(C) 2012 Shaun L. Winterton. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Australasian spider flies (Diptera: Acroceridae) are reviewed, with all eight currently recognized genera diagnosed and figured. The panopine genus Panops Lamarck, 1804 from Australia and Indonesia is revised with four new species described, increasing the total number of species in the genus to nine: Panops aurum sp. n., Panops danielsi sp. n., Panops jade sp. n. and Panops schlingeri sp. n. Five species of Panops are redescribed: Panops austrae Neboiss, 1971, Panops baudini Lamarck, 1804, Panops boharti (Schlinger, 1959), comb. n., Panops conspicuus (Brunetti, 1926) and Panops grossi (Neboiss, 1971), comb. n. The monotypic genera Neopanops Schlinger, 1959 and Panocalda Neboiss, 1971 are synonymized with Panops. Keys to genera of Australasian Acroceridae and species of Panops, Helle Osten Sacken, 1896 and Australasian Pterodontia Gray, 1832 are included.
cybertaxonomy, spider parasitoid
Spider flies (also known as small-headed flies) (Diptera: Acroceridae) are a distinctive group of lower brachyceran flies characterized by unusual adult body shape and highly specialized larval biology as parasitoids of spiders. Adults are recognized as important pollinators of angiosperms (Fig. 1), frequently as strong fliers with greatly elongate mouthparts for feeding in long corolla flowers, although some species have reduced or even vestigial mouthparts`(
Panops baudini Lamarck feeding on Daviesia croniniana F.Muell. (Fabaceae), photographed during September in Boorabbin National Park, Western Australia. Photograph by Dan Schoknecht (Western Australian Museum).
The Australasian acrocerid fauna comprises all three subfamilies, although represented by relatively few genera. Two acrocerine genera (Ogcodes Latreille, 1797 and Pterodontia Gray, 1832) are found throughout the region, and are considered cosmopolitan throughout all major biogeographic regions. Philopotinae are represented by an endemic genus in New Zealand (Helle Osten Sacken, 1896) (
Panopinae are well represented in the Australasian region.Six genera are described previously from New Zealand (Apsona Westwood, 1876), Indonesia (Neopanops Schlinger, 1959) and Australia (Panocalda Neboiss, 1971, Panops Lamarck, 1804, Mesophysa Macquart, 1838 and Leucopsina Westwood, 1876) (
Terminology follows
| 1 | Postpronotal lobes greatly enlarged, contiguous along midline to form collar for head | Philopotinae, 2 |
| – | Postpronotal lobes not greatly enlarged, widely separate along midline | 3 |
| 2 | Wing with cells d, br, bm, and cu-p present, venation relatively complete (Fig. 3A) | Helle Osten Sacken, 1896 (New Zealand) |
| – | Wing with only cell br present, venation reduced (Fig. 3B) | Schlingeriella Gillung & Winterton, 2011 (New Caledonia) |
| 3 | Antenna usually styliform or rod-like with multiple terminal setae; wing venation reduced: at most three radial veins present, cells d and basal r4+5 merged or absent (Figs 3C, D); tibiae without spines (except Pterodontia) | Acrocerinae, 4 |
| – | Antenna with elongate flagellum, cylindrical or flattened, without terminal styliform seta; wing venation complete: four radial veins present, cells d and basal r4+5 separate (Figs 2A–D); at least some tibiae with an apical spine on outer margin (absent in Apsona) | Panopinae, 5 |
| 4 | Eye apilose, without setae; venation reduced with many veins absent or poorly defined, almost all cells weakly formed or absent; tibial spines absent (Figs 3C, 63–64) | Ogcodes Latreille, 1797 (Cosmopolitan) |
| – | Eye pilose; all wing veins well defined to wing margin, discal cell and basal portion of r4+5 merged into single closed cell; tibial spines present (Figs 3D, 65–66) | Pterodontia Gray, 1832 (Cosmopolitan) |
| 5 | Eye strongly pilose; antennal flagellum slender and tapered to apex; tibial spines absent (Figs 2A, 4–6) | Apsona Westwood, 1876 (New Zealand) |
| – | Eye apilose or weakly pilose; antennal flagellum thickened to apex; tibial spines present (Australia) | 6 |
| 6 | Eye apilose, or sparsely or partially pilose; wing hyaline; crossvein 2r-m joining to stem R4+5 (Fig. 2D) | Panops Lamarck, 1804 |
| – | Eye always apilose; wing at least partially infuscate, particularly along anterior margin; crossvein 2r-m joining to vein R5 (Fig. 2B–C) | 7 |
| 8 | Dorsal profile of abdomen with swollen, rounded tergites; without transverse yellow band on tergite 3; not wasp-like in appearance (Figs 12–16) | Mesophysa Macquart, 1838 |
| – | Dorsal profile of abdomen with truncated tergites raised along posterior margins; transverse yellow band on tergite 3; distinctly wasp-like in appearance (Figs 7–11) | Leucopsina Westwood, 1876 |
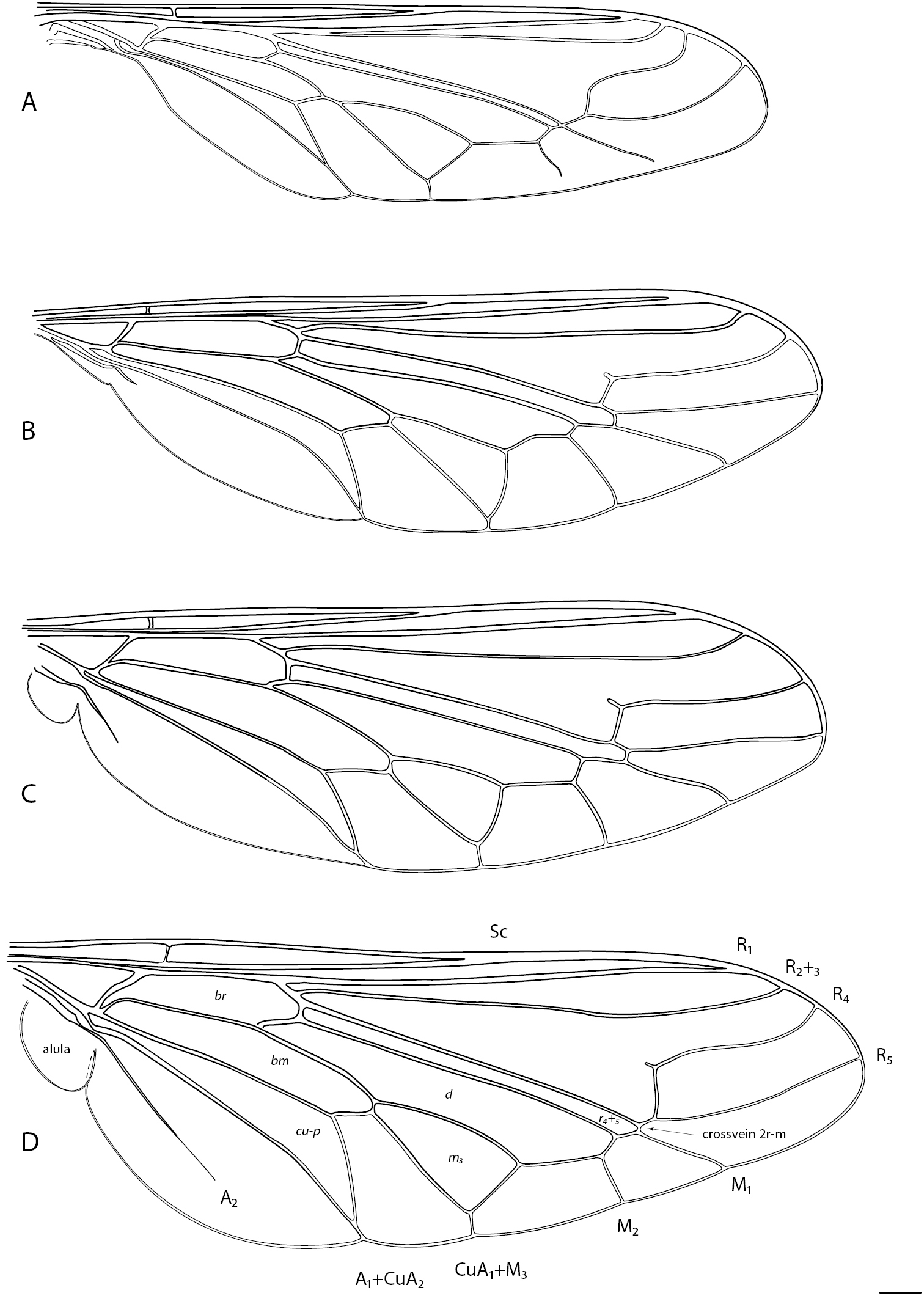
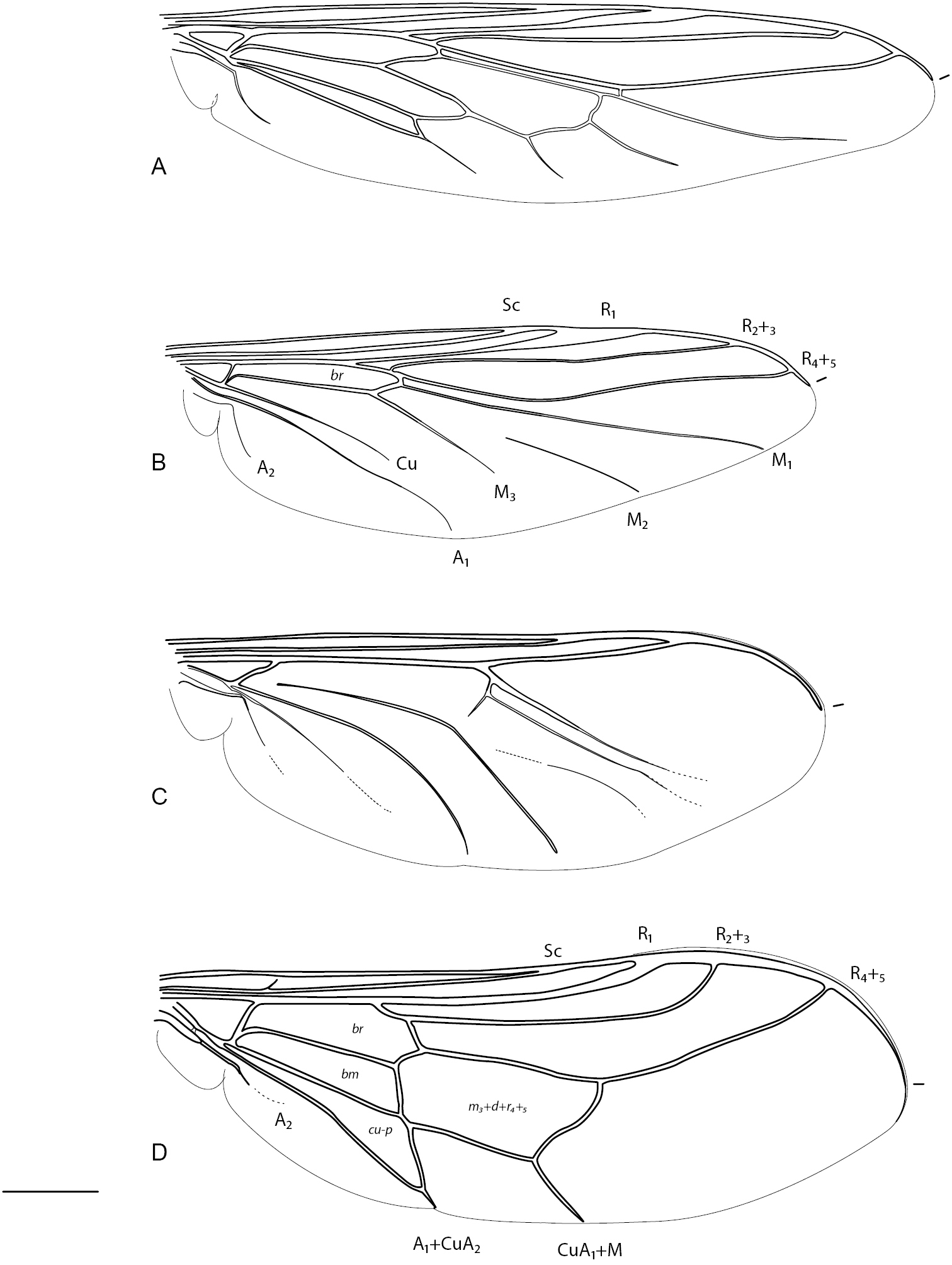
Acroceridae wings. Panopinae: A Apsona muscaria Westwood B Leucopsina odyneroides Westwood C Mesophysa tenaria Neboiss D Panops jade sp. n. Scale line = 0.2 mm.
Acroceridae wings. Philopotinae: A Helle rufescens Brunetti B Schlingeriella irwini Gillung & Winterton. Acrocerinae C Ogcodes basalis Walker D Pterodontia davisi Paramonov (female). Scale line = 0.2 mm.
Panops Lamarck, 1804: 263.
Usually large and densely pilose, body shape never arched; antennal flagellum elongate cylindrical to paddle-shaped, sometimes tapered but never stylate, usually lacking terminal setae; postpronotal lobes never meeting medially; wing venation complete to wing margin (rarely reduced), cells m3, d, bm and basal r4+5 typically present, closed distally; tibial spines present (rarely absent); larvae exclusively parasitoids of mygalomorph spiders.
Apsona Westwood, 1876; Leucopsina Westwood, 1876; Mesophysa Macquart, 1838; Panops Lamarck, 1804.
http://species-id.net/wiki/Apsona
Figs 2A, 4–6Body length: 7–9 mm. Colouration metallic green; head width slightly smaller than thorax width, hemispherical; postocular ridge and occiput rounded; three ocelli; posterior margin of eye rounded; eye pilose (dense); position of antenna on frons nearer to ocellar tubercle; eyes contiguous above and below antennal base; palpus present; proboscis longer than head length; flagellum shape elongate, tapered apically, apex lacking terminal setae; scapes separate; subscutellum not enlarged, barely visible; tibial spines absent; pulvilli present; wing hyaline, markings absent; costa circumambient, costal margin straight apically in both sexes; humeral crossvein present; radial veins curved towards wing anterior margin; R1 not inflated distally; pterostigma and cell r1 membranous, not ribbed; R2+3 present; R4+5 present as forked petiolate veins; cell r4+5 bisected by 2r-m, basal cell narrow elongate, closed; 2r-m very short, joining M1 to stem R4+5; R4 without spur vein; medial vein compliment with M1, M2 and M3 present (M3 fused with CuA1); discal cell closed completely; M1 and M2 usually not reaching wing margin; cell m3 present; CuA1 joining M3, petiolate to wing margin; CuA2 fused to A1 before wing margin, petiolate; wing microtrichia absent; anal lobe well developed; alula absent; abdominal tergites smooth, rounded; abdomen shape greatly rounded, inflated, conical posteriorly.
Apsona muscaria Westwood, male, lateral view [700415]. Body length = 8.0 mm.
Apsona muscaria Westwood, male, oblique view [700418]. Body length = 8.0 mm.
Apsona muscaria Westwood, male, anterior view [700419]. Body length = 8.0 mm.
Apsona muscaria Westwood, 1876.
Apsona is a monotypic genus endemic to New Zealand and can be readily differentiated from all other Panopinae based on the lack of tibial spines. Apsona shows little relationship to the rest of the Australasian Panopinae and shows remarkable similarity to the New World genus Eulonchus Gerstaecker, 1856, sharing numerous characteristics such as metallic green colouration, antennal shape, dense eye pilosity, elongate mouthparts, eyes contiguous below antennal base and absence of an alula (
http://species-id.net/wiki/Leucopsina
Figs 2B, 7–11Body length: 9.0 mm [male], 12.0 mm [female]. Colouration black and yellow [wasp mimic]; head slightly smaller than thorax width, shape hemispherical; postocular ridge and occiput rounded; three ocelli, anterior ocellus reduced in size (female) or absent (male); posterior margin of eye emarginate; eye apilose; position of antennae on head adjacent to ocellar tubercle; male frons width above antennal base not contiguous, eyes contiguous below antennal base; palpus present; proboscis greater than head length; flagellum shape elongate, cylindrical; apex lacking terminal setae; scapes separate; subscutellum enlarged; tibial spines present; pulvilli present; wing markings present (infuscate anteriorly); costa circumambient (weaker along anal margin); costal margin straight; humeral crossvein present; radial veins straight; R1 not inflated distally; pterostigma and cell r1 membranous, not ribbed; R2+3 present; R4+5 originating separately from cell r4+5 (or at same point); cell r4+5 bisected by 2r-m, basal cell narrow elongate, closed; 2r-m joining M1 to R5; R4 with spur vein; medial vein compliment: M1, M2 and M3 present (M3 fused with CuA1); discal cell closed completely; medial veins reaching wing margin; cell m3 present; CuA1 joining M3, petiolate to margin; CuA2 fused to A1 before wing margin, petiolate; wing microtrichia absent; anal lobe well-developed; alula weakly developed; abdominal tergites smooth, rounded, tergites raised along posterior margins; abdomen constricted anteriorly.
Leucopsina odyneroides Westwood, male, lateral view [700421]. Body length = 9.0 mm.
Leucopsina odyneroides Westwood, male, dorsal view [700423]. Body length = 9.0 mm.
Leucopsina odyneroides Westwood, male, anterior view [700426]. Body length = 9.0 mm.
Leucopsina odyneroides Westwood, female, lateral view [700436]. Body length = 12.0 mm.
Leucopsina odyneroides Westwood, female, dorsal view [700447]. Body length = 12.0 mm.
Leucopsina burnsi (Paramonov, 1957); Leucopsina odyneroides Westwood, 1876.
Leucopsina is an endemic Australian genus of contrastingly coloured yellow and black flies, with distinct sexual dimorphism between males and females; male having more pronounced constriction of the abdomen anteriorly. The body colouration, darkening of the costal wing margin and abdominal waist allows members of this genus to be convincing wasp mimics (
http://species-id.net/wiki/Mesophysa
Figs 2C, 12–16Body length: 8.0–10.0 mm [male], 9.0–11 mm [female]. Colouration non-metallic, usually matte greenish hue; head size slightly smaller than thorax width; shape hemispherical; postocular ridge and occiput rounded; three ocelli; posterior margin of eye emarginate; eye apilose; antennae positioned on head adjacent to ocellar tubercle; eyes not contiguous above antennal base, contiguous below antennal base; palpus present; proboscis greater than head length; flagellum shape elongate, cylindrical (flattened), truncated apically [more pronounced in male]; scapes separate; flagellum apex lacking terminal setae; subscutellum not enlarged, barely visible; tibial spines present; pulvilli present; wing infuscate, markings present; costa circumambient (weaker along anal margin); costal margin straight apically; humeral crossvein present; radial veins straight; R1 not inflated distally; pterostigma and cell r1 membranous, not ribbed; R2+3 present; R4+5 originating separately from cell r4+5; cell r4+5 bisected by 2r-m, basal cell narrow elongate, closed; 2r-m, joining M1 to R5; R4 with spur vein; medial vein compliment with M1, M2 and M3 present (M3 fused with CuA1); discal cell closed completely; medial veins reaching wing margin; cell m3 present; CuA1 joining M3, petiolate to margin; CuA2 fused to A1 before wing margin, petiolate to margin; wing microtrichia absent; anal lobe well developed; alula well developed; abdominal tergites smooth, rounded; abdomen shape rounded, cylindrical, similar width to thorax or constricted anteriorly (male), tergites raised along posterior margins.
Mesophysa tenaria Neboiss, male, lateral view [700448]. Body length = 10.0 mm.
Mesophysa tenaria Neboiss, male, oblique view [700450]. Body length = 10.0 mm.
Mesophysa tenaria Neboiss, male, anterior view [700452]. Body length = 10.0 mm.
Mesophysa tenaria Neboiss, female, lateral view [700453]. Body length = 11.0 mm.
Mesophysa tenaria Neboiss, female, oblique view [700454]. Body length = 11.0 mm.
Mesophysa flavipes (Latreille, 1811); Mesophysa ilzei Neboiss, 1971; Mesophysa tenaria Neboiss, 1971; Mesophysa ultima Neboiss, 1971.
Mesophysa is an endemic eastern Australian genus closely related to Leucopsina. They share a similar habitus with narrowing of the abdomen anteriorly (more pronounced in Leucopsina), apilose eyes, infuscate wings and flagellum shape, as well as the crossvein 2r-m joining to R5 rather than to the stem R4+5. This genus can be differentiated from Leucopsina by the lack of black and yellow markings. Mesophysa has been considered a synonym of Panops by some authors (
http://species-id.net/wiki/Panops
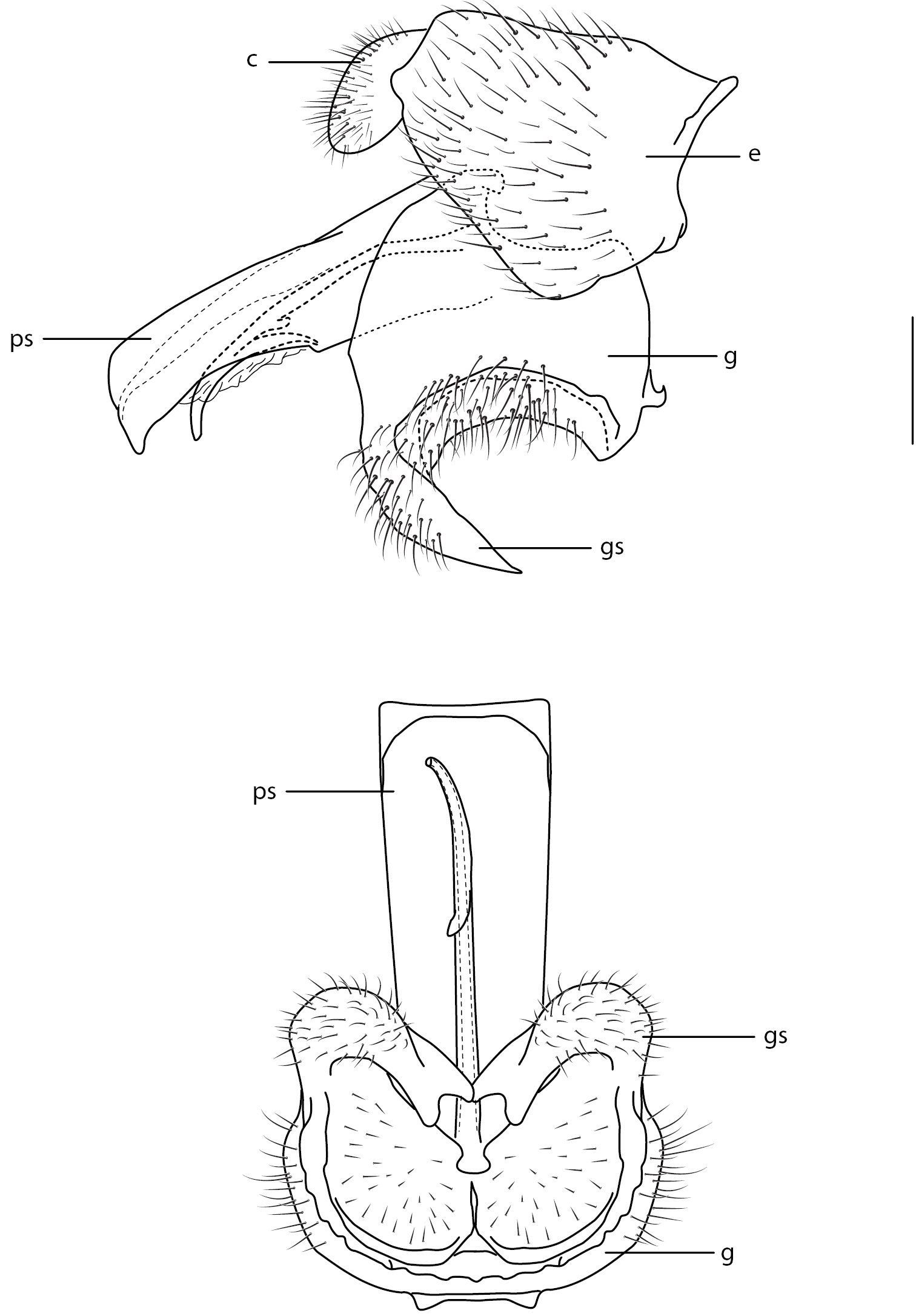
Figs 1, 2D, 17–55Body length: 8.0–12.5 mm [male], 9.5–14.5 mm [female]. Colouration non-metallic or metallic; head slightly smaller than thorax width, shape hemispherical; postocular ridge and occiput rounded; three ocelli, anterior ocellus reduced in size or absent; posterior margin of eye emarginate; eye apilose or pilose (sparse) (sometimes localized dorsally); position of antennae on head adjacent to ocellar tubercle; eyes not contiguous above antennal base, contiguous below antennal base; palpus present; proboscis length variable, less than or greater than head length; flagellum shape elongate, slightly tapered (female) or elongate, cylindrical (male); flagellum apex lacking terminal setae; scapes separate; subscutellum not enlarged, barely visible; tibial spines present; pulvilli present; wing hyaline, markings absent; costa circumambient (weaker along anal margin); costal margin at pterostigma straight; humeral crossvein present; R1 not inflated distally; pterostigma and cell r1 membranous, not ribbed; vein R2+3 present; R4 and R5 present as forked petiolate veins; radial veins straight towards wing apex, slightly angled anteriorly; cell r4+5 bisected by 2r-m, basal cell narrow elongate, closed; 2r-m joining M1 to stem R4+5; R4 with or without spur vein; medial vein compliment with M1, M2 and M3 present; discal cell closed completely; medial veins reaching wing margin; cell m3 present; CuA1 joining M3, petiolate to wing margin; CuA2 fused to A1 before wing margin, petiolate to margin; wing microtrichia absent; anal lobe well developed; alula well developed; abdominal tergites smooth, rounded; abdomen shape greatly rounded, inflated (larger in female). Male genitalia (Fig. 17) typical for Panopinae and varying little between species: gonostylus fused with gonocoxite and non-articulated, but with lightly sclerotized areas ventrally indicating flexion of gonostylus with gonocoxite; gonostylus as ventrally curved process with cup-like ventromedial surface; aedeagus consisting of flattened quadrangular, or cylindrical, parameral sheath with ventral rod-like structure with apical gonopore; ejaculatory apodeme poorly developed.
Panops baudini Lamarck. A male genitalia, lateral view B same, ventral view. Scale line = 0.2 mm. Abbreviations: c cercus; e epandrium; g gonocoxite; gs gonostylus; ps parameral sheath of aedeagus.
Panops aurum sp. n.; Panops austrae Neboiss, 1971; Panops baudini Lamarck, 1804; Panops boharti (Schlinger, 1959) comb. n.; Panops conspicuus (Brunetti, 1926); Panops danielsi sp. n.; Panops grossi (
Panops is the type genus for the subfamily Panopinae and includes some large metallic coloured species. The genus is endemic to Australia and neighbouring Papua region of Indonesia. The original concept of the genus was expanded to include species from the New World by some authors, but these have subsequently been placed in the separate and distantly related genus Lasia Wiedemann, 1824 (e.g. Lasia metallica Rondani, 1863; Lasia ocelliger (Wiedemann, 1830)).
Panops baudini keys to two couplets as the eye pilosity is extremely minute in some individuals and may be overlooked. Females are unknown for Panops boharti comb. n. and Panops aurum sp. n., whilst males are unknown for Panops schlingeri sp. n.
| 1 | Eye sparsely pilose (Fig. 39) or pilosity localized dorsally (Fig. 44) | 2 |
| – | Eye completely apilose (Fig. 18) | 5 |
| 2 | Proboscis elongate, length greater than head height (Fig. 18) | 3 |
| – | Proboscis very short, hardly projecting from oral cavity, shorter than head height (Figs 44, 50) | 4 |
| 3 | Postpronotal lobe dark, concolourous with rest of pleuron (widely distributed in Australia) (Figs 25–30) | Panops baudini Lamarck, 1804 |
| – | Postpronotal lobe yellow, pleuron greenish (Queensland) (Figs 39–43) | Panops danielsi sp. n. |
| 4 | Eye extending posteriorly beyond widest part of head; eye with sparse, minute pile of uniform length across eye (length subequal to width of lateral ocellus); ocellar tubercle not touching margin of eye; palpus as long or longer than proboscis (Papua) (Figs 31–33) | Panops boharti (Schlinger, 1959), comb. n. |
| – | Eye not extending posteriorly beyond widest part of head; eye pilose on dorsal-lateral region only, pile denser and more elongate (length much greater than width of lateral ocellus); ocellar tubercle touching margin of eye; palpus half as long as proboscis (South Australia) (Figs 44–47) | Panops grossi (Neboiss, 1971), comb. n. |
| 5 | Proboscis short, hardly projecting from oral cavity | 6 |
| – | Proboscis elongate, length equal to, or greater than head height | 7 |
| 6 | Postpronotal lobes dark yellow; femora dark brown, rest of legs cream (Northern Territory) (Figs 53–55) | Panops schlingeri sp. n. |
| – | Postpronotal lobes and legs dark, concolourous with rest of body (Queensland) (Figs 48–52) | Panops jade sp. n. |
| 7 | Postpronotal lobes pale, contrasting with rest of thorax (Figs 34–38) | Panops conspicuus (Brunetti, 1926) |
| – | Postpronotal lobes dark, concolourous with rest of thorax (Figs 21, 28) | 8 |
| 8 | Body metallic, thorax green, abdomen violet; margin of lower calypter relatively dark (Figs 21–24) (Western Australia) | Panops austrae Neboiss, 1971 |
| – | Thorax mostly glossy black, abdomen often with extensive red-brown to purple laterally; margin of lower calypter relatively pale (Figs 19, 26) | 9 |
| 9 | Face above clypeus apilose; body covered with white setal pile; male distiphallus broad, spatulate (widely distributed in Australia) (Figs 25–30) | Panops baudini Lamarck, 1804 |
| – | Face above clypeus with gold setal fringe; body covered with yellow-gold setal pile; male distiphallus narrow (Figs 18–20) (Western Australia) | Panops aurum sp. n. |
urn:lsid:zoobank.org:act:3864CACB-368C-4770-88E8-8346544EBED7
http://species-id.net/wiki/Panops_aurum
Figs 18–20Holotype male, AUSTRALIA: Western Australia: Darlington, 450 ft., E.S. Ross, D.Q. Cavagnaro, 5.ix.1962 [-31.901, 116.081] (CAS).
Eye apilose; proboscis longer than head height; body non-metallic; antennae red-brown; parafacial with yellow marginal pile; postpronotal lobe concolourous with rest of thorax; legs dark yellow, femora brown-black.
Body length: 11.0 mm (male). Headwitheye apilose; ocellar tubercle raised laterally; medial ocellus absent; occiput brown-black, occipital pile yellow, postocular ridge and gena overlain with grey pubescence; clypeus length equal to oral cavity, brown-black; palpus yellow; margin of oral cavity (parafacial) densely pilose (yellow); proboscis longer than head height; flagellum apex of uniform width, truncated apically, flagellum red-brown; scape and pedicel brown. Thorax with postpronotal lobe brown-black; scutum black, scutal vestiture dense yellow-gold pile; scutellum black; pleuron black; coxae black; femora brown-black, apices dark yellow; tibiae dark yellow; tarsi dark yellow; lower calypter white with dark yellow margin; wing hyaline, venation dark; vein R4 without spur vein. Abdomen shape rounded globose, much larger than thorax, colour orange-red to yellow, dark markings anteriorly and medially, vestiture dense elongate pile, yellow anteriorly, brown posteriorly on tergites 2–5.
Panops aurum sp. n., male, lateral view [700495]. Body length = 11.0 mm.
Panops aurum sp. n., male, dorsal view [700496]. Body length = 11.0 mm.
Panops aurum sp. n., male, anterior view [700497]. Body length = 11.0 mm.
The specific epithet is derived from the Latin, aurum – gold; referring to the distinctive golden setal pile on the head and thorax.
Panops aurum sp. n. is known only from a single male specimen from Western Australia. The fringing yellow setae around the oral cavity and yellow pile on the thorax are distinctive for the species.
http://species-id.net/wiki/Panops_austrae
Figs 21–24Holotype female, AUSTRALIA: Northern Territory: nr. Mount Olga [-25.3, 130.73], C.A., Paul Genery, ix.1960, picked up dead in sand (Type- T.4177) (NMV).
AUSTRALIA: Western Australia: male, Wialki [-30.483, 118.117], R. P. McMillan, 12.x.1983 (WAM); male, W of Norseman, Eucalyptus woodland, dry gully to salt lake, Malaise trap, C. Lambkin et al., ANIC bulk sample 2184, 1-17.xi.2003 271m [-32.186, 121.721] (ANIC).
Eye apilose; proboscis equal to head height; body metallic green-blue; antennae yellow-brown; parafacial without marginal pile; postpronotal lobe concolourous with rest of thorax; legs black.
Body length: 8.0–10.0 mm (male), 14.5 mm (female). Head with eye apilose; ocellar tubercle relatively flat, medial ocellus present; occiput metallic green-blue, occipital pile dense, white; postocular ridge and gena overlain with grey pubescence; clypeus length equal to oral cavity, brown-black; palpus white or black; margin of oral cavity (parafacial) glabrous; proboscis equal or slightly longer than head height; flagellum dark yellow-orange, suffused with brown, apex in male tapered, narrow apically; scape and pedicel brown or dark yellow. Thorax postpronotal lobe green; scutum metallic green or metallic blue, scutal vestiture dense white pile; scutellum metallic blue-green; pleuron metallic green or metallic blue; coxae black with metallic blue iridescence; femora black; tibiae black or brown; tarsi black; lower calypter white, with brown margin; wing hyaline (male) or slightly infuscate (female), venation dark; vein R4 with spur vein. Abdomen shape rounded globose, much larger than thorax (female) or rounded to conical, not larger than thorax (male), colour metallic green or metallic blue violet, vestiture as minute setae, dense white-silver elongate setae along anterior margin of tergites 2–5.
Panops austrae Neboiss, male, lateral view (partially denuded) [700499]. Body length = 8.0 mm.
Panops austrae Neboiss, male, dorsal view [700502]. Body length = 8.0 mm.
Panops austrae Neboiss, male, anterior view [700498]. Body length = 8.0 mm.
Panops austrae Neboiss, female, dorsal view [700508]. Body length = 14.5 mm.
Panops austrae is a large, metallic coloured species similar to Panops jade sp. n. and Panops schlingeri sp. n. It is easily distinguished from these species by the longer proboscis and dense white thoracic pile. This species is known from remote, arid regions of the Northern Territory and Western Australia.
http://species-id.net/wiki/Panops_baudini
Figs 1, 17, 25–30 Panops baudini Lamarck. Neotype female, AUSTRALIA: New South Wales: Asquith (nr, Sydney), 10.x.1962, A.L. Dyce (ANIC) (designated by
Mesophysa marginata Macquart. Type female, [no label data] (MHN). See discussion by
Epicerina nigricornis Macquart. Type male, AUSTRALIA: “2/47 Tasmanie J. Verreaux 1847” (MNHN). See discussion by
Panops lamarckianus Westwood. Type male, AUSTRALIA: Queensland: Moreton Bay, 1859 (OUMNH).
Mesophysa australasiae Thomson. Type male, AUSTRALIA: New South Wales: Sydney, Kinb. (NHRS). See discussion by
AUSTRALIA: Queensland: male, female, Isla Gorge National Park, [-25.183, 149.966] 12.ix.1992, 320m, G. Daniels (GDCB); male, Isla Gorge National Park, [-25.183, 149.966] 11.ix.1992, 320m, R. Eastwood (GDCB); 32 km S Theodore, [-25.166, 150.000], 13.ix.1992, 300m, G. Daniels (GDCB); 2 males, female, 43 km WSW Millmerran, [-27.983, 150.933], 21.ix.1986, G. & A. Daniels (GDCB); 2 females, Lake Broadwater, nr. Dalby, [-27.361, 151.102], site 8, 27.ix.1986, G. & A. Daniels (GDCB); male, Gayndah, Masters (NMV). New South Wales: female, Sydney swamps (NMV); male, Sydney, 17.x.1932, G.M. Goldfinch (ANIC); female, Ku-ring-gai Chase National Park [-33.651, 151.201], 2.x.1972, A. & G. Daniels (GDCB); 2 males, Goondera Ridge, Royal National Park [-34.122, 151.063], 24.x.1976, G. & A. Daniels (GDCB). Victoria: female, Mitta Mitta River, 8km NW of Dartmouth Dam [-36.566, 147.55], 30.x.1976, A. A. Calder (NMV). Western Australia: 3 males, W of Norseman, Eucalyptus woodland, dry gully to salt lake, Malaise trap, C. Lambkin et al., ANIC bulk sample 2184, 1-17.xi.2003 271m [-32.186, 121.721] (ANIC); male, Wongan Hills area [-30.871, 116.771], Greg Guérin, on flowers of Microcorys (CAS); female, East Yuna Nature Reserve, 34 km WNW Mullewa [-28.42, 115.42], 23–24.ix.1983, C. & T. Houston, 559-17, on flowers of ?Helipterum (WAM); female Australia, Boorabbin Rock National Park [-31.23, 120.16], W Coolgardie, 26.ix.2005, L. Packer (CNC) [not examined but identity confirmed by B. Sinclair].
Diagnosis. Eye minutely pilose; proboscis longer than head height; body black (with faint blue iridescence in western population); antennae red-brown to black; parafacial with marginal pile; postpronotal lobe concolourous with rest of thorax; femora black with pale apices, rest of leg dark yellow to white with black on tibiae; abdomen red or yellow laterally; distiphallus broad apically.
Body length: 9.5–12.5 mm (male), 11.0–14.0 mm (female). Head with eye sparsely pilose with minute setae (appears apilose); ocellar tubercle raised laterally or relatively flat; medial ocellus reduced; occiput brown-black, occipital pile white, sparse; postocular ridge and gena overlain with grey pubescence; clypeus length equal to oral cavity, brown-black; palpus white or yellow; margin of oral cavity (parafacial) pilose; proboscis longer than head height; flagellum red-brown to black; scape and pedicel brown. Thorax with postpronotal lobe brown-black; scutum black, scutal vestiture dense white pile; scutellum black; pleuron black (thorax with slight bluish iridescence in western populations); coxae black; femora black or brown-black, apices dark yellow; tibiae predominantly black with dark yellow to white (apically); tarsi dark yellow to white; lower calypter white, with yellow margin; wing hyaline (male) or slightly infuscate (female); venation dark; vein R4 with spur vein, rarely without. Abdomen shape rounded globose, much larger than thorax, colour highly variable, orange-red to yellow, dark markings anteriorly and medially, or dark yellow, brown anteriorly on tergites 2–6, vestiture as extensive short white-silver pile, longer laterally.
Panops baudini Lamarck (western form), male, lateral view [700505]. Body length = 9.5 mm.
Panops baudini Lamarck (western form), male, oblique view [700509]. Body length = 9.5 mm.
Panops baudini Lamarck (western form), male, anterior view [700510]. Body length = 9.5 mm.
Panops baudini Lamarck (eastern form), female, lateral view [700512]. Body length = 12.0 mm.
Panops baudini Lamarck (eastern form), female, oblique view [700513]. Body length = 12.0 mm.
Panops baudini Lamarck (eastern form), female, anterior view [700514]. Body length = 12.0 mm.
The type for the genus, Panops baudini is the most commonly represented species in collections. This species is distributed in Queensland, New South Wales, Victoria and Western Australia. The apex of the aedeagus is broad and quadrangular in this species (Fig. 17) while in all other species it is much narrower. The record from Tasmania is apparently erroneous (
http://species-id.net/wiki/Panops_boharti
Figs 31–33Holotype male, INDONESIA: Papua: Cyclops Mountains, Sabron, 930 ft. [-2.509, 140.523], iv.1936, L. E. Cheesman, B. M. 1936-271 (BMNH).
Eye pilose; eye extends posteriorly beyond maximum head width; proboscis very short, not extending beyond oral cavity; body brown and yellow; antennae yellow; parafacial without marginal pile; postpronotal lobe cream with brown spot; legs yellow, femora brown with yellow apices; lower calypter cream with brown margin.
Body length: 9.0 mm (male). Head with eye sparsely pilose, slightly denser and elongate laterally; eye extends posteriorly beyond maximum head width; ocellar tubercle relatively flat; medial ocellus present; occiput cream, brown suffusion laterally; occipital pile white, sparse; flagellum yellow, apex uniform width, truncated apically; scape and pedicel dark yellow; clypeus minute, yellow-brown; palpus yellow; margin of oral cavity (parafacial) glabrous; proboscis not extending beyond oral cavity. Thorax with postpronotal lobe cream, brown suffusion dorsally; scutum brown, cream posterolaterally; scutal vestiture dense brown and white, matching respective scutal markings; scutellum brown with bluish iridescence, cream laterally; pleuron cream with brown markings; coxae cream with brown markings; femora cream with brown on middle half; tibiae dark yellow; tarsi dark yellow; lower calypter white, brown marginally on membrane; wing hyaline, venation brownish, pale yellow distally along costa and radial veins; vein R4 with spur vein. Abdomen rounded globose, slightly larger than thorax, colour dark yellow, brown on tergites 3–6, vestiture minute setae, dense white-silver elongate setae along anterior margin of tergites 2–5.
Panops boharti (Schlinger) comb. n., male, lateral view [700515]. Body length = 9.0 mm.
Panops boharti (Schlinger) comb. n., male, dorsal view [700517]. Body length = 9.0 mm.
Panops boharti (Schlinger) comb. n., male, anterior view [700522]. Body length = 9.0 mm.
Panops boharti comb. n.was described by
http://species-id.net/wiki/Panops_conspicuus
Figs 34–38Holotype female, AUSTRALIA: Western Australia: Kalamunda [-31.974, 116.058], 14.iii–14.iv.1914, R.E. Turner, 1914-349 (BMNH).
AUSTRALIA: Victoria: male, female, Kiata [-36.366, 141.791], R. Oldfield, X 4172, captured as copulating pair (NMV). Western Australia: female, Boulder Rock [-32.133, 116.166], 15.iii.1981, M.J. Smart, Jarrah Forest, 300m, hovering 2–3 m above ground, taken at rest on leaf (WAM); 4.5 km E Lake Monger on Wanarra Road [-29.544, 116.775], 7.v.2008, T.F. Houston and E. G. Cunningham, 1266-1 (WAM).
Eye apilose; proboscis longer than head height; body colour and shape sexually dimorphic: male black with slender body, female yellow and brown with globose abdomen; antennae yellow-brown to red-brown with black suffusion; parafacial without marginal pile; postpronotal lobe yellow; legs yellow with brown medially on femora and tibiae.
Body length: 11.0 mm (male), 12.0–13.0 mm (female). Head with eye apilose; ocellar tubercle raised laterally; medial ocellus present; occiput colour brown-black (male) or brown with dark yellow spot laterally (female); occipital pile yellow; postocular ridge and gena glabrous; clypeus shorter than oral cavity; yellow-brown; palpus yellow; margin of oral cavity (parafacial) glabrous; proboscis longer than head height; flagellum dark yellow, suffused with brown (female) or red with black suffusion (male), apex in male tapered, narrow apically; scape and pedicel brown. Thorax with postpronotal lobe yellow; scutum black (male) or yellow and brown (markings variable) (female); scutal vestiture dense white pile or dense yellow-gold pile; scutellum black or brown; pleuron brown; coxae brown; femora brown-black, apices dark yellow; tibiae dark yellow or dark yellow, suffused with brown; tarsi dark yellow; lower calypter white, with dark yellow margin; wing hyaline (male) or slightly infuscate (female), venation dark; vein R4 with spur vein. Abdomen shape rounded globose, much larger than thorax (female) or cylindrical along length (male), colour orange-yellow or brown-black, vestiture elongate yellow pile (whitish in male).
Panops conspicuus (Brunetti), male, lateral view [700525]. Body length = 11.0 mm.
Panops conspicuus (Brunetti), male, oblique view [700527]. Body length = 11.0 mm.
Panops conspicuus (Brunetti), male, anterior view [700528]. Body length = 11.0 mm.
Panops conspicuus (Brunetti), female, lateral view [700529]. Body length = 13.0 mm.
Panops conspicuus (Brunetti), female, oblique view [700530]. Body length = 13.0 mm.
Panops conspicuus is recorded from arid regions of southwest Western Australia and Western Victoria. There is dramatic sexual dimorphism in both body colouration and shape in this species, with males very similar to species of Mesophysa. Panops conspicuus can be differentiated from other Panops species by the bright yellow postpronotal lobes, elongate mouthparts, yellow and brown colouration (female), and apilose eyes. Females of this species are similarly coloured to females of Panops grossi comb. n., a species which also displays dramatic sexual dimorphism.
urn:lsid:zoobank.org:act:3FAB3406-C6A4-42CC-9ABC-B82BCB22FDE8
http://species-id.net/wiki/Panops_danielsi
Figs 39–43Holotype male, AUSTRALIA: Queensland: 3km SW Fox Ck. x-ing [crossing], ‘Wolverton’ [-13.104, 142.970], 13.iv.1989, G. and A. Daniels (AMS).
AUSTRALIA: Queensland: female, male, same data as holotype (GDCB) (CAS); female, 7 km NNW Coen, [-13.844, 143.163], 17.iv.1989, G. and A. Daniels (GDCB); female, 26 km W ‘Fairview’, [-15.535, 144.154], 20.iv.1989, G. and A. Daniels (GDCB).
Eye uniformly sparse pilose; proboscis longer than head height; body dark yellow and brown, with metallic green-blue iridescence; antennae red-brown or black; parafacial with marginal pile; postpronotal lobe dark yellow; legs dark yellow and brown.
Body length: 11.0 mm (male), 10.5–12.0 mm (female). Head with eye sparsely pilose, uniformly distributed, setae minute; ocellar tubercle raised laterally; medial ocellus absent; occiput metallic green-blue; occipital pile yellow; postocular ridge and gena overlain with grey pubescence; flagellum apex in male uniform width, truncated apically, narrower in female, red-brown (male) or black (female); scape and pedicel dark yellow; clypeus length equal to oral cavity, brown-black; palpus yellow; margin of oral cavity (parafacial) pilose; proboscis longer than head height. Thorax with postpronotal lobe yellow; scutum glossy black (with metallic iridescence), dark yellow marginally; scutal vestiture dense yellow-gold pile; scutellum brown, dark yellow medially; pleuron brown with metallic iridescence; coxae black or brown; femora brown-black, apices dark yellow; tibiae dark yellow, suffused with brown; tarsi dark yellow; lower calypter white, with yellow margin; wing hyaline, venation dark; vein R4 with spur vein. Abdomen shape rounded globose, much larger than thorax (female) or rounded to conical, not larger than thorax (male), colour black with metallic green iridescence (female) or dark yellow, brown anteriorly on tergites 2–6 (male), vestiture extensive white-silver elongate setae, brown posteromedially on tergites 3–5 (female) or erect dark pile (male).
Panops danielsi sp. n., male, lateral view [700531]. Body length = 11.0 mm.
Panops danielsi sp. n., male, anterior view [700532]. Body length = 11.0 mm.
Panops danielsi sp. n., female, lateral view [700533]. Body length = 12.0 mm.
Panops danielsi sp. n., female, oblique view [700534]. Body length = 12.0 mm.
Panops danielsi sp. n., female, anterior view [700535]. Body length = 12.0 mm.
This species is named in honour of the collector of this species, Greg Daniels.
Panops danielsi sp.n. is known only from Far Northern Queensland. This species is closely related to Panops baudini as both species have similar shaped mouthparts and pilose eyes. Panops danielsi sp. n. can be distinguished by the more evident eye pilosity, yellow postpronotal lobes and body colouration.
http://species-id.net/wiki/Panops_grossi
Figs 44–47Holotype female, AUSTRALIA: Northern Territory: Koolpinyah, 21.iv.1916 [-12.331, 131.148] G. F. Hill, (in copula) (SAM).
‘Allotype’. AUSTRALIA: Northern Territory: same data as holotype (SAM).
Eye pilose dorsally only, relatively dense and elongate; proboscis shorter than head height; body colour and shape sexually dimorphic: male metallic olive green, female yellow and brown, globose; antennae yellow; parafacial without marginal pile; postpronotal lobe and legs concolourous with rest of body.
Body length: 9.0 mm (male), 12.0 mm (female). Head eye pilose dorsally only, dense and relatively elongate; occiput olive green, occipital pile dense white (male) or yellow (female); postocular ridge and gena overlain with grey pubescence; ocellar tubercle raised laterally or relatively flat; medial ocellus absent; clypeus shorter than oral cavity, yellow-brown; palpus black; margin of oral cavity (parafacial) glabrous; proboscis not extending beyond oral cavity; flagellum yellow, apex in male uniform width, truncated apically; scape and pedicel brown. Thorax with postpronotal lobe yellow (female) or green (male); scutum metallic olive green or yellow-orange; scutal vestiture dense white or yellow-gold pile; scutellum metallic olive green or orange-yellow with brown suffusion; pleuron orange or metallic olive green; coxae brown; femora brown-black, apices dark yellow; tibiae brown; tarsi brown; lower calypter white, brown marginally on membrane or white, with dark yellow margin; wing hyaline or slightly infuscate, venation dark; vein R4 without spur vein. Abdomen shape with male rounded, not larger than thorax, metallic olive green, vestiture dense short pile, longer laterally; female rounded globose, much larger than thorax (female), orange-yellow (female), vestiture elongate yellow pile.
Panops grossi (Neboiss) comb. n., male, lateral view [700536]. Body length = 9.0 mm.
Panops grossi (Neboiss) comb. n., male, dorsal view [700537]. Body length = 9.0 mm.
Panops grossi (Neboiss) comb. n., male, anterior view [700538]. Body length = 9.0 mm.
Panops grossi (Neboiss) comb. n., female, oblique view [700539]. Body length = 12.0 mm.
Panops grossi comb. n.was described by
urn:lsid:zoobank.org:act:96D0BD2A-0C81-4BCE-BB32-671D1C2D901C
http://species-id.net/wiki/Panops_jade
Figs 2D, 48–52Holotype male, AUSTRALIA: Queensland: Isla Gorge National Park [-25.183, 149.966], 3.x.1991, 320 m, G. Daniels (AMS).
AUSTRALIA: Queensland: female, Isla Gorge National Park [-25.183, 149.966], 3.x.1991, 320 m, G. Daniels (CAS); female, Isla Gorge National Park [-25.183, 149.966], 14.ix.1992, 320 m, G. Daniels (AMS).
Eye apilose; proboscis shorter than head height; body metallic green-blue to violet iridescence; antennae red-brown; parafacial with marginal pile; postpronotal lobe concolourous with rest of thorax; legs black with metallic blue-violet iridescence.
Body length: 11.5 mm (male), 11.5–12.0 mm (female). Head with eye apilose; ocellar tubercle relatively flat; medial ocellus present; occiput metallic green-blue, occipital pile white, sparse; postocular ridge and gena overlain with grey pubescence; clypeus length equal to oral cavity, black with blue-green suffusion; palpus black; margin of oral cavity (parafacial) pilose; proboscis extending beyond oral cavity, but shorter than head height; flagellum apex in male tapered, slightly rounded apically, red-brown; scape and pedicel red-brown. Thorax with postpronotal lobe blue-violet; scutum metallic blue-violet, green posteromedially; scutellum metallic blue-violet; coxae and femora with metallic blue-violet iridescence; tibiae black; tarsi black; lower calypter white with brown margin; wing hyaline, venation dark; vein R4 with spur vein. Abdomen shape rounded globose, much larger than thorax, colour metallic green or blue-violet iridescent, vestiture extensive white-silver short pile, longer laterally.
Panops jade sp. n., male, lateral view [700540]. Body length = 11.5 mm.
Panops jade sp. n., male, dorsal view [700541]. Body length = 11.5 mm.
Panops jade sp. n., female, lateral view [700542]. Body length = 12.0 mm.
Panops jade sp. n., female, dorsal view [700543]. Body length = 12.0 mm.
Panops jade sp. n., female, anterior view [700545]. Body length = 12.0 mm.
This beautifully coloured species is named after my daughter, Jade Tanya Winterton, whose name also describes the deep green colouration found in this species.
Panops jade sp. n. is a distinctive species with extensive green to blue-violet iridescence, particularly in the female. It is similar to the western Australian species, Panops austrae, but is distinguished by the length of the mouthparts, leg colour and different vestiture pattern on the abdomen. Panops jade sp. n. is known only from Isla Gorge National Park in southern Queensland. Both males and females are recorded from Spinifex grass (Triodia sp.), presumably at rest.
urn:lsid:zoobank.org:act:03D163A1-D1DA-4810-8D88-77F76D5CC490
http://species-id.net/wiki/Panops_schlingeri
Figs 53–55Holotype female, AUSTRALIA: Northern Territory: 9 km NE of Mudginbarry H.S. (on scarp), 10.vi.1973, D. H. Colless [-12.310, 132.579] (ANIC).
AUSTRALIA: Northern Territory: female, 8 km SSW of Oenpelli Mission 7.vi.1973, J. Cardale [-12.381, 133.024] (ANIC).
Eye apilose; proboscis shorter than head height; body metallic green-blue iridescence; antennae orange; parafacial without marginal pile; postpronotal lobe dark yellow; legs dark yellow, femora brown-black with yellow apices.
Body length: 9.5–11.0 mm (female only). Head with eye apilose; ocellar tubercle relatively flat; medial ocellus present; occiput metallic green-blue, occipital pile white, dense; postocular ridge and gena overlain with grey pubescence; clypeus shorter than oral cavity, brown-black; palpus black; margin of oral cavity (parafacial) glabrous; proboscis not extending beyond oral cavity; flagellum orange; scape and pedicel dark red-yellow. Thorax with postpronotal lobe yellow; scutum metallic green to blue iridescent; scutal vestiture dense white pile; scutellum metallic blue-green; pleuron metallic green to blue iridescent; coxae brown-black with metallic blue iridescence; femora brown-black, apices dark yellow; tibiae dark yellow; tarsi dark yellow; lower calypter white, with dark yellow margin; wing hyaline, venation dark; vein R4 without spur vein. Abdomen shape rounded globose, much larger than thorax, dark with metallic green to blue iridescence, vestiture as dense short pile, longer laterally.
Panops schlingeri sp. n., female, lateral view [700546]. Body length = 11.0 mm.
Panops schlingeri sp. n., female, oblique view [700547]. Body length = 11.0 mm.
Panops schlingeri sp. n., female, anterior view [700548]. Body length = 11.0 mm.
I am honoured to name this species after the world-renowned Acroceridae taxonomist Dr. Evert Irving Schlinger.
Panops schlingeri sp. n. is known only from two female specimens collected in the Northern Territory. This species is differentiated easily by the green-blue iridescence on the body and dark yellow postpronotal lobes.
Philopota Wiedemann. Schlinger, 1971: 186.
Body shape slightly to strongly arched and never densely pilose; small to medium sized; antennal flagellum stylate; postpronotal lobes enlarged and meeting medially to form collar behind head; tibial spines absent; wing costal vein ending at wing apex, never circumambient; wing venation highly variable, ranging from relatively complete with cells cu-p, bm br, d and basal r4+5 present, to highly reduced with only cell br present; cell m3 absent; veins R4 and R5 always present as single vein R4+5; cubital and medial veins not reaching posterior wing margin; larvae exclusively parasitoids of araneomorph spiders.
Helle Osten Sacken, 1896; Schlingeriella Gillung & Winterton, 2011.
http://species-id.net/wiki/Helle
Figs 3A, 56–59Body length: 4.0–6.0 mm [male], 6.0–7.0 mm [female]. Body shape strongly arched; colouration non-metallic (brown or black); head size slightly narrower than thorax width, shape sub-spherical; postocular ridge and occiput rounded; three ocelli, anterior ocellus reduced in size; posterior margin of eye rounded; eye apilose; position of antennae on head near middle of frons; eyes contiguous above antennal base, not contiguous below antennal base; palpus present; proboscis greater than head length; flagellum stylate, apex with terminal seta; postpronotal lobes enlarged, medially contiguous to form collar; subscutellum enlarged; legs not elongated; wing markings absent; costa ending near wing apex, costal margin straight; humeral crossvein absent; radial veins straight or curved towards wing anterior margin; R1 inflated distally at pterostigma; pterostigma and cell r1 membranous, not ribbed; R2+3 present; R4+5 angled anteriorly approximately midway; cell r4+5 bisected by 2r-m, basal cell very narrow elongate, closed; 2r-m joining M1 to R4+5; cell r4+5 present, narrow elongate, closed (open apically when 2r-m rarely absent); crossvein 2r-m present (rarely absent);R4 without spur vein; medial vein compliment with M1, M2 and M3 present (M3 fused with CuA1); discal cell closed completely; medial veins not reaching wing margin; CuA1 joining M3, petiolate to margin; CuA2 fused to A1 before wing margin, petiolate; wing microtrichia absent; anal lobe well developed; alula well developed; abdominal tergites smooth, rounded; abdomen shape elongate, narrow cylindrical or conical (male), or rounded and inflated (female).
Helle longirostris (Hudson), male, lateral view [700556]. Body length = 5.0 mm.
Helle longirostris (Hudson), female, lateral view [700557]. Body length = 5.5 mm.
Helle rufescens Brunetti, male, lateral view [700558]. Body length = 8.5 mm.
Helle rufescens Brunetti, female, lateral view [700559]. Body length = 7.0 mm.
Helle longirostris (Hudson, 1892); Helle rufescens Brunetti, 1926.
Helle is an endemic genus to New Zealand that is closely related to Schlingeriella, the only other philopotine genus in the region (
| 1 | Body colour brown-black, sometimes with metallic iridescence, scutum without dark markings (Figs 56–57) | Helle longirostris (Hudson, 1892) |
| – | Body colour yellowish-orange, scutum with dark longitudinal stripes, narrower anteriorly (Figs 58–59) | Helle rufescens Brunetti, 1926 |
urn:lsid:zoobank.org:act:99EAC1BE-4A6F-43E0-B61A-6460BF68694E
http://species-id.net/wiki/Schlingeriella
Figs 3B, 60–62Body length: 2.4–4.0 mm [male], 4.4–6.0 mm [female]. Body shape arched; body colouration non-metallic dark brown; head width much smaller than thorax (female) or slightly smaller than thorax (male); head spherical; postocular ridge and occiput extended posteriorly into slight ridge; posterior margin of eye rounded; eyes bare; position of antennae on head near middle of frons, slightly nearer to mouthparts; eyes contiguous above antennal base, not contiguous below; palpus present; proboscis longer than head; antennal flagellum stylate, apex with terminal seta; thorax with postpronotal lobes enlarged, medially contiguous to form collar; subscutellum enlarged; legs not greatly elongated; pulvilli present; wing hyaline, markings absent; costa ending in radial field; costal margin straight in both sexes; humeral crossvein absent; radial veins meeting wing margin before wing apex; R1 inflated distally at pterostigma; R2+3 present; R4+5 slightly curved anteriorly midway; veins M1, M2 and M3 present; discal cell absent; medial veins reaching wing margin (or nearly so); crossvein 2r-m absent; Cu reduced, not reaching wing margin; anal lobe not enlarged; alula well developed; abdomen smooth, rounded, cylindrical in shape, similar width to thorax (male) or greatly rounded, inflated (female).
Schlingeriella irwini Gillung & Winterton, male, lateral view [700560, 693079]. Body length = 2.4 mm.
Schlingeriella irwini Gillung & Winterton, female, lateral view [700561, 693080]. Body length = 4.4 mm.
Schlingeriella irwini Gillung & Winterton, female, anterior view [700562]. Body length = 4.4 mm.
Schlingeriella irwini Gillung & Winterton, 2011.
Schlingeriella is differentiated from other Philopotinae by medial veins mostly reaching the wing margin, R1 inflated apically, reduced wing venation (i.e. absence of all wing cells except cell br), elongate mouthparts and apilose eyes. See results of
Acrocera Meigen 1803: 266.
Small to medium sized, densely pilose to apilose, body rarely arched; antennal flagellum stylate; postpronotal lobes widely separated, never medially contiguous; wing venation highly variable, ranging from complete with cells cu-p, bm br, d, m3 and basal r4+5 present, to highly reduced with few closed cells; humeral crossvein rarely well developed; tibial apical spines absent (rarely present); larvae exclusively parasitoids of araneomorph spiders.
Ogcodes Latreille, 1797; Pterodontia Gray, 1832
http://species-id.net/wiki/Ogcodes
Figs 3C, 63–64 Synonymy and usage restricted to Australasian region fauna only; see
Body length: 3.0–5.0 mm [male], 4.0–8.0 mm [female]. Body shape not arched, colouration black, yellow or white, non-metallic; head much smaller than thorax width, shape sub-spherical; postocular ridge and occiput rounded; two or three ocelli, anterior ocellus sometimes absent; posterior margin of eye rounded; eye apilose; position of antennae on head adjacent to mouthparts; eyes contiguous above antennal base, not contiguous below antennal base; palpus absent; proboscis apparently absent; flagellum shape stylate; apex with terminal setae (or single seta); antenotum not collar-like behind head; subscutellum enlarged; tibial spines absent; pulvilli present; wing hyaline, markings absent; costa ending near wing apex, costal margin straight; humeral crossvein absent; radial veins straight; R1 inflated or not inflated distally; pterostigma and cell r1 membranous, not ribbed; only two radial veins present, R2+3 absent, R4+5 not reaching wing margin; medial vein compliment with M1, M2 and M3 present, or two M veins present; discal cell weakly formed or absent; medial veins not reaching wing margin; cell m3 absent; CuA1 absent; CuA2 separate from A1, ending just before wing margin; crossvein 2r-m absent; wing microtrichia absent; anal lobe well developed; alula well developed; abdominal tergites smooth, rounded (rarely with tubercles in fossil species); abdomen shape greatly rounded, inflated.
Ogcodes sp., male, lateral view [700563]. Body length = 9.0 mm.
Ogcodes sp., female, lateral view [700564]. Body length = 5.0 mm.
Ogcodes is a distinctive and cosmopolitan genus and the most species-rich in the family. Thirty-four species in two subgenera (Ogcodes and Protogcodes Schlinger, 1960) are listed by
Ogcodes is in need of revision and no recent keys to species have been published for the region. The most recent revision of the genus was by
http://species-id.net/wiki/Pterodontia
Figs 3D, 65–66Synonymy and usage list restricted to Australasian region fauna only.
Body length: 3.0–7.0 mm [male], 4.0–10.0 mm [female]. Body shape not arched. Body colouration non-metallic; head much narrower than thorax width; shape nearly spherical; postocular ridge and occiput rounded; three ocelli; posterior margin of eye rounded; eye pilose (dense); antennae located adjacent to mouthparts; eyes contiguous above antennal base, not contiguous below antennal base; palpus absent; proboscis greatly reduced; flagellum stylate, apex with terminal setae (multiple); antenotum shape not collar-like behind head; subscutellum not enlarged, barely visible; tibial spines present; pulvilli present; wing markings absent; costa circumambient; wing costal margin straight or with anterior projection (males); humeral crossvein present or reduced; radial veins curved or angled towards wing anterior margin; R1 inflated distally at pterostigma (especially in male); pterostigma and cell r1 membranous, not ribbed; R2+3 present; R4+5 present as single vein; basal cell r4+5 (portion basal to bisecting 2r-m) merged with discal cell to form composite cell comprising d+r4+5; cell m3 absent; medial vein compliment usually a single M vein fused with CuA1, petiolate to margin, sometimes with second medial vein originating from cell d+r4+5; CuA2 fused to A1 before wing margin, petiolate, rarely open to wing margin; wing microtrichia absent; anal lobe well developed; alula present or absent, rarely well developed; abdominal tergites smooth, rounded; abdomen shape greatly rounded, inflated.
Pterodontia davisi Paramonov, male, dorsal view [700565]. Body length = 7.0 mm.
Pterodontia mellii Erichson, female, lateral view [700566]. Body length = 11.0 mm.
Pterodontia davisi Paramonov, 1957; Pterodontia longisquama Sabrosky, 1947; Pterodontia mellii
| 1 | Thorax black, or yellow suffused with dark brown to black ventrally, abdomen yellow to red laterally on segments 2–4; mid and hind femora brown to black; lower calypter hyaline medially, relatively small (< ½ length of wing) (Western Australia, Tasmania, New South Wales, Queensland) (Fig. 66) |
Pterodontia melli |
| – | Thorax and abdomen completely brown to black; all legs uniformly yellow to white; lower calypter relatively large (> ½ length of wing), uniformly brown | 2 |
| 2 | Wing brown infuscate (Papua New Guinea) | Pterodontia longisquama Sabrosky, 1947 |
| – | Wing hyaline (Queensland, New South Wales) (Fig. 65) |
Pterodontia davisi |
Pterodontia is a cosmopolitan genus containing 19 valid species, three of which are recorded from the Australasian region (
Some species of Pterodontia have greatly enlarged and sclerotized lower calypters, appearing somewhat like a second pair of wings (e.g. Pterodontia davisi). Males in this genus typically have sclerotized projections on the costal margin of the wing. Characteristics which diagnose this genus from other acrocerids include head very small relative to thorax width, tibial spines present, cells m3, d and basal r4+5 fused to form a single cell, eyes densely pilose, antennae adjacent to the ocellar tubercle and mouthparts reduced. Contrary to other authors, Pterodontia has been placed previously in Panopinae by
Thank you to Greg Daniels (Brisbane, Australia), Chris Grinter and Norman Penny (CAS), Erica McAllister (BMNH), Terry Houston (WAM), Ken Walker (NMV), Peter Hudson (South Australian Museum) and David Yeates (Australian National Insect Collection) for loan of specimens. Thank you to Evert Schlinger and F. Christian Thompson for access to unpublished bibliographical and nomenclatural information on Acroceridae that were used in part to compile lists of nomenclatural acts and usage. Much of this research was based on years of dedicated work, both published and unpublished, by E.I. Schlinger on Acroceridae of the world; I am grateful for the opportunity for consolidate some of this work and publish some of the results herein. Thank you also to Dan Schoknecht (WAM) and Terry Houston for providing the image of the living Panops baudini and identification of the host plant, respectively. Thank you to Jéssica Gillung, Torsten Dikow and Bradley Sinclair for their comments on the draft manuscript. Thank you also to F. Christian Thompson for providing his expertise on the etiquette of not publishing. This research was supported by the Australian Biological Resource Study (ABRS-209-48). Statements and viewpoints expressed herein do not necessarily reflect the opinion of ABRS.