(C) 2012 Erik J. van Nieukerken. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A grapevine leafminer Antispila oinophylla van Nieukerken & Wagner, sp. n., is described both from eastern North America (type locality: Georgia) and as a new important invader in North Italian vineyards (Trentino and Veneto Region) since 2006. The species is closely related to, and previously confused with Antispila ampelopsifoliella Chambers, 1874, a species feeding on Virginia creeper Parthenocissus quinquefolia (L.) Planchon., and both are placed in an informal Antispila ampelopsifoliella group. Wing pattern, genitalia, and DNA barcode data all confirm the conspecificity of native North American populations and Italian populations. COI barcodes differ by only 0–1.23%, indicating that the Italian populations are recently established from eastern North America. The new species feeds on various wild Vitis species in North America, on cultivated Vitis vinifera L. in Italy, and also on Parthenocissus quinquefolia in Italy. North American Antispila feeding on Parthenocissus include at least two other species, one of which is Antispila ampelopsifoliella. Morphology and biology of the new species are contrasted with those of North American Antispila Hübner, 1825 species and European Holocacista rivillei (Stainton, 1855). The source population of the introduction is unknown, but cases with larvae or pupae, attached to imported plants, are a likely possibility. DNA barcodes of the three European grapevine leafminers and those of all examined Heliozelidae are highly diagnostic. North American Vitaceae-feeding Antispila form two species complexes and include several as yet unnamed taxa. The identity of three out of the four previously described North American Vitaceae-feeding species cannot be unequivocally determined without further revision, but these are held to be different from Antispila oinophylla. In Italy the biology of Antispila oinophylla was studied in a vineyard in the Trento Province (Trentino-Alto Adige Region) in 2008 and 2009. Mature larvae overwinter inside their cases, fixed to vine trunks or training stakes. The first generation flies in June. An additional generation occurs from mid-August onwards. The impact of the pest in this vineyard was significant with more than 90% of leaves infested in mid-summer. Since the initial discovery in 2006, the pest spread to several additional Italian provinces, in 2010 the incidence of infestation was locally high in commercial vineyards. Preliminary phylogenetic analyses suggest that Antispila is paraphyletic, and that the Antispila ampelopsifoliella group is related to Coptodisca Walsingham, 1895, Holocacista Walsingham & Durrant, 1909 and Antispilina Hering, 1941, all of which possess reduced wing venation. Vitaceae may be the ancestral hostplant family for modern Heliozelidae.
Invasive species, new species, Vitaceae, viticulture, COI, leafmines, venation, genitalia, Holocacista rivillei, Coptodisca, Antispilina, Phyllocnistis vitegenella, phylogeny
There are several cases known of leafmining Lepidoptera developing into important agricultural pests, such as Phyllocnistis citrella Stainton, 1856 (Gracillariidae) on citrus, now a worldwide problem (
Lepidopteran leafminers of grapevine (Vitis vinifera L.) have not yet developed into serious pests in Europe, although one North American species did recently invade European vineyards; Phyllocnistis vitegenella Clemens, 1859 (Lepidoptera: Gracillariidae) became established in Italy and elsewhere in Europe around 1995 (
In the summer of 2007, leafmines similar to those caused by Holocacista rivillei were observed in a vineyard in northeastern Italy (Borgo Valsugana, Trento province). However, the initial gallery mine was immediately enlarged into a larger blotch, indicating a different species. Adults reared from these mines differed from Holocacista rivillei in size and wing pattern. On their external characters they were identified as belonging to the genus Antispila Hübner, [1825], but not to one of the two species currently known in Europe, i.e., Antispila treitschkiella (Fischer von Röslerstamm, 1843) and Antispila metallella ([Denis & Schiffermüller], 1775) (
The family Heliozelidae (superfamily Adeloidea) comprises 123 described species in 12 genera (
The grapevine family Vitaceae comprises an important group of hosts for the genus Antispila worldwide; out of 32 Antispila species for which host plants are known, 13 feed on Vitaceae, of which at least ten are associated with the genus Vitis (Table 1). Several more unnamed species are also associated with Vitaceae. In North America, Heliozela aesella Chambers, 1877 makes galls in leaves and shoots on Vitis (
Heliozelidae species associated with Vitaceae, type country and hostplant species. For the American species where the identity is not fully established we added [cf] between genus and species name.
| Species | Type country | Hostplants | source |
|---|---|---|---|
| Antispila oinophylla | USA | Vitis aestivalis, Vitis labrusca, Vitis riparia, Vitis vinifera, Vitis vulpina, [Parthenocissus] | this paper |
| Antispila ampelopsifoliella Chambers, 1874 | USA | Parthenocissus quinquefolia |
|
| Antispila sp. “vitis1” | (USA) | Vitis aestivalis | this paper |
| Antispila [cf] isabella Clemens, 1860 | USA | Vitis aestivalis, Vitis labrusca, Vitis riparia |
|
| Antispila sp. “vitis2” | (USA) | Vitis aestivalis, Vitis riparia | this paper |
| Antispila viticordifoliella Clemens, 1860 | USA | Vitis vulpina |
|
| Antispila cf viticordifoliella Clemens, 1860 | (USA) | Parthenocissus quinquefolia | this paper |
| Antispila voraginella Braun, 1927 | USA | Vitis arizonica |
|
| Antispila ampelopsia Kuroko, 1961 | Japan | Ampelopsis brevipedunculata, Vitis flexuosa |
|
| Antispila inouei Kuroko, 1987 | Japan | Vitis coignetiae, Vitis labruscana |
|
| Antispila iviella Kuroko, 1961 | Japan | Parthenocissus tricuspidata |
|
| Antispila orbiculella Kuroko, 1961 | Japan | Ampelopsis brevipedunculata |
|
| Antispila tateshinensis Kuroko, 1987 | Japan | Vitis coignetiae |
|
| Antispila uenoi Kuroko, 1987 | Japan | Vitis coignetiae, Vitis labruscana |
|
| Antispila argostoma Meyrick, 1916 | India | Cayratia trifolia |
|
| Antispila aristarcha Meyrick, 1916 | India | Vitis sp. |
|
| Antispila isorrhythma Meyrick, 1926 | India | Vitis sp. |
|
| Antispila species | Indonesia, Borneo | Leea indica | EJvN |
| Antispila species | Australia | Cissus antarctica |
|
| Holocacista rivillei Stainton, 1855 | Malta | Vitis vinifera | see text |
| Heliozela aesella Chambers, 1877 | USA | Parthenocissus quinquefolia, Vitis vulpina, Vitis sp. |
|
Antispila oinophylla adults were collected from Borgo Valsugana for sequencing and larvae were collected and reared for morphological studies. Holocacista rivillei and Phyllocnistis vitegenella adults were also collected from northeastern Italy (Appendix B). To obtain material of the new speciesand related species from North America for comparison, various Antispila mines and larvae were collected by EJvN and CDo during a field trip September-October 2010 in the states of Georgia and Tennessee and by EJvN in September 2011 (partly with DLW) in Connecticut, Massachusetts, Vermont and New York state. Other material included in the taxonomic and DNA analyses was collected by DLW, who has been collecting and rearing Antispila and other leafminers from across North America for three decades (Appendix B). Further material was studied or borrowed from the following collections.
Abbreviations for depositories:
ANSP Academy of Natural Sciences in Philadelphia, Pennsylvania, USA
CNC Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada
DLW Research collection of David L. Wagner, Storrs, Connecticut, USA
MCZ Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA
RMNH Netherlands Centre for Biodiversity Naturalis, former Leiden Zoology collections, Leiden, Netherlands.
UMDC University of Maryland, College park, USA
UPI University of Padova, Department of Environmental Agronomy and Crop Science, Italy
ZMUO Zoological Museum University of Oulu, Finland.
RearingCollected leaves were kept in polystyrene jars or bags, with some moss and or tissue added, until the larvae had prepared the shields. It was often necessary to remove the cut/out shields from the leaves, which were then removed from the breeding jars and dried as vouchers. Breeding jars were kept during winter in an outbuilding, and brought indoors in March, where they were kept until emergence of adults. Specimens collected during fall 2011 were still in hibernation diapause when this manuscript was accepted.
MorphologyMethods for preparation of the genitalia follow
The Distribution Map for North America was prepared with DMap 7.0 (
DNA was extracted destructively from larvae or adult specimens preserved in 96% or 100% ethanol or extracted in a non-destructive fashion from the abdomen of voucher specimens, which were then used to prepare genitalic dissections (protocol in
A 665 bp or a 658 bp fragment of the mitochondrial COI gene was amplified using the following primers: in Padua LCO1490 and HCO2198 (
In Padova, amplification was carried out in 20ml volumes containing 2ml from the nucleic acid extract, 200mM dNTPs, 0.5mM of each primer, 4mM 10x PCR buffer, 2.5 mM MgCl2 and one unit of Taq polymerase (Promega). The reaction was performed in an INC PTC-100 thermal controller (MJ Research Inc.). Amplification conditions were as follows: the first period of denaturation was 94°C for 5 min, followed by 38 cycles of denaturation at 94°C for 1 min, annealing at 48°C for 1 min, and extension at 72°C for 1 min; the final extension cycle had a step at 72°C for 5 min. A negative control with no template was included for each series of amplifications, to detect instances of contamination. The amplified products were separated on a 1% agarose gel and visualized under UV following staining with Sybr Safe (Invitrogen). PCR products were purified with the ExoSAP-IT kit (Amersham Biosciences).
In Leiden, amplification was performed in volumes of 25 µl. The PCR cycle consisted of 3 min initial denaturation at 94°C, 15 sec cycle denaturation at 94°C, 30 sec cycle at 50°C, 40 sec cycle extension at 72°C for 40 cycles. After all cycles had finished, a final extension was performed at 72°C for 5 min. The amplified products were separated on a 1% agarose gel and visualized under UV following staining with ethidium bromide.
The sequencing at Padova was performed at the BMR Genomics Service (Padova, Italy) in an ABI PRISM automatic DNA sequencer (Applied Biosystems), in both forward and reverse direction, but for some samples only in forward direction. In Leiden PCR clean-up and sequencing was outsourced to MACROGEN on an ABI 3730XL, all samples were sequenced in both forward and reverse direction. The chromatograms were checked with Sequencher (Gene Codes Corporation) and the resulting sequences were aligned by eye in BIOEDIT 7.0.9.0 (
Neighbor-joining (NJ) trees based on DNA barcode sequences of all available specimens were reconstructed with Paup* 4.0b10 (
Phylogenetic trees based on maximum parsimony were generated with PAUP using a heuristic search, 1, 000 replicates, with tree-bisection-reconnection (TBR) as the branch-swapping algorithm. A bootstrap analysis was run with TNT (
A Bayesian Analysis was carried out with the same dataset. Model selection was performed using jModeltest 0.1.1 (
The sequence data generated and used in this study have been deposited in the public BOLD database (project “Antispila Vine introduction” [ANTVI] and GenBank (Appendix B).
Field observationsSurveys were carried out from 2007 to 2011 to investigate the Antispila oinophylla distribution in northeastern Italy. We sampled commercial vineyards but also isolated vine rows and plants of Virginia creeper, Parthenocissus quinquefolia.
Observations on Antispila oinophyllaphenology and behaviour were carried out in 2008 and 2009 in Borgo Valsugana (Trentino Regione). The vineyard was planted with a Chardonnay cultivar and was trained with the local “pergola” system. The vineyard received a number of fungicide treatments but insecticides were not applied. In 2008, a total of 180 leaves (30 plants, six leaves per vine) were sampled six times during the season, from May to September. In 2009, a total of 100 leaves (five replicates of ten plants, two leaves per plant) taken from the mid part of the shoots were sampled across ten dates, from May to September. In both years the number of mines produced by Antispila oinophyllalarvae was assessed on each leaf. In 2009, active mines containing living larvae were distinguished from those vacated by the larvae (mines with larval cut-outs).
Results IdentificationTo identify the new Italian Antispila, we checked all descriptions of the Vitaceae miners, as well as all other known Antispila species. Unfortunately, outside Europe, genitalia have been illustrated and described only for Japanese species of Antispila, including all five Vitaceae miners (
Antispila Hübner, [1825]: 419. Type species Antispila stadtmuellerella Hübner, [1825]: 419 (a junior synonym of Antispila metalella ([Denis & Schiffermüller], 1775), subsequent designation by ICZN
urn:lsid:zoobank.org:act:F58A029E-A856-4414-B4EA-D7CAA6151948
http://species-id.net/wiki/Antispila_oinophylla
Figs 1–6, 9–29, 62, 63Holotype ♂, USA: Georgia, Murray Co., Chattahoochee Nat. Forest, E of Chatsworth, GA rd 52, 523 m, 34.74066N, 84.71852W, hardwood forest along highway, leafmines on Vitis aestivalis var. aestivalis, 14.x.2010, EvN2010266, emerged 14.iv–4.v.2011, E.J. van Nieukerken & C. Doorenweerd, Genitalia slide EJvN 4204, RMNH.INS.24204 (RMNH).
32♂, 31♀. Italy: 1♂, 3♀ (all dissected), Trento, Borgo Valsusana, leafmines 2007, on Vitis vinifera, emerged 1.iii–26.iv.2008, M. Baldessari; 3♀, same locality, 13.viii.2008; 10♂, 1♀ (1♂ RMNH.INS.23920 dissected & DNA barcode), same locality, 18.viii.2008; 17♂, 18♀ (1♂ RMNH.INS.24038, 1♀ RMNH.INS.24039 dissected & DNA barcode), same locality, 29.vi.2009, leafmines on Vitis vinifera, EvN no 2009903, emerged in Leiden, 14.vii–6.viii.2009, M. Baldessari (all RMNH). Canada: 1♂, Ontario, Ottawa, mines on Vitis, rearing 57–112, emerged 31.iii.1958, Freeman & Lewis (CNC); 1♀, Quebec, Hull, mines on Vitis, rearing 55–228, emerged 26.vi.1956, T.N. Freeman (CNC). USA: 1♂, Connecticut, Tolland Co., Mansfield, 22.viii.1989, leafmines on Vitis, DLW89H37 breeding, emerged 4.v.1990, D.L. Wagner (DLW); 1♀ (dissected), Connecticut, Windham Co., Hampton, 916 Pudding Hill Rd., leafmines on Vitis 1–5.ix.1988, DLW 88J7, emerged 20.vii.1989, D.L. Wagner (DLW); 1♂ 1 ♀, Georgia, same data as holotype; 1♀, Georgia, Murray Co., Chattahoochee Nat. Forest, Cohutta Overlook, 730 m, 34.785356N, 84.627323W, shrub in forest clearing, leafmines on Vitis aestivalis var. bicolor, 14.x.2010, EvN2010270, emerged 19.iv.2011, E.J. van Nieukerken & C. Doorenweerd (RMNH); 1♀ (dissected, EvN 4211), Kentucky, [Covington], bred, [19th century], Chambers, “pseudotype, ” MCZ Type 1367 (MCZ); 1♂, 1♀ (♂ dissected), Vermont, Chittenden Co., South Burlington, leafmines on Vitis 11.viii.1988, DLW 88H23, emerged 30.iii–15.v.1989, D.L. Wagner (DLW).
(all in RMNH). Italy: leafmines & larvae, Borgo Valsusana, 29.vi.2009, on Vitis vinifera, EvN no 2009903, M. Baldessari. USA: 1 larva, Connecticut, Tolland Co., Storrs campus, on Vitis labrusca, 185 m, 8.ix.2011, EvN2011168, B. Gagliardi; leafmines and larvae (being reared), Connecticut, New London Co., Connecticut College Arboretum, 34 m, 41.37929N, 72.11121W, on Vitis labrusca, 10.ix.2011, EvN2011193, E.J. van Nieukerken; leafmines and larvae (being reared), Connecticut, New Haven Co., West Rock Ridge SP, 125 m, 41.33353N, 72.96423W, on Vitis aestivalis var aestivalis, 10.ix.2011, EvN2011198, E.J. van Nieukerken; leafmines & larvae (DNA barcode RMNH.INS.18394), Georgia, same data as holotype; leafmines & larvae (DNA barcode RMNH.INS.18392), Georgia, Murray Co., Chattahoochee Nat. Forest, Cohutta Overlook, 730 m, 34.78535N, 84.62732W, shrub in forest clearing, leafmines on Vitis aestivalis var. bicolor, 14.x.2010, EvN2010270, E.J. van Nieukerken & C. Doorenweerd (RMNH); leafmines & 2 larvae (DNA barcode RMNH.INS.18533), Massachusetts, Berkshire Co., Beartown State forest, SW margin, 480 m, 42.19814N, 73.28928W, on Vitis riparia, 12.ix.2011, EvN2011208, E.J. van Nieukerken; leafmines & larvae (DNA barcode RMNH.INS.18558), New York, Essex Co., Hwy 9N, 3.5 km WSW Keeseville, 142 m, 44.49233N, 73.52042W, on Vitis riparia, 14.ix.2011, EvN2011237, E.J. van Nieukerken; leafmines & larvae (DNA barcode RMNH.INS.18555), New York, Essex Co., Wilsboro, Noblewood Park, 62 m, 44.35216N, 73.36435W, on Vitis riparia, 14.ix.2011, EvN2011244, E.J. van Nieukerken; leafmines & larvae (DNA barcodes RMNH.INS.18298, 18300), Tennessee, Blount Co., NP Great Smoky Mts, Rich Mountain Gap, 619 m, 35.64557N, 83.80537W, rich forest on limestone ridge, leafmines on Vitis vulpina, 2.x.2010, EvN2010119, E.J. van Nieukerken & C. Doorenweerd (RMNH); mine and larva, (DNA barcode LGSME035–06), Tennessee, Cocke Co., Cosby, ATBI house, 35.77771N, 83.21359W, on Vitis sp. 12.viii.2006, DLW 2006H55, D.L. Wagner (DLW); leafmines & larvae (being reared and DNA barcode RMNH.INS.18669), Vermont, Addison Co., Button Bay SP, Lake Champlain borders, 44 m, 44.18154N, 73.36892W, on Vitis riparia, 16.ix.2011, EvN2011253, E.J. van Nieukerken.
In North America, at least four other species have an apical silver spot (together forming the ampelopsifoliella group): Antispila ampelopsifoliella , Antispila voraginella, which has a darker head, an unnamed species from Vitis (here Antispila “vitis2”) and Antispila hydrangaeella Chambers, 1874. The latter, which is closely similar in appearance, can be separated by the greater number of white flagellomeres at the antennal tip (six segments) and feeds on Hydrangea arborescens L. (Hydrangeaceae). Dissection of genitalia is needed to distinguish Antispila oinophylla from other members of the ampelopsifoliella group. Male genitalia are characterised by the long carinal spine at the phallotrema and several other details; female genitalia differ by the number of cusps on the ovipositor from at least Antispila ampelopsifoliella.
In Europe, Antispila oinophylla differs from all other Heliozelidae with a similar forewing colour pattern (species of Antispila, Antispilina and Holocacista) by the presence of a small silvery spot in the apical part of forewing and the distinctly white head. Some Elachistidae are superficially similar, but differ in long-pointed and upcurved palpi, longer antennae and more elongate habitus.
The leafmine of Antispila oinophylla differs from that of Holocacista rivillei by its short initial gallery, which is later usually completely incorporated into the blotch, whereas the initial gallery of Holocacista rivillei mines is usually as long as or longer than the blotch, and remains intact. In Eastern North America other Vitis-feeding Antispila do not show the concentric arrangement of frass that is typical for Antispila oinophylla – particularly in thinner leaves –and the mines are often larger. Mines of Antispila cf isabella and related species are much larger, and also have much larger cut-outs, 5 mm or longer. Since not all Vitis miners have been comprehensively studied, mine identification cannot yet be relied on.
Adult (Figs 1–5). Head face and vertex covered with appressed, strongly metallic, silvery-white scales, more prominently raised in male. Palpi porrect, white; base of proboscis covered with white scales. Antenna fuscous, apical 1 or 2 flagellomeres white. Labial palp silvery white, slightly upturned. Thorax lead-coloured, shiny, contrasting with forewings. Legs grey, tarsi mostly yellowish white, especially on undersides. Forewing dark fuscous with silver-golden patterning; an outwardly oblique fascia from 1/8 of posterior margin to 1/4 of costa, narrowing towards costa; triangular (dorsal) spot at middle of posterior margin, reaching to middle of wing, smaller triangular costal spot just beyond middle, sometimes touching dorsal spot; small, silvery subapical spot in middle of wing at 3/4; fringe line distinct. Terminal fringe paler. Hindwing pale grey. Abdomen lead-coloured, including vestiture on external genitalia.
Antispila oinophylla, adult habitus. 1 Male holotype, RMNH.INS.24204 2 Female paratype, RMNH.INS.24039, Italy, Borgo Valsusana. 3–5 Alive male, Georgia, paratype, emerged 29.iv.2011.
Measurements: male: forewing length 2.5–2.8 mm (2.6 ± 0.10, n=11), wingspan 5.5–6.2 mm, 25–31 antennal segments (29.1 ± 1.9, n=11); female: forewing length 2.3–2.8 mm (2.5 ± 0.16, n=10), wingspan 4.8–5.6 mm, 25–29 antennal segments (27.2 ± 1.4, n=8).
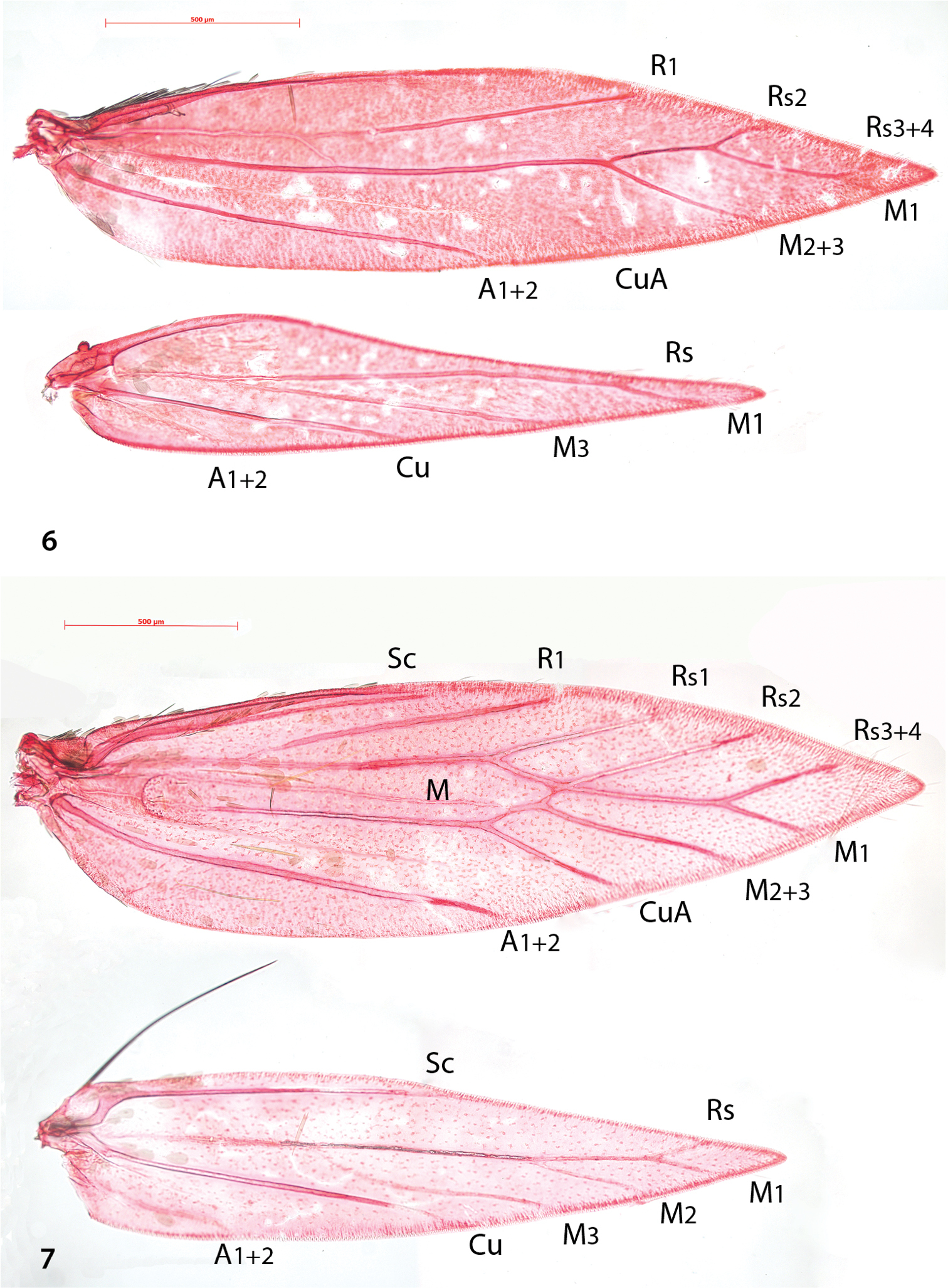
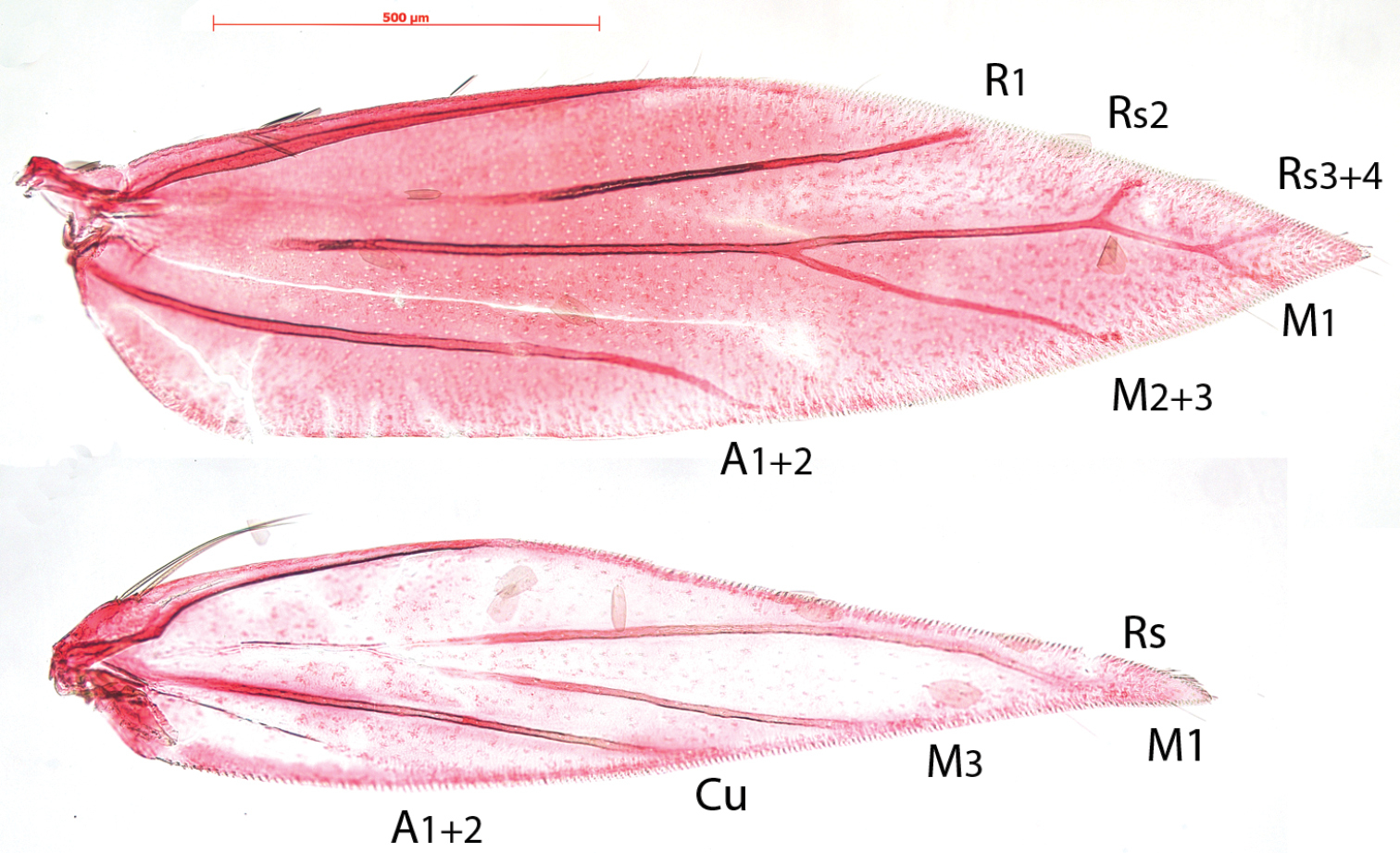
Venation (Fig. 6). Forewing with Sc barely visible. R1 a separate vein, connected by persistent trachea to Rs+M stem. Rs+M terminating in five branches, interpreted as Rs2 (possibly with 1) to costa, Rs3+4 to costa just before apex, M1 to dorsum just beyond apex, M2+3 to dorsum and a weakly developed CuA. A1+2 a strong separate vein. Hindwing with Sc barely or not visible, Rs+M a strong vein, bifurcate from ca. 1/4th, upper vein ending in two branches: Rs and M1, lower vein single (M3); Cu and A1+2 separate veins.
Compared to the complicate venation of many other Antispila species, including the type species Antispila metalella, (example in Fig. 7, Antispila treitschkiella) venation reduced with loss of forewing cell, separate M stem and connection between R1 and Rs, loss of Rs1 and in hindwing loss of M2. The venation more closely resembles that of Holocacista rivillei (Fig. 8), which is even more reduced and also lacks Cu in the forewing.
Antispila, venation. 6 Antispila oinophylla, male, Italy, RMNH.INS.24257 7 Antispila treitschkiella, male, Netherlands, Leiden, RMNH.INS.24258.
Holocacista rivillei, venation.Female, Italy, RMNH.INS.24259.
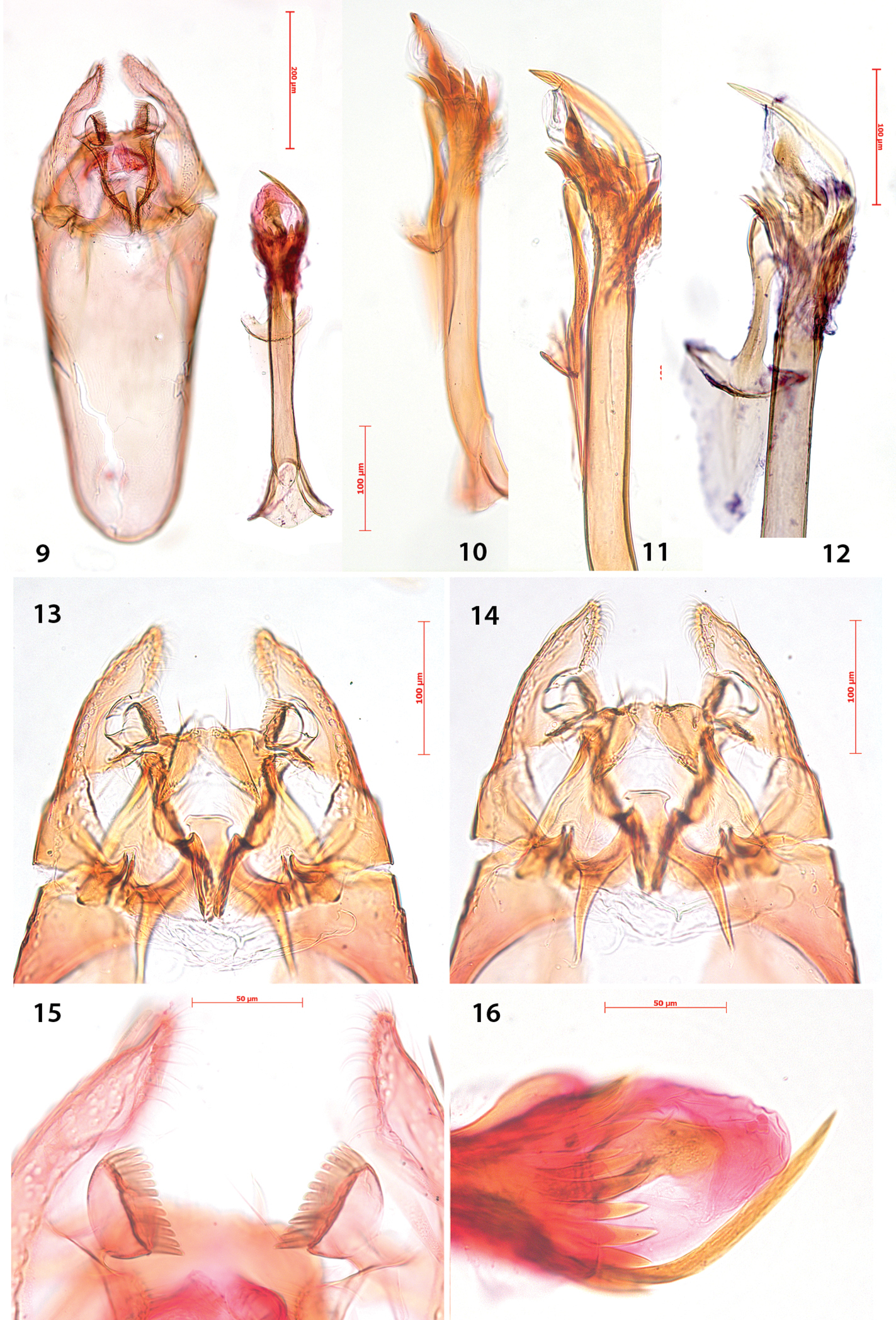
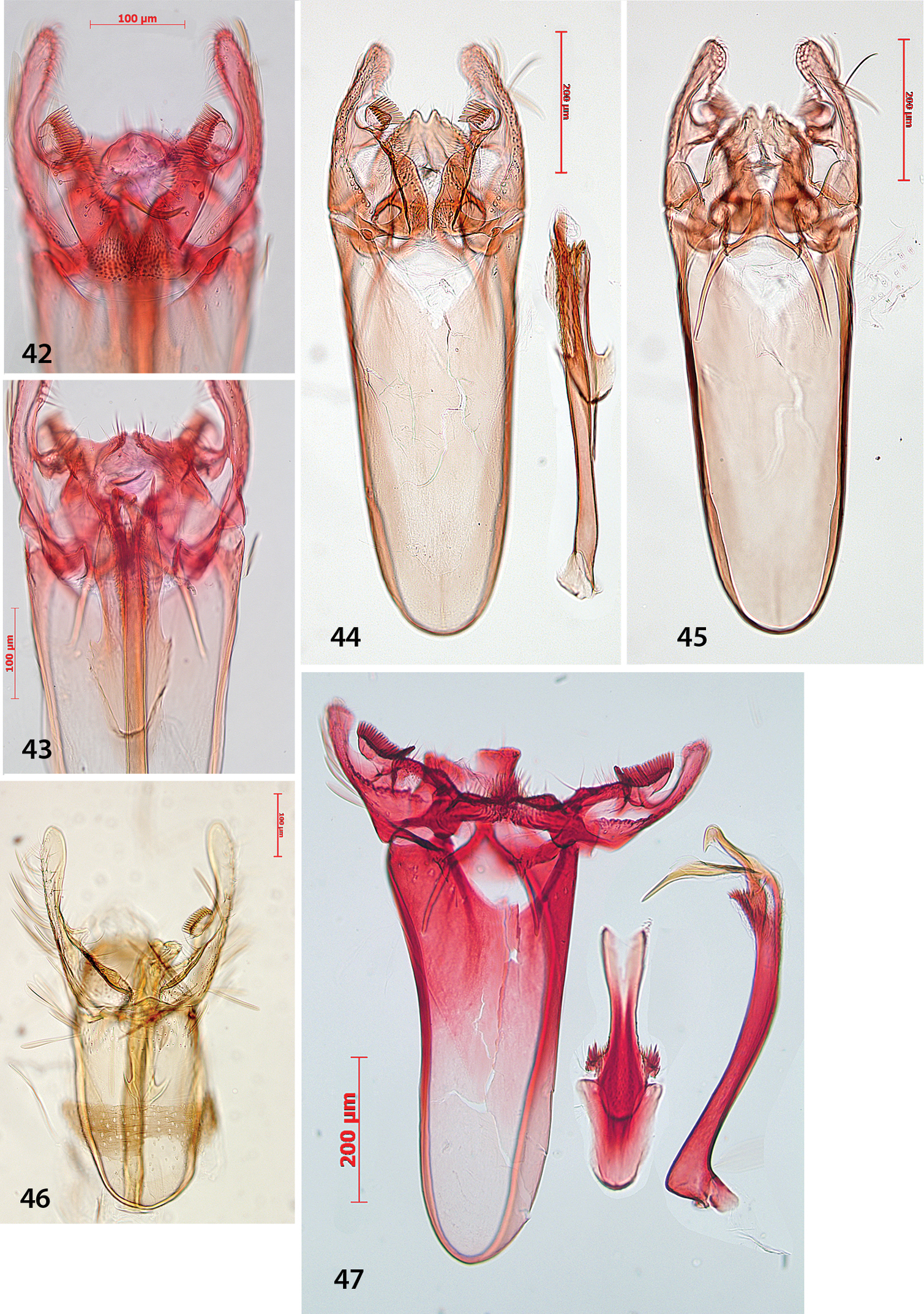
Male genitalia (Figs 9–16). Uncus bar-shaped, with two large setae dorsally. Vinculum very long, anteriorly rounded, posteriorly shallowly bilobed. Valva more or less triangular, pecten on pedicel, with 10–13 comb teeth (Fig. 15); inner margin of valva with setose lobe anterior to pecten pedicel; basally with a triangular protuberance, almost touching that of other valva; transtilla with trapezoid medial plate, sublateral processes relatively short. Juxta anteriorly spade-shaped, about half as long as phallus. Phallus long, anteriorly much widened, at phallotrema with a comb of about 10–12 strong teeth and at left side a very long curved process (Figs 10–12, 16).
Antispila oinophylla, male genitalia.Paratype, Italy, RMNH.INS.23920 (9, 15, 16), Paratype, Italy, RMNH.INS.15247 (12), Holotype, RMNH.INS.24204 (10, 11, 13–14). 9 Complete genitalia with separate phallus in ventral view 10–12 Phallus and juxta in ventro-lateral view 15–16 Complex of tegumen, uncus, valvae and transtilla 15 Detail of valval tips and pectinifers 16 Detail of spines near phallotrema.
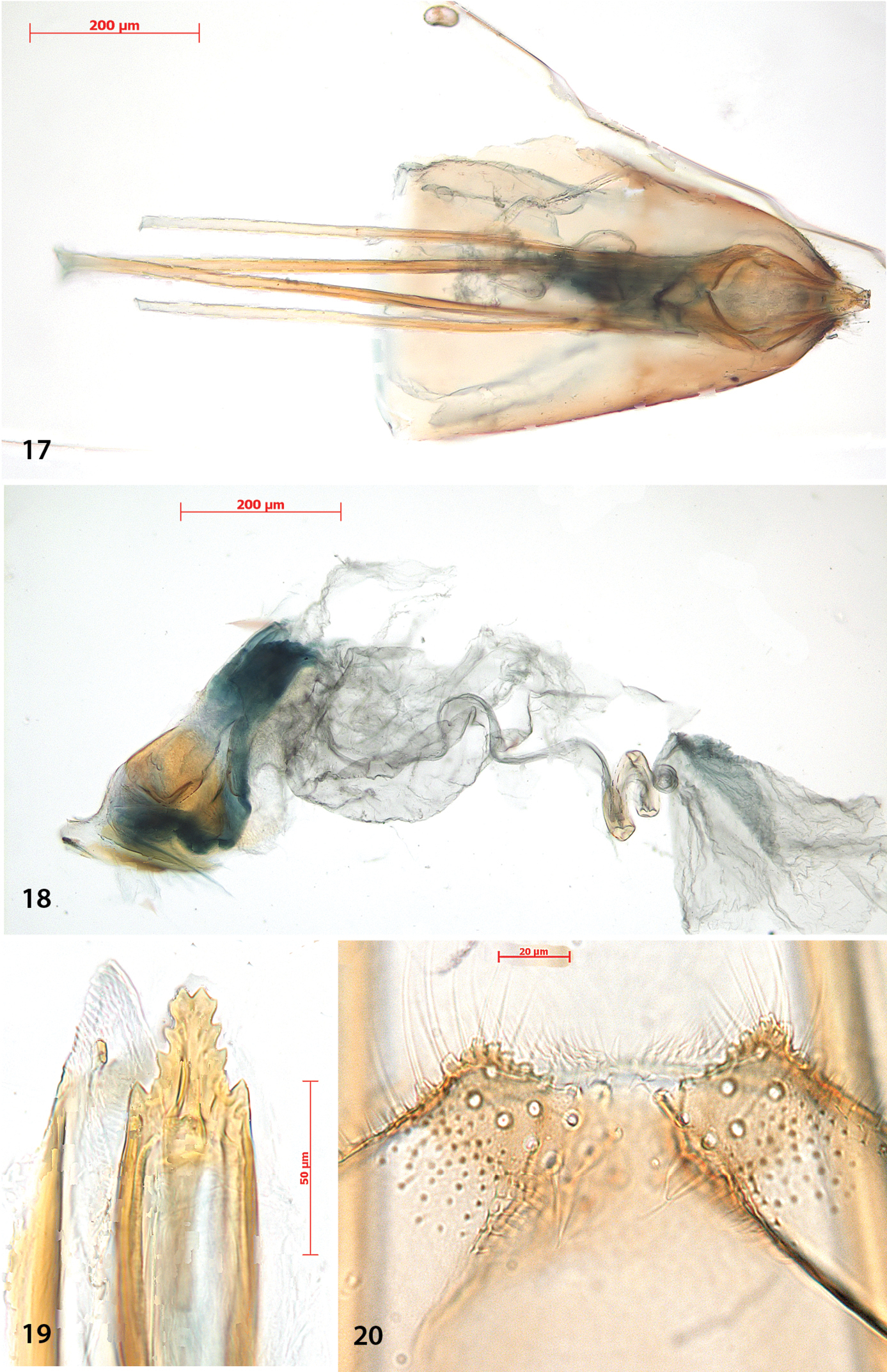
Female genitalia (Figs 17–20). Ovipositor with 4–5 cusps at either side (Fig. 19). S8 medially indented, with many papillate setal sockets. Vestibulum with broad, indistinct sclerotization and no spines (Fig. 18).
Antispila oinophylla, female genitalia. 17 Terminal segmentsandapophyses, ventral view, paratype, EJvN4211, USA, Kentucky (pseudotype ampelopsifoliella) 18 Internal genitalia, lateral view, showing sclerotisation in vestibulum, paratype, EJvN4206, USA, Connecticut 19 Ovipositor tip, dorsal view, EJvN4206 20 Detail of S8, ventral view, paratype, Italy, RMNH.INS.15244.
Host plants.In North America reared from or found as larva on summer grape Vitis aestivalis Michx., both var. aestivalis and var. bicolor Deam, fox grape Vitis labrusca L., riverbank grape Vitis riparia Michx. and frost grape Vitis vulpina L. Literature records of Antispila “ampelopsifoliella” from Vitis or grape likely refer to this species (
Leafmines (Figs 21–28). The egg is inserted on the underside of a leaf, usually within 1–2 mm from a vein. The mine starts as a rather straight or slightly contorted gallery towards the vein, usually forms a right angle and often follows the vein for a short distance, then again turns away from the vein where it expands into a blotch. The gallery portion, of variable length, is usually later incorporated into the blotch mine. The frass is linear, usually occupies the complete mine width, but occasionally is deposited in a thin line (Fig. 27). In the blotch much of the blackish-brown frass is deposited close to the origin in semicircular concentric frass lines. This characteristic pattern is best seen in thin shade leaves (e.g., Figs 25, 26); in sun-exposed leaves the frass pattern is often obscured. The whole mine occupies as a rule an area of less than 10 × 10 mm; only in thin leaves are mines appreciably larger. The larva cuts out an elliptic case of about 3.2–4.0 mm long.
Antispila oinophylla, life history: leafmines on several species of Vitis and different localities. 21, 23, 24 Italy, Borgo Valsusana, Vitis vinifera, 25.vi.2009 22 USA: Vermont, Button Bay SP, Vitis riparia 16.ix.2011 25 USA:Tennessee, NP Great Smoky Mts, Vitis vulpina, 2.x.2010, mine in shade leaf 26, 28 USA: Georgia, type locality, Vitis aestivalis var. aestivalis, 14.x.2010 27 USA: Vermont, Button Bay SP, Vitis riparia, 16.ix.2011, DNA barcode, RMNH.INS.18589.
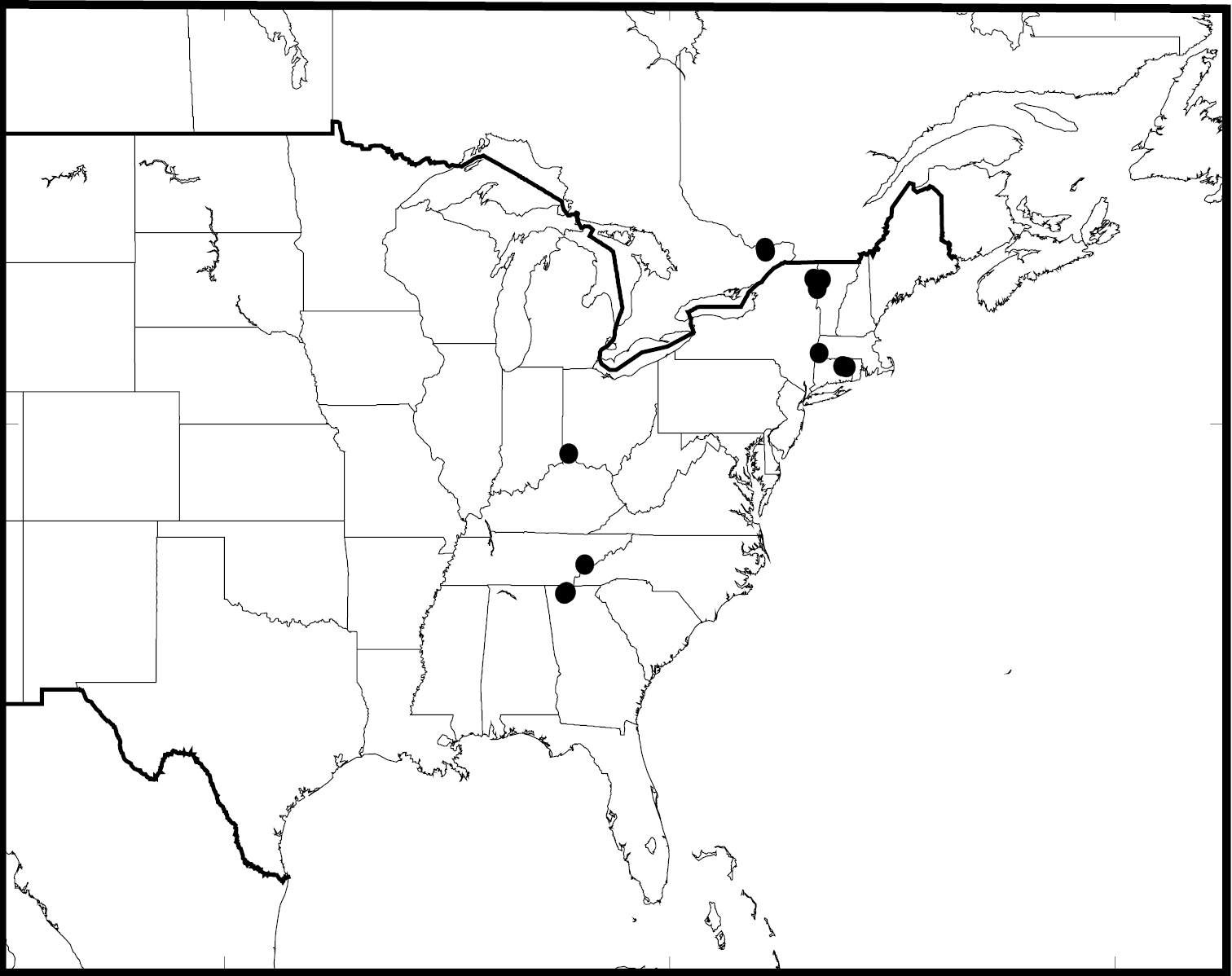
(Fig. 29, 62). In North America, Antispila oinophylla is known with certainty (material cited) from Canada: Ontario, Quebec; USA: Connecticut, Georgia, Kentucky, New York, Tennessee, and Vermont. Records under Antispila ampelopsifoliella from Maine, Missouri, and Ohio (
Antispila oinophylla, distribution in North America.
The epithet oinophylla, a noun in apposition, is from the Greek οινος (oinos = wine) and φυλλον, plural φυλλα (phyllon, phylla = leaf), “wine leaves, ” because the larva lives in the leaves of the grapevine from which wine is made.
Four species feeding on Vitaceae have been named previously from North America. No name-bearing types are available for three species, only for Antispila voraginella is a holotype extant. The latter is clearly different from Antispila oinophylla, and restricted to western North America. For the eastern species Antispila isabella, Antispila viticordifoliella and Antispila ampelopsifoliella, we have only the original descriptions and subsequent interpretations to establish identities. The fact that our preliminary sampling of DNA barcodes for grape-feeding Antispila show great diversity, complicates matters further. Below, we will discuss these three species in the chronological order of their descriptions.
Antispila isabella was described from mines on “Isabella grape” (a cultivar of Vitis labrusca) and adults (
Antispila viticordifoliella was also described by Clemens in 1860, from mines on “wild grapes” only, differing by a smaller case (shield) and a larva “without dots.” Although the foodplant was not explicitly mentioned by Clemens, from the species name it is evident that the host must have been Vitis cordifolia Michx. (a synonym of Vitis vulpina). In fact his very brief description could fit the mines of Antispila oinophylla, but subsequently the name has always (e.g.
Antispila ampelopsifoliella:Chambers(1874a: 168) only briefly described the mine and larva from “Ampelopsis quinquefolia” [= Parthenocissus quinquefolia] (and stated that he “never succeeded in breeding it.”). Only a month later he described the moth under the name “Antispila ampelopsisella” [sic, considered as a subsequent incorrect spelling], writing: “Since that paper was placed in the hands of the Editor, many months ago, I have succeeded in rearing it from the mine [from Parthenocissus]” (
“Last summer I found its leaves [referring to a Vitis species] mined by a larva closely resembling that of Antispila ampelopsifoliella, supra, and which I suspect to be the same. ….. From it I bred the species described below, which I do not now name, as it may prove to be identical with Antispila ampelopsifoliella.” (
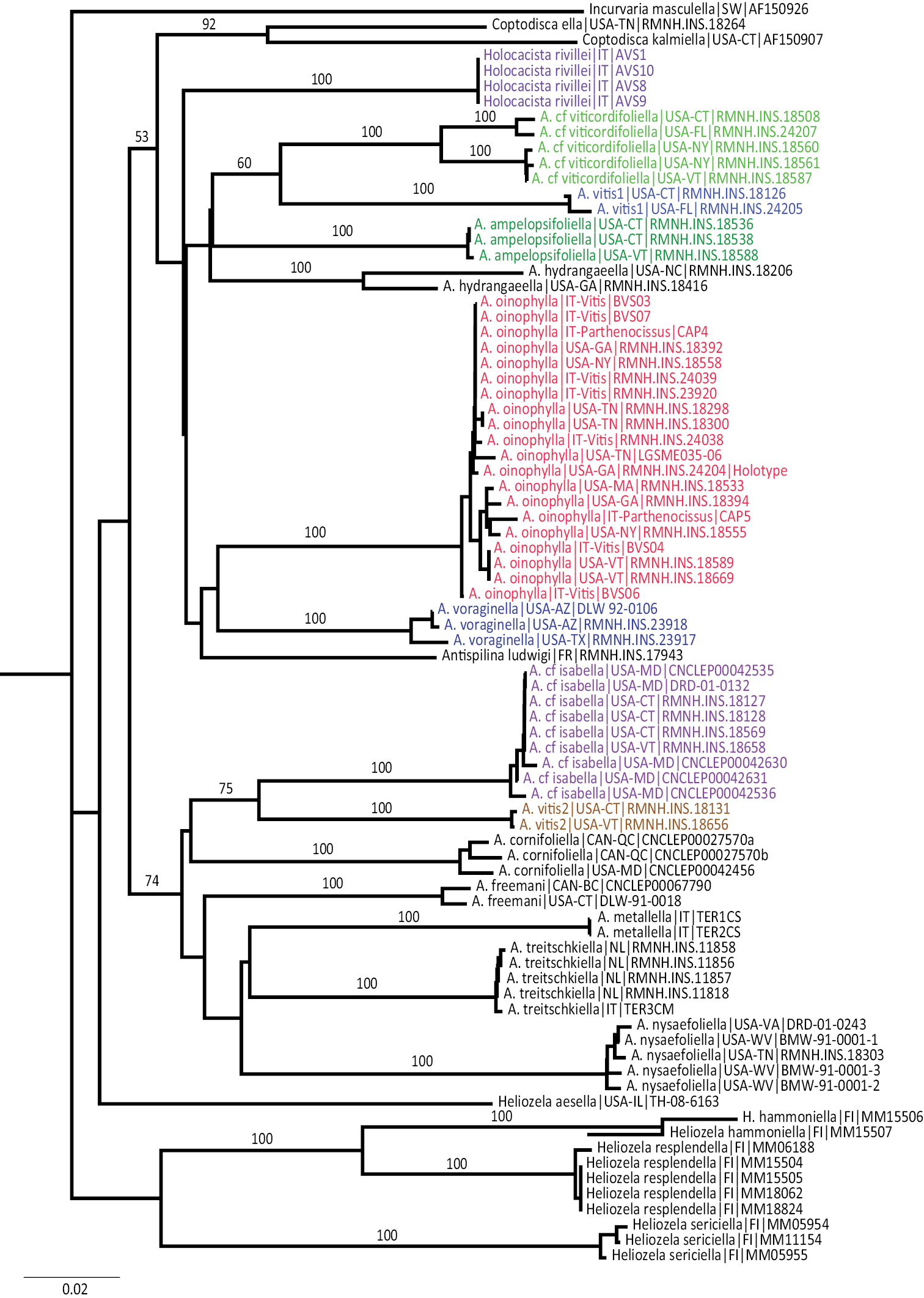
Neighbor-joining tree for heliozelid COI barcodes, based on uncorrected pairwise distances. Numbers on branches are bootstrap values, 10, 000 replicates. Vitaceae-feeding clusters are coloured differently, others in black. Labels include species name or informal name, codes for country and state (in North America) and sample numbers (Genbank numbers for sequences taken from Genbank).
In Chambers’ collection at MCZ there are three specimens under the name Antispila ampelopsifoliella that probably served as the basis for the adult description. These specimens were termed pseudotypes (
We restrict here the usage of the name Antispila ampelopsifoliella to the species feeding on Parthenocissus, with an apical spot (The generic name for Parthenocissus quinquefolia was Ampelopsis at the time Chambers described the species.) Although we have not obtained a DNA barcode form such an adult, the fact that an adult from the other cluster on this host (see below) does not have such a spot and is tentatively identified as Antispila cf viticordifoliella, we can associate Antispila ampelopsifoliella adults with one of the larval types. When adults are available for all barcode clusters, we suggest that a neotype be selected from material reared from Parthenocissus from the vicinity of Covington, Kentucky, to fix the identity of Chambers’ name.
Barcode analysis
Neighbor-joining trees of all sequenced barcodes, both based on Kimura 2P distances and uncorrected distances give highly similar results in topology and branch lengths, we illustrate here the last one (Fig. 30). All species clusters have a bootstrap value of 100, and within-species variation is usually low or absent. We caution, however, that for several species, such as Antispila treitschkiella or Holocacista rivillei most sequences are from just one or two populations. Two species clusters show large intraspecific distances: the two specimens of Antispila hydrangaeella have 5.22% K2P distance and 4.99% uncorrected pairwise distance, and the species tentatively named Antispila cf viticordifoliella forms two clusters with around 4% distance in both methods. Although the mines of these clusters look superficially the same we have not studied the adults of one cluster, so it is possible that these clusters represent separate species.
We have 20 sequences representing Antispila oinophylla, seven of which are 100% identical, five from Italy (including one from Parthenocissus) and two from North America (RMNH.INS.18392 from Georgia and RMNH.INS.18558 from New York). The others are very similar, with at most five nucleotides differing from those of the core group (RMNH.INS.18394 from Georgia). The genetic distance varies from 0 to 1.23% K2P distance (1.22% uncorrected). The differences occur in 16 different positions, of which six cases are found in more than one specimen (e.g., a G instead of A in position 82 combined with a T in 316; the seven specimens forming a “clade” in Fig. 30 with RMNH.INS.18533 and BVS04; position 550: C instead of T; four specimens forming the “clade” in Fig. 30 with RMNH.INS.18533, position 634 a T instead of A in RMNH.INS.18298 and RMNH.INS.18300, both from Tennessee). Several haplotypes are found both in Italy and North America. The largest distance is between two North American specimens, one from Georgia and one from Tennessee (RMNH.INS.18394 and LGSM035–06). The genetic distance to the closest congeneric species Antispila voraginella is large: more than 10%.
Phylogenetic analyses
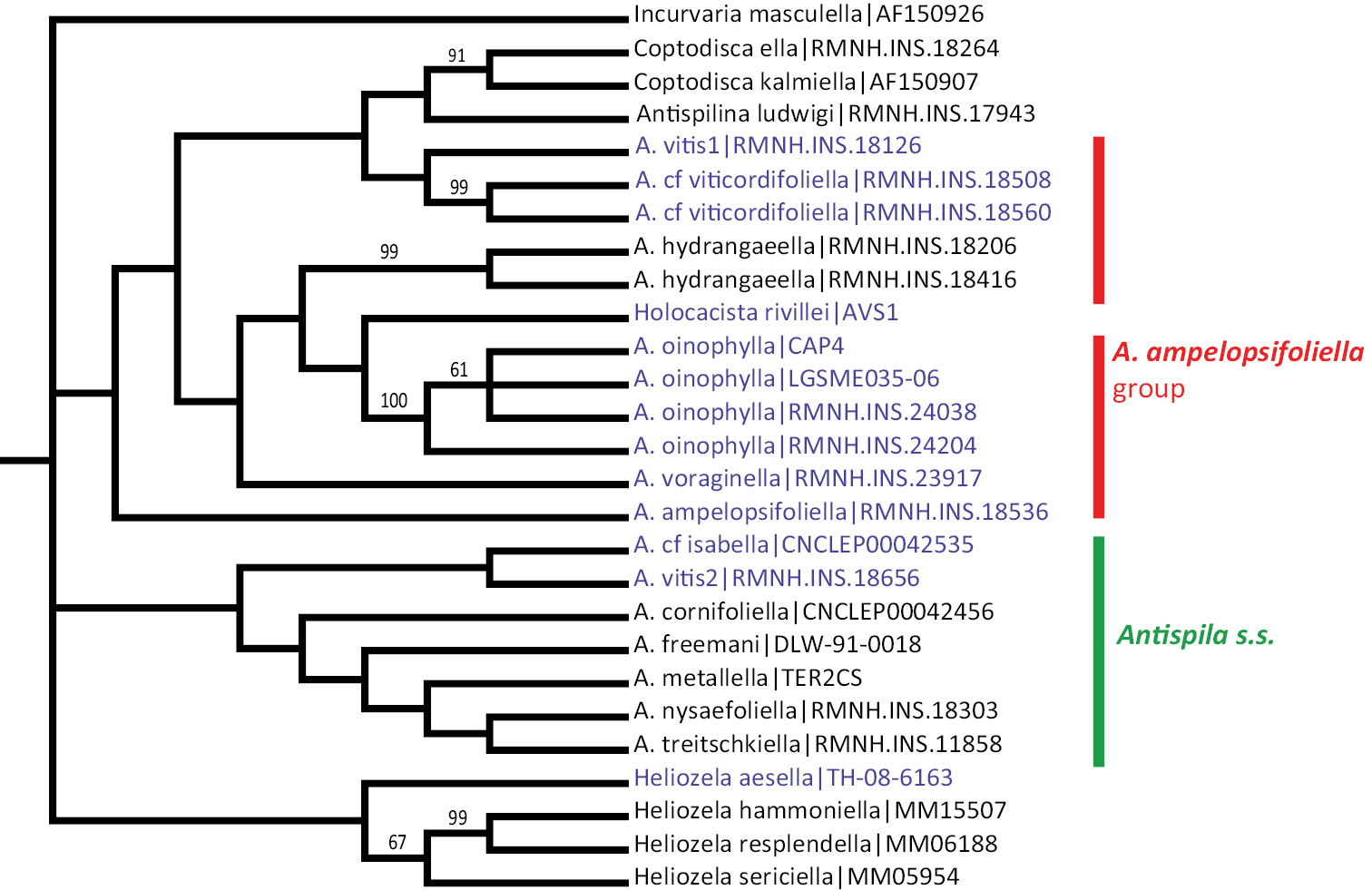
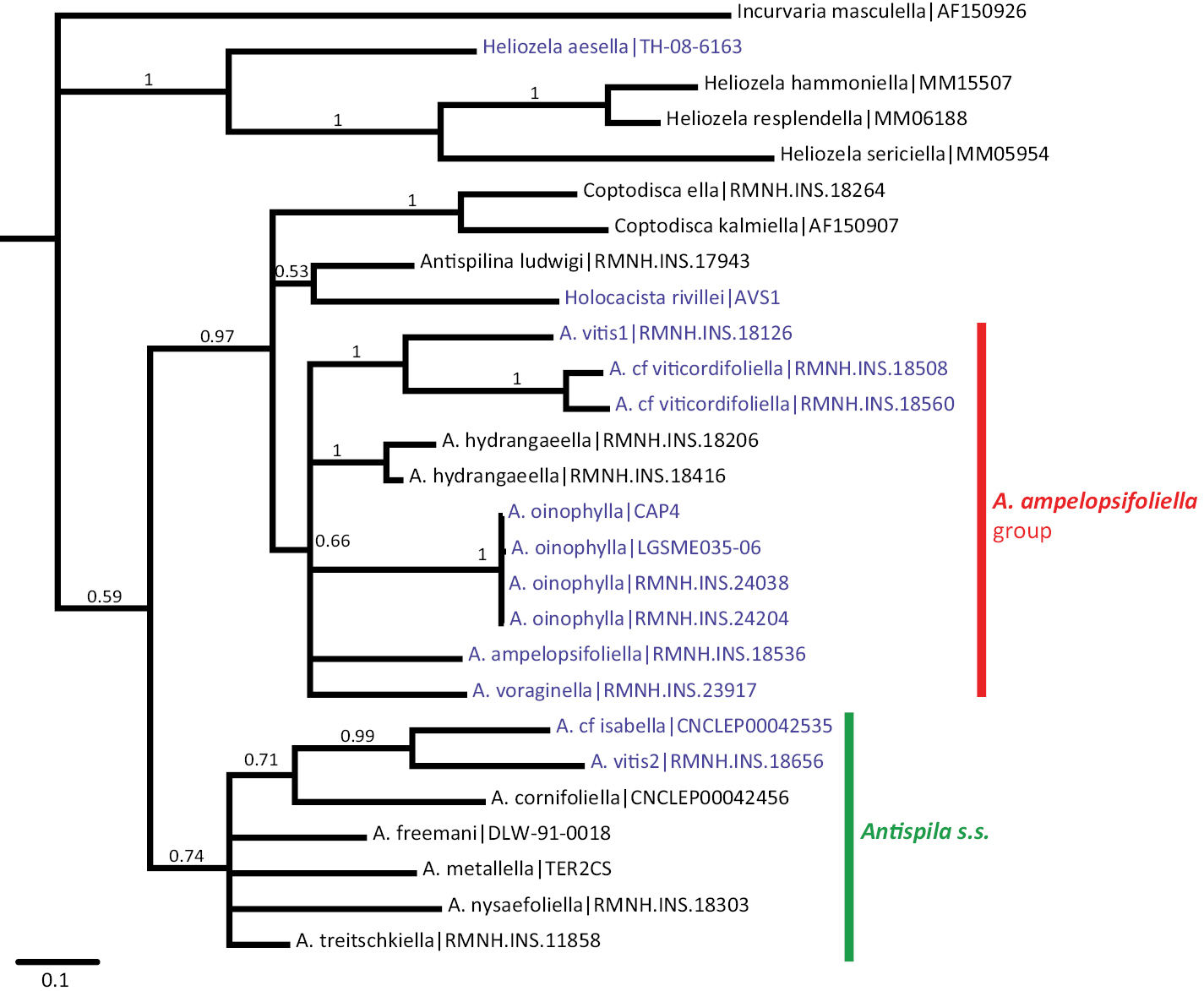
The maximum parsimony analysis of the barcode sequences resulted in three shortest trees, of which the 50% majority rule tree is illustrated (Fig. 31). The semi-strict tree differs only in the position of Heliozela aesella, which forms a polytomy with the three main heliozelid clades in Figure 31. Of the 658 characters, 243 characters are parsimony informative. Bootstrap values are taken from the TNT analysis. The two Bayesian analyses of the same dataset showed few differences, we here illustrate the consensus tree based on three partitions (Fig. 32).
Cladogram, 50% majority rule consensus of three shortest trees from maximum parsimony analysis of COI sequences. CI = 0.361, RI = 0.456, RC = 0.168. Figures are bootstrap values from a TNT analysis (10, 000 bootstrap replicates). Purple-coloured taxa are feeding on Vitaceae. The semi-strict tree differs only in the position of Heliozela aesella (see text).
Cladogram from Bayesian analysis on three partition dataset. Figures are posterior probabilities. Purple-coloured taxa are feeding on Vitaceae.
Both cladograms are rather similar. Antispila oinophylla forms a highly supported clade. Clades for Heliozela, a core Antispila grouping and a clade with several smaller genera and the Antispila ampelopsifoliella group were recovered, with strong support in the Bayesian analysis for thelatter clade (0.97) and for Heliozela (1) and less support for core Antispila (0.74). Within the core Antispila clade, the two Vitaceae species form a clade, well supported in the Bayesian tree, nested in or sister to the Cornaceae-feeding species.
The Bayesian analysis recovered a monophyletic Antispila ampelopsifoliella group. In both analyses this group clusters with the small genera Coptodisca, Holocacista and Antispilina. These all share the reduced venation as described here for Antispila oinophylla. Relative positions of these small genera and the two clades of Antispila vary amongst various analyses. In the Bayesian tree there is low support for a clade of Antispilina and Holocacista. In none of the analyses was Heliozelidae recovered as a monophyletic group.
Vitaceae-feeding taxa are indicated in the cladograms by a purple colour. If these cladograms correctly represent the phylogenetic history of the Heliozelidae, it appears that Vitaceae were the ancestral hosts for the family.
Comparative notes to other speciesBelow we will briefly treat the other Vitaceae miners amongst North American and European Heliozelidae and one other closely related species, in order to distinguish them from Antispila oinophylla. As there are several more Antispila species in North America than currently described, this is a preliminary treatment until a thorough revision can be completed. Because we have not yet been able to link some larval barcode clusters to their associated adults, the number of leafmine types described below is higher than the number of adult “species”. Material examined for each of these “taxa” is listed in the Appendix A.
http://species-id.net/wiki/Antispila_ampelopsifoliella
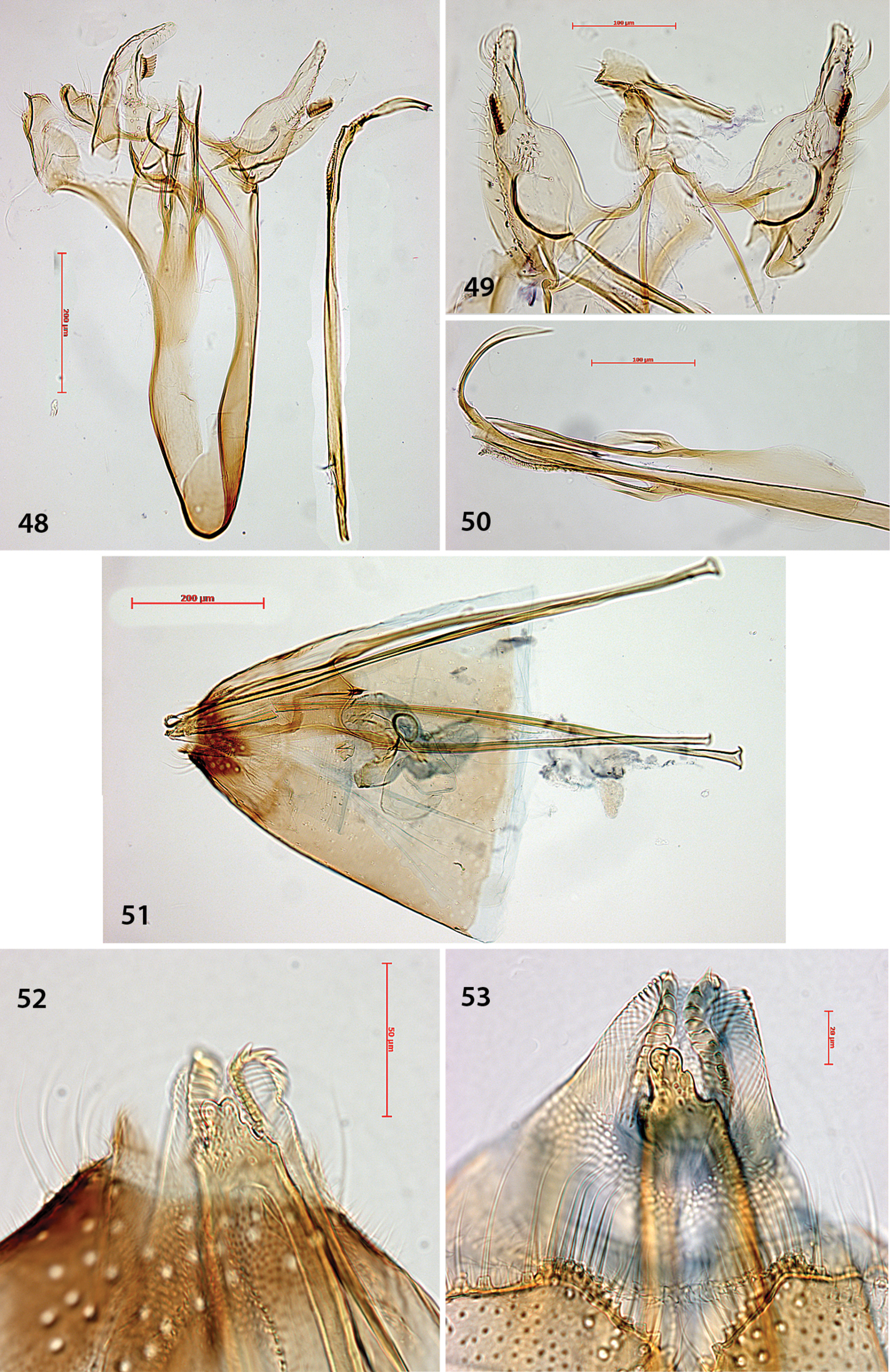
Figs 35, 42, 43, 53, 56We can not separate Antispila ampelopsifoliella (Fig. 35) from Antispila oinophylla based on external characters: it may average a bit smaller, but our samples are too few in number to make statistical comparisons. In the male genitalia (Figs 42–43), uncus not bilobed; valva with pecten with ca. 11–13 comb spines, base of valva with rounded lobe, not triangular; juxta rather wide, with lateral groups of spines; phallus with much shorter terminal spines and a comb of rather short triangular spines near phallotrema. Female genitalia (Fig. 53): ovipositor only with 3 cusps at either side. Vestibulum with some spines.
Holocacista and Antispila adult habitus in dorsal or lateral(40, 41)view. 33 Holocacista rivillei, male, Italy 34 Antispila voraginella, male, USA: Arizona, genitalia slide EJvN3918 35 Antispila ampelopsifoliella, female, USA, Vermont: Salisbury, genitalia slide JCK15220 36 Antispila hydrangaeella, female, USA: Georgia, Chattahoochee NF 37 Antispila cf viticordifoliella, female, Canada: Ottawa 38, 39 Antispila cf isabella, male, upper and underside (39) with androconial scales, USA: Connecticut, Mansfield, DLW90J8 40 Antispila “vitis1”, female, USA: Florida, genitalia slide EJvN4205 41 Antispila cf viticordifoliella, female, USA: Florida, genitalia slide EJvN4207. Arrows indicate white tipped antennae in Antispila hydrangaeella and cf viticordifoliella.
Antispila species, male genitalia. 42–43 Antispila ampelopsifoliella, USA, New York state, genitalia slide EJvN4200 44–45 Antispila voraginella, USA, holotype, genitalia slide EJvN3916 46 Antispila cf isabella, USA: Kentucky, Morehead, genitalia slide CNC MIC1859 47 Antispila hydrangaeella, USA: North Carolina, NP Great Smoky Mts., genitalia slide EJvN4198.
Holocacista rivillei, male and female genitalia, Antispila ampelopsifoliella, female genitalia(53). 48–50 Male genitalia, Italy, slides RMNH.INS.15248, 15250, 15251 51–52Female genitalia, slide RMNH.INS.15252 53 Ovipositor and tergum 8, genitalia slide JCK15220.
Hostplant: Parthenocissus quinquefolia.
(Fig. 56). Egg usually inserted in leaf under- or upperside close to a vein, mine starting with a relatively long contorted gallery with thin broken frass, or when it runs along margin in a straighter course, later abruptly enlarged into elongate blotch or wide gallery; frass dispersed in middle. The early narrow gallery may be as long as the elongate blotch. The mine can be found in any part of the leaf. Larva yellowish white, black head, cut-out ca 3.5–4 mm long. The mine resembles that of Antispila hydrangaeella. It was most frequently found in the larger and thinner ground leaves of Virginia creeper.
Holocacista rivillei and Antispila species, life history. 54–55 Holocacista rivillei on Vitis vinifera, Italy: Rovereto 56 Antispila ampelopsifoliella on Parthenocissus quinquefolia, USA: Button Bay SP, 16.ix.2011 57 Antispila cf viticordifoliella on Phyllonorycter quinquefolia, same locality 58, 60 Antispila cf isabella on Vitis riparia, USA: Button Bay SP, 16.ix.2011 59, 61Antispila “vitis2” on Vitis riparia, samelocality. In the last four photos also parts are visible of gallery mines of Phyllocnistis vitegenella.
Eastern North America, confirmed from USA: Connecticut, Kentucky, New York, Vermont and Canada: Ontario.
http://species-id.net/wiki/Antispila_voraginella
Figs 34, 44, 45Adult (Fig. 34) very similar to and about same size as Antispila oinophylla, but head and thorax covered with brassy shining scales rather than silver. In male genitalia (Figs 44–45) uncus clearly bilobed, valva with fewer pecten spines: 8–10, triangular lobe absent; transtilla with narrower central plate and phallus with rather different set of spines: the long one of oinophylla absent, and row along phallotrema less comb-like, whereas there is a row of many spines along both sides. Female genitalia not examined.
Hostplant: Vitis arizonica. Seems to be univoltine, larvae found in June–July northward; through September in monsoonal areas to south; adults emerging the following spring April to June.
Mine illustrated by Powell and Opler (2009: plate 59:7). Mines rather different from those of Antispila oinophylla: larvae usually gregarious with mines forming large pale blotches.
Evidently allopatric to Antispila oinophylla and only recorded from the Rocky Mountains: Utah, Arizona and West Texas.
Fig. 40
From this barcode cluster we have just two females from Florida (Fig. 40, one barcoded) and one larva from Connecticut, of which it is unclear to what type mine it belongs. The female is indistinguishable externally from Antispila oinophylla. Almost certainly this represents another new species.
http://species-id.net/wiki/Antispila_hydrangaeella
Figs 36, 47DNA barcodes suggest that two species might be involved, and leafmines from a population in North Carolina (Smoky Mts NP) and northern Georgia do show some differences. Described adults and larvae are from the Georgia population.Externally, adult Antispila hydrangaeella (Fig. 36) is extremely similar to the other species of the Antispila ampelopsifoliella group, but it differs by the last six antennal segments being white and by genitalia and hostplant data. In male genitalia (Fig. 47) uncus only shallowly bilobed; valva with long pecten with more comb spines: ca. 20, triangular lobe absent, at base of valva beardlike setation; juxta rather wide, with groups of spines laterally; phallus with two very long terminal spines and many small spines near phallotrema, not forming a comb. Female genitalia not examined.
Hostplant: Hydrangea arborea.
One type (North Carolina) with long gallery mines, often following a vein, ending in a blotch with greenish to brown frass. The mines from Georgia with early gallery mine much contorted in a small area, with black frass, ending in elongate mine with blackish dispersed frass.
USA: Georgia, Illinois, Kentucky, North Carolina, presumably widespread in eastern United States.
http://species-id.net/wiki/Antispila_viticordifoliella
In the interpretation of this species by
Hostplant: Vitis vulpina. Leafmines not described in detail.
USA: Kentucky, Pennsylvania. Many records are unreliable and often refer to the isabella complex.
http://species-id.net/wiki/Antispila_cf_viticordifoliella
Figs 37, 41, 57Two females (Figs 37, 41), reared from Parthenocissus mines, match Chambers’ (1874a) description of Antispila viticordifoliella adults. Because the possibility exists that two species with similar externals, feeding respectively on Vitis and Parthenocissus, are involved here, we cannot decide whether the Parthenocissus miner is conspecific with viticordifoliella or not, before we have studied genitalia and/or DNA barcodes from specimens originating from both hostplants (to date we have only barcodes from Parthenocissus miners and no males from either form). Moreover, there is a deep split in the barcodes from Parthenocissus miners, here tentatively identified as Antispila cf viticordifoliella, one cluster from New York and Vermont, the other from Connecticut and Florida. We did not see differences in mine or larva between these clusters, and thus tentatively regard them as one species.
Hostplant: Parthenocissus quinquefolia.
(Fig. 57). Egg often inserted on leaf margin, position often hard to find, rarely near midrib, mine without a gallery at the start, an elliptic elongate blotch mine, often running along or near leaf margin; frass sometimes grouped in a clump, more typically spread in an irregular broad line. Larva yellow with almost black head, cut-out ca 3.5–4 mm long. This mine was most frequently seen in thicker leaves borne from climbing shoots.
Canada: Ontario. USA: Connecticut, Florida, New York, Vermont.
http://species-id.net/wiki/Antispila_cf_isabella
Figs 38, 39, 46, 58, 60 (59, 61 Antispila “vitis2”)Under this name there is probably a complex of species, often with conspicuous androconial scales in males. Among the barcodes we distinguish two clusters, here tentatively named as Antispila cf isabella and Antispila “vitis2”. The adults described here do not necessarily belong to one of the described mine types.
Moths (Figs 38–39) of this species complex are easily distinguished from the Antispila ampelopsifoliella group by the missing apical spot on forewing and larger average size. Moreover males have conspicuous yellow or brown androconial scales on forewing underside (Fig. 39). The venation is also more complete (as in Fig. 7).
Male genitalia were examined of one of the species (Fig. 46) the valva is more elongate, and the pecten includes 10–13 teeth. Phallus lacks larger spines at phallotrema, but has many scale-like, small spines, and posteriorly possesses an asymmetric broad lobe; anteriorly not widened. Other individuals have not been examined; as noted above, the group is in need of revision.
Hostplant: Vitis aestivalis, Vitis labrusca [incl. “Isabella” grapes], Vitis riparia.
Mines of Antispila cf isabella (Figs 58, 60) are relatively large mines, with the egg deposited near a vein. No gallery visible, mine a large blotch, with a roundish patch of reddish frass near beginning, probably attached to upper epidermis, and dispersed black frass throughout mine. Cut-out large, around 5 mm long.
Mines of Antispila “vitis2” (Figs 59, 61) also start on a vein, without gallery, and are relatively compact blotches, with frass concentrated in a mushroom shape or reversed triangular near beginning of mine. Cut-out large, around 4.8 mm.
Canada: Ontario. USA: Connecticut, Georgia, Kentucky, New York, Pennsylvania, Vermont.
Both COI sequences and external sexual secondary characters show that more species are involved. We have tentatively named the most common form as Antispila cf isabella, and research of types or material from the collections of Clemens and Chambers is needed for establishing the identities of these names.
http://species-id.net/wiki/Holocacista_rivillei
Figs 8, 33, 48–52, 54, 55Moth (Fig. 33) much smaller than Antispila species, with 3.5–4 mm wingspan. Forewing pattern without apical spot, costal spots further away from wingbase than dorsal spots. Male genitalia (Figs 48–50) with slightly bilobed uncus, valva more elongate, pecten with 8–10 teeth; juxta with pair of lateral teeth; phallus extremely slender and long, ending posteriorly in long curved spine and row of small spines below that. Juxta bilobed apically. Venation reduced, rather similar to that of Antispila oinophylla (see Fig. 8).
Hostplant: Vitis vinifera.
(Figs. 54–55). Mine beginning with relatively long, slender gallery, later a small blotch with small cut-outs. Cocoons often attached to stems or leaves.
Southern Europe, western and Central Asia: Spain, France, Italy, Malta, Slovenia, Croatia, Bulgaria, Greece, Ukraine, Turkey, SE Russia, Georgia, Kazakhstan, Uzbekistan, Turkmenistan (
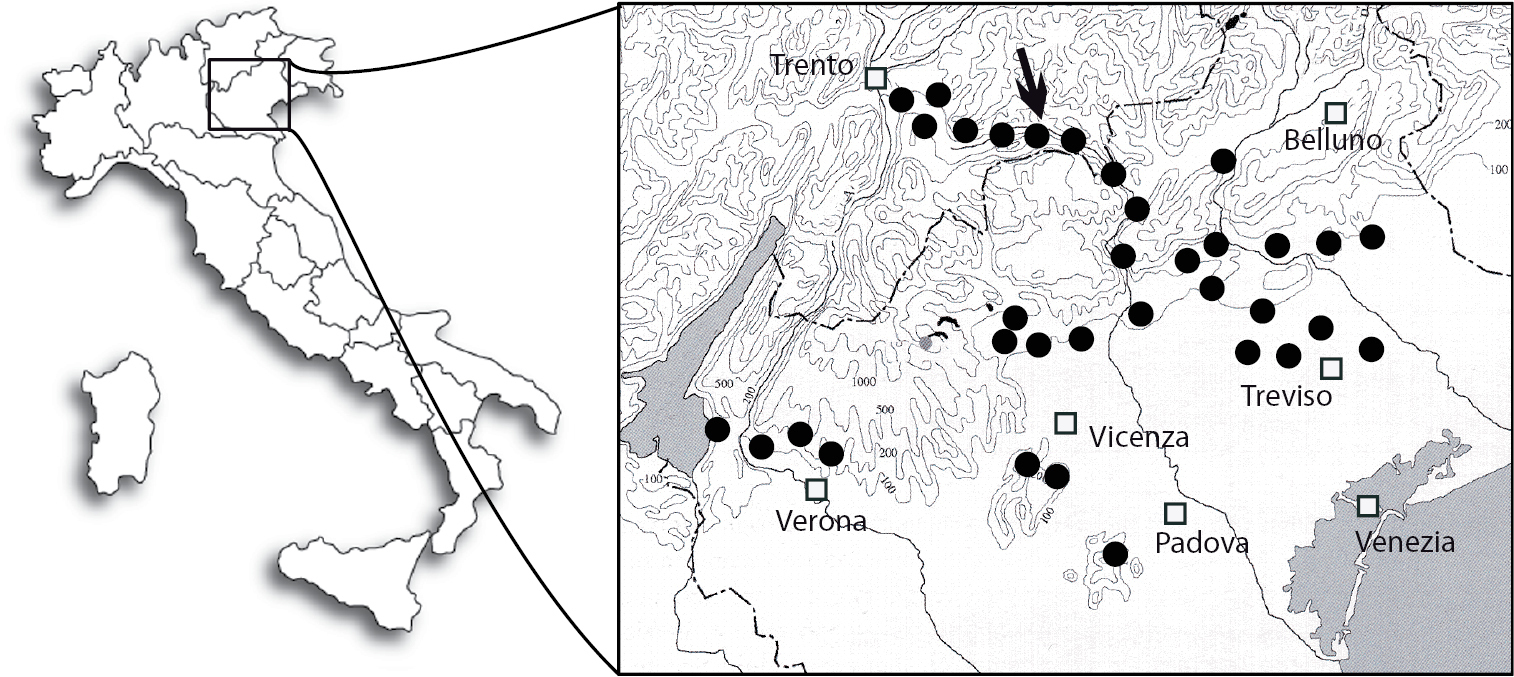
In Italy, Antispila oinophylla was detected for the first time in the summer of 2007 in a vineyard located in Valsugana (Borgo Valsugana, Trento province, Trentino-Alto Adige Region). Additional surveys conducted in the late summer of 2007 revealed its occurrence also in the neighbouring Vicenza and Belluno provinces (Veneto Region), particularly in neglected vineyards. In 2008, the distribution of the species did not differ greatly in the Trento province; elsewhere the insect was recorded in commercial vineyards of three provinces of the Veneto Region (Vicenza, Belluno and Treviso), sometimes at significant densities. In a number of vineyards Antispila oinophylla occurred together with Phyllonorycter vitegenella, rarely with Holocacista rivillei. In 2009 and 2010, a dense infestation was detected in commercial vineyards located in the Vicenza province (Breganze), about 80 km south of Borgo Valsugana. In this area severe symptoms had been detected as early as 2006 but they were misidentified as being caused by Holocacista rivillei. Since viticulture of this area is much more extensive than that around Borgo Valsugana, it is likely that Antispila oinophylla was introduced first in the Vicenza province and dispersed from there to the other areas. Also in 2010 the species was recorded in the Verona province, 90 km west of Breganze (E. Marchesini, pers. comm.). The distribution of Antispila oinophylla in Italy in 2010 is presented in Fig. 62.
Map showing the distribution of Antispila oinophylla in Italy up to 2010 (filled circles = sites of occurrence; arrow = site of collection specimens for sequencing).
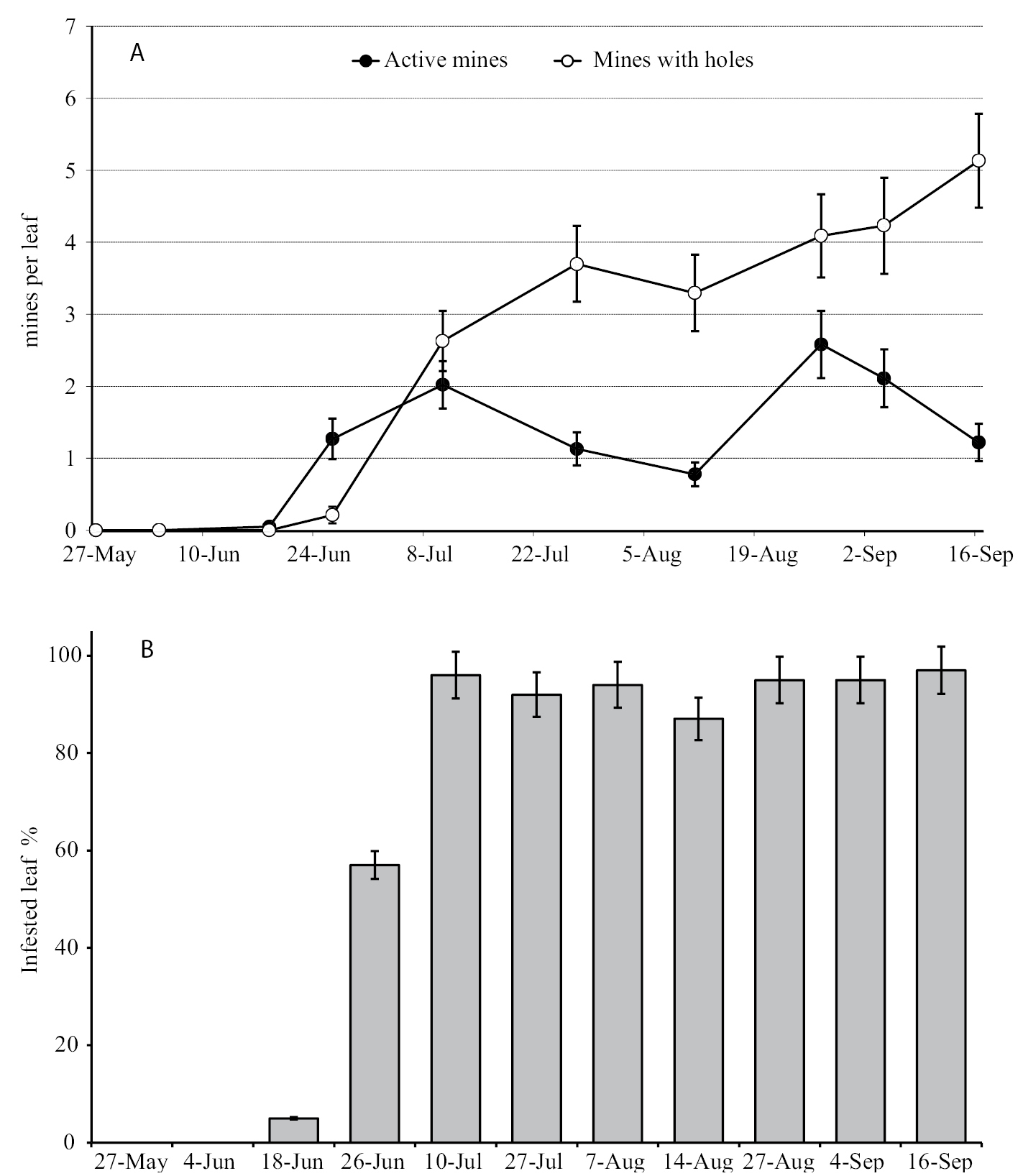
Observations carried out in winter 2008 showed that fully fed, final instar larvae of Antispila oinophylla overwintered inside their cases, fixed to the vine trunks or training stakes. Most larvae pupated in May and the first adults were seen in early June. Mines were detected first in the second half of June. Larvae of the penultimate instar cover the internal surface of the mine with a thin layer of silk, cut away an oval leaf section from both the upper and lower leaf surfaces, and then formed a case by joining the excised leaf sections with silk. Case-bearing larvae move slowly on the leaf surface and then descend with a silken thread until they contact a trunk, training stake, or other solid object to which they affix their case. In the experimental vineyard, the first cases were observed in the first half of July. An additional generation occurred from the second half of August onwards. In 2008, 86.9% of the leaves were infested with a density of 3.26 ± 0.25 (mean ± standard error) mines per leaf by the end of the first generation. In late summer, by the end of the second generation, 95.6% of leaves were infested with an average of 5.44 ± 0.37 mines per leaf.
Observations carried out during 2009 in the same vineyard, confirmed the existence of two generations. Adults were detected from early June to early July. The first mines were observed in mid-June and the first cases in late June (Fig. 63). Larval densities of the first generation peaked in early July, and by late July most mines had been abandoned by the larvae. Mines of the second generation were visible beginning in the second half of August. In the first generation, 96% of leaves were infested with an average of 4.6 ± 0.53 mines per leaf. In the second generation 97% of leaves were mined with an average density of 6.67 ± 0.72 mines per leaf. Active larvae were found until mid-October.
Incidence of the Antispila oinophylla infestation at Borgo Valsugana (Trento province, Italy) in 2009 expressed as A the number of mines per leaf and B the percentage of infested leaves (mean ± SE).
Identification of the unknown leafminer proved to be difficult. Many groups of Microlepidoptera remain poorly studied taxonomically. Even in North America,
We emphasize here the importance of combining traditional morphological descriptions with the additional dataset of DNA sequences for taxonomic groups whose identification is particularly difficult and mainly based on the description of genitalia.
An interesting observation is that we did not find any occurrence of Antispila oinophylla on Parthenocissus in its natural habitat in North America, although it utilizes that host in Italy. We found Antispila oinophylla mines on Vitis growing intertwined with Parthenocissus vines, that harboured two different species of Antispila, all occurring within a few centimetres of each other. Despite this sympatry, we did not find any indication of host shifts.
PhylogenyWhile it is generally inadvisable to rely solely on DNA barcodes for phylogenetic inferences, several recent studies suggest that some phylogenetic information could be taken from both the sequences themselves or translated amino acids (
Another interesting result from our provisional phylogenetic analyses is the hypothesis that Vitaceae may form the ancestral hostplants for modern Heliozelidae. For the basal genus Plesiozela Karsholt & Kristensen, 2003 no host plant information is available (
Antispila oinophylla is the first alien species of Heliozelidae introduced into Europe (
Factors leading to the introduction of Antispila oinophylla in Italy are unknown. Antispila oinophylla is the most recent Nearctic insect species reported to be damaging grapevines in Italy (first in Europe). Its invasion follows those of Phyllocnistis vitegenella (
The presence of several North American haplotypes of the DNA barcode in Italian material of Antispila oinophylla may indicate that the introduction could have involved more than a single introduction event.
InfestationEarly observations, carried out during 2007 and 2008 in the Trento province, showed that the incidence of infestation by Antispila oinophylla was significant in vineyards not treated with insecticides. By 2009 significant infestation levels were observed in several commercial vineyards in the Trentino and Veneto Regions despite the application of insecticides. Phyllocnistis vitegenella is also increasingly important in commercial vineyards in northeastern Italy. Native parasitoids showed some effects in keeping Phyllonorycter vitegenella below economic thresholds (
We thank Claudia Savio, Isabel Marinez-Saňudo, Alberto Pozzebon (Department of Environmental Agronomy and Crop Science, Padova, Italy), René Glas, Frank Stokvis and Dick Groenenberg (NCB Naturalis, Leiden, Netherlands) for their kind assistance in field trials and molecular laboratory work. Jean-François Landry (Agriculture Canada, Ottawa, Canada), Jason Weintraub (Academy of Natural Sciences in Philadelphia, Pennsylvania, USA) and Philip D. Perkins (Museum of Comparative Zoology, Harvard University, Cambridge, USA) loaned us material of American Antispila species. Richard L. Brown (Mississippi State University, Starkville, USA) is acknowledged for hospitality and assistance during our fieldwork in the Southeast USA. Paul Super and Keith Langdon (Smoky Mountains National Park, USA) were very helpful during fieldwork in the Great Smoky mountains. The National Park Service (USA) is acknowledged for permits to conduct scientific research. Jean-François Landry, Donald R. Davis (Smithsonian Institution, Washington DC, USA), Charles and Kim Mitter (University of Maryland, USA), Marko Mutanen (Oulu, Finland), Rodolphe Rougerie and Paul Hebert (Biodiversity Institute of Ontario, Guelph, Canada) kindly gave us permission to use unpublished DNA barcodes of various Heliozelidae. Sjaak Koster (NCB Naturalis) made several genitalia preparations, and Kees van den Berg (NCB Naturalis) assisted in many ways. We are indebted to Chris Owen (University of Connecticut, Storrs, USA) for his assistance with our Bayesian analyses. Many of the people mentioned above helped us further shape the paper by contributing to discussions on the various drafts.
Material studied for comparison with Antispila oinophylla
Antispila ampelopsifoliella
Canada: 2♂, 2♀ (2♂, 1♀ dissected), Ontario, Normandale, mines on Parthenocissus, rearing 57–157, emerged 16–23.iii.1958, Freeman & Lewis (CNC). USA: 1♂ (dissected), New York, St. Lawrence Co., Oak Point, leafmines on Phyllonorycter quinqueguttella, 12–17.viii.1988, DLW 88N41, emerged 14.iii.1989, D.L. Wagner (DLW); 1♀, (dissected), Vermont, Salisbury, Bryant Mtn. 16 Pudding Hill Rd., leafmines on Phyllonorycter quinqueguttella, 10–11.ix.1987, DLW 87J10, emerged 29.iv.1988, D.L. Wagner (DLW).
Leafmines and larvae (barcodes RMNH.INS.18536, 38), USA: Connecticut, Windham Co, Windham airport, Mansfield Hollow SP, 88 m, 9.ix.2011, EvN2011178, E.J. van Nieukerken & D.L. Wagner; Leafmines and larvae: New York, Essex Co, 3 km N Keene, Lacy Rd, 250 m, 14.ix.2011, EvN 2011233, E.J. van Nieukerken; Leafmines and larvae (barcode RMNH.INS.18588), Vermont, Addison Co., Button Bay SP, Lake Champlain borders, 44 m, 16.ix.2011, EvN2011254, E.J. van Nieukerken. All onParthenocissus quinqueguttella and in RMNH.
Antispila “vitis1”
USA: Larva(barcode RMNH.INS.18126), Connecticut, Tolland Co., Mansfield, 30.viii.2009, leafmines on Vitis, DLW 2009H285, D.L. Wagner (DLW, RMNH); 2♀ (barcode RMNH.INS.24205), Florida, Ocala Co., Ocala, Anthony, 18.vi.2006, leafmines on Vitis aestivalis, DLW 2006F32vii.2006, D.L. Wagner & T. Dickel (DLW).
Antispila voraginella
USA: 1♀, Arizona, Cochise Co., Huachuca Mts., Carr Canyon, Sierra Vista, Vitis, 4.vi.1987 [probably 1986], DLW 86F123, emerged 20.iv.1987, R. Wielgus (DLW); 1♂, Arizona, Cochise Co., Huachuca Mts., Miller canyon, Vitis arizonica, 31.vii.1986, DLW 86G15, emerged 23.v.1987, D.L. Wagner (DLW); 3♂, 3♀ (2♂ dissected and DNA barcode RMNH.INS.23918), Arizona, Santa Cruz Co., Sycamore Cany.S of Ruby, leafmines Vitis arizonica, 23.vii.1991, DLW 91G18, emerged 3.vi.1992, D.L. Wagner (DLW); 2♂, 1♀ (1♂ dissected and DNA barcode RMNH.INS.23917), Texas: Culberson Co., Guadalupe Mts NP, McKittrick canyon, leafmines Vitis 30–31.vii.1989, DLW 89G108, emerged 14–23.iv.1990, D.L. Wagner (DLW); 1♂ (holotype) 2♀ (paratypes), Utah: [Washington County], Zion Canyon, leafmines Vitis arizonica, 24.vii.[1925], B. 1206, emerged 12–14.iv.[1926], A.F. Braun (ANSP).
Antispila hydrangaeella
USA: 27♂ ♀ (1♂ dissected), leafmines & larvae (DNA barcode RMNH.INS.18416), Georgia, Gilmer Co., Chattahoochee Nat. Forest, Barnes Creek Picknick Area, 760 m, hardwood forest, leafmines on Hydrangea arborescens, 14.x.2010, EvN 2010279, emerged 3–18.iv.2011, E.J. van Nieukerken & C. Doorenweerd (RMNH); 29♂ ♀, leafmines & larvae (DNA barcode RMNH.INS.18206), North Carolina, Haywood Co., NP Great Smoky Mts, Big Creek area, 573 m, hardwood forest along river, leafmines on Hydrangea arborescens, 28.ix.2010, EvN 2010073, emerged 31.iii–9.iv.2011, E.J. van Nieukerken & C. Doorenweerd (RMNH).
Antispila cf viticordifoliella
Canada: 1♀, Ontario, Ottawa, 14.iv.1971, “Vir. creeper” [Parthenocissus], 70–48, G.C. Lewis (CNC). USA: Leafmines and larvae (barcode RMNH.INS.18508), USA: Connecticut, Windham Co, Windham airport, Mansfield Hollow SP, 88 m, leafmines on Parthenocissus quinqueguttella, 9.ix.2011, EvN2011178, E.J. van Nieukerken & D.L.Wagner (RMNH); 1♀ (barcode RMNH.INS.24207), Florida, Miami-dade Co., Key Biscane, Cape Florida SP, leafmines on Parthenocissus quinqueguttella, 19.iv.2002, DLW 2002D6, emerged 22.v.2002, R. Wagner & D.L. Wagner (DLW); Leafmines and larvae(barcodes RMNH.INS.18560–61), USA: New York, Essex Co, Hwy 9N, 3.5 km WSW Keeseville, 142 m, 14.ix.2011, EvN 2011238, E.J. van Nieukerken; Leafmines and larvae(barcodes RMNH.INS.18587), Vermont, Addison Co., Button Bay SP, Lake Champlain borders, 44 m, 16.ix.2011, EvN2011254, E.J. van Nieukerken. All onParthenocissus quinqueguttella and in RMNH.
Antispila cf isabella
Canada: 2♀ (dissected, MIC1862), Ontario, Simcoe, emerged 4.vi.1965, leafmines on Vitis, T.N. Freeman (CNC); 1♂, Ontario, Simcoe, emerged 10.iii.1971, leafmines on Vitis, T.N. Freeman (CNC); USA: 1♂, Connecticut, Windham Co., Hampton, 916 Pudding Hill Rd., leafmines on Vitis, 22.viii.1989, DLW 89H47, emerged 18–20.iii.1990, D.L. Wagner (DLW); Leafmines and larvae(barcodes RMNH.INS.18127–28), Connecticut, Tolland Co., Mansfield, 30.viii.2009, leafmines on Vitis, DLW 2009H285, D.L. Wagner (DLW, RMNH); 1♂, larvae and leafmines, Georgia, Murray Co., Chattahoochee Nat. Forest, E of Chatsworth, GA rd 52, 523 m, leafmines on Vitis aestivalis var. aestivalis, 14.x.2010, EvN2010266, emerged 14.iv–4.v.2011, E.J. van Nieukerken & C. Doorenweerd (RMNH); 1♂, (dissected, MIC1859), Kentucky, Morehead, emerged 3.vi.1963, leafmines on Vitis, T.N. Freeman (CNC); Leafmines and larvae(barcodes). Vermont, Addison Co., Button Bay SP, Lake Champlain borders, 44 m, 44.18154N, 73.36892W, on Vitis riparia, 16.ix.2011, EvN2011253, E.J. van Nieukerken (RMNH); 1♂, Vermont, Chittenden Co., South Burlington, 11.viii.1988, DLW 88H23, emerged 30.iii.1989, D.L. Wagner (DLW)
Antispila “vitis2”
USA: Leafmines and larvae(barcode RMNH.INS.18131), Connecticut, Tolland Co., Mansfield, 30.viii.2009, leafmines on Vitis, DLW 2009H285, D.L. Wagner (DLW, RMNH); Leafmines and larvae(barcode RMNH.INS.18656), Vermont, Addison Co., Button Bay SP, Lake Champlain borders, 44 m, leafmines on Vitis riparia, 16.ix.2011, EvN2011253, E.J. van Nieukerken (RMNH).
Holocacista rivillei
Italy: 28 adults (4♂, 1♀ dissected), Trento, Borghetto, Vitis vinifera, 2007, emerged i-ii.2008, M. Baldessari (RMNH).
List of samples used for the DNA barcoding. (doi: 10.3897/zookeys.170.2617.app2) File format: Excel spreadsheet (xls).
Explanation note: List of samples used for the DNA barcoding analysis with collection site and BOLD and GenBank accession numbers. *GenBank sequences from
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Nieukerken EJ, Wagner DL, Baldessari M, Mazzon L, Angeli G, Girolami V, Duso C, Doorenweerd C (2012) Antispila oinophylla new species (Lepidoptera, Heliozelidae), a new North American grapevine leafminer invading Italian vineyards: taxonomy, DNA barcodes and life cycle. ZooKeys 170: 29–77. doi: 10.3897/zookeys.170.2617.app2