(C) 2010 Wilson R. Lourenço. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new genus and species of scorpion belonging to the family Pseudochactidaeare described based on four specimens collected in the Tien Son cave at the Phong Nha - Ke Bang National Park, Quang Binh Province, Vietnam. The new species represents a true troglobitic element, the first one known for the family Pseudochactidae. This represents the third known record of a pseudochactid, and the first from Vietnam.
Scorpion, Vietnam, Phong Nha - Ke Bang National Park, karst cave system, new genus and species, troglobitic element
One of the most remarkable scorpions described during the last 30 years is Pseudochactas ovchinnikovi
Gromov, 1998, discovered in an isolated mountainous region of
southeastern Uzbekistan and southwestern Tajikistan in Central Asia.
Although this scorpion shares some features with buthid and nonbuthid
scorpions, it is remarkable because it displays a number of characters
unique among recent (extant) scorpions, including a distinct
trichobothrial pattern. This led
Subsequently, authors have not reached a consensus
regarding the phylogenetic position of this enigmatic scorpion. Based on
its peculiar trichobothrial pattern,
Shortly after these publications, a second genus and species belonging to the family Pseudochactidae, Troglokhammouanus steineri Lourenço, 2007, was described from karst caves in Laos (
Since the description of Troglokhammouanus steineri (
The Southeast Asia or Indochina tectonic plate forms the
core of the geological structure of southeastern Asia. This plate
comprises the countries of Vietnam, Laos, Cambodia and western
Thailand, but according to
The Southeast Asia plate originated during the
Proterozoic. It became detached during Palaeozoic and drifted northward.
The carbonate platforms were developed during the Devonian-Late
Palaeozoic. The Palaeozoic history of detachment and collision is quite
speculative. The equivalent of Caledonian orogeny, followed by the
formation of the Palaeotethys Ocean is quite possible. Climate records
indicate major differences between Sibumasu, Indochina and South China
during the Late Palaeozoic. During the Triassic, as a result of the
Indosinian orogeny and closure of the Palaeotethys Ocean, the Southeast
Asian plate joined the Asian continent (
Tien Son Cave, internal view, showing second author searching for scorpions.
In Central Vietnam, the dominant geological feature is
the Truong Son Range. This string of mountains and plateaus, also known
as the Annamite Mountain Range, is roughly 1200 km long and 50–75 km
wide, intersected by passes and lowlands. Most of its hills lie between
elevations of 500–2000 m, and for much of its distance they run
parallel to the central coastline, straddling the border with Laos.
Central Vietnam’s Truong Son Range is a transitional region between the
subtropical communities of the North and the tropical ones of the South,
and it harbours many endemic species (
Site in the cave where scorpions were found.
The Phong Nha-Ke Bang karst is the oldest major karst area in Asia. It has been subject to massive tectonic changes and comprises a series of rock types that are interbedded in complex ways. Probably as many as seven different major levels of karst development have occurred as a result of tectonic uplift and changing sea levels, thus the karst landscape of PNKB is extremely complex with high geodiversity and many geomorphic features of considerable significance. There is also strong evidence that sulphuric dissolution and hydrothermal action have played an important role in shaping the general landscape and the caves, though this has not yet been properly assessed.
Modern Phong Nha-Ke Bang is a result of five stages in
the Earth’s crustal development and movement: Late Ordovician - Early
Silurian Stage (about 450 My), Middle-late Devonian Stage (about 340
My), Carboniferous-Permian (about 300 My), Mesozoic Orogenic stage,
and Cenozoic stage (
Phong Nha - Ke Bang (Vietnamese: Vườn quốc gia Phong Nha-Kẻ Bàng)
is now a national park and UNESCO World Heritage Site in the Bố Trạch
and Minh Hóa Districts of central Quang Binh Province, in north-central
Vietnam, about 500 km south of Hanoi. The park borders the Hin Namno
Nature Reserve in the province of Khammouan, Laos (
Phong Nha-Ke Bang area is noted for its cave systems with a total length of about 126 km; only 20 caves have been surveyed by Vietnamese and British scientists; 17 of these are located in the Phong Nha area and three in the Ke Bang area. Before discovery of Son Doong Cave, Phong Nha held several world cave records, as it has the longest underground river, as well as the largest caverns and passageways. The park derived its name from Phong Nha cave, the most beautiful of all.
Like northern Central Vietnamin general and Quang Binh Province in particular, the climate in this national park is tropical, hot, and humid. The annual mean temperature ranges from 23 to 25 °C, with extremes of 41°C in the summer and a 6°C in the winter. The hottest months in this region are from June to August, with an average temperature of 28°C, and the coldest months from December to February with an average temperature of 18°C. Annual rainfall is 2000–2500 mm, and 88% of the rainfall occurs from July to December. With more than 160 rainy days per year, no month is without rain. Mean annual relative humidity is 84% in forests.
Tien Son Cave, where the new scorpion was found is
located in Son Trach Commune, Bố Trạch District. The entrance is
located 1 km from Phong Nha Cave, at an altitude of 200 m. Tien Son
Cave is 980 m in length. A 10 m deep hole is situated 400 m from the
entrance, after which a 500 m long underground cave is open exclusively
to professional scientists. Like Phong Nha Cave, this cave features
spectacular stalactites and stalagmites. According to British
speleologists, Tien Son Cave was created tens of millions years ago,
when a water current holed this limestone mountain in Ke Bang. Following
a series of movements of rocks, this mass was levered or lowered,
blocking the current and creating what is now Tien Son Cave, while the
flow of the underground river was redirected to Phong Nha Cave. Although
Phong Nha and Tien Son Caves are located next to each other, there are
no passages linking them (
Scorpions were collected by the second author, while
exploring the caves with the help of standard electric torches.
Scorpions were found under some heavy flat rocks, about 200 m from the
main cave entrance. Measurements and illustrations were made using a
Wild M5 stereo-microscope with a drawing tube and an ocular micrometer.
Measurements follow those of
Family Pseudochactidae Gromov, 1998
Cheliceral movable finger with three denticles
(medial, subdistal, external distal) on dorsal edge; external distal
denticle smaller than internal distal denticle. Anterior margin of
carapace depressed with a moderate concavity, posterior margin
shallowly recurved. Lateral ocelli absent. Pair ofcircumocular sutures
with a broad U-shaped configuration (diagnostic for family), only
vestigial and incomplete in the posterior region to median ocular
tubercle. Median ocelli absent; median tubercle represented by a smooth
depressed zone. Anterosubmedial carinae absent from zone limited by
circumocular sutures. Type D trichobothrial pattern (
The generic name is a combination of Viet (for Vietnamese) and bocap (scorpion in Vietnamese language).
Vietbocap canhi sp. n.
as for the genus.
male holotype; female and two male paratypes. Vietnam, Quang Binh Province, north-central Vietnam, Bố Trạch - Minh Hóa District, Phong Nha - Ke Bang National Park, Tien Son Cave (17°32'N; 106°16'E), mid section of cave (200 m from cave entrance), 16/V/2010 (D.-S. Pham). Holotype and female paratype are deposited in the collection of the Muséum national d’Histoire naturelle, Paris. The other paratypes are deposited in the collections of the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi.
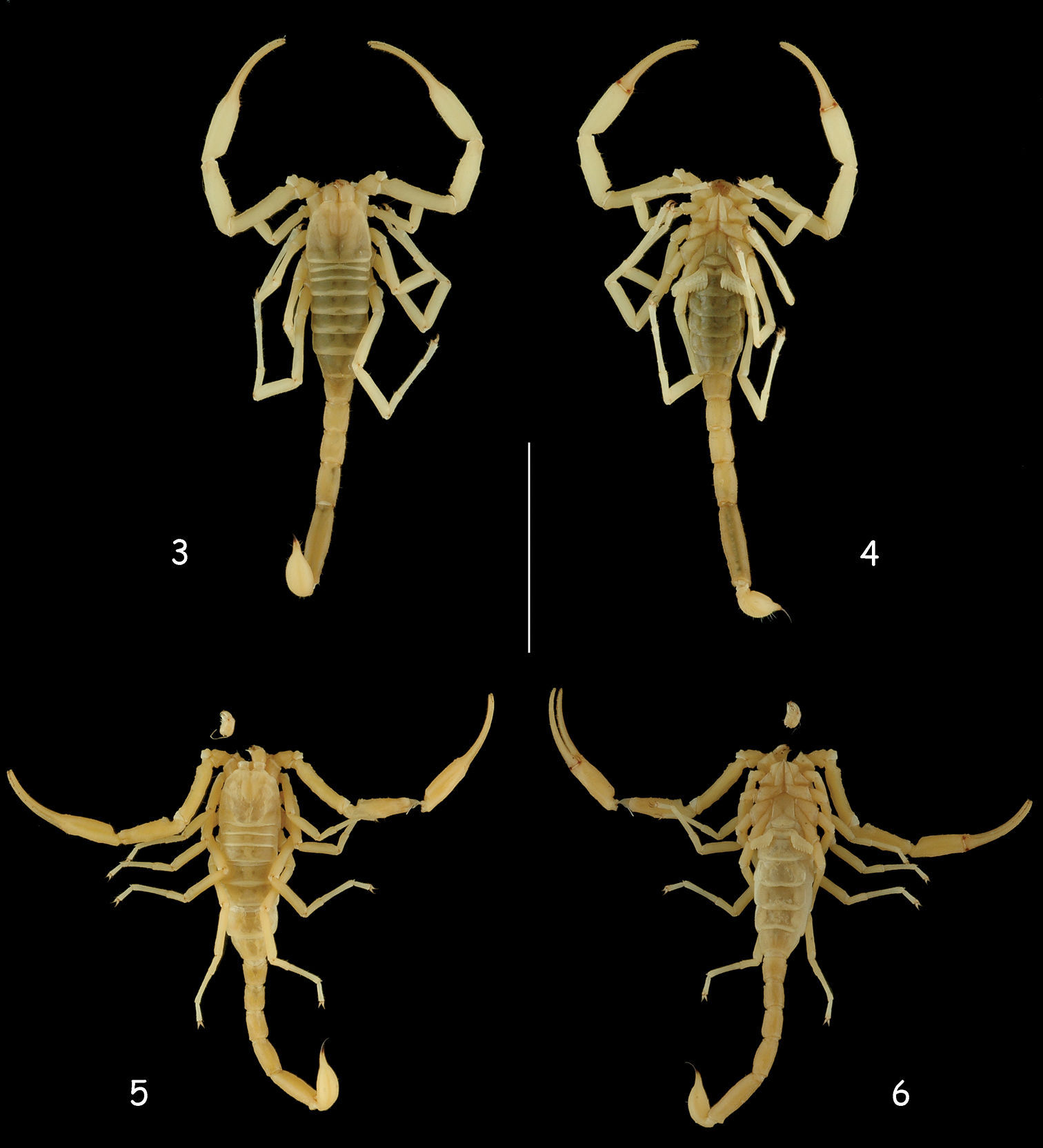
Vietbocap canhi sp. n., male holotype and female paratype, dorsal and ventral aspects. Scale bar = 10 mm.
In honour of Dr. Le Xuan Canh, Director of the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, for his support of scorpion research in Vietnam.
based on the male holotype and paratypes (measurements given in mm after the description).
Colour. General coloration yellowish to pale yellow; cheliceral teeth, telson tip and rows of granules on pedipalp fingers reddish-yellow to dark reddish.
Morphology. Chelicerae: dorsal edge of fixed
finger, with four denticles (basal, medial, subdistal, distal);
ventral edge with 4–5 very reduced denticles; movable finger with three
denticles (medial, subdistal, external distal) on dorsal edge,
without basal denticles; ventral edge with 4–5 reduced denticles;
external distal denticle smaller than internal distal denticle; ventral
aspect offingers and manus with numerous macrosetae. Carapace. Anterior
margin depressed with a moderately marked concavity. Lateral ocelli
absent. Median ocular tubercle represented by a smooth depressed zone;
median ocelli absent; interocular furrow obsolete. One pair of vestigial
circumocular sutures with a broad U-shaped configuration, incomplete
behind median ocular tubercle. Anteromedian and posteromedian furrows
shallow; posterolateral furrow shallow, weakly curved; posteromarginal
furrow narrow, very shallow. Carapace almost totally smooth, except
for some isolated granules anteriorly; acarinate; anterosubmedial
carinae absent from the zone internal to circumocular sutures. Pedipalp
segments apilose. Femur with five discernible carinae, all weak to
vestigial; intercarinal surfaces smooth. Patella with 5–6 discernible
carinae; ventrointernal carinae with some spinoid granules; intercarinal
surfaces smooth. Chela with only vestigial carinae, rounded and
smooth. Fixed and movable fingers strongly curved; dentate margins each
with median denticle row comprising eight oblique granular sub-rows;
each sub-row comprising several small granules and internal and external
accessory granules. Trichobothria:Orthobothriotaxic, Type D (
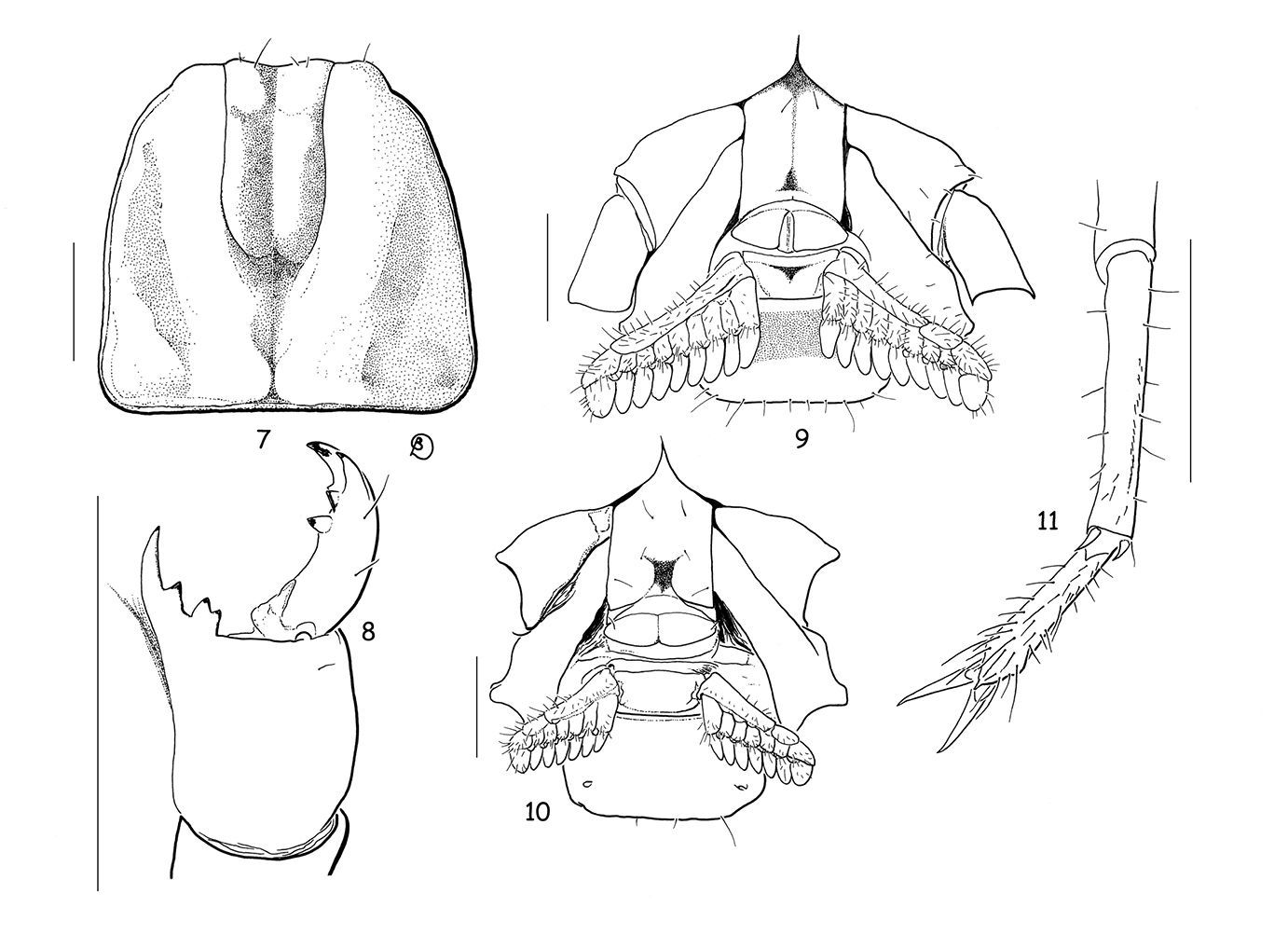
Vietbocap canhi sp. n. (M=male, F=female). 7 Carapace, dorsal aspect (M) 8 Chelicera, dorsal aspect (F) 9–10 Ventral aspect, showing sternum, genital operculum, pectines and sternite III (M & F) 11 Leg IV, showing absence of tibial spur and telotarsi with spinular setae (F). Scale bars = 1 mm.
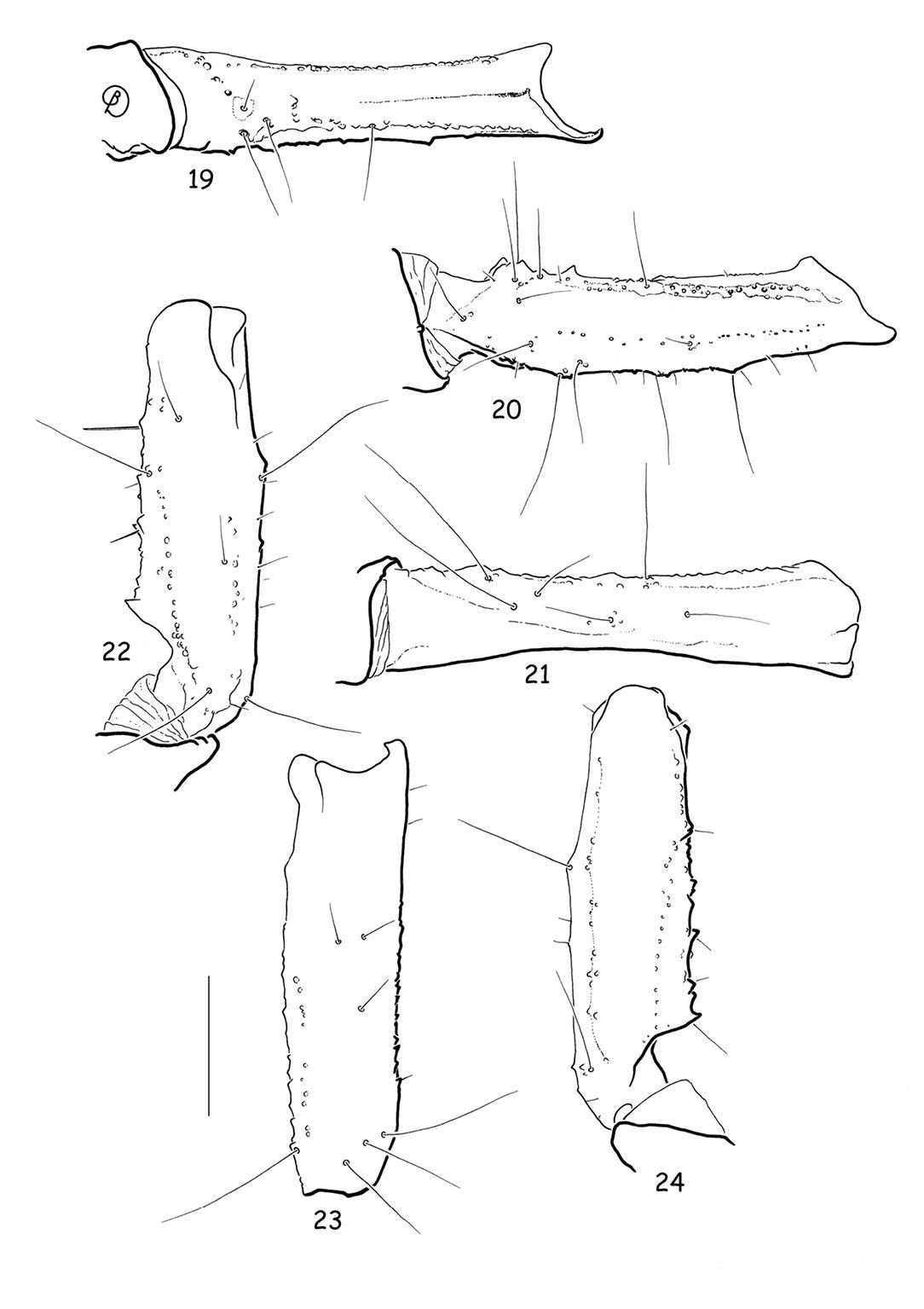
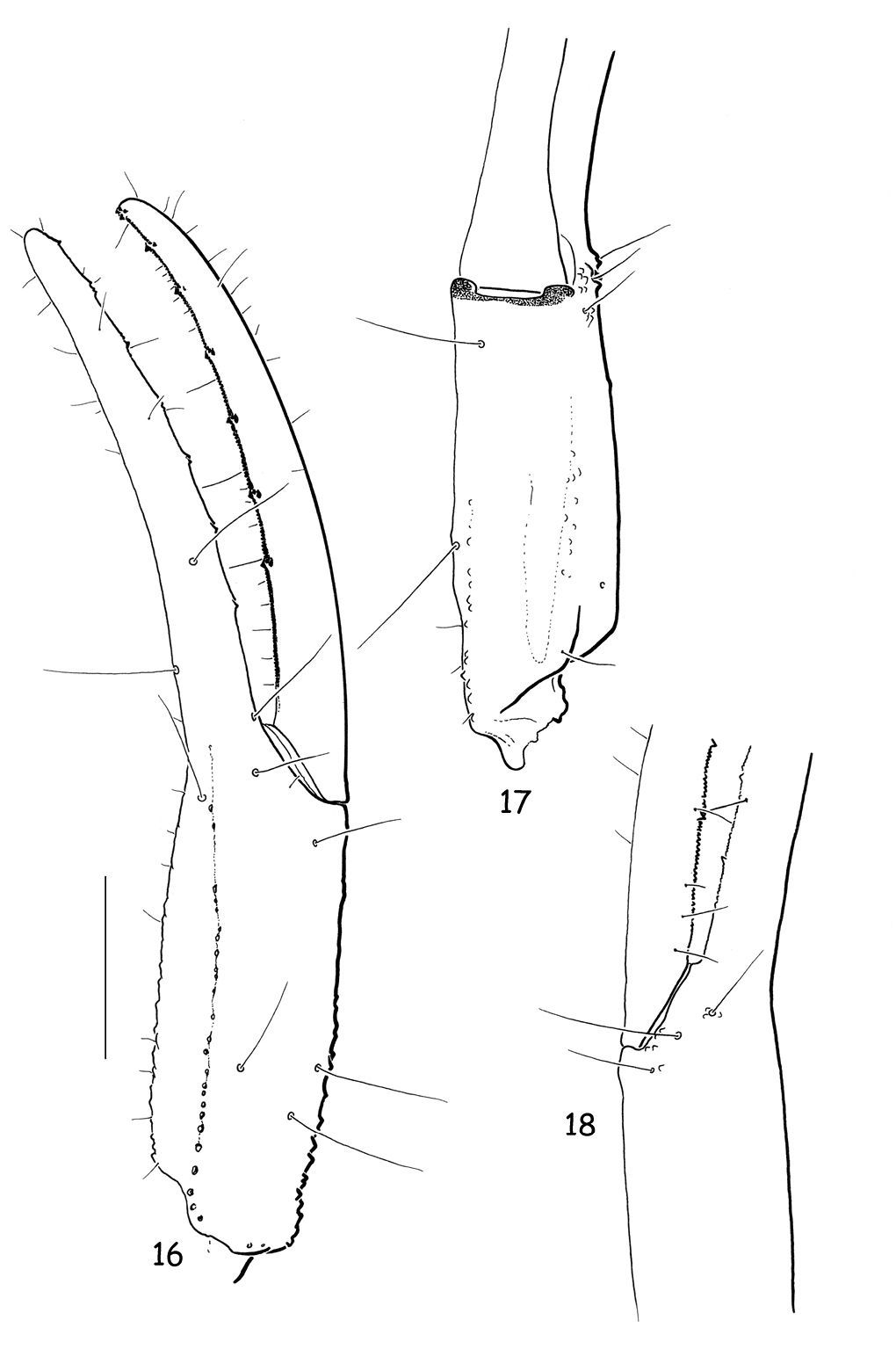
Vietbocap canhi sp. n. (M=male, F=female). 12–13 Metasomal segment V and telson, lateral and ventral aspects (M) 14 Idem female 15 Movable finger of pedipalp chela with subrows of granules (M). Scale bars = 1 mm.
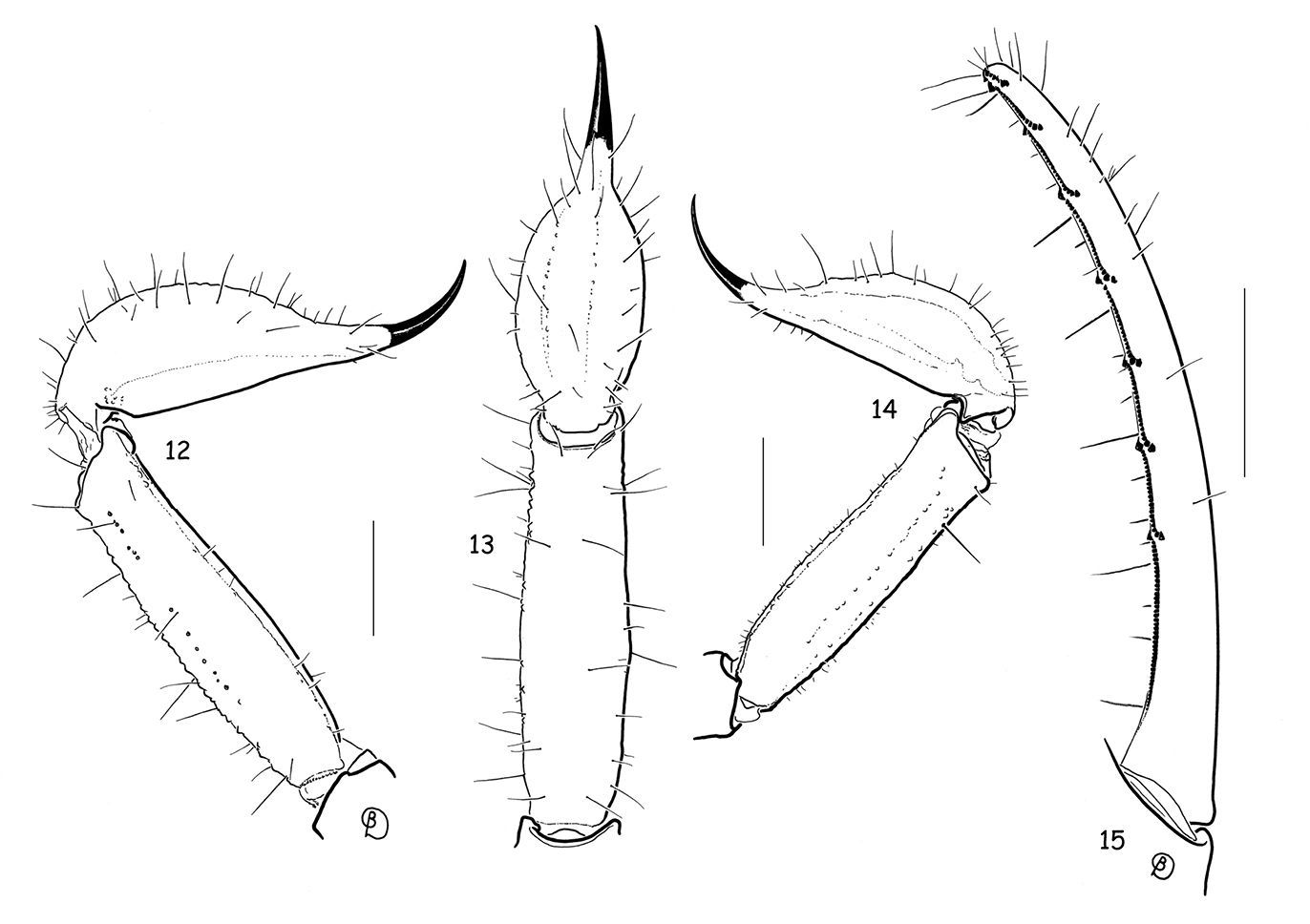
Vietbocap canhi sp. n., female paratype. Trichobothrial pattern. Chela, dorso-external, ventral and internal aspects. Scale bar = 1 mm.
Vietbocap canhi sp. n., female paratype. Trichobothrial pattern. 19–21 Femur, internal, dorsal and external aspects 22–24 Patella, dorsal, external and ventral aspects. Scale bar = 1 mm.
Only known from the type locality.
Total length 22.4/21.3. Carapace: length 2.9/2.8; anterior width 2.0/1.8; posterior width 3.2/2.9. Mesosoma length 5.5/6.4. Metasomal segments: I, length 1.2/1.0, width 1.4/1.2; II, length 1.4/1.2, width 1.3/1.0; III, length 1.5/1.4, width 1.2/0.9; IV, length 2.1/1.7, width 1.1/0.8; V, length 3.9/3.2, width 1.1/0.8, depth 0.9/0.8. Telson length 3.9/3.6; vesicle length 2.4/2.2, width 1.3/1.0, depth 1.2/0.9. Pedipalp: femur length 3.8/3.1, width 0.9/0.7; patella length 3.6/3.2, width 1.1/0.9; chela length 7.1/5.8, width 1.2/1.0, depth 1.0/0.9; movable finger length 4.2/3.9.
| 1 | Median and lateral ocelli present; leg tibial spurs present | 3 |
| 2 | Median and lateral ocelli absent; leg tibial spurs absent | Vietbocap canhi sp. n. |
| 3 | Circumocular sutures incomplete; peg sensillae of pectines rounded | Troglokhammouanus steineri Lourenço, 2007 |
| – | Circumocular sutures complete; peg sensillae of pectines spatular | Pseudochactas ovchinnikovi Gromov, 1998 |
We are most grateful to Bernard Duhem (MNHN, Paris) for preparing the illustrations; to Elise-Anne Leguin (MNHN, Paris) for the preparation of the plates; to Thomas Ziegler (Cologne, Germany) for information on the ecology of the Phong Nha - Ke Bang National Park region; to Mark Judson (MNHN, Paris) for corrections to the manuscript and to Victor Fet (Marshall University, Huntington, USA) for his useful comments to the text. Finally, the second author wishes to acknowledge the Nagao Natural Environment Foundation of Japan and also the GTZ Vietnam for their support of his field studies.