(C) 2010 Massimo Olmi. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Esagonatopus floridensis sp. n. is described from Florida, Oklaloosa County (USA). A revision of the three Nearctic species of Esagonatopus Olmi, 1984 is presented. New data on geographic distribution, morphologic variability and opposite sexes of Esagonatopus niger (Fenton, 1924) and Esagonatopus perdebilis (Perkins, 1907) are given. A key to the Nearctic species of Esagonatopus is presented.
Taxonomy, Esagonatopus floridensis, USA, key, Dryinidae
Dryinidae (Hymenoptera: Chrysidoidea) are parasitoids of Hemiptera Auchenorrhyncha (
In 2009 and 2010 we have examined additional specimens of Esagonatopus from the United States, Canada and Mexico and have found a new species described herein. This material made it possible to revise the entire group of Nearctic species and provide new data on geographical distribution, morphologic variability and opposite sexes of Esagonatopus niger (Fenton, 1924) and Esagonatopus perdebilis (Perkins, 1907).
Material and methodsThe descriptions follow the terminology used by (
The treatments of Esagonatopus niger and Esagonatopus perdebilis are updated by adding new localities and morphological variations to the descriptions reported by
In the figures of male genitalia the right half was removed.
In the text ! means that the specimen was examined personally by the authors.
The specimens studied in this paper are deposited in the following collections:
AMNH American Museum of Natural History, New York, U.S.A.
BNC Benoît Nusillard’s collection, Montboucher sur Jabron (France).
BPBM Bernice P. Bishop Museum, Honolulu, Hawaii, U.S.A.
CDAE California State Collection of Arthropods, Department of Food and Agriculture, Sacramento, California, U.S.A.
CNC Canadian National Collection of Insects, Ottawa, Canada.
DEUK Department of Entomology, College of Agriculture, University of Kentucky, Lexington, Kentucky, U.S.A.
EMG Entomology Museum, University of Georgia, Athens, Georgia, U.S.A.
LACM Natural History Museum of Los Angeles County, Los Angeles, California, U.S.A.
MOLC Massimo Olmi’s collection, c/o Department of Plant Protection, University of Tuscia, Viterbo, Italy.
MZLU Zoological Institute, Lund, Sweden.
PMA Provincial Museum of Alberta, Edmonton, Alberta, Canada.
RDHC Robert D. Haines’ collection, Visalia, California, U.S.A.
SEMC Natural History Museum, University of Kansas, Lawrence, Kansas, U.S.A.
TAMU Department of Entomology, Texas A. & M. University, College Station, Texas, U.S.A.
UCR Department of Entomology, University of California, Riverside, California, U.S.A.
USNM National Museum of Natural History, Washington, D.C., U.S.A.
Systematic Accounts Female: apterous; pronotum crossed by a strong
transverse impression; enlarged claw with distal apex pointed, with a
small subapical tooth, without lamellae, with bristles or peg-like
hairs; antenna without rhinaria (sensu
Nearctic, Neotropical.
Cicadellidae (
Six.
Key to the Nearctic species of Esagonatopus
Females
| 1 | Meso-metapleural suture distinct and complete | Esagonatopus floridensis sp. n. |
| – | Meso-metapleural suture obsolete | 2 |
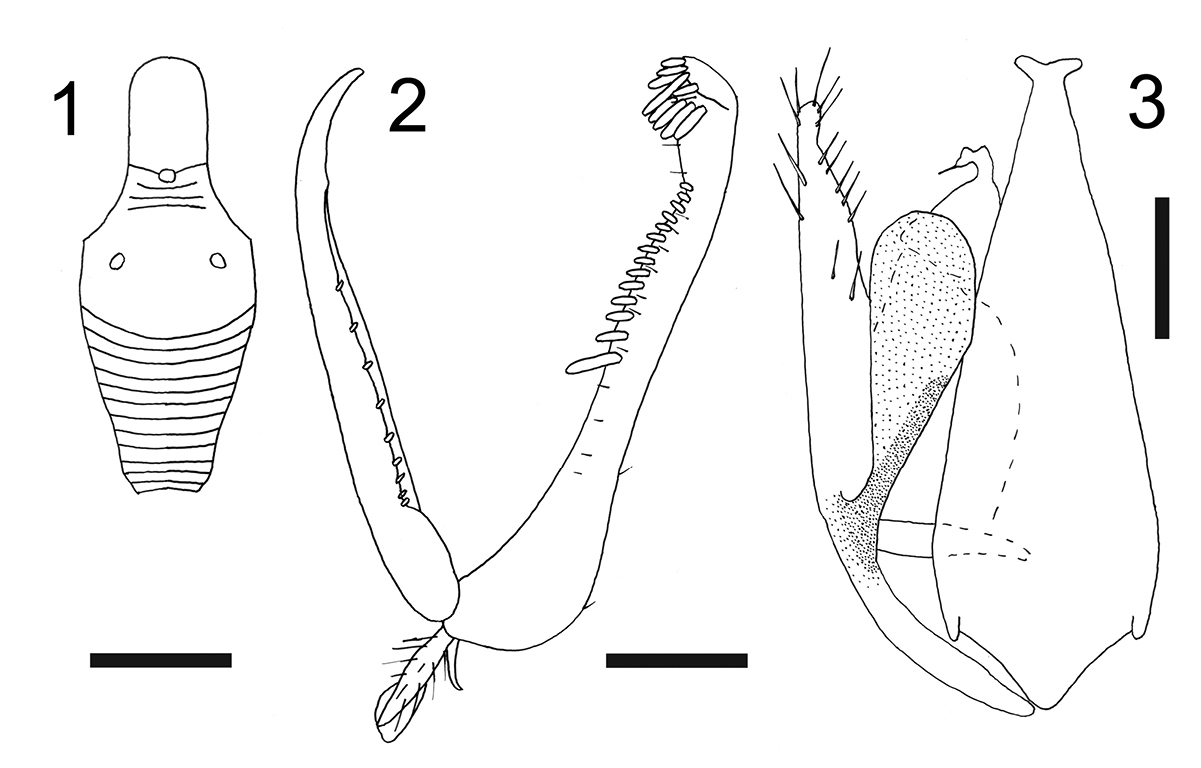
| 2 | Metanotum with lateral rounded protrusions (Fig. 1) | Esagonatopus niger (Fenton) |
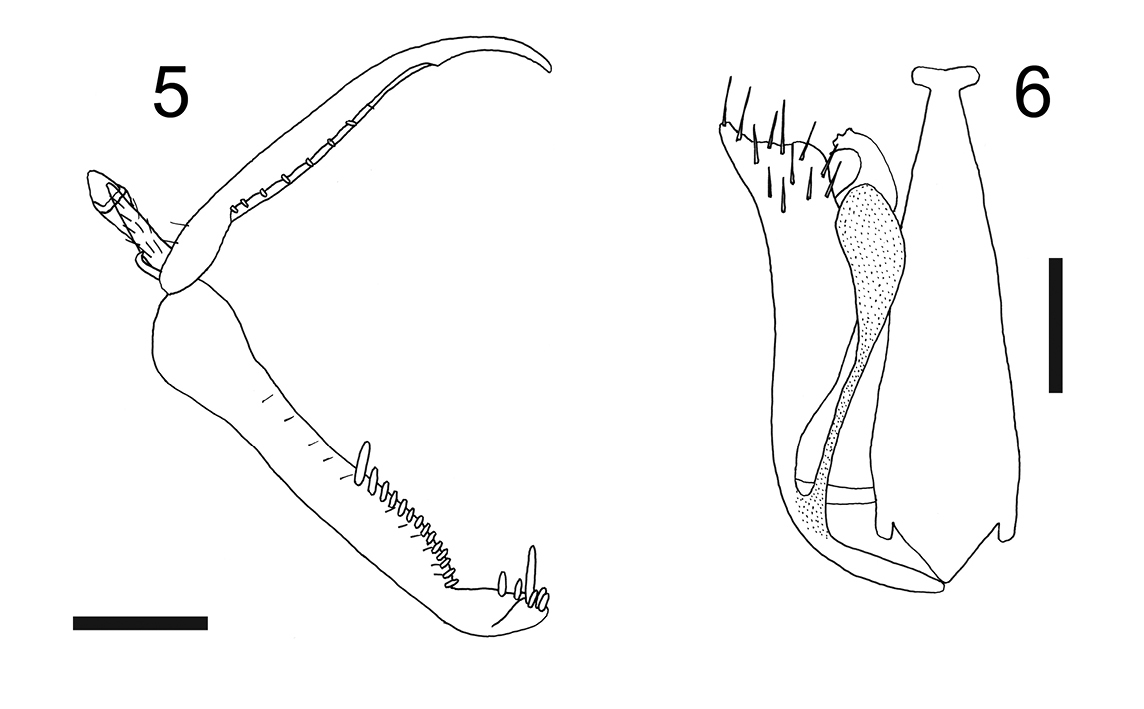
| – | Metanotum laterally not protruding (Fig. 4) | Esagonatopus perdebilis (R. Perkins) |
Males (unknown in Esagonatopus floridensis)
| 1 | Notauli posteriorly separated; dorsal process of parameres broader (Fig. 3) | Esagonatopus niger (Fenton) |
| – | Notauli posteriorly meeting; dorsal process of parameres very slender and narrow (Fig. 6) | Esagonatopus perdebilis (R. Perkins) |
Figs 1, 2, 3
Type: Holotype, female, USA: Iowa, Story Co., Ames, 8.vii.1923, C.J. Drake coll., ex Scaphoideus sp. probably immistus Say (USNM!). Further specimens examined: CANADA: Ontario: Marmora (CNC!); Near Windsor, Ojibway Park (PMA!); Ottawa (MZLU!); Walpole Island (MZLU!); S Minonico (CNC!); St. Davids (CNC! AMNH!). MEXICO: Mexico (New record): Chapultepec (USNM!). Morelos: Cuernavaca (USNM!). U SA.: Arizona: Johnson Co., McKay Bog, 9 mi. NE Clarksville (CNC!). California (New record): Tulare Co., Ash Mountain, Kaweah Power Station (RDHC!); Contra Costa Co., Moraga (CDAE!); Imperial Co., Niland (USNM!); Riverside Co., Menifee Valley, 33°39’N; 117°13’W (UCR!). Florida (New record): Liberty Co., Torreya State Park (CNC!). Georgia (New record): Rabun Co., Clayton (LACM!). Kentucky: Fayette Co., Lexington (DEUK!); Robertson Co. (Freytag, 1977). New York: Schuyler Co., Valois (BNC!); Yates Co., Dresden (BNC!); Ontario Co., Geneva (BNC!). North Dakota: Walsh Co., Grafton (AMNH!); Ramsey Co. (AMNH!). Pennsylvania: Dauphin Co., Harrisburg (USNM!). Virginia (New record): Louisa Co., 6.5 Km S of Cuckoo (AMNH!).
Female with meso-metapleural suture obsolete; metanotum with lateral rounded protrusions (Fig. 1). Male with notauli posteriorly separated; dorsal process of parameres broad (Fig. 3).
Female: apterous; length 2.3–3.0 mm. Head black or ferruginous, except anterior region of face, clypeus and mandibles yellow; occasionally head completely yellow or with a black transverse band on vertex. Antenna brown, except segment 1 testaceous, or yellow and segments 8–10 darkened; occasionally antennae totally yellow. Mesosoma usually black, occasionally completely yellow, or ferruginous, with irregular dark spots. Petiole black. Gaster usually brown-ferruginous; occasionally testaceous. Legs completely yellowish red, or with fuscous areas. Occasionally body completely yellow or testaceous, with petiole black. Antenna clavate; antennal segments in following proportions: 8:5:15:9:6:6:5:5:5:7. Head excavated, shiny, smooth, with vertex without sculpture and face and occiput granulated; frontal line complete; occipital carina absent; POL = 1.5; OL = 2; OOL = 7.5. Palpal formula 6/2. Pronotum crossed by a strong transverse impression, shiny, with anterior collar smooth and without sculpture, disc granulated and lateral regions sculptured by longitudinal striae. Scutum shiny, with few longitudinal striae, without lateral pointed apophyses. Scutellum flat, shiny, without sculpture. Meso-metapleural suture obsolete. Metanotum transversely striate, not hollow behind scutellum. Metathorax + propodeum shiny, granulated, with lateral rounded protrusions (Fig. 1). Mesopleura, metapleura and posterior surface of propodeum strongly transversely striate. Fore tarsal segments in following proportions: 13:2:5:16:23. Enlarged claw (Fig. 2) with a small subapical tooth and a row of 7–9 peg-like bristles. Segment 5 of fore tarsus (Fig. 2) with two rows of approximately 16–17 lamellae or one row of 13–15 lamellae (with proximal lamellae longer than medial and distal lamellae); distal apex with a group of 7–18 lamellae. Tibial spurs 1/0/1.
Male: fully winged; length 2.0 mm. Head black, except mandibles testaceous. Antenna brown. Mesosoma and petiole black. Gaster and legs brown. Antenna hairy, filiform; antennal segments in following proportions: 4:5:7:6:6:6:6:5.5:5.5:10. Antennal segment 3 less than three times as long as broad (2.8). Head shiny, granulated; occiput excavated; temples distinct; frontal line absent; occipital carina absent; POL = 6; OL = 2; OOL = 4. Palpal formula 6/2. Scutum shiny, granulated. Notauli complete, posteriorly separated; minimum distance between notauli shorter than greatest breadth of posterior ocelli (2:3). Scutellum and metanotum shiny, smooth, without sculpture. Propodeum completely reticulate rugose. Forewing hyaline, without dark transverse bands; stigmal vein with distal part longer than proximal part (17:8). Dorsal process of the parameres (Fig. 3) broadened. Tibial spurs 1/1/2.
Figures 1–3. Esagonatopus niger. 1 Scutum and metathorax + propodeum (in dorsal view) of a female specimen from Mexico, Cuernavaca 2 Chela of holotype 3 Male genitalia of a specimen from New York, Geneva. Scale bar 0.31 mm for 1, 0.12 mm for 2 and 0.06 mm for 3.
Cicadellidae (
Figs 4, 5, 6
Types: Lectotype (designated by
Female with meso-metapleural suture obsolete; metanotum laterally not protruding (Fig. 4). Male with notauli posteriorly meeting; dorsal process of parameres very slender and narrow (Fig. 6).
Female: apterous; length 2.9–3.1 mm. Completely yellow-testaceous, except petiole black; antenna yellow, except segments 4–10 or 8–10 darkened. Antenna clavate; antennal segments in following proportions: 7:4.5:11:6:5:5:5:4.5:4:6. Head excavated, shiny, smooth, with vertex without sculpture and anterior region of face and occiput granulated. Palpal formula 6/2. Pronotum crossed by a strong transverse impression, shiny, without sculpture. Scutum shiny, without sculpture, without lateral pointed apophyses. Scutellum flat, without sculpture. Meso-metapleural suture obsolete. Metanotum transversely striate, not hollow behind scutellum. Metathorax + propodeum shiny, without sculpture, without lateral rounded protrusions (Fig. 4). Posterior surface of propodeum strongly transversely striate. Fore tarsal segments in following proportions: 10:2:3:13:19; Enlarged claw (Fig. 5) with a small subapical tooth and a row of 7–9 peg-like bristles. Segment 5 of fore tarsus (Fig. 5) with 1–2 rows of approximately 13–15 lamellae; distal apex with a group of 9–11 lamellae. Tibial spurs 1/0/1.
Male: fully winged; length 1.6 mm. Head black, except mandibles testaceous; antenna brown; mesosoma black; gaster brown-black; legs brown, except articulations, fore tibiae and fore tarsi testaceous. Antenna hairy, filiform; antennal segments in following proportions: 4:4.5:6:5:5:4:4:5:4:7; antennal segment 3 about three times as long as broad (6:2). Head dull, granulated, laterally with two shiny and smooth areas situated between posterior ocelli and eyes and surrounded by very low keels; frontal line absent; occipital carina absent; occiput concave; temples distinct; POL = 4.5; OL = 2; OOL = 3. Palpal formula 6/2. Scutum dull, granulated. Notauli complete, posteriorly meeting. Scutellum and metanotum shiny, very finely punctate, without sculpture among punctures. Propodeum dull, reticulate rugose, without keels. Forewing hyaline, without dark transverse bands; marginal cell open; distal part of stigmal vein longer than proximal part (10:6). Dorsal process of the parameres (Fig. 6) slender, much shorter than parameres, with distal apex broadened. Tibial spurs 1/1/2.
Figure 4. Esagonatopus perdebilis. Female specimen from Arizona, Nogales (from
Figures 5, 6. Esagonatopus perdebilis. 5 Chela of a female specimen from Arizona, Nogales 6 Male genitalia of a specimen from Mexico, Michoacan, near Huahua. Scale bar 0.12 mm for 5 and 0.08 mm for 6.
Cicadellidae (
urn:lsid:zoobank.org:act:926B0805-93C4-4D90-B8C7-F08B8C938B26
Figs 7, 8, 9Floridensis from Florida, where this species was collected.
Holotype, female, USA: Florida, Oklaloosa Co., 2 mi N Holt, 31.v.1991, J.B. Woolley coll. (TAMU (it will be transferred to USNM)!).
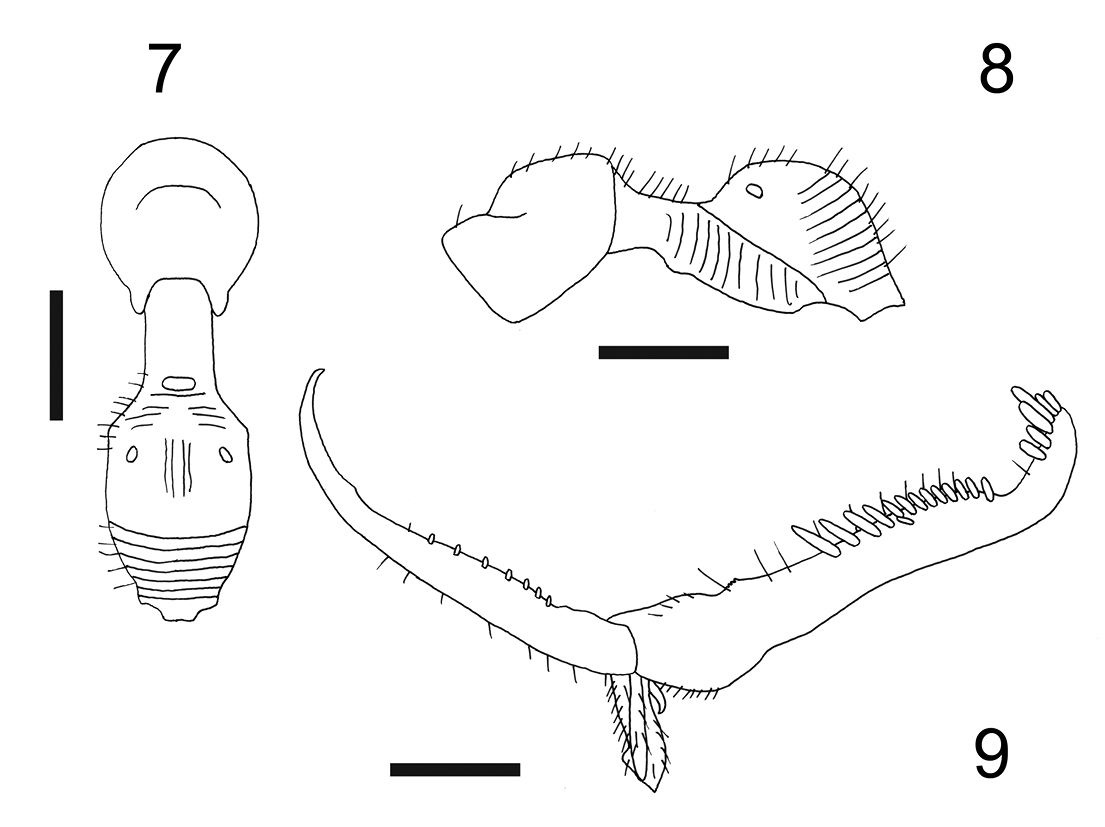
Female with meso-metapleural suture distinct and complete; metanotum with lateral pointed protrusions. Male unknown.
Female: apterous; length 2.7 mm. Head brown, except anterior region of face, clypeus and mandibles testaceous; antenna brown-testaceous; mesosoma brown, except scutum testaceous-yellow; petiole and gaster black; legs testaceous, except part of coxae and clubs of femora brown. Antenna clavate; antennal segments in following proportions: 9:4:11:6:5:4.5:4:4:4:5. Head excavated, shiny, smooth, without sculpture, except occiput granulated; frontal line complete; occipital carina incomplete, shortly present behind and on the sides of posterior ocelli; POL = 1; OL = 2; OOL = 7; greatest breadth of posterior ocelli about as long as POL. Palpal formula 6/2. Pronotum crossed by a strong transverse impression, shiny, with anterior collar smooth and without sculpture, disc sculptured by weak longitudinal striae. Scutum shiny, with few longitudinal striae, without lateral pointed apophyses. Scutellum flat, shiny, without sculpture. Meso-metapleural suture distinct and complete. Metanotum transversely striate, not hollow behind scutellum, with sides protruding; lateral protrusions pointed (Fig. 7). Metathorax + propodeum (Fig. 8) dull, with anterior surface weakly sculptured by many longitudinal striae; disc with a track of a median longitudinal furrow. Mesopleura, metapleura and posterior surface of propodeum strongly transversely striate. Fore tarsal segments in following proportions: 11:2.5:4:14:22. Enlarged claw (Fig. 9) with a small subapical tooth and a row of 7 peg-like hairs + 1 hair. Segment 5 of fore tarsus (Fig. 9) with 2 rows of 1 + 13 lamellae (with proximal lamellae longer than medial and distal lamellae); distal apex with a group of about 6 lamellae. Tibial spurs 1/0/1.
Male: unknown.
Figures 7–9. Esagonatopus floridensis sp. n. 7 Mesosoma in dorsal view 8 Mesosoma in lateral view 9 Chela. Scale bar 0.36 mm for 7, 0.38 mm for 8 and 0.14 mm for 9.
unknown.
Many thanks to the curators of the institutions sending material on loan. This study was supported by a grant of the University of Tuscia: 60%.