(C) 2010 Peter Jäger. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Palystes kreutzmanni sp. n. is described from habitats close to Kleinmond, in the Western Cape Province, South Africa. Spiders of this new species live in the typical fynbos vegetation of the Western Cape region. They build retreats between apical leaves of Leucadendron bushes. The systematic position of Palystes kreutzmanni sp. n. is discussed. Male and female show characters of different species groups, especially the female copulatory organ seems to be unique within the genus Palystes L. Koch, 1875.
Taxonomy, systematics, relationships, species groups, new species
The genus Palystes L. Koch 1875 was revised by
During an expedition in South Africa the junior author collected spiders of a species of the subfamily Palystinae
Simon, 1897 in Western Cape Province. It is described as a new species
and its relationships are discussed. A short identification key is
provided as partial update of that of
Measurements are in millimetres, arising points of tegular appendages are given as clock-position of the left palp in ventral view. Leg and palp measurements are given as: total (femur, patella, tibia, metatarsus, tarsus). Leg spination is given as: prolateral, dorsal, retrolateral, ventral (the latter digit may be omitted in the case of absence of ventral spines). Female copulatory organs were treated with 96% lactic acid. Material is stored in 70% denatured ethanol.
Abbreviations: ALE anterior lateral eyes, AME anterior median eyes, DK field numbers of Dirk Kunz, PJ subsequent numbers of Sparassidae examined by Peter Jäger, PLE posterior lateral eyes, PME posterior median eyes, RTA retrolateral tibial apophysis, SD tissue sample numbers from arachnology collection SMF.
Museum collections
ISAM Iziko South African Museum, Cape Town (Margie Cochrane)
PPRI Plant Protection Research Institute, Pretoria (Ansie Dippenaar-Schoeman
SMF Senckenberg Research Institute, Frankfurt (Peter Jäger)
Holotype male (PJ 3261), South Africa, Western Cape Province, W of Kleinmond, 34°20'8.69"S, 18°58'32.45"E, 197 m altitude, fynbos vegetation, retreat between apical leaves of Leucadendron sp., by hand, by day, Esther van der Westhuizen and Dirk Kunz leg. 15.V.2004, DK 48, SD 224–225 (SMF). Paratypes: 1 female (PJ 3262), same data as for holotype, except for 34°20'9.708"S, 18°58'32.7"E, 193 m altitude, DK 50, SD 273–274 (SMF). 1 female (PJ 3266), same data as for preceding specimen except for DK 54, SD 233–234 (ISAM). 1 female (PJ 3265), same data as for preceding specimen except for DK 52, SD 229–230 (PPRI). 1 female (PJ 3264), same data as for preceding specimen except for DK 53, SD 231–232 (SMF). 1 female (PJ 3263), same data as for preceding specimen except for DK 51, SD 2226–227 (SMF). 1 female (PJ 3267), same data as for preceding specimen except for 34°20'7.7274"S, 18°58'31.9074"E, 192 m altitude, DK 47, SD 228 (SMF).
Medium sized Sparassidae, body length of males: 12.7, of females: 13.7–17.0. Males (Figs 1–5) similar to those of Palystes lunatus-group (sensu
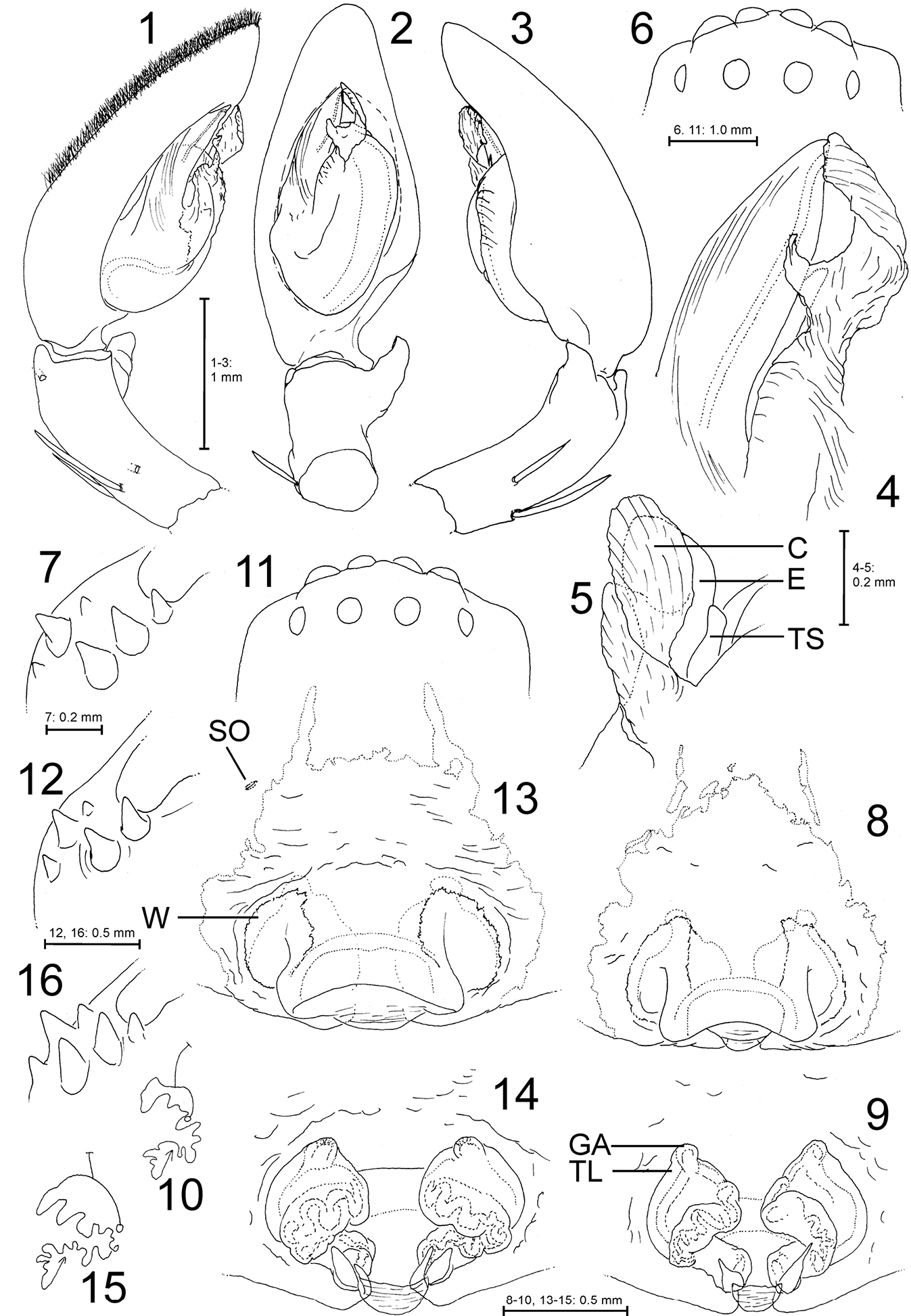
Palystes kreutzmanni sp. n. from South Africa, Western Cape Province, Kleinmond (1–7 Holotype male, PJ 3261 8–12 Paratype female, PJ 2362 13–15 Paratype female, PJ 3267 16 Paratype female, PJ 3263). 1–5 Palp (1 prolateral, 2 ventral, 3 retrolateral, 4 detail of embolus and conductor, ventral, 5 same, retrolatero-distal). 6, 11 Eye arrangement, dorsal 7, 12, 16 Left chelicera, ventral 8, 13 Epigyne, ventral 9, 14 Vulva, dorsal 10, 15 Schematic course of internal duct system (circle – copulatory orifice, T – glandular appendages, arrow – fertilisation duct in direction of uterus externus). Abbreviations: C – conductor, E – embolus, GA – glandular appendage, SO – slit sense organ, TL – transparent layer, TS – tegular sclerite, W – transparent “window”.
In honour of Mr Jürgen Kreutzmann for supporting the systematic research, description of biodiversity and nature conservation in South Africa; noun in genitive case.
(holotype, PJ 3261). Prosoma length 6.4, prosoma width 5.1, anterior width of prosoma 2.8, opisthosoma length 6.3, opisthosoma width 3.4. Eyes (Fig. 6): AME 0.39, ALE 0.48, PME 0.35, PLE 0.31, AME–AME 0.15, AME–ALE 0.04, PME–PME 0.39, PME–PLE 0.41, AME–PME 0.45, ALE–PLE 0.29, clypeus height at AME 0.16, clypeus height at ALE 0.21. Spination: Palp: 131, 001, 2121; legs: femur I–III 323, IV 332; patella I–IV 101; tibia 2226; metatarsus I–III 2024, IV 3036. Ventral metatarsus IV distally with median spine embedded in scopula. Leg formula: 2143. Measurements of palp and legs: Palp 7.5 (2.5, 0.9, 1.6, -, 2.5), leg I 29.5 (8.4, 3.5, 8.0, 7.4, 2.2), leg II 30.3 (8.9, 3.5, 8.3, 7.4, 2.2), leg III 23.2 (7.1, 2.8, 6.1, 5.3, 1.9), leg IV 28.0 (8.9, 2.8, 7.2, 7.0, 2.1). Cheliceral furrow with 3 anterior and 3 posterior teeth (Fig. 7).
Palp as in diagnosis. Dorsal scopula covering two apical third of cymbium (Fig. 1). Basal cymbium strongly truncated (Fig. 2). Tibia distinctly shorter than cymbium. Embolus arising in a 8.30-o’clock-position, conductor almost centrally from tegulum. Sperm duct running broad and submarginally at retrolateral tegular margin, narrowing distinctly in S-curve prolaterally and further on its way to subapical opening at embolus. Both margins of embolus slightly convex (Figs 2, 4). Conductor membranous, basally twisted and apically folded.
Colouration in ethanol (Figs 17–19): Reddish-brown with pattern consisting of white and red hairs and dark markings. Dorsal shield with two lateral longitudinal bands narrowing anteriorly and dark triangular pattern in front of fovea. Indistinct narrow bright median line in front of fovea. Clypeus with transversal band of dense bright hairs. Sternum dark reddish brown, without pattern. Labium dark reddish-brown proximally, with distal bright lip. Gnathocoxae brighter, especially at inner margins. Ventral and retrolateral coxae bright yellowish brown, prolateral sides dark brown. Chelicerae dark reddish brown, with two short longitudinal bands consisting of white hairs, one frontal and one lateral. Palpal femora yellowish brown, rest reddish brown as other appendages. Legs with small spine patches consisting of white hairs, tibiae annulated. Ventral femora I and II with white patch in distal half consisting of white hairs (more distinct in live specimens: Fig. 22). Dorsal tarsi with small longitudinal dark patch in distal half. Dorsal opisthosoma with solid black patch above heart surrounded by bright lanceolate area extending to spinnerets: This brighter area bordered especially in posterior half by darker part. Lateral opisthosoma becoming brighter to ventral side. Ventral opisthosoma with red patch between epigastric furrow and spinnerets becoming blackish anterior and posterior and including one pair of small white patches in the middle and four longitudinal lines of tiny muscle sigillae; further bright dots situated laterally of the red patch. For colouration of live specimen see Fig. 22.
(paratype, PJ 3262). Prosoma length 6.5, prosoma width 5.2, anterior width of prosoma 3.4, opisthosoma length 8.0, opisthosoma width 5.3. Eyes (Fig. 11): AME 0.39, ALE 0.40, PME 0.31, PLE 0.30, AME–AME 0.16, AME–ALE 0.07, PME–PME 0.41, PME–PLE 0.39, AME–PME 0.40, ALE–PLE 0.37, clypeus height at AME 0.21, clypeus height at ALE 0.25. Spination: Palp: 131, 101, 2121, 1014; legs: femur I–III 323, IV 332; patella I 001, II 0(1)01, III–IV 001; tibia 2126; metatarsus I–III 2024, IV 3036. Ventral metatarsi III–IV with distal median spine embedded in scopula. Leg formula: 2143. Measurements of palp and legs: Palp 7.5 (2.2, 1.1, 1.7, -, 2.5), leg I 24.1 (6.9, 3.2, 6.3, 5.8, 1.9), leg II 24.3 (7.2, 3.2, 6.4, 5.7, 1.8), leg III 18.3 (5.7, 2.5, 4.6, 4.1, 1.4), leg IV 22.6 (7.2, 2.5, 5.7, 5.5, 1.7). Cheliceral furrow with 3 anterior and 2 posterior teeth (Fig. 12, but see “Variation”, Fig. 16). Palpal claw with 6 teeth.
Copulatory organ as in diagnosis. Epigynal field
as long as wide with narrow anterior bands. No slit sense organs present
close to epigynal field (but see “Variation”, Fig. 13).
Bright transparent areas (“windows”) situated anterolaterally of median
septum. Median septum containing a large blunt cavity, most likely for
accommodating the male RTA during copulation process (Figs 8, 13; as observed, e.g., in Heteropoda spp., Jäger unpubl.). Internal duct system with additional external layer (Fig. 9: TL) concealing the original profile of the ducts.
Colouration in ethanol (Figs 20–21): As in male but in general with less distinct pattern. Pattern of dorsal shield of prosoma barely recognisable, heart patch of dorsal opisthosoma indistinct. Legs with fewer and shorter white hairs. Prolateral coxae bright yellowish brown. Chelicerae without lateral bright longitudinal band.
Females (n=6): Spination: femur III 323(2), patellae I 101/001, II–IV 101/001/000. Chelicerae with 3 posterior teeth (Fig. 16).
Colouration: in general there were distinct differences in contrast and strength of the pattern. Dorsal opisthosoma with patch above heart only marginally black, uniformly grey or with same colour as surrounding areas; in one female (PJ 3264) one lateral pair of small white patches was included in the middle of the heart. Ventral opisthosoma with paired white patches and white dots varying in size, shape and position. In one female (PJ 3266) an additional small unpaired white median patch was present between the paired patches and the spinnerets. Pattern of prolateral coxae varying from entirely yellowish brown to having dark patches to a different degree with stronger markings in anterior coxae. Frontal longitudinal white bands of chelicerae varying in length.
Copulatory organ: In one female (PJ 3267) one slit sense organ was present (Fig. 13). Internal duct system may be more compact and right and left half more separated (Fig. 14). The median septum may be broader (Fig. 13) or exhibiting a median bulge in the concave posterior margin.
Palystes kreutzmanni sp. n. from South Africa, Western Cape Province, Kleinmond (17–19 Holotype male, PJ 3261 20–21 Paratype female, PJ 3263).
Palystes kreutzmanni sp. n. from South Africa, Western Cape Province, Kleinmond (22 Holotype male, PJ 3261 23 Fynbos vegetation at the type locality 24 Leucadendron sp., predominant plant at the type locality 25 Retreat of Palystes kreutzmanni sp. n. between apical leaves of Leucadendron sp.).
Only known from the type locality (Figs 23–24).
Retreats were built between apical leaves of Leucadendron plants (Fig. 25). Spiders were resting here during the day time.
Palystes kreutzmanni sp. n. cannot be assigned to any of the species groups listed in
Number of tibial spines should differentiate between two genera (
Considering all these observations Palystes kreutzmanni sp. n. may be one species with mixed apomorphic character states, or characters previously used for differentiating species groups within Palystinae were symplesiomorphic. However, in both cases only a thorough revision of all taxa in question can help to understand character evolution and phylogenetic position of species and genera, and finally placing the new species correctly.
In the identification key of
| 16(15) | Tibial apophysis three-lobed; embolus straight; conductor elongate, straight (Cape Town, Stellenbosch, Somerset West and Bredashorp districts, western Cape, South Africa) | Palystes castaneus |
| – | Tibial apophysis entire; embolus recurved through 90° over bulb; conductor short, bowl shaped | 17 |
| (leading to Palystes martinfilmeri and Palystes stilleri) | ||
| – | Tibial apophysis entire; embolus short, straight, pointed; conductor short, slightly bowl-shaped (western Cape, South Africa) | Palystes kreutzmanni sp. n. |
Females key out at #7. At this point a trichotomy should be inserted:
| 7(2) | Sternum entirely black; femora I–II mottled without distinct markings; opisthosoma laterally mottled, without distinct markings, ventrally with black bell-shaped mark or black-framed yellow panel between black crescent and spinnerets; septum posteriorly produced laterally (western Cape, South Africa) | 8 |
| (leading to Palystes stilleri, Palystes martinfilmeri and Palystes castaneus) | ||
| – | Sternum usually with two mesally interrupted transverse dark bars (sometimes faint, sometimes with an additional short longitudinal bar mesally); femora I–II ventrally with clear white spots; opisthosoma laterally with clear white spots, ventrally rich red to orange-red with distinct clear white spots between black crescent and spinnerets (indistinct in specimens from Amatola Mountains, eastern Cape, South Africa); septum with transverse or posteriorly produced median lobe | 10 |
| (leading to Palystes crawshayi, Palystes stuarti, Palystes lunatus, Palystes perornatus, Palystes leppanae and Palystes karooensis) | ||
| – | Sternum dark reddish brown to black without transverse bars; femora I–II ventrally with one single white distal patch ventrally and bright spine patches (indistinct in ethanol); opisthosoma laterally with indistinct irregular pattern, ventrally dark red to black with indistinct white spots mostly in anterior half; septum posteriorly produced laterally, copulatory ducts with cover of sclerotised layer concealing glandular appendages (western Cape, South Africa) | Palystes kreutzmanni sp. n. |
Dirk Kunz received support on his expedition to South Africa from various people and institutions: Ansie Dippenaar-Schoeman (Pretoria) and Norman Larsen (Cape Town) organised the field work, Esther van der Westhuizen (Butterfly World, Western Cape) helped with collecting spiders in the Western Cape Province. The German Academic Exchange Service (DAAD) provided financial support. Additionally, the manuscript benefited from comparison with other Palystinae species examined during visits of the senior author in the Natural History Museum London (NHM, Paul Hillyard, Janet Beccaloni), Museum National d’Histoire Naturelle, Paris (MNHN, Christine Rollard), Zoological Museum of the University, Berlin (ZMB, Jason Dunlop), Hungarian Natural History Museum Budapest (HNHM, Sandór Mahunka, Tamás Szűts), and Zoological Museum of the University, Copenhagen (ZMUC, Nicolaj Scharff). Helpful efforts by the curators, grants by the European Community (Access to Research Infrastructure action of the Improving Human Potential Programme) as well as steady support from Ansie Dippenaar-Schoeman (Pretoria) are gratefully acknowledged. Peter Croeser (Pietermaritzburg), Cristina Rheims (Sao Paulo) and Jeremy Miller (Leiden) helped with checking the manuscript.