F phallus, ventral G sternum VII posteromesal process, ventral. 5. Notidobiella parallelipipeda Schmid. Female genitalia, segments IX, X, dorsal.
CHILE: Ñuble, Recinto, 4–6.iii.1968, Flint and Peña, 1 male, 1 female (pinned) (NMNH); Linares, El Castillo, Malcho, E Parral, 750 m, 8–10.i.1988, L.E. Peña, 1 male, 1 female (pinned) (NMNH).
urn:lsid:zoobank.org:act:6203A2A2-CB58-418F-A731-8C31A2243E7D
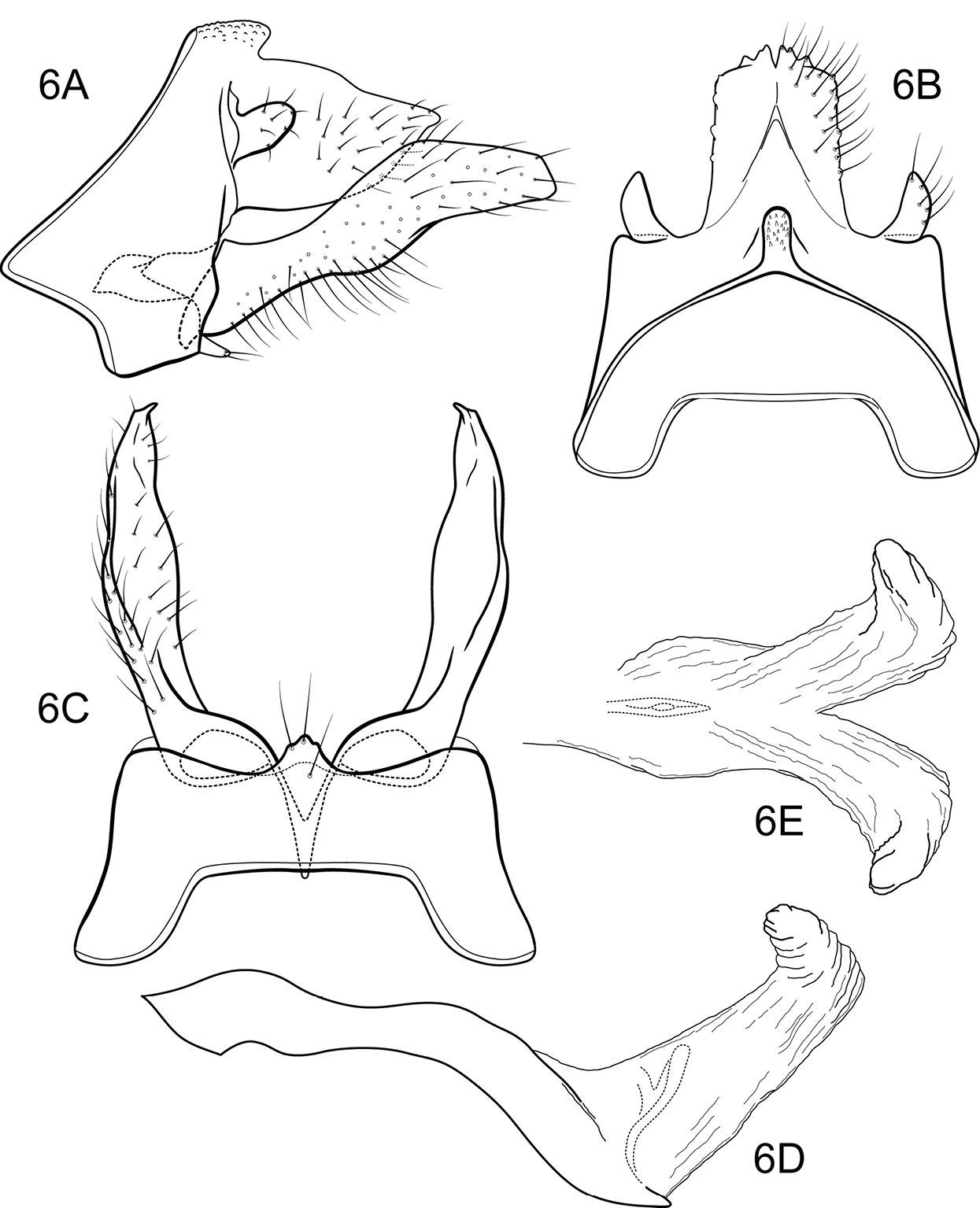
Figs 6–7This is the smallest species in the genus. Its wings are short and broad with venation typical for the genus except forewing crossveins r and s are absent, leaving the discoidal cell open (Fig. 7). Its genitalia are the most atypical in the genus in that the inferior appendages are not broadly spatulate, but sinuous in shape and uniform in width. Tergum IX bears a short triangular, posteromesal process, rather than short, paired processes, as found in the other species. Otherwise, the genitalia are typical for the genus.
Adult. Forewing length 4.5–5.0 mm male (n=8). Color faded, overall pale stramineous (specimens in alcohol); forewings colorless, almost transparent, denuded. Sternum VII of male with broad, fingernail-like, posteromesal process.
Male genitalia (Fig. 6). Segment IX with anterior margin acutely produced ventrolaterally; tergum IX narrow, ridge-like; sternum IX with short, triangular, posteromesal process, bearing apical setae. Tergum X simple, subquadrate in lateral view, with slight dorsomesal excavation, setose. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, elongate, narrow throughout length, without mesal process on ventromesal margin; in ventral view, apex acute, slightly incurved. Phallic apparatus simple, tubular, slightly curved from base to apex; endophalic membranes prominent, with paired apical membranous lobes; elongate, lightly sclerotized band internally (perhaps the phallotremal sclerite).
Unknown.
Notidobiella amazoniana, sp. n. Male genitalia A segments IX, X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D phallus, lateral E apex of phallus, dorsal.
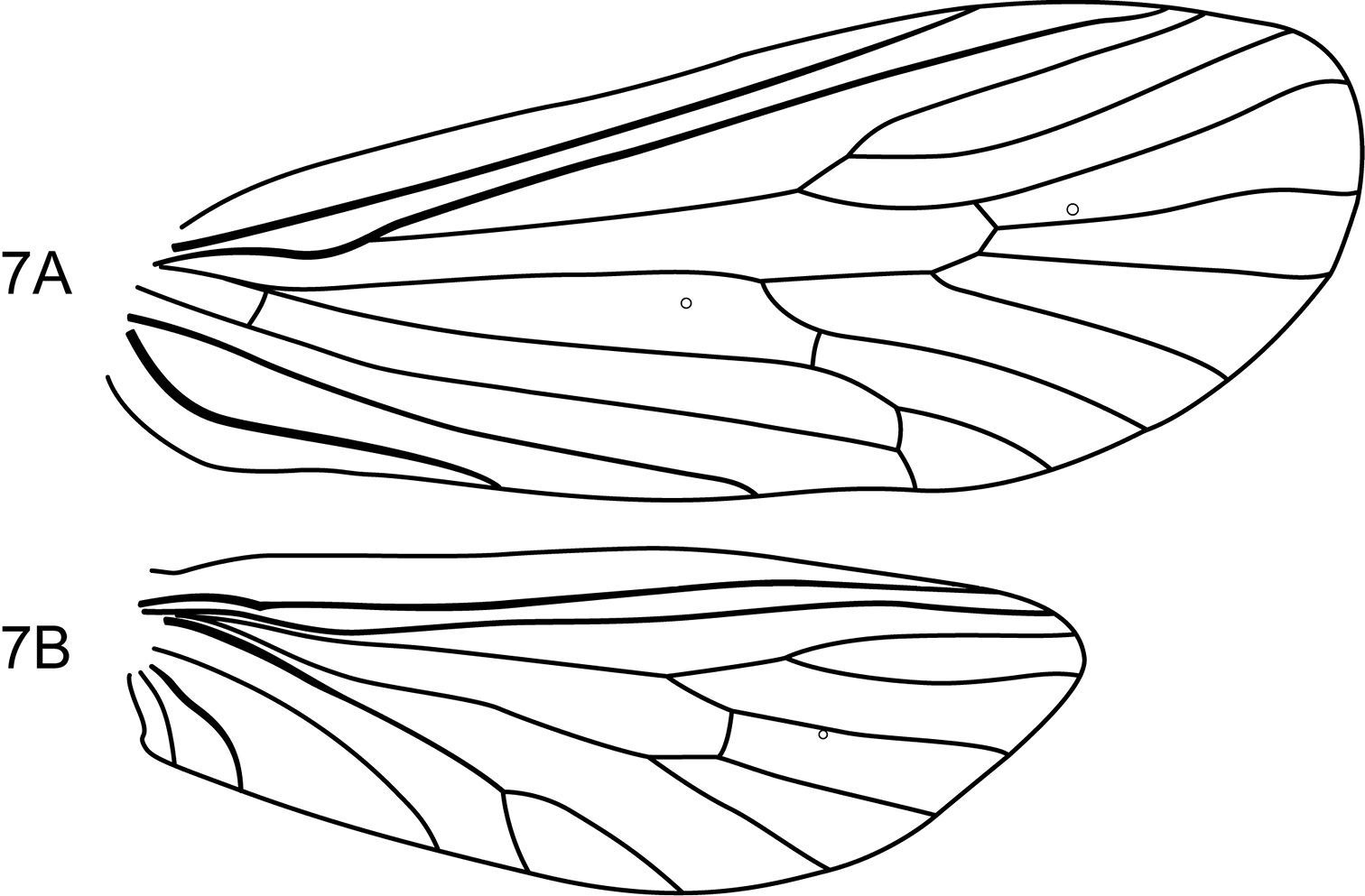
Notidobiella amazoniana, sp. n. Wings A forewing B hind wing.
BRAZIL: Amazonas: AM 010, km 246, 15–16.vii.1979, J. Arias (alcohol) (UMSP000131226) (INPA).
BRAZIL: Amazonas: same data as holotype, 3 males (alcohol) (UMSP), 4 males (alcohol) (NMNH).
Named for the state and region where the specimens were collected, which represents a significant northern extension of the range of the genus.
urn:lsid:zoobank.org:act:3365A48E-060D-4925-AC4B-B8AD7E74944F
Figs 8–10This new species is most similar to Notidobiella chacayana in the overall shape and structure of the inferior appendages. Both species possess an elongate mesal process on the ventromesal margin of the inferior appendage. In Notidobiella ecuadorensis sp. n., the ventromesal process is also present, but is shorter and broader in ventral view; in the other 2 Chilean species, Notidobiella inermis and Notidobiella parallelipipeda, the ventromesal processes are either very reduced (Notidobiella inermis) or long (Notidobiella parallelipipeda), but not nearly as long as in Notidobiella brasiliana sp. n. Setting Notidobiella brasiliana sp. n., apart from all of its congeners is the pair of elongate posteromesal processes on sternum IX; in all other species these processes are much shorter and broader. Furthermore, forewing crossveins r and s are absent, leaving the discoidal cell open (Fig. 10).
Adult. Forewing length 7.0 mm male (n=1); 7.9–8.2 mm female (n=4). Color medium to dark brown, palps and legs light brown; forewings dark brown with scattered golden hairs, pale golden spot on anal margin at about midlength. Sternum VII of male with broad, fingernail-like, posteromesal process.
Male genitalia (Fig. 8). Segment IX with anterior margin broadly produced mesolaterally; tergum IX narrow, elevated, mound-like; sternum IX with pair of prominent, elongate, posteromesal processes, bearing long apical setae. Tergum X simple, triangular in lateral view, with slight dorsomesal excavation, setose. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, broadly spatulate, narrow basally, with elongate mesal process on ventromesal margin. Phallic apparatus simple, tubular, slightly curved from base to apex; endophalic membranes prominent, but simple; phallotremal sclerite not apparent.
Female genitalia (Fig. 9). Tergum VIII quadrate; pleural membranes extensive, highly folded; sternum VIII broad, anterior margin with apodemal ridge, extending dorsolaterally; posterolateral corners rounded, heavily setose, especially posteriorly. Tergum IX with heavily setose, posterolateral lobes, rounded in dorsal and lateral views; without lateral ridge; sternum IX highly membranous, the membranes with parallel pleats or folds; tergum IX semimembranous dorsally. Tergum X with short, bifurcate, setose projection. Internal vaginal sclerites complex (no discernable differences among the species); bursa copulatix subspherical, semisclerotized; pair of small, oval sclerites lying in membranes above vaginal sclerites (position variable).
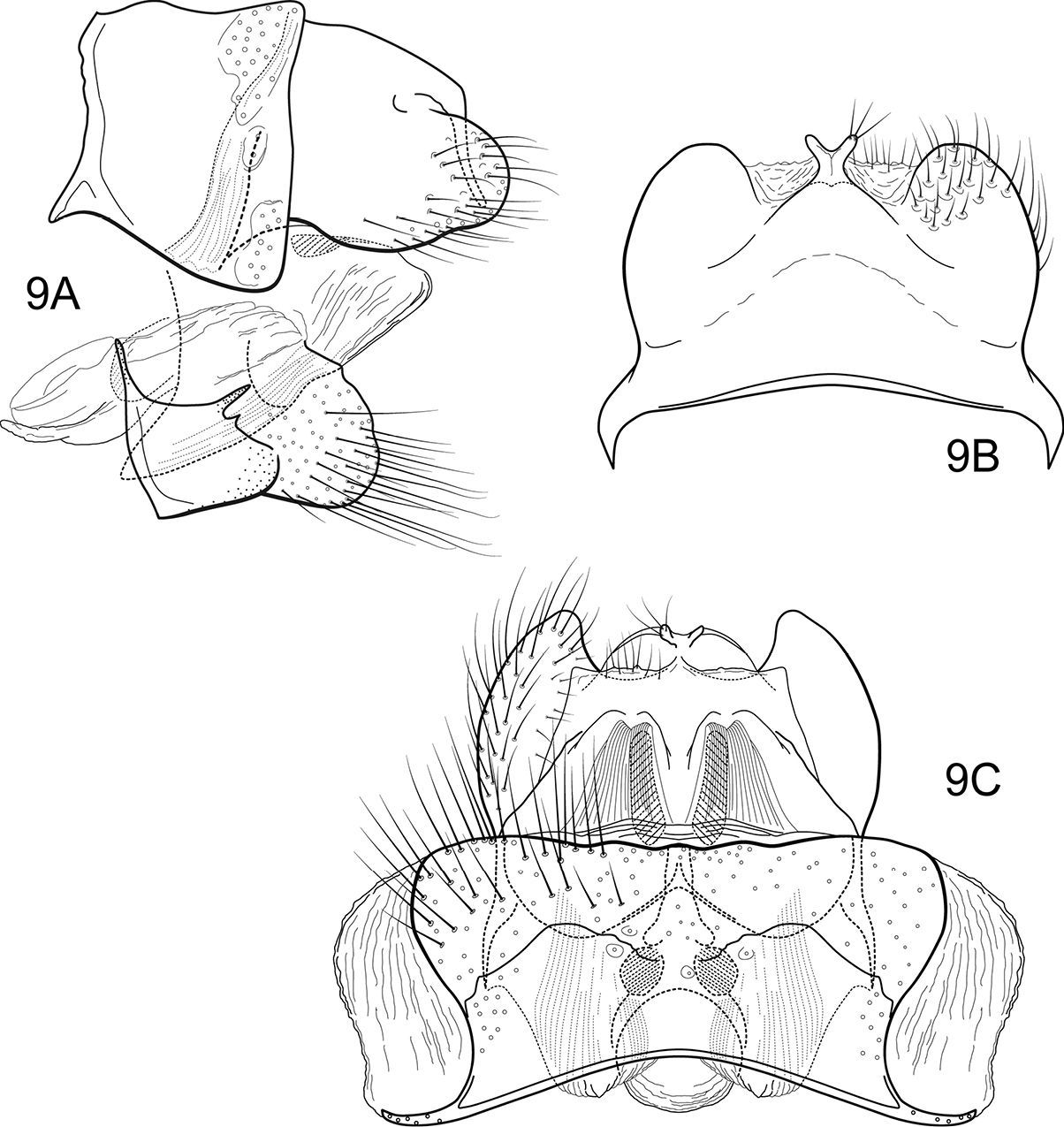
Notidobiella brasiliana, sp. n. Male genitalia A segments VII-X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D phallus, lateral E sterna VII, VIII, ventral.
Notidobiella brasiliana, sp. n. Female genitalia A segments VIII-X, lateral B segments IX, X, dorsal C segments VIII-X, ventral.
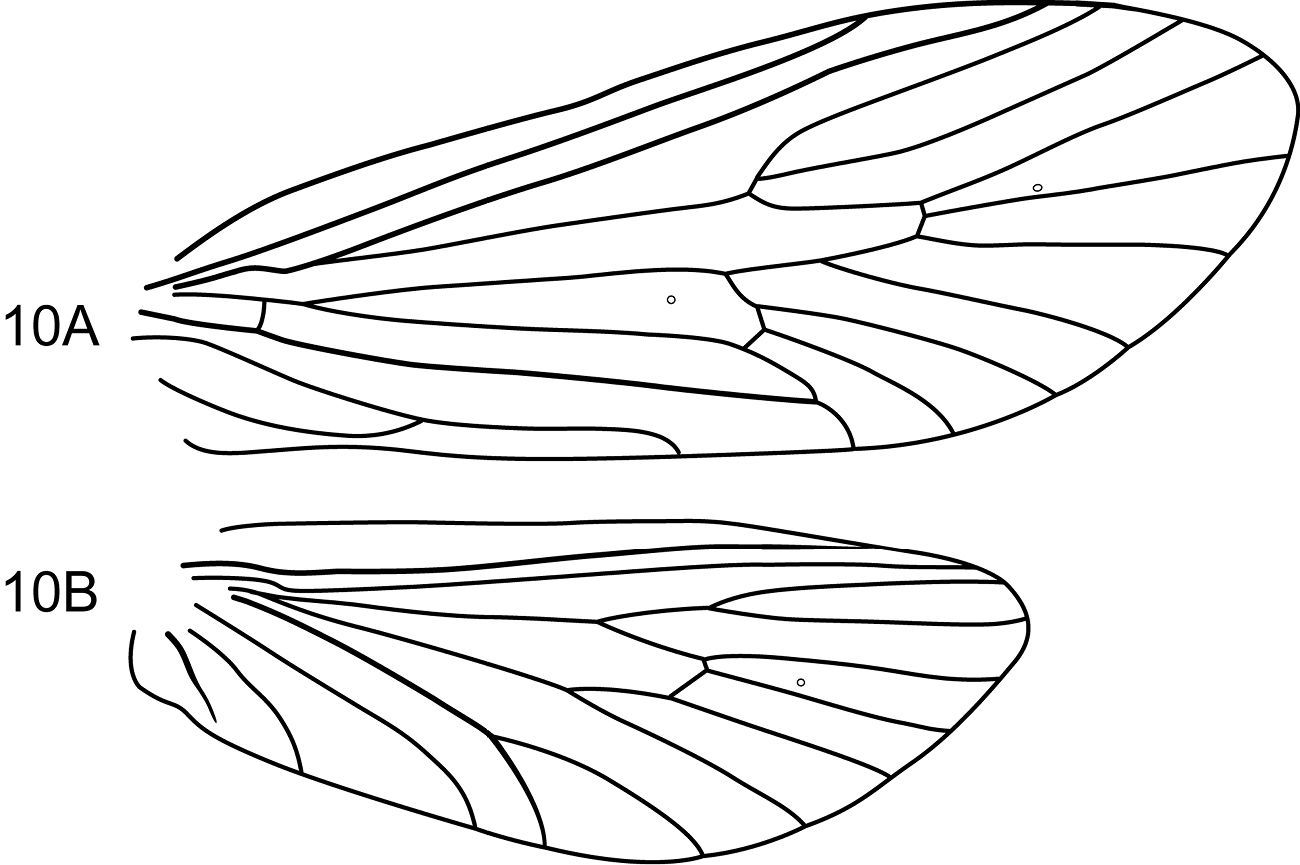
Notidobiella brasiliana, sp. n. Wings A forewing B hind wing.
BRAZIL: São Paulo: Parque Estadual de Campos do Jordão, 1st order trib. to Rio Galharada, 22°41.662'S, 45°27.783'W, el 1530 m, 14–16.ix.2002, Blahnik, Prather, Huamantinco (pinned) (UMSP000086351) (MZUSP).
BRAZIL: São Paulo: Parque Estadual de Campos do Jordão, Rio Galharada, 22°41.662'S, 45°27.783'W, el 1530 m, 13–15.ix.2002, Blahnik, Prather, Melo, Huamantinco, 2 females (alcohol) (MZUSP); same data as holotype, 2 females (pinned) (UMSP).
Named for Brazil, the country of the type specimens, which represents a significant northeastward extension of the range of the genus.
Figs 11–12
This Chilean species is most similar to Notidobiella brasiliana because of the similarly shaped inferior appendages, with their similar elongate mesal processes. It differs from that species in the much shorter posteromesal processes of sternum IX.
Adult. Forewing length 6.8–7.5 mm male (n=3); 7.2–9.0 mm female (n=3). Color brown, palps and legs stramineous; forewings brown, with scattered golden setae. Sternum VII of male with broad, fingernail-like, posteromesal process.
Male genitalia (Fig. 11). Segment IX with anterior margin produced ventrolaterally; tergum IX slightly elevated, mound-like; sternum IX with pair of short, posteromesal processes, bearing long apical setae. Tergum X simple, triangular in lateral view, with dorsomesal excavation, setose. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, broadly spatulate, narrow basally, with elongate mesal process on ventromesal margin. Phallic apparatus simple, tubular, curved from base to apex; endophalic membranes prominent, but simple; phallotremal sclerite not apparent.
Female genitalia (Fig. 12). Tergum VIII quadrate; pleural membranes extensive, highly folded; sternum VIII broad, anterior margin with apodemal ridge, extending dorsolaterally; posterolateral corners rounded, heavily setose, especially posteriorly. Tergum IX with heavily setose, posterolateral lobes, rounded to subtriangular in dorsal view; with lateral, microsetose, elevated ridge; sternum IX highly membranous, the membranes with parallel pleats or folds; dorsally tergum IX semimembranous. Tergum X with short setose projection. Internal vaginal sclerites complex (no discernable differences among the species); bursa copulatix subspherical, semisclerotized; pair of small, oval sclerites lying in membranes above vaginal sclerites (position variable).
Notidobiella chacayana Schmid. 11 Male genitalia A segments IX, X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D phallus, lateral E phallus, ventral. 12. Notidobiella chacayana Schmid. Female genitalia, segments IX, X, dorsal.
CHILE: Cauquenes, Tregualeme, 35°56'S, 72°43'W, 11–12.xii.1993, C. and O. Flint Jr., 1 male, 1 female (pinned) (NMNH); X Región de los Lagos, Isla de Chiloé, Río Verde, 1.9 km W Puntra, 42°07.078'S, 73°50.364'W, el. 40 m, 3.ii.2005, Holzenthal, Blahnik, Chamorro, 2 males, 2 females (pinned) (UMSP); XIV Región de los Ríos, Monumento Nacional Alerce Costero, unnamed trib., trail to Alerce Milenario, 40°11.874'S, 73°26.217'W, el. 895 m, 5.ii.2008, Holzenthal, Pauls, Mendez, 1 male (pinned) (UMSP).
urn:lsid:zoobank.org:act:20CB7DC2-73DF-4D4D-9593-0A87C589BABB
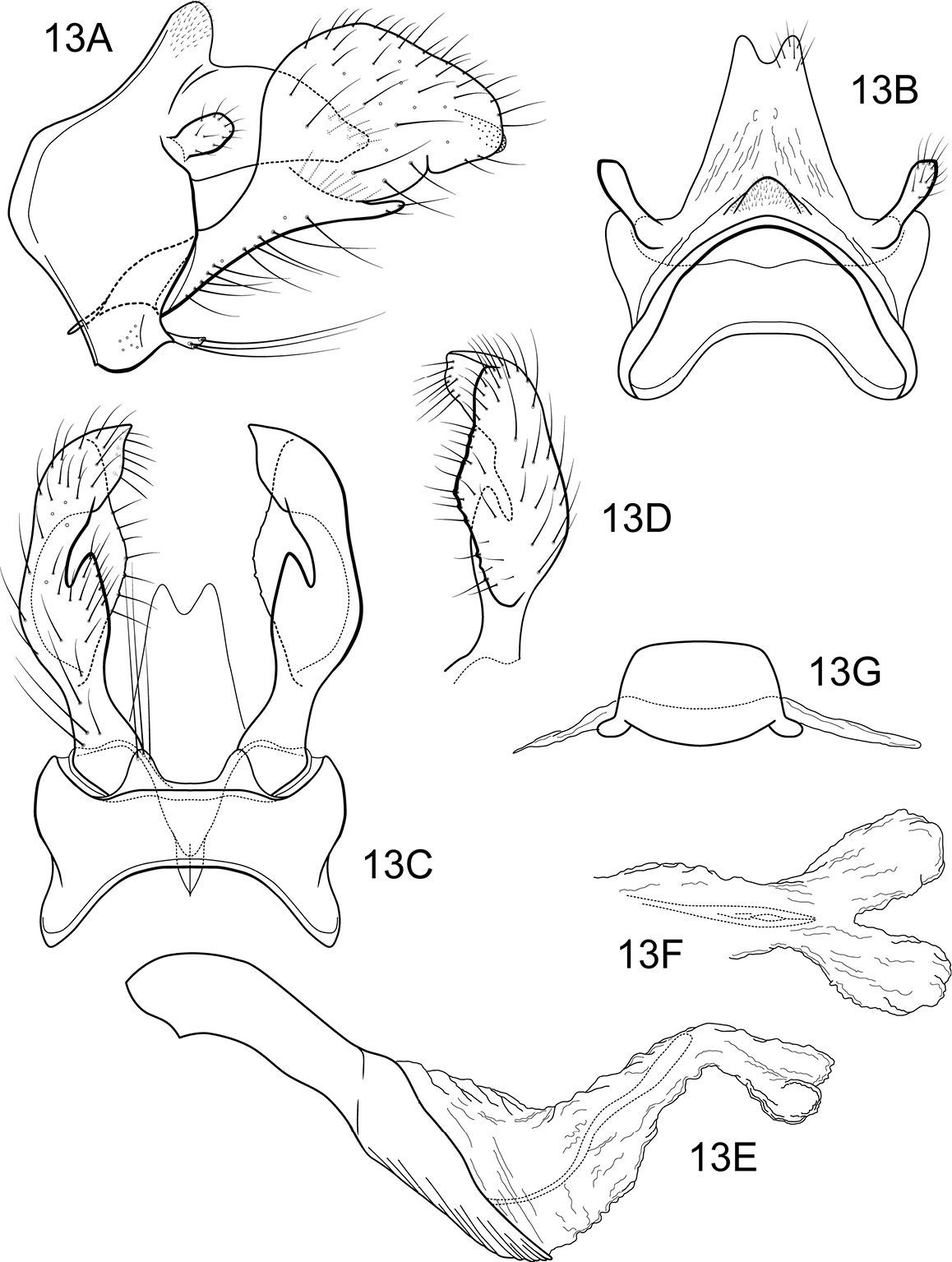
Figs 13–14The combination of broadly spatulate inferior appendage, thumb-like mesal process on the ventromesal margin of the inferior appendage, and short posteromesal processes on sternum IX separate this species from its congeners. The wing venation (Fig. 14) is similar to that of the type species.
Adult. Forewing length 6.2 mm (n=1). Color faded, overall yellowish-brown (specimen in alcohol); forewings stramineous, denuded. Sternum VII of male with broad, fingernail-like, posteromesal process.
Male genitalia (Fig. 13). Segment IX with anterior margin broadly produced midlaterally; tergum IX narrow, elevated, mound-like; sternum IX with pair of short, triangular, posteromesal processes, bearing very long apical setae. Tergum X simple, triangular in lateral view, with slight dorsomesal excavation, setose apically. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, very broadly spatulate, narrow basally, with short, thumb-like mesal process on ventromesal margin. Phallic apparatus simple, tubular, relatively straight from base to apex; endophalic membranes prominent, with paired apical membranous lobes; elongate, lightly sclerotized band internally (perhaps the phallotremal sclerite).
Unknown.
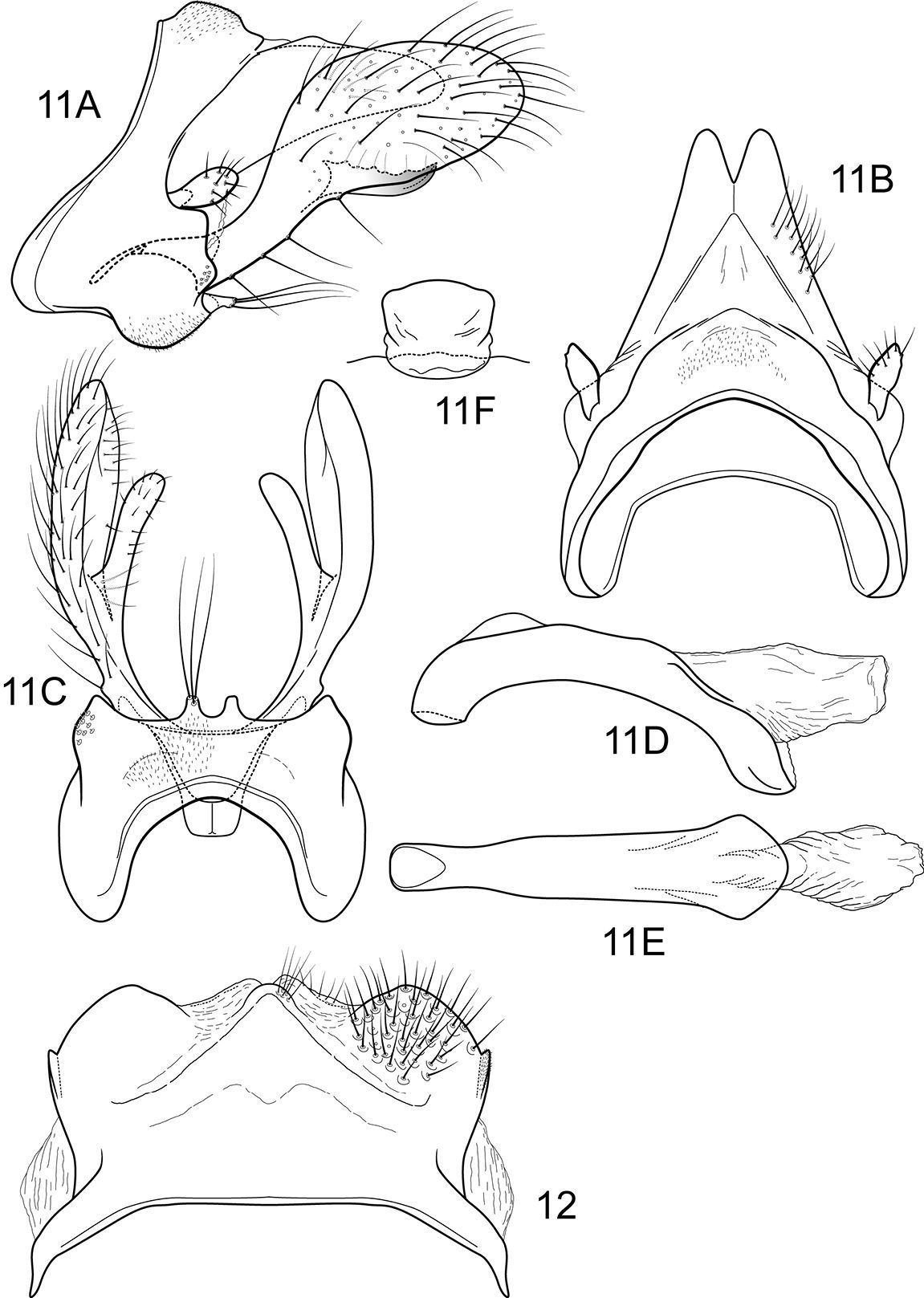
Notidobiella ecuadorensis, sp. n. Male genitalia A segments IX, X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D inferior appendage, dorsal E phallus, lateral F phallus apex, dorsal G sternum VII posteromesal process, ventral.
Notidobiella ecuadorensis, sp. n. Wings A forewing B hind wing.
male, ECUADOR: Pastaza: Puyo, 1–7.ii.1976, Spangler et al. (alcohol) (UMSP000208470) (NMNH).
Named for Ecuador, the country of the holotype, which represents a significant northern extension of the range of the genus.
Figs 15–16
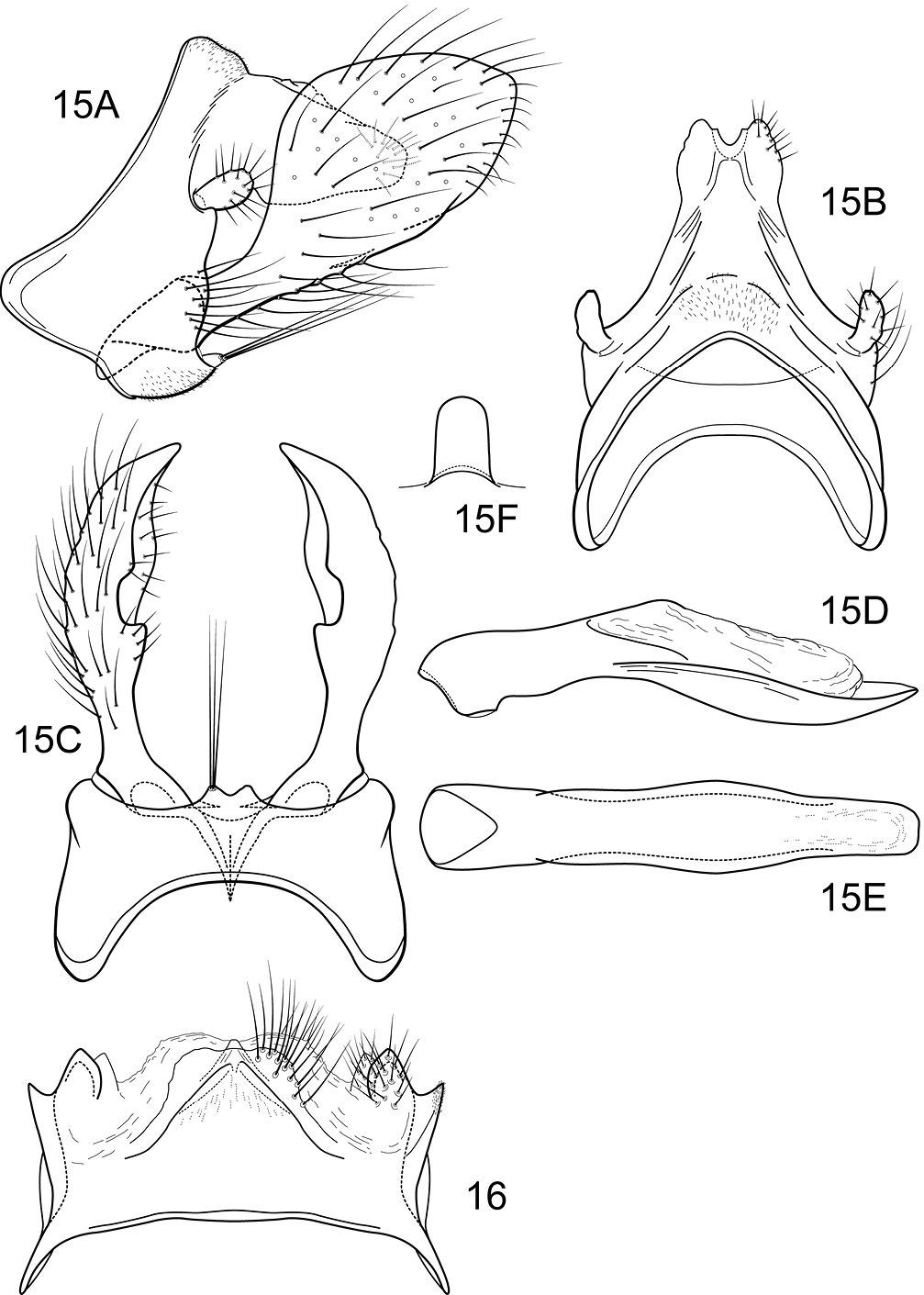
Notidobiella inermis shares with Notidobiella ecuadorensis broadly spatulate inferior appendages with short, thumb-like mesal processes, but differs in details of the shape of the inferior appendages, as illustrated, and in the possession of a narrow posteromesal process on sternum VII.
Adult. Forewing length 6.0–6.5 mm male (n=2); 8.0 mm female (n=2). Color brown, palps and legs stramineous; forewings brown, with scattered golden setae. Sternum VII of male with narrow, fingernail-like, posteromesal process.
Male genitalia (Fig. 15). Segment IX with anterior margin produced ventrolaterally; tergum IX narrow; sternum IX with pair of short, posteromesal processes, bearing long apical setae. Tergum X simple, subquadrate in lateral view, with slight dorsomesal excavation, setose. Preanal appendage short, ovate, setose. Inferior appendage prominent, heavily setose, very broadly spatulate, narrow basally, with short, thumb-like mesal process on ventromesal margin. Phallic apparatus simple, tubular, relatively straight from base to apex; endophalic membranes prominent, but simple; phallotremal sclerite not apparent.
Female genitalia (Fig. 16). Tergum VIII quadrate; pleural membranes extensive, highly folded; sternum VIII broad, anterior margin with apodemal ridge, extending dorsolaterally; posterolateral corners rounded, heavily setose, especially posteriorly. Tergum IX with heavily setose, posterolateral lobes, subovate, small, bilobed in dorsal and lateral views; with lateral, microsetose, elevated ridge; sternum IX highly membranous, the membranes with parallel pleats or folds; dorsally tergum IX with sclerotized ridge. Tergum X with broad heavily setose projection. Internal vaginal sclerites complex, no discernable differences among the species; bursa copulatix subspherical, semisclerotized; pair of small, oval sclerites lying in membranes above vaginal sclerites (position variable).
Notidobiella inermis Flint. 15 Male genitalia A segments IX, X, inferior appendages, lateral B segments IX, X, dorsal C segment IX, inferior appendages ventral D phallus, lateral E phallus, ventral F sternum VII posteromesal process, ventral. 16. Notidobiella chacayana Schmid. Female genitalia, segments IX, X, dorsal.
CHILE: Llanquihue, Salto Chamiza, Correntosa, 100 m, 19.i.1987, C.M. and O.S. Flint, Jr., 1 male, 2 females (pinned) (NMNH); Llanquihue, El Chinque, N Correntosa (S Volcán Calbuco), 300 m, 20–25.i.1980, 1 male paratype (pinned) (NMNH).
| 1 | Inferior appendage narrow basally, broadly spatulate apically, with mesal process on ventromesal margin (Figs 4A, 8A); abdominal sternum IX with pair of posteromesal processes (Figs 4C, 8C); forewing length 6–8 mm | 2 |
| – | Inferior appendage elongate, narrow throughout length, without mesal process on ventromesal margin (Figs 6A, C); abdominal sternum IX with single, short, triangular, posteromesal process (Fig. 6C); forewing length 4.5–5 mm | Notidobiella amazoniana sp. n. |
| 2(1) | Inferior appendage mesal process elongate (Figs 4C, 8C) | 3 |
| – | Inferior appendage mesal process short, thumb-like (Figs 13C, 15C) | 5 |
| 3(2) | Posteromesal processes of abdominal sternum IX short (Figs 6C, 13C); distribution: Chilean subregion (Chile) | 4 |
| – | Posteromesal processes of abdominal sternum IX elongate (Fig. 8C); distribution: Brazilian subregion (southeastern Brazil) | Notidobiella brasiliana sp. n. |
| 4(3) | Spatulate apex of inferior appendage broadly ovate (Fig. 11A) | Notidobiella chacayana Schmid |
| – | Spatulate apex of inferior appendage parallel sided (Fig. 4A) | Notidobiella parallelipipeda Schmid |
| 5(2) | Abdominal tergum IX highly elevated, mound-like (Fig. 13A); ventromesal process of abdominal sternum VII broad (Fig. 13G); distribution: Brazilian subregion (Ecuador) | Notidobiella ecuadorensis sp. n. |
| – | Abdominal tergum IX not elevated (Fig. 15A); ventromesal process of abdominal sternum VII narrow (Fig. 15F); distribution: Chilean subregion (Chile) | Notidobiella inermis Flint |
As defined most recently by
Four Trichoptera families are representative of a temperate Gondwanan pattern: Helicophidae, Kokiriidae, Philorheithridae, and Tasimiidae,
each family with genera endemic to Australia/New Zealand/New
Caledonia, southern South America, or Madagascar (no genera are
shared) (
Genera (number of included species) in the families Helicophidae, Kokiriidae, Philorheithridae, and Tasimiidae and their regional distributions, including references to recent works inferring or discussing phylogenetic relationships among genera.
| Family, genus (# species) | Distribution |
|---|---|
|
Helicophidae ( | |
| Alloecella Banks (3) | SE Australia, Tasmania |
| Alloecentrella Wise (4) | New Zealand |
| Alloecentrellodes Flint (2) | Chile |
| Austrocentrus Schmid (3) | Chile, Argentina |
| Eosericostoma Schmid (2) | Chile, Argentina |
| Helicopha Mosely (21) | Australia, Tasmania, New Caledonia |
| Heloccabus Neboiss (1) | E Australia |
| Microthremma Schmid (8) | Chile |
| Pseudosericostoma Schmid (1) | Chile |
| Zelolessica McFarlane (2) | New Zealand (incl. Stewart Island) |
|
Kokiriidae ( | |
| Kokiria McFarlane (1) | New Zealand |
| Mecynostomella Kimmins (7) | New Caledonia |
| Pangulia Navás (2) | Chile |
| Tanjistomella Neboiss (1) | SE Australia |
| Taskiria Neboiss(3) | SE Australia, Tasmania |
| Taskiropsyche Neboiss (1) | Tasmania |
|
Philorheithridae ( | |
| Afrorheithrus Weaver, Gibon, and Chvojka (3) | Madagascar |
| Aphilorheithrus Mosely (4) | SE Australia, Tasmania |
| Austrheithrus Mosely (3) | SE Australia, Tasmania |
| Kosrheithrus Mosely (3) | SE, SW Australia, Tasmania |
| Mystacopsyche Schmid (2) | Chile, Argentina |
| Philorheithrus Hare (6) | New Zealand |
| Psilopsyche Ulmer (3) | Chile, Argentina |
| Ramiheithrus Neboiss (2) | SE Australia, Tasmania |
| Tasmanthrus Mosely (3) | Tasmania |
| Tasimiidae (no phylogenetic assessment available) | |
| Charadropsyche Flint (1) | Chile |
| Tasiagma Neboiss (2) | SE Australia, Tasmania, Lord Howe Island |
| Tasimia Mosely (5) | SE Australia, Tasmania |
| Trichovespula Schmid (1) | Chile |
While not wholly endemic to the region, other caddisfly
families contain a diverse temperate Gondwana fauna including, most
notably, Hydrobiosidae (reviewed by
The family Sericostomatidae contains temperate Gondwanan components, including 5 endemic South African/Malagasy genera (Aclosma, Aselas, Cheimacheramus, Petroplax, Rhoizema) and 4 endemic South American genera (Grumicha, Myotrichia, Notidobiella, Parasericostoma, excluding Chiloecia, nomen dubium).
As indicated above, the family includes other genera endemic to the
Nearctic and West Palearctic regions. Sericostomatids are absent from
the Australasian region (all Australasian species previously assigned to
Sericostomatidae have been transferred to other families, see
The presence of Helicophidae and Hydrobiosidae in Eocene Baltic amber (
Evidence suggests that the contemporary distribution of the Patagonian and Australasian temperate Gondwanan Trichoptera fauna reflects a past dispersal corridor between Australia and southern South America via Antarctica (
The now widespread occurrence of Notidobiella
in temperate southern Chile and tropical South America (Ecuador,
southeast Brazil, Amazonian Brazil) suggests a more recent dispersal of
the genus to northern tropical South America from Patagonia and its
subsequent diversification. The data from insects analyzed by
As confirmed by
Other than in a few studies, phylogenetic hypotheses are
lacking for most of the taxa reviewed above. Phylogenies of Southern
Hemisphere caddisfly taxa inferred from molecular data are even fewer
(e.g.,
We are grateful to Dr. Oliver S. Flint, Jr., Smithsonian Institution, for generously providing most of the material described in this paper. Thanks to Desi Robertson, Karl Kjer, Robin Thomson, Steffen Pauls, Christy Geraci, Ivailo Stoyanov, and 2 anonymous reviewers for discussion and helpful comments on the manuscript. This material is based on work supported by the National Science Foundation grant no. DEB 9971885 and the Minnesota Agricultural Experiment Station project no. AES0017017.