(C) 2010 Christopher G. Majka. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Mycetophagidae (hairy fungus beetles) of the Maritime Provinces of Canada are surveyed. Seven species in the genera Mycetophagus, Litargus, and Typhaea are found in the region. Six new provincial records are reported including Mycetophagus punctatus and Mycetophagus flexuosus, whichare newly recorded in the Maritime Provinces. The distribution of all species is mapped, colour habitus photographs of all species are figured, and an identification key to species is provided. The discussion notes that four of the species found in the region are apparently rare, possibly due to the history of forest management practices in the region; a situation similar to that of a significant proportion of other saproxylic beetles found in the Maritime Provinces.
Coleoptera, Mycetophagidae, Mycetophaginae, Mycetophagus, Litargus, Typhaea, aritime Provinces, Canada, biodiversity, fungus beetles, rare species
The Mycetophagidae
(hairy fungus beetles) are a family of relatively small, fungus-eating
beetles. Only five genera and 26 species are known in North America,
15 of which have been recorded in Canada (
Acronyms (largely following
ACNS Agriculture and Agri-Food Canada, Kentville, Nova Scotia, Canada
ACPE Agriculture and Agri-Food Canada, Charlottetown, Prince Edward Island, Canada
CBU Cape Breton University, Sydney, Nova Scotia, Canada
CGMC Christopher G. Majka Collection, Halifax, Nova Scotia, Canada
CNC Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada
DHWC David H. Webster Collection, Kentville, Nova Scotia, Canada
JCC Joyce Cook Collection (now at the New Brunswick Museum, Saint John, New Brunswick, Canada)
JOC Jeffrey Ogden Collection, Truro, Nova Scotia, Canada
KIC Kent Island Collection, Bowdoin College, Brunswick, Maine, USA
NSAC Nova Scotia Agricultural College, Bible Hill, Nova Scotia, Canada
NSMC Nova Scotia Museum, Halifax, Nova Scotia, Canada
NSNR Nova Scotia Department of Natural Resources Insectary, Shubenacadie, Nova Scotia, Canada
RMC Richard Migneault Collection, Edmundson, New Brunswick, Canada
Abbreviations: FIT, flight intercept trap.
IdentificationAn identification key to species [adapted from
| 1 | Epipleural fold of elytra concave; Litargus Erichson | Litargus tetraspilotus (Fig. 9) |
| – | Epipleural fold of elytra horizontal and flat | 2 |
| 2 | Eyes transverse, sinuate anteriorly | Mycetophagus Hellwig |
| – | Eyes more rounded, not sinuate anteriorly; Typhaea Curtis | Typhaea stercorea (Fig. 8) |
| 1 | Antennae gradually widening towards apex with the last 3, 4, or 5 antennomeres before the apical one more or less serrate and slightly asymmetrical; subgenus Mycetophagus (s. str.) | 2 |
| – | Antennae with a 4- or 5-segmented club, strongly to feebly differentiated from preceding antennomeres; antennomeres bilaterally symmetrical | 4 |
| 2(1) | Apical antennomere longer than 2 preceding combined; length 4.6–6.3 mm | Mycetophagus punctatus (Fig. 3) |
| – | Apical antennomere shorter than or as long as 2 preceding combined; length 3.6 mm or less | 3 |
| 3(2) | Pale elytral markings reaching or crossing suture from basal 1/5 to 1/2 of elytra | Mycetophagus flexuosus (Fig. 4) |
| – | Pale elytral markings not attaining suture | Mycetophagus serrulatus (Fig. 5) |
| 4(1) | Antennae with a 5-segmented club; subgenus Ilendus Casey; length 3.2–4.7 mm | Mycetophagus pluripunctatus (Fig. 6) |
| – | Antennae with a 4-segmented club; subgenus Parilendus Casey; length 3.3–4.0 mm | Mycetophagus quadriguttatus (Fig. 7) |
* Note: elytral markings on Mycetophagus species are variable.
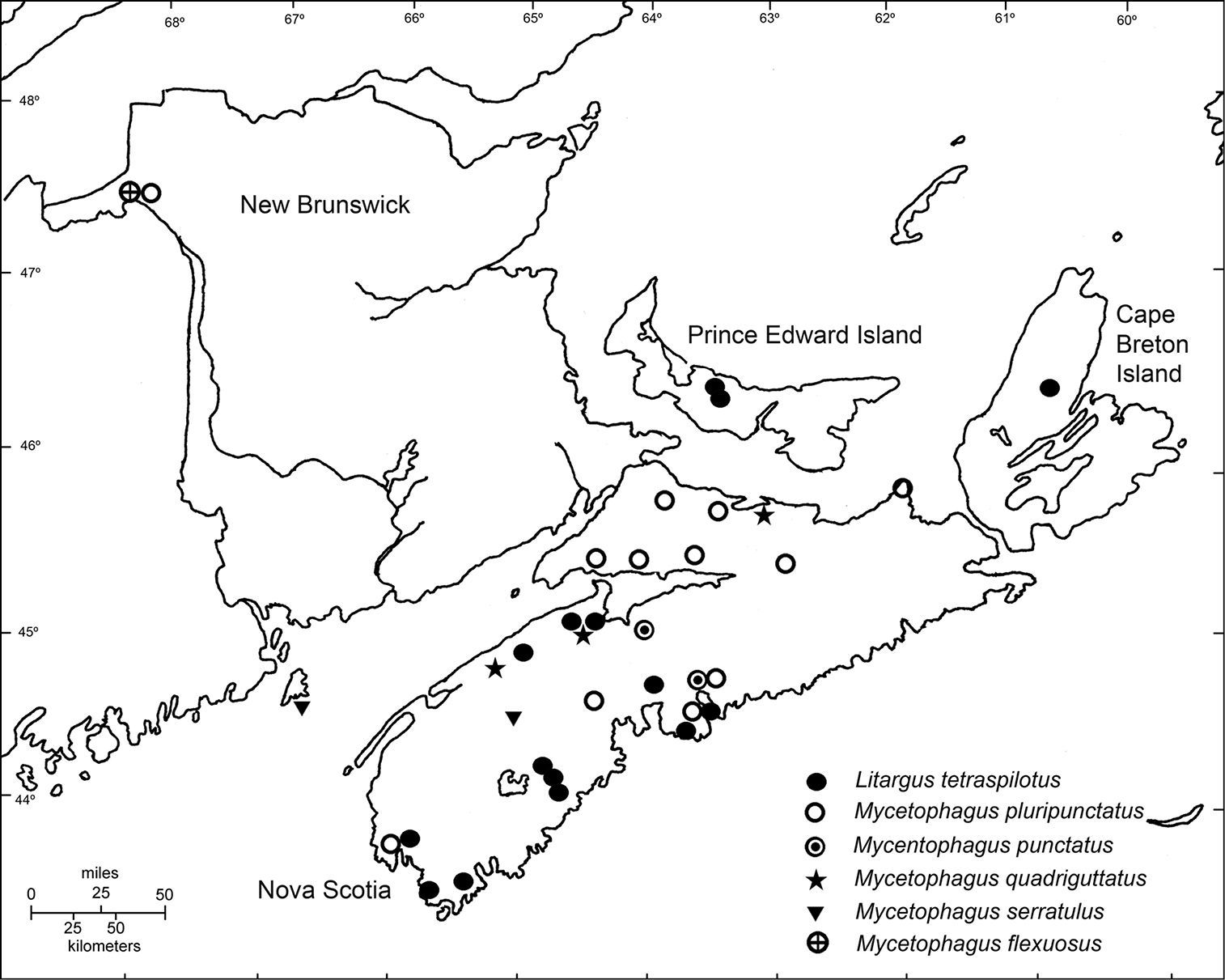
ResultsIn the course of this survey 175 specimens of Mycetophagidae were examined – 8 from New Brunswick, 149 from Nova Scotia, and 18 from Prince Edward Island. Included were specimens of seven species in three genera. Mycetophagus flexuosus Say is newly recorded in the Maritime Provinces from New Brunswick; Mycetophagus punctatus Say is newly recorded in the Maritime Provinces from Nova Scotia; Mycetophagus serrulatus Casey is newly recorded in New Brunswick; Mycetophagus pluripunctatus LeConte is newly recorded in New Brunswick; Mycetophagus quadriguttatus Müller is newly recorded in Nova Scotia; and Litargus tetraspilotus LeConte is newly recorded in Prince Edward Island – a total of five new provincial records, two of which are newly recorded in the region. Four species are known from New Brunswick, six from Nova Scotia, and two from Prince Edward Island (Table 1).
Mycetophagidae fauna of the Maritime Provinces of Canada
| NB | NS | PE | Distribution in NE North America | ||
|---|---|---|---|---|---|
| Mycetophaginae | |||||
| Mycetophagus Hellwig | |||||
| subgenus Mycetophagus Hellwig | |||||
| Mycetophagus flexuosus Say | 1 | MA, ME, NB, NH, NY, ON, QC, VT | |||
| Mycetophagus punctatus Say | 1 | CT, MA, ME, NH, NS, NY, ON, QC, VT | |||
| Mycetophagus serrulatus Casey | 1 | 1 | NB, NH, NS, NY, ON, QC, VT | ||
| subgenus Ilendus Casey | |||||
| Mycetophagus pluripunctatus LeConte | 1 | 1 | MA, ME, NB, NH, NS, NY, ON, QC, VT | ||
| subgenus Parilendus Casey | |||||
| Mycetophagus quadriguttatus Müller * | 1 | 1 | MA, ME, NB, NH, NS, NY, ON, QC, VT | ||
| Typhaea stercorea (Linnaeus) † | 1 | 1 | 1 | MA, ME, NB, NH, NS, NY, ON, PE, QC, RI, VT | |
| Litargus tetraspilotus LeConte | 1 | 1 | MA, ME, NH, NS, NY, ON, PE, QC, RI, VT | ||
| totals | 4 | 6 | 2 | ||
Notes: * Holarctic species; † adventive Palaearctic species; NB New Brunswick; PE Prince Edward Island; NS Nova Scotia.
Distribution in northeastern North America: for the purposes of this treatment, northeastern North America is taken to consist of the following jurisdictions: CT Connecticut; LB Labrador; MA Massachusetts; ME Maine; NB New Brunswick; NF insular Newfoundland; NH New Hampshire; NS Nova Scotia; NY New York; ON Ontario; PE Prince Edward Island; PM Saint-Pierre et Miquelon; QC Québec; RI Rhode Island; and VT Vermont.
Distribution of Litargus tetraspilotus, Mycetophagus pluripunctatus, Mycetophagus punctatus, Mycetophagus quadriguttatus, Mycetophagus serrulatus, and Mycetophagus flexuosus in the Maritime Provinces of Canada.
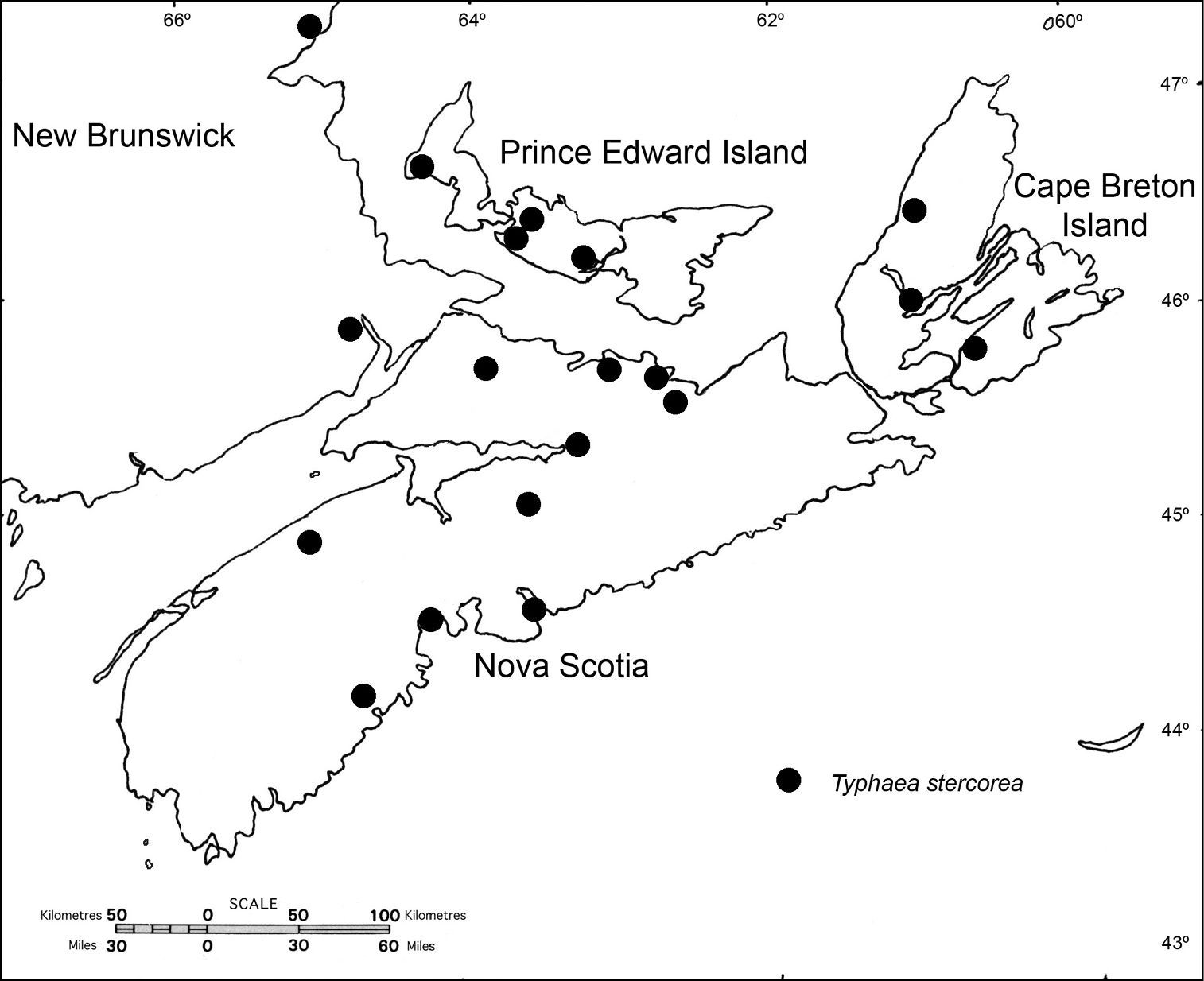
Distribution of Typhaea stercorea in the Maritime Provinces of Canada.
Dorsal habitus photograph of Mycetophagus punctatus. Length: 4.6–6.3 mm. Photo credit: Guy A. Hanley.
NEW BRUNSWICK: Madawaska County: Edmundston, 47°22.285'N; 68°14.663'W, 14 August 2010, R. Migneault, in polypore on dead aspen log (1, RMC); Edmundston, 47°22.285'N; 68°14.663'W, 22 August 2010, R. Migneault, in polypore on dead aspen log (1, RMC).
Mycetophagus flexuosus is newly recorded in the Maritime Provinces from New Brunswick (Fig. 1).
Dorsal habitus photograph of Mycetophagus flexuosus. Length: 3.0–4.6 mm. Photo credit: Tom Murray.
NOVA SCOTIA: Halifax Co.: Soldier Lake, 7 June 2005, J. Ogden, spruce beetle trap (1, NSNR); Hants Co.: Smileys Park, 6 July 2005, J. Ogden, spruce beetle trap (1, NSNR).
Mycetophagus punctatus
Say is newly recorded in the Maritime Provinces from Nova Scotia. Both
specimens were collected in the central mainland of Nova Scotia (Fig. 1). The species is common under loose bark and on fungi (
NEW BRUNSWICK: Charlotte Co.: Grand Manan archipelago, Kent Island, 23 July 2012, M. Steck, balsam fir forest, sweeping (1, KIC). NOVA SCOTIA: Annapolis Co.: Durland Lake, 21 June 2003, P. Dollin, hemlock/balsam fir/black spruce forest (120+ years), bracket fungi on white birch (1, NSMC).
Mycetophagus serrulatus Casey is newly recorded in New Brunswick. The species was reported from Nova Scotia by
Dorsal habitus photograph of Mycetophagus serrulatus. Length: 1.3–3.6 mm. Photo credit: Christopher G. Majka.
NEW BRUNSWICK: Madawaska County: Edmundston, 47°22.285'N; 68°14.663'W, 22 August 2010, R. Migneault, in polypore on dead aspen log (1, RMC). NOVA SCOTIA: Antigonish Co.: Cape George Point, 23 June1993, M. LeBlanc, funnel trap (1, NSMC); Colchester Co.: Kemptown, 1 June 1995, 28 June 1995, C. Corkum, young deciduous forest, FIT (2, NSMC); Upper Bass River, 18 May 1995, C. Corkum, old deciduous forest, FIT (1, NSMC); Upper Bass River, 3 June 1995, C. Corkum, old deciduous forest, FIT (1, NSMC); Cumberland Co.: East Leicester, 2 June 1995, C. Corkum, old deciduous forest, FIT (1, NSMC); East Leicester, 14 June 1995, C. Corkum, old deciduous forest, FIT (1, NSMC); East Leicester, 15 June 1995, C. Corkum, old deciduous forest, FIT (1, NSMC); Fox River, 17 May 1995, C. Corkum, young deciduous forest, FIT (1, NSMC); Fox River, 3 June 1995, C. Corkum, young deciduous forest, FIT (1, NSMC); Harrington River, 13 July 1995, C. Corkum, young deciduous forest, FIT (1, NSMC); Wentworth, 21 May-5 July 1965, B. Wright, sugar maple forest, window trap (1, NSMC); Halifax Co.: Halifax, 1 December 1986, B. Wright (1, NSMC); Soldier Lake, 30 July 2004, D. MacDonald, spruce beetle trap (1, NSNR); Lunenburg Co.: Card Lake, 2-15 June, 1997, D.J. Bishop, red spruce/hemlock forest (old growth), FIT (1, NSMC); Yarmouth Co.: Wellington, 23-29 August 1992, J. & F. Cook, mixed forest (1, JCC).
Mycetophagus pluripunctatus LeConte is newly recorded in New Brunswick. The species was reported from Nova Scotia by
Dorsal habitus photograph of Mycetophagus pluripunctatus. Length 3.2–4.7 mm. Photo credit: Nicholas Gompel.
NOVA SCOTIA: Annapolis Co.: Paradise, 11 June 2005, K. Webster, spruce beetle trap (1, NSNR); Colchester Co.: Balmoral Mills, 19 June 1974, B. Wright, grist mill (1, NSMC); Kings Co.: Kentville, 10 August 2005, D.H. Webster, compost heap, moldy corncobs (1, DHWC).
Mycetophagus quadriguttatus Müller is newly recorded in Nova Scotia (Fig. 1). The species was reported from New Brunswick by
Although
Dorsal habitus photograph of Mycetophagus quadriguttatus. Length 3.3–4.0 mm. Photo credit: Christopher G. Majka.
Eighty-two specimens (NB=6, NS=66, PE=12) were examined. The earliest records from each province are: NEW BRUNSWICK: Northumberland Co.: Tabusintac, 13 June 1939, 26 July 1939, W.J. Brown (2, CNC). NOVA SCOTIA: Colchester Co.: Truro, 4 March 1919, collector not recorded (8, NSAC). PRINCE EDWARD ISLAND: Prince Co.: Central Bedeque, 29 July 1954, F.M. Cannon (1, ACPE).
Typhaea stercorea (Linnaeus) was reported from New Brunswick, Nova Scotia, and Prince Edward Island by
The dates of earliest detection are given above: New Brunswick (1939), Nova Scotia (1919), and Prince Edward Island (1954). Typhaea stercorea is widespread in Europe, having been recorded in every country and region in the continent (
Dorsal habitus photograph of Typhaea stercorea. Length: 2.2–3.2 mm. Photo credit: Tim Moyer.
NOVA SCOTIA: Cape Breton Co.: East Bay, 9 September 2003, C.W. D’Orsay (1, CBU); Colchester Co.: Bible Hill, 8 July 2004, K.R. Aikens, pasture, sweep (1, CBU); Bible Hill, 14 June 2005, S.M. Townsend, sweep (3, CBU); Debert, 9 June 1994, J. Ogden (1, NSNR); Masstown, 7 September 2002, C.G. Majka, marshy swamp (1, CGMC); Shubenacadie, 26 August 1997, J. Ogden (1, NSNR); Digby Co.: Brier Island, Pond Cove, 9 August 2004, J. Ogden & K. Goodwin, knapweed, sweep (3, JOC); Brier Island, Pond Cove, 10 August 2004, J. Ogden & K. Goodwin, sweep (1, JOC); Brier Island, Westport, 9 August 2004, J. Ogden & K. Goodwin, grassland, sweep (2, JOC); Halifax Co.: Big Indian Lake, 16 July 2003, P. Dollin, Picea rubens forest (80-120 years), in rotting mushroom (1, NSMC); Point Pleasant Park, 15 August 2000, 7 September 2000, C.G. Majka, mixed forest (2, CGMC); Point Pleasant Park, 9 September 2000, 2 June 2002, 23 July 2002, C.G. Majka, coniferous forest (3, CGMC); Point Pleasant Park, 12 May 2001, 10 June 2001, 25 May 2002, C.G. Majka, coniferous forest, on Picea rubens (6, CGMC); Point Pleasant Park, 19 May 2001, 29 May 2001, C.G. Majka, coniferous forest, on Pinus strobus (4, CGMC); 29 July 2001, 18 August 2001, Point Pleasant Park, C.G. Majka, mixed forest (2, CGMC); Point Pleasant Park, 9 May 2002, C.G. Majka, coniferous forest, on Abies balsamea (1, CGMC); Point Pleasant Park, 9 June 2002, C.G. Majka, mixed forest, on Aralia hispida (1, CGMC); Point Pleasant Park, 7 July 2002, C.G. Majka, seashore (1, CGMC); Point Pleasant Park, 14 September 2002, C.G. Majka, marsh, on herbaceous vegetation (1, CGMC); Point Pleasant Park, 30 June 2004, C.G. Majka, coniferous forest, on Pinus sylvestris (2, CGMC); West Dover, 7 September 2003, C.G. Majka, coastal barrens, heaths (1, CGMC); Kings Co.: Aldershot, 5 August 1949, 2 August 1949, 10 August 1949, 20 August 1949, 16 May 1950, H.T. Stultz (5, ACNS); Greenwich, 29 May 1958, H.T. Stultz (1, ACNS); Kingston, 30 June 2002, C.G. Majka, sandy pine barren (1, CGMC); Queens Co.: Eight Mile Lake, 11 August 2003, P. Dollin, Picea rubens forest (40-80 years), in vegetation, sweep (1, NSMC); Little Ponhook Lake, 1 August 1993, B. Wright, in oak apple galls (3, NSMC); Ponhook Lake nr. Greenfield, 13 July 1993, J. Cook, ultraviolet light trap (2, JCC); Shelburne Co.: Clyde River Road, 16 July 1992, S. & J. Peck, forest, car net (1, JCC); Forbes Point, 9 July 2007, R. Gorham, grass/alders (4, CGMC); Victoria Co.: Cape Breton Highlands: Kelly Rd, 24 June 2005, J. Ogden, malaise trap (1, NSNR); Yarmouth Co.: Moses Lake, 8 km N of Argyle, 17-22 July 1993, J. & T. Cook, mixed forest, FIT (1, JCC). PRINCE EDWARD ISLAND: Queens Co.: Cavendish, 19 July 2001, C.G. Majka, coastal vegetation (1, CGMC); Princeton-Wharburton Road, 19 August 2002, C.G. Majka, old field (3, CGMC); St. Patricks, 18 August 2002, C.G. Majka, old field (1, CGMC); St. Patricks, 29 June 2003, C.G. Majka, mixed forest (1, CGMC).
Litargus tetraspilotus LeConte is newly recorded from Prince Edward Island.
In the Maritime Provinces Litargus tetraspilotus
has been collected in many habitats including coniferous, deciduous,
and mixed forests, seashores, coastal barrens, grasslands, marshy
areas, a sandy pine barren, and an old field ecosystem. Specimens have
been collected on the foliage of white pine (Pinus strobus L.), jack pine (Pinus sylvestris L.), red spruce (Picea rubens Sarg.), balsam fir (Abies balsamea (L.) Mill.), on deciduous, and herbaceous vegetation, on bristly sarsaparilla (Aralia hispida Vent.), and in a rotting mushroom.
Dorsal habitus photograph of Litargus tetraspilotus. Length: 1.8–2.0 mm. Photo credit: Christopher G. Majka.
Typhaea stercorea and Litargus tetraspilotus are abundant and widely distributed in the Maritime Provinces. Mycetophagus pluripunctatus appears to be uncommon but widely distributed on the mainland of Nova Scotia. The other four species of mycetophagids – Mycetophagus punctatus, Mycetophagus flexuosus, Mycetophagus serrulatus, and Mycetophagus quadriguttatus
– are all represented by a handful of specimens or less. They would all
appear to qualify as “apparently rare” saproxylic beetles as defined by
In general, mycetophagids have received rather little attention by researchers in North America, and the bionomics of many species have not been carefully investigated. Certainly this is true in the Maritime Provinces and additional fieldwork in the region is required to ascertain more about their distribution, abundance, bionomics, and ecological role in the habitats that they inhabit.
Sincere thanks to Søren Bondrup-Nielsen (Acadia University), Susan Westby (Agriculture and Agri-Food Canada, Kentville), Christine Noronha and Mary Smith (Agriculture and Agri-Food Canada, Charlottetown), Meredith Steck and Nathaniel Wheelwright (Bowdoin College), David McCorquodale, Kathleen Aikens, Clayton D’Orsay, and Sheena Townsend (Cape Breton University), Yves Bousquet (Canadian National Collection of Insects, Arachnids, and Nematodes), DeLancey Bishop and Joyce Cook (Carleton University), Philana Dollin (Dalhousie University), Jean Pierre Le Blanc (Nova Scotia Agricultural College), Jeffrey Ogden (Nova Scotia Department of Natural Resources), Rebecca Gorham, Richard Migneault, and David Webster for making specimens available for this study. Thanks to Nicholas Gompel, Guy A. Hanley, and Tim Moyer, and Tom Murray for providing habitus photographs. Thanks to David Christianson, Calum Ewing, and Andrew Hebda at the Nova Scotia Museum for continuing support and encouragement. Two anonymous reviewers provided many helpful comments to an earlier version of the manuscript. This work has been assisted by the Board of Governors of the Nova Scotia Museum.