(C) 2011 Evgeniy Zinovyev. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The distribution of beetles at the end of the Middle Pleninglacial (=terminal Quaternary) was examined based on sub-fossil material from the Ural Mountains and Western Siberia, Russia. All relevant localities of fossil insects have similar radiocarbon dates, ranging between 33, 000 and 22, 000 C14 years ago. Being situated across the vast territory from the southern Ural Mountains in the South to the middle Yamal Peninsula in the North, they allow latitudinal changes in beetle assemblages of that time to be traced. These beetles lived simultaneously with mammals of the so-called “mammoth fauna” with mammoth, bison, and wooly rhinoceros, the often co-occurring mega-mammalian bones at some of the sites being evidence of this. The beetle assemblages found between 59° and 57°N appear to be the most interesting. Their bulk is referred to as a “mixed” type, one which includes a characteristic combination of arcto-boreal, boreal, steppe and polyzonal species showing no analogues among recent insect complexes. These peculiar faunas seem to have represented a particular zonal type, which disappeared since the end of the Last Glaciation to arrive here with the extinction of the mammoth biota. In contrast, on the sites lying north of 60°N, the beetle communities were similar to modern sub-arctic and arctic faunas, yet with the participation of some sub-boreal steppe components, such as Poecilus ravus Lutshnik and Carabus sibiricus Fischer-Waldheim. This information, when compared with our knowledge of synchronous insect faunas from other regions of northern Eurasia, suggests that the former distribution of beetles in this region could be accounted for both by palaeo-environmental conditions and the impact of grazing by large ruminant mammals across the so-called “mammoth savannas”.
Carabidae, Coleoptera, sub-fossil beetles, fauna change, insect assemblages
One of the main tasks of any zoological investigation is the study of the influence of environmental factors on the structure of communities, including changes in insect faunas. These changes may be estimated from modern faunas. But it is necessary to study such factors, which could define the specific structure of insect communities in the past.
With respect to research on palaeo-entomological processes, it is extremely difficult to estimate the character of external influences on the structure of communities, because there are no real opportunities to inspect them directly. It is only possible to make reconstructions, which are based on the analysis of sub-fossil insect assemblages found in Quaternary strata. The term “sub-fossil” means, that insect remains are presented in these layers by isolated chitin fragments not yet fossilized. Present ecological requirements of these species can be extrapolated to the period of the past investigated; the conclusions of which can be compared with results of palaeo-botanical analysis and studies of mega and small mammals. The comparison of these conclusions allows a reconstruction to be made of palaeoenvironmental conditions prevailing in the given territory in the analyzed period of the past.
The aim of this study is to try to explain peculiarities of the insect faunas in relation with the paleoenvironmental conditions of the terminal phase of the Late Pleistocene and estimate the factors possibly determining the composition of insect species in the past, including the influence of the large herbivorous mammals.
MaterialsTo this end, I took some synchronous sites situated in
the vast territory from the Jamal peninsula in the North up to
vicinities of Ekaterinburg city in the South. Radiocarbon dating
confirmed the synchrony of these sites. The period of investigations
covers the end of the Late Pleistocene including terminal phase of
Middle Pleninglacial period and the beginning of the Late Pleninglacial
or Late Glacial Maximum (LGM). Chronologically this time corresponds to
the end of Maritime Isotope Stage (MIS) 3 and the beginning of MIS 2;
33, 000–22, 000 years Before Present (BP). This period is considered by
geologists as the most severe time of the Late Pleistocene and
characterized by a cooler-than-present climate which fluctuated heavily
on time scales of a few thousand years (
The work is based on sub-fossil material obtained from 13
sites scattered over the large territory of the Ural Mountains and West
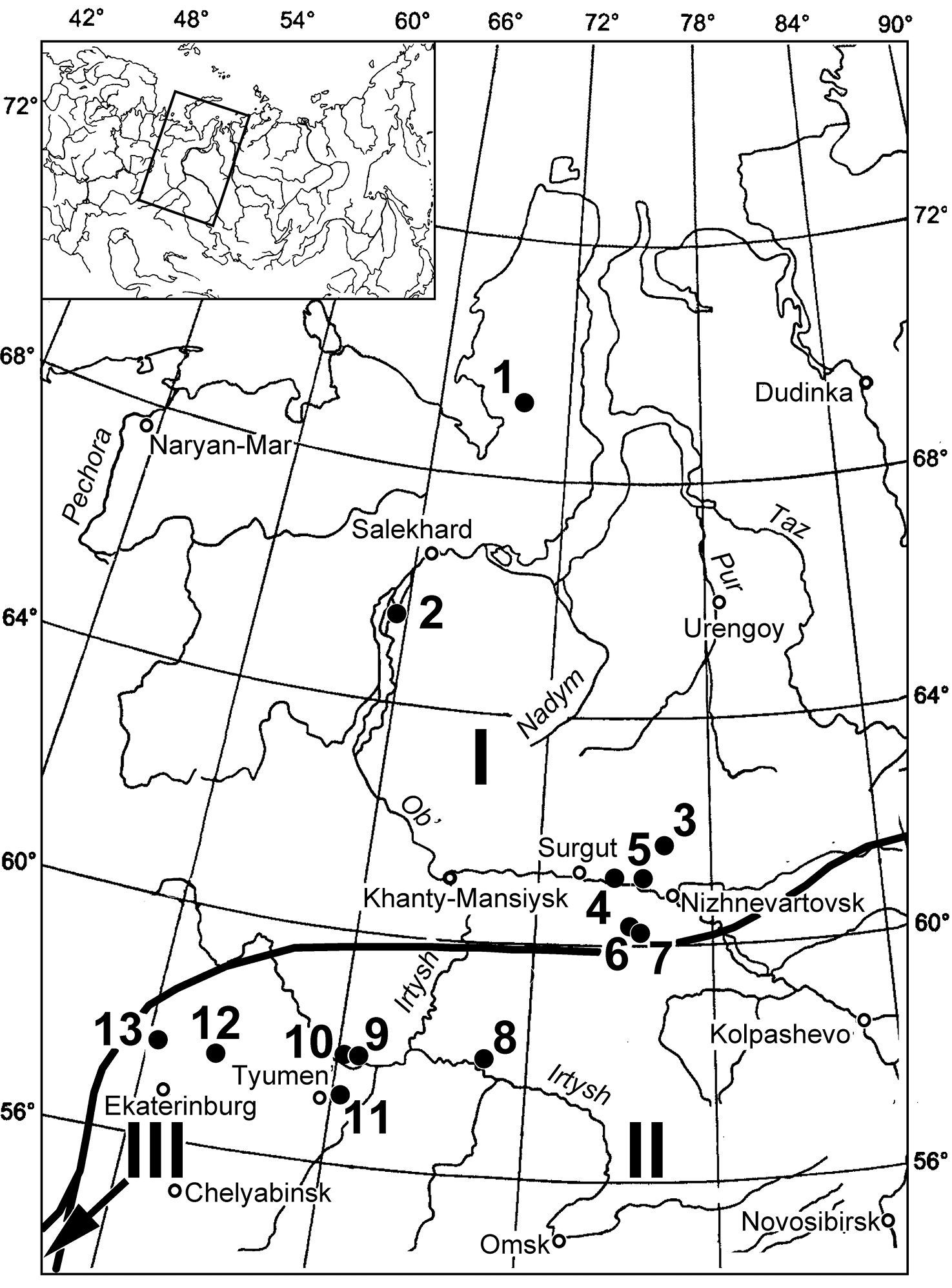
Siberia (Figure 1; Table 1).
Sub-fossil insect remains were found in deposits exposed both in
quarries and in river banks. Field sampling was done using the standard
techniques in
Geographical location of the study sites in the Ural Mountains and Western Siberia. Numbers of sites: 1 Syoyakha-Mutnaya 2 430 km from Ob 3 Aganskiy uval-1290/2 4 Mega 5 Lokosovo 6 Kul’egan-2247 Point I 7 Kul’egan -2247 Point II 8 Skorodum 9 Andriyshino 10 Nizhnyaya Tavda 11 Mal’kovo 12 Nikitino 13
Shurala. Bold line - Borders of vegetation types, reconstructed for
the beginning of MIS 2 on the basis of palynological data: I periglacial tundra II periglacial steppe and forest-steppe III boreal forest and parklands (after
Chronological position of the study sites dated by the end of the Middle Pleninglacial period and associated with the “mammoth fauna”
| Sites where sub-fossil insect faunas were found | Coordinates | Radiocarbon data, years before present (yr. BP). The abbreviation of organization and laboratory number of this data are given in parenthesis | |||
|---|---|---|---|---|---|
| N | E | ||||
| 1. | Syoyakha-Mutnaya (V.I.Nazarov, unpublished data) | 72°25' | 66°48' | 30, 700±1100 (UPI-716) | |
| 2. | 430 km of Ob | 64°25' | 70°50' | 24, 000±1500 (IPAE-63) | |
| 3. | Aganskiy uval-1290/2 | 61°22' | 76°45' | 23, 300±575 (IPAE-95) | |
| 4. | Mega-2172 | 60°56' | 72°20' | 33, 100±2300 (IOAN 132) | |
| 60°56' | 72°20' | 26, 285±590 (SOAN 982) | |||
| 5. | Lokosovo | 60°40' | 71°32' | 22, 930±650 (SOAN 956) | |
| 6. | Kul’egan-2247 Point I | 60°25' | 75°50' | 21, 815±225 (SOAN-6837) | |
| 7. | Kul’egan -2247 Point II | 60°25' | 75°50' | 26, 730±250 (LOIA-8663) | |
| 8. | Skorodum | 57°47' | 70°58' | 26, 500±550 (SOAN 4538) | |
| 9. | Andriyshino | 57°41' | 66°08' | approximately 30, 000 C14 yr. BP | |
| 10. | Nizhnyaya Tavda | 57°41' | 66°12' | 27, 400±335 (SOAN 4534) | |
| 57°41' | 66°12' | 24, 820±750 (SOAN 4535) | |||
| 11. | Mal’kovo | 56°25' | 66°10' | 31, 800±350 (GIN-5338) | |
| 12. | Nikitino | 57°34' | 63°17' | 24, 480±550 (SOAN 4537) | |
| 57°34' | 63°17' | 28, 460±800 (SOAN 4536) | |||
| 13. | Shurala | Plant detritus | 57°28' | 60°15' | 27, 600±150 (Ki-15505) |
| Mammalian bone | 57°28' | 60°15' | 36, 700±250 (Ki-15512) | ||
All studied insect faunas occurred in the interval between 33, 000 and 22, 000 C14 yr. BP (Table 1), the terminal phase of the Middle Pleninglacial (MIS 3). These studies cover the vast territory between 67° and 57°N. I tried to trace elements of latitudinal zonality and estimate factors affecting natural ecosystems and insect faunas.
According to the classification by
Only faunas of the arctic type were found at the sites lying north of 61°N latitude (sites 1–3 in Table 1). The main characteristics of these faunas are:
1. Dominance or sub-dominance of arctic species – Curtonotus alpinus, Pterostichus costatus, Pterostichus sublaevis and the rove beetle Tachinus cf. arcticus (Table 2).
2. Dominance or sub-dominance of sub-arctic species of the sub-genus Cryobius and the species Pterostichus pinguedineus, Pterostichus ventricosus, Diacheila polita, Curtonotus torridus (Table 2).
3. Single occurrences of sub-boreal steppe species – Carabus sibiricus, leaf beetles Chrysolina perforata, Chrysolina aeruginosa. Only one elytrum of a specimen of Poecilus ravus was found in the Aganskyi uval-1290/2 site (61°22'N, 76°45'E).
Entomo-complexes referred to as “arctic” allow the reconstruction of severe environmental conditions similar to the modern arctic tundra, characterized by a cold climate with temperatures of July +12°C, January -27°C, the distribution of open landscapes and the absence of wood.
Between 61° and 59°N, the fossil beetle faunas of the sub-arctic type are similar to the recent communities of the south tundra and forest tundra (sites 4–7 in Table 1). The main characteristics of these faunas are:
1. Presence of arctic species – Curtonotus alpinus, Pterostichus costatus and the rove beetle Tachinus cf. arcticus (but in fewer quantities than in arctic faunas).
2. Dominance of sub-arctic species presented by the sub-genus Cryobius of genus Pterostichus, Pterostichus pinguedineus, Curtonotus torridus, Diacheila polita (Table 2).
3. Occurrence of sub-boreal steppe species – Carabus sibiricus, Poecilus ravus, the weevil Stephanocleonus eruditus, and the carrion beetle Aclypaea sericea.
4. Presence of single xylophagous beetles associated with larch or spruce, the weevil Callirus albosparsus, and the bark beetle Phoelotribus spinulosus.
Insect assemblages referred to as belonging to the “sub-arctic” type, are similar to modern insect faunas from the southern part of the contemporary Sub-arctic. Presumably, reconstructed landscapes look like modern south tundra or forest tundra with the presence of single trees, such as larch or spruce. The thermal regime is probably characterized by several temperatures: July +13° – +14°C, January -25° – -26°C. These reconstructions are confirmed by palaeo-botanical data.
The faunas from sites situated south of 59°N are of a “mixed” type characterized by species combinations not presently found together; insect complexes of the majority of these localities resemble each other, with main features:
1. Dominance or sub-dominance of weevils Otiorhynchus similar to Otiorhynchus politus.
2. Presence of arctic and sub-arctic species – Pterostichus (Cryobius) spp., Curtonotus alpinus, the carrion beetle Aclypaea sericea.
3. Presence of sub-boreal steppe and sub-alpine insects – Poecilus (Derus) spp., Cymindis mannerheimi, Pseudotaphoxenus dauricus.
4. Occurrence of some halophylous beetles – Pogonus spp., darkling beetles Belopus spp.
5. Occurrence of xylophagous beetles (e.g., the bark beetle Phoelotribus spinulosus).
These faunas have no analogues among modern insect
complexes, and may be classified as indicative of tundra steppe,
although their species composition differs from that known from relict
tundra steppe communities found today in Eastern Siberia and described
by
Species of beetles found in the study sites associated with the “mammoth fauna”
| Type of range* | Taxon | Sites (see Table 1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| COLEOPTERA: | ||||||||||||||
| Carabidae | ||||||||||||||
| a-sb | Carabus sibiricus F.-W. | + | + | + | ||||||||||
| a-sb | Carabus cf. sibiricus F.-W. | + | ||||||||||||
| Carabus (Trachycarabus) sp. | + | |||||||||||||
| a | Carabus truncaticollis Esch. | + | ||||||||||||
| a | Carabus cf. truncaticollis Esch. | + | ||||||||||||
| sa | Carabus cf. odoratus F.-W. | + | ||||||||||||
| Carabus (Morphocarabus) sp. | + | |||||||||||||
| Carabus sp. | + | + | + | + | + | + | ||||||||
| p | Nebria rufescens Sturm | + | ||||||||||||
| sa | Nebria nivalis Payk. | + | + | |||||||||||
| Nebria sp. | + | |||||||||||||
| sa | Pelophila borealis Payk. | + | + | + | + | + | + | + | + | |||||
| p | Elaphrus riparius L. | + | ||||||||||||
| p | Notiophilus cf. aestuans Motsch. | + | ||||||||||||
| p | Nebria cf. aquaticus L. | + | + | + | + | + | + | + | ||||||
| b | Nebria reitteri Spaeth | + | + | |||||||||||
| b | Nebria biguttatus F. | + | ||||||||||||
| Nebria sp. | + | + | + | + | + | |||||||||
| sa | Blethisa catenaria Brown. | + | + | + | + | |||||||||
| p | Blethisa multipunctata L. | + | ||||||||||||
| sa | Diacheila polita Fald. | + | + | + | + | + | + | + | + | + | + | + | ||
| sa | Diacheila arctica Gyll. | + | + | |||||||||||
| p | Elaphrus riparius L. | + | + | + | + | |||||||||
| sa | Elaphrus lapponicus Gyll. | + | ||||||||||||
| b | Elaphrus angusticollis R. F. Sahlb. | + | + | + | ||||||||||
| b | Elaphrus cf. angusticollis R. F. Sahlb. | + | ||||||||||||
| Elaphrus sp. | + | + | ||||||||||||
| p | Lorocera pilicornis F. | + | ||||||||||||
| p | Clivina fossor L. | + | + | + | ||||||||||
| p | Dyschiriodes cf. globosus Hbst. | + | ||||||||||||
| Dyschiriodes sp. | + | + | + | |||||||||||
| b | Trechus secalis Payk. | + | ||||||||||||
| b | Trechus rivularis Gyll. | + | + | |||||||||||
| b | Bembidion striatum F. | + | + | |||||||||||
| b | Bembidion velox L. | + | ||||||||||||
| sa | Bembidion captivorum Net. | + | ||||||||||||
| sa | Bembidion scandicum Lindr. | + | ||||||||||||
| b | Bembidion ovale Motsch. | + | ||||||||||||
| sa | Bembidion umiatense Lindr. | + | ||||||||||||
| sa | Bembidion cf. umiatense Lindr. | + | + | + | ||||||||||
| b | Bembidion infuscatum Dej. | + | + | |||||||||||
| b | Bembidion cf. infuscatum Dej. | + | ||||||||||||
| sa | Bembidion grapei Gyll. | + | + | |||||||||||
| sa | Bembidion cf. grapei Gyll. | + | + | |||||||||||
| b | Bembidion scopulinum Kby. | + | ||||||||||||
| b | Bembidion deletum Serv. | + | ||||||||||||
| b | Bembidion cf. deletum Serv. | + | ||||||||||||
| p | Bembidion cf. tetracolum Say | + | ||||||||||||
| Bembidion (Ocydromus) sp. | + | + | + | + | + | + | ||||||||
| sa | Bembidion fellmanni Mnnh. | + | ||||||||||||
| sa | Bembidion cf. fellmanni Mnnh. | + | + | + | ||||||||||
| Bembidion (Plataphodes) sp. | + | + | + | |||||||||||
| p | Bembidion obliquum Ol. | + | ||||||||||||
| Bembidion (Bembidionetolitzkya) sp. | + | |||||||||||||
| Bembidion sp. | + | + | + | + | + | + | + | |||||||
| sb | Pogonus cf. punctatulus Dej. | + | ||||||||||||
| sb | Pogonus cf. cumanus Lutschn. | + | ||||||||||||
| sb | Pogonus cf. meridionalis Dej. | + | + | |||||||||||
| sb | Pogonus cf. transfuga Chaud. | + | ||||||||||||
| sb | Pogonus sp. | + | + | + | + | + | ||||||||
| p | Patrobus septentrionis Dej. | + | + | + | + | + | + | |||||||
| p | Pogonus assimilis Chd. | + | + | |||||||||||
| sb | Poecilus major Motsch. | + | + | |||||||||||
| sb | Pogonus cf. major Motsch. | + | + | |||||||||||
| sb | Pogonus ravus Lutshn. | + | + | + | + | + | + | + | + | |||||
| sb | Pogonus cf. ravus Lutshn. | + | + | + | ||||||||||
| sb | Pogonus hanhaicus Tsch. | + | ||||||||||||
| sb | Pogonus cf. hanhaicus Tsch. | + | + | + | ||||||||||
| sb | Pogonus (Derus) sp. | + | + | |||||||||||
| t | Pogonus lepidus Leske | + | ||||||||||||
| Pogonus (s.str.) sp. | + | + | ||||||||||||
| p | Pterostichus nigrita F. | + | + | |||||||||||
| b | Pogonus mannerheimi Dej. | + | ||||||||||||
| b | Pogonus maurusiacus Mnnh | + | ||||||||||||
| b | Pogonus cf. maurusiacus Mnnh | + | ||||||||||||
| sa | Pogonus parens Tsch. | + | ||||||||||||
| Pogonus (Eosteropus) sp. | + | + | ||||||||||||
| sa | Pogonus montanus Motsch. | + | + | |||||||||||
| sa | Pogonus cf. montanus Motsch. | + | ||||||||||||
| sa | Pogonus kokeili ssp. archangelicus Popp. | + | ||||||||||||
| sa | Pogonus tundrae Tsch. | + | + | + | + | |||||||||
| sa | Pogonus cf. tundrae Tsch. | + | + | |||||||||||
| sa | Pogonus cf. abnormis J.R.Sahlb. | + | ||||||||||||
| Pogonus (Petrophilus) sp. | + | + | + | + | ||||||||||
| sa | Pogonus agonus Horn. | + | + | |||||||||||
| a | Pogonus vermiculosus Men. | + | + | + | + | + | + | |||||||
| a | Pogonus cf. cancellatus Motsch. | + | ||||||||||||
| a | Pogonus costatus Men. | + | + | + | + | + | ||||||||
| a | Pogonus sublaevis J.R.Sahlb. | + | + | + | + | |||||||||
| sa | Pogonus tareumiut Ball. | + | ||||||||||||
| sa | Pogonus cf. tareumiut Ball. | + | ||||||||||||
| sa | Pogonus theeli Maekl. | + | ||||||||||||
| sa | Pogonus cf. theeli Maekl. | + | ||||||||||||
| sa | Pogonus middendorfii J.Sahlb. | + | + | |||||||||||
| sa | Pogonus cf. middendorffi J.R.Sahlb. | + | + | + | ||||||||||
| sa | Pogonus ventricosus Esch. | + | + | + | + | |||||||||
| sa | Pogonus cf. ventricosus Esch. | + | + | + | ||||||||||
| sa | Pogonus pinguedineus Esch. | + | + | |||||||||||
| sa | Pogonus cf. pinguedineus Esch. | + | + | + | + | + | + | |||||||
| sa | Pogonus cf. nigripalpis Popp. | + | + | |||||||||||
| sa | Pogonus negligens Sturm. | + | + | + | + | + | + | |||||||
| sa | Pogonus cf. negligens Sturm | + | + | |||||||||||
| sa | Pogonus brevicornis Kby. | + | + | + | + | + | + | |||||||
| sa | Pogonus cf. brevicornis Kby | + | + | |||||||||||
| sa | Pogonus (Cryobius) sp. | + | + | + | + | + | + | + | + | + | + | + | ||
| b | Pogonus diligens Sturm | + | + | + | + | |||||||||
| b | Pogonus cf. diligens Sturm | + | + | |||||||||||
| b | Pogonus cf. strenuus Panz. | + | + | |||||||||||
| b | Pogonus (Phonias) sp. | + | + | + | ||||||||||
| Pogonus sp. | + | + | + | + | + | + | ||||||||
| sa | Stereocerus haematopus Dej. | + | + | + | + | + | ||||||||
| sa | Stereocerus cf. haematopus Dej. | + | ||||||||||||
| sa | Stereocerus rubripes Motsch. | + | ||||||||||||
| sa | Stereocerus cf. rubripes Motsch. | + | + | |||||||||||
| sa | Stereocerus sp. | + | ||||||||||||
| Platynus sp. | + | + | ||||||||||||
| sa | Agonum alpinum Motsch. | + | ||||||||||||
| p | Agonum cf. versutum Sturm | + | ||||||||||||
| p | Agonum ericeti Panz. | + | + | |||||||||||
| p | Agonum micans Nic. | + | ||||||||||||
| p | Agonum cf. gracile Gyll. | + | ||||||||||||
| Agonum (Europhilus) sp. | + | |||||||||||||
| Agonum sp. | + | + | + | + | + | + | ||||||||
| b | Synuchus vivalis Payk. | + | ||||||||||||
| sb | Pseudotaphoxenus dauricus F.-W. | + | ||||||||||||
| sa | Amara quenseli Shoenh. | + | + | |||||||||||
| a | Agonum glacialis Mnnh. | + | + | |||||||||||
| sa | Agonum erratica Duft. | + | + | |||||||||||
| b | Agonum minuta Motsch. | + | ||||||||||||
| sa | Agonum interstitialis Dej. | + | + | + | + | |||||||||
| b | Agonum brunnea Gyll . | + | + | + | + | + | ||||||||
| b | Agonum cf. brunnea Gyll. | + | ||||||||||||
| Agonum (Bradytus) sp. | + | |||||||||||||
| Agonum (Celia) sp. | + | |||||||||||||
| Agonum sp. | + | + | + | + | + | |||||||||
| sa | Curtonotus hyperboreus Dej. | + | ||||||||||||
| a | Curtonotus alpinus Payk. | + | + | + | + | + | + | |||||||
| a | Curtonotus cf. alpinus Payk. | + | ||||||||||||
| sa | Curtonotus torridus Panz. | + | + | + | + | + | ||||||||
| sa | Curtonotus cf. torridus Panz. | + | + | + | + | |||||||||
| sb | Curtonotus dauricus Motsch. | + | ||||||||||||
| Curtonotus sp. | + | + | + | + | + | + | ||||||||
| sa | Harpalus nigritarsis C.R.Sahlb. | + | + | + | + | |||||||||
| sa | Harpalus cf. nigritarsis C.R.Sahlb. | + | + | |||||||||||
| sb | Harpalus cf. pulvinatus Men | + | ||||||||||||
| Harpalus sp. | + | + | ||||||||||||
| sa | Dicheirotrichus mannerheimi R. F. Sahlb. | + | + | + | + | |||||||||
| sb | Cymindis mannerheimi Gebl. | + | + | + | ||||||||||
| sa | Cymindis macularis F.-W. | + | + | |||||||||||
| b | Cymindis cf. rivularis Motsch. | + | ||||||||||||
| Cymindis sp. | + | + | ||||||||||||
| Carabidae indet. | + | + | + | |||||||||||
| Dytiscidae: | ||||||||||||||
| Agabus (Gaurodytes) sp | + | + | + | + | + | + | ||||||||
| Agabus sp. | + | + | + | + | + | |||||||||
| Hydroporus sp. | + | + | + | + | ||||||||||
| Dytiscidae indet. | + | + | ||||||||||||
| Gyrinidae: | ||||||||||||||
| Gyrinus sp. | + | + | ||||||||||||
| Hydrophilidae: | ||||||||||||||
| p | Hydrobius fuscipes L. | + | + | + | ||||||||||
| p | Helophorus cf. nubilis F. | + | ||||||||||||
| sa | Helophorus obscurellus Popp. | + | ||||||||||||
| sa | Helophorus cf. obscurellus Popp. | + | ||||||||||||
| Helophorus sp. | + | + | + | + | + | + | + | |||||||
| ?Helophorus sp. | + | |||||||||||||
| Cercyon sp. | + | + | + | + | + | + | + | |||||||
| Histeridae: | ||||||||||||||
| Margarinotus sp. | + | |||||||||||||
| Catopidae: | ||||||||||||||
| Catops sp. | + | + | + | + | + | + | + | |||||||
| Colon sp. | + | |||||||||||||
| Silphidae: | ||||||||||||||
| sb | Aclypaea sericea Zoubk. | + | ||||||||||||
| sb | Aclypaea bicarinata Gebl. | + | + | |||||||||||
| p | Aclypaea opaca L. | + | + | + | + | + | + | |||||||
| Thanatophilus sp. | + | + | + | |||||||||||
| Liodidae: | ||||||||||||||
| Agathidium sp. | + | + | + | + | + | + | ||||||||
| Anisotoma sp. | + | |||||||||||||
| Liodes sp. | + | + | + | + | + | |||||||||
| Staphylinidae: | ||||||||||||||
| p | Acidota crenata Mnnh. | + | ||||||||||||
| p | Acidota cf. cruentata Mnnh. | + | ||||||||||||
| Acidota sp. | + | |||||||||||||
| Olophrum sp. | + | + | + | + | ||||||||||
| Omaliinae gen. sp. | + | + | + | + | + | + | + | + | ||||||
| a | Tachinus cf. arcticus Maekl. | + | + | + | + | + | + | + | ||||||
| Omaliinae gen. sp. | ||||||||||||||
| Ocypus sp. | + | |||||||||||||
| Oxythelinae gen. sp. | + | + | ||||||||||||
| Tachinus sp. | + | + | + | + | + | + | ||||||||
| ?Mycetoporus sp. | + | |||||||||||||
| ? Philonthus sp. | + | |||||||||||||
| Tachyporinae gen. sp. | + | + | ||||||||||||
| Stenus sp. | + | + | + | + | ||||||||||
| Lathrobium sp. | + | + | + | + | ||||||||||
| Paederinae gen sp. | + | + | + | |||||||||||
| Quedinus sp. | + | + | + | |||||||||||
| p | Scaphisoma sp. | + | ||||||||||||
| Staphylinidae indet. | + | + | + | + | + | |||||||||
| Scarabaeidae: | ||||||||||||||
| t | Aphodius distinctus Müll. | + | ||||||||||||
| t | Acidota cf. distinctus Müll. | + | + | + | + | |||||||||
| t | Acidota cf. melanostictus W.Schm. | + | + | + | ||||||||||
| t | Acidota cf. fossor L. | + | + | |||||||||||
| t | Acidota cf. brevis Er. | + | + | |||||||||||
| t | Acidota cf. rufipes L. | + | ||||||||||||
| Acidota sp. | + | + | + | + | + | + | + | |||||||
| p | Aegialia abdita Nikritin | + | + | + | + | + | ||||||||
| Helodidae: | ||||||||||||||
| Cyphon sp. | + | + | ||||||||||||
| ?Cyphon sp. | ||||||||||||||
| Dermestidae: | ||||||||||||||
| Dermestidae indet. | + | |||||||||||||
| Byrrhidae: | ||||||||||||||
| Byrrhus sp. | + | + | + | + | ||||||||||
| sb | Porcinolus murinus F. | + | + | |||||||||||
| sa | Morychus viridis Kuzm. et Kor. | + | + | + | + | |||||||||
| sa | Morychus cf. viridis Kuzm. et. Kor. | + | ||||||||||||
| Morychus sp. | + | + | + | |||||||||||
| Simplocaria sp. | + | + | + | + | + | + | ||||||||
| Curimopsis sp. | + | |||||||||||||
| Byrrhidae gen. sp. | + | + | ||||||||||||
| Anobiidae: | ||||||||||||||
| p | Caenocara bovistae Hoffm. | + | + | |||||||||||
| Heteroceridae: | ||||||||||||||
| Heteroceris sp. | + | |||||||||||||
| Elateridae: | ||||||||||||||
| p | Hypnoidus cf. rivularis Gyll. | + | ||||||||||||
| Hypnoidus sp. | + | + | ||||||||||||
| Nitidulidae: | ||||||||||||||
| Nitidulidae gen. indet. | + | + | + | |||||||||||
| Cryptophagidae: | ||||||||||||||
| Cryptophagidae indet. | + | |||||||||||||
| Erotylidae: | ||||||||||||||
| Erotylidae indet. | + | |||||||||||||
| Coccinellidae: | ||||||||||||||
| b | Scymnus sp. | + | ||||||||||||
| sa | Hippodamia arctica Schneider | + | ||||||||||||
| b | Coccinella trifasciata L. | + | ||||||||||||
| b | Coccinella cf. hieroglyphica L. | + | ||||||||||||
| b | Coccinella sp. | + | ||||||||||||
| Latridiidae: | ||||||||||||||
| Latridiidae gen.sp. | + | + | ||||||||||||
| Oedemeridae: | ||||||||||||||
| Oedemeridae gen. sp. | + | |||||||||||||
| Anthicidae: | ||||||||||||||
| Anthicidae gen.sp. | + | |||||||||||||
| Tenebrionidae: | ||||||||||||||
| sb | Belopus sp. | + | ||||||||||||
| Chrysomelidae: | ||||||||||||||
| Donacia sp. | + | + | ||||||||||||
| sb | Chrysolina perforata Gebl. | + | ||||||||||||
| sb | Chrysolina cf. perforata Gebl. | + | ||||||||||||
| sb | Chrysolina cf. aeruginosa Fald. | + | ||||||||||||
| a | Chrysolina cf. cavigera J.R.Sahlb. | + | ||||||||||||
| a | Chrysolina cf. subsulcata Esch. | + | + | |||||||||||
| sa | Chrysolina septentrionalis Men. | + | ||||||||||||
| sa | Chrysolina cf. septentrionalis Men | + | ||||||||||||
| p | Chrysolina cf. graminis L. | + | ||||||||||||
| Chrysolina sp. | + | + | + | + | + | + | ||||||||
| a | Chrysomela cf. taimyrensis L. Medv. | + | ||||||||||||
| Chrysolina sp. | + | + | + | + | + | + | + | |||||||
| sb | Colaphellus sophiae Schall. | + | + | + | ||||||||||
| p | Hydrothassa hannoverana F. | + | ||||||||||||
| Phaedon sp. | + | + | ||||||||||||
| p | Plagoiodera versicolora Laich. | + | + | |||||||||||
| Crosita sp. | + | + | ||||||||||||
| Phratora sp. | + | |||||||||||||
| Chalcoides sp. | + | |||||||||||||
| ?Chalcoides sp. | + | |||||||||||||
| ?Chaetocnema sp. | + | |||||||||||||
| Altica sp. | + | |||||||||||||
| Alticinae gen. sp. | + | |||||||||||||
| Chrysomelidae indet. | + | + | ||||||||||||
| Erirhinidae: | ||||||||||||||
| p | Tournotaris bimaculatus F. | + | + | + | + | + | + | + | + | + | ||||
| b | Tournotaris ochoticus Kor. | + | + | |||||||||||
| b | Tournotaris cf. ochoticus Kor. | + | ||||||||||||
| p | Notaris aethiops F. | + | + | + | + | + | ||||||||
| Notaris sp. | + | + | + | + | ||||||||||
| Curculionidae: | ||||||||||||||
| sb | Otiorhynchus unctuosus Germ. | + | ||||||||||||
| b | Otiorhynchus politus Gyll. | + | + | + | + | |||||||||
| b | Otiorhynchus cf. politus Gyll. | + | + | + | + | + | + | |||||||
| sb | Otiorhynchus wittmeri Legalov | + | ||||||||||||
| sb | Otiorhynchus cf. wittmeri Legalov | + | ||||||||||||
| sa | Otiorhynchus cf. arcticus F. | + | ||||||||||||
| p | Otiorhynchus ovatus L. | + | ||||||||||||
| p | Otiorhynchus cf. ovatus L. | + | ||||||||||||
| Otiorhynchus sp. | + | + | + | + | + | + | ||||||||
| sa | Sitona cf. ovipennis ssp. borealis Kor. | + | + | |||||||||||
| Sitona sp. | + | + | ||||||||||||
| b | Chlorophanus cf. sibiricus Gyll. | + | ||||||||||||
| Chlorophanus sp. | + | |||||||||||||
| ?p | Phyllobius cf. crassipes Motsch et Phyllobius maculicornis Germ. | + | ||||||||||||
| Phyllobius sp. | + | + | + | + | + | + | ||||||||
| Strophosoma sp. | + | |||||||||||||
| sb | Eusomus ovulum Germ. | + | ||||||||||||
| a-sb | Coniocleonus ferrugineus Fahr | + | + | + | ||||||||||
| a-sb | Coniocleonus cf. ferrugineus Fahr | + | ||||||||||||
| Coniocleonus sp. | + | + | + | |||||||||||
| a-sb | Stephanocleonus eruditus Fast. | + | ||||||||||||
| Stephanocleonus sp. | + | + | ||||||||||||
| sb | Bothynoderes foveocollis Gebl. | + | ||||||||||||
| Cleoninae indet. | + | + | + | + | + | + | ||||||||
| p | Hypera rumicus L. | + | ||||||||||||
| p | Hypera cf. ornata Cap. | + | + | + | ||||||||||
| p | Hypera elongata Pk. | + | ||||||||||||
| Hypera sp. | + | + | + | + | + | + | + | + | + | + | ||||
| sa | Lepyrus nordenskjoldi Faust. | + | + | |||||||||||
| sa | Lepyrus cf. nordenskjoldi Faust | + | + | |||||||||||
| sa | Lepyrus cf. arcticus Pk. | + | ||||||||||||
| Lepyrus sp. | + | + | + | + | ||||||||||
| b | Trichalophus maeklini Faust | + | ||||||||||||
| Trichalophus sp. | + | |||||||||||||
| p | Phytobius cf. velaris Gyll. | + | + | |||||||||||
| b | Callirus albosparsus Boh. | + | ||||||||||||
| b | Callirus sp. | + | ||||||||||||
| b | Pissodes sp. | + | + | |||||||||||
| Bagous sp. | + | + | ||||||||||||
| ?Limnobaris sp. | + | |||||||||||||
| b | ?Magdalis sp. | + | ||||||||||||
| b | Rhyncholus ater L. | + | ||||||||||||
| Anthonomus sp. | + | + | ||||||||||||
| p | Dorytomus cf. imbecillus Faust | + | ||||||||||||
| Dorytomus sp. | + | + | + | + | ||||||||||
| p | Ceutorrhynchus cf. erysimi F. | + | ||||||||||||
| Callirus sp. | + | + | + | |||||||||||
| p | Isochnus saliceti Müll | + | ||||||||||||
| sa | Isochnus arcticus Kor. | + | + | |||||||||||
| Rhynchaenus sp. | + | + | + | + | + | |||||||||
| Curculionidae indet. | + | + | ||||||||||||
| Brentidae: | ||||||||||||||
| sa | Hemitrichapion tschernovi T.-M. | + | + | + | + | |||||||||
| Hemitrichapion sp. | ||||||||||||||
| p | Mesotrichapion cf. punctirostre Gyll. | + | + | |||||||||||
| p | Betulapion simile Kby | + | + | |||||||||||
| p | Hemitrichapion cf. simile Kby. | + | ||||||||||||
| Cyanapion sp. | + | |||||||||||||
| Brentidae gen.sp. | + | + | + | + | + | + | + | + | ||||||
| Scolytidae: | ||||||||||||||
| b | Phoelotribus spinulosus Rey. | + | + | + | + | |||||||||
| b | Polygraphus sp. | + | ||||||||||||
At first, these faunas suggest cooler than present climatic conditions, which is confirmed by the occurrence of sub-arctic species (Pterostichus (Cryobius) cf. pinguedineus, Pterostichus ventricosus, Curtonotus torridus and the arctic species (Curtonotus alpinus). This shows their southward distribution relative to their modern ranges.
As evidence of the lack of dense forest, is an absence of such typically boreal beetles as Calathus micropterus, Pterostichus adstricus, Pterostichus oblongopunctatus and others inhabiting the forest litter; at present they are widely distributed in the vast territories of West Siberia. Single boreal species are rare in the “mixed” faunas and are represented mainly by bark beetles (for example, Phoelitribus spinulosus, associated with spruce). The presence of sub-boreal beetles, inhabiting modern East-Siberian steppes (Poecilus ravus, Poecilus hanhaicus) and sub-alpine grasslands (Cymindis mannerheimi) could indicate open landscapes. An abundance of weevils of the genus Otiorhynchus may be explained by the wide distribution of herbal meadow vegetation. These faunas differ from contemporary insect steppe communities by the lack of darkling beetles, occurring in modern steppes and forest steppes (Oodescelis polita, Crypticus quisquilius, Platyscelis hypolita, Opatrum riparium etc.); it is possible that their absence was a result of cold climatic conditions.
At the same time, the presence of halophylous species, such as ground beetles of the genus Pogonus, darkling beetles of the genus Belopus, may indicate local soil salinity. At present these halophilic species are distributed southwards from 56–57°N and are very rare between 57–58°N, situated in sites with “mixed” faunas. Moreover, I am not aware of the presence of halophilic species of the genera Pogonus and Belopus in Central and East Siberia.
It may be assumed that such faunas inhabited open communities which can be defined as “cool grasslands” with a presence of rarefied forests or of single trees and local soil salinity. Similar conclusions have been drawn from palaeo-botanical data obtained at the same sites: they show a dominance of herbal vegetation with an abundance of cereals, wormwoods and chenopodiaceous plants.
The occurrence of some boreal faunas in single sites (Niznyaya Tavda, C14 27, 400 ± 335 yr. BP) do not contradict the overall distribution of open landscapes, and show the presence of isolated patches of forest vegetation, like in modern forest steppes.
Therefore, entomological data show that at the time of the terminal phase of Middle Pleninglacial the following types of landscapes were distributed in the territories of the Ural Mountains and West Siberia: the northern part of the region north of 61°N was dominated by open landscapes similar to modern tundra, between 64 and 62°N - similar to forest tundra and between 59 and 57°N – non-analogue landscapes, which may be defined as “open grasslands” or savannas with a presence of rarefied forests.
The main influence on the natural ecosystems came
from palaeo-environmental factors. The Middle Weichselian Interstadial
was characterized by a continental and a cooler-than-present climate
with low winter temperatures and a wide distribution of permafrost; the
resulting development of large ice sheets caused a strong drying effect.
Decreasing sea levels provided the opening of sea shelves and the
connection between Europe and the British Isles, and the Beringian
Bridge between Siberia and Alaska. Cold and dry climatic conditions
reconstructed for main territories of Europe, even for the
Mediterranean region (
It is necessary to define which factors might prevent the distribution of woods in the period of the Late Pleistocene studied. The main environmental factor is climate as a combination of thermal regime, precipitation, insulation, etc. At present I can suggest that severe climatic conditions similar to the palaeo-environment of the terminal phase end of the Middle Pleninglacial in the Central part of North Eurasia between 59° and 57°N are presented in the inner parts of Central and East Siberia. However, the modern conditions of cool and continental climate cannot avert the present distribution of woodland vegetation in this area. I suggest that not only climatic factor prevented of the distribution of woods in the central part of northern Eurasia between 59° and 57°N. Apart from climate, other factors might influence Pleistocene ecosystems; these factors may have impeded reforestation and stimulate the distribution of open landscapes.
The influence of mammoths and other large herbivorous
mammals representing the “mammoth fauna” is probably large. It is known
that vast areas of the continent were occupied by mammals belonging to
the mammoth complex at that time (
Firstly, in many sites fossil insects were found along with mammoth remains (Mammuthus primigenius) and other large herbivorous mammals (teeth, tusks, fragments of cranium, etc.) (
Secondly, in the majority of sites fragments of dung beetles of the genus Aphodius, were found which suggests the presence of mammoths and other large herbivorous mammals in the same landscapes (
According to the literature, mammoths and other mega mammals such as woolly rhinoceros (Coelodonta antiquitatis), giant deer (Megaloceros giganteus), reindeer (Rangifer tarandus), wild ox (Ovibos moschatus), primitive bison (Bison priscus)and some others may be considered as an additional factor, which influenced Late Pleistocene ecosystems (
Mammoths and other mammals were indicators of certain communities, and preserved specific ecosystems (
1. Destruction of undergrowth and feeding impeded reforestation and might preserve herbal communities.
2. The hooves of mammoths destroyed the moss turf; as a result, moss cover disappeared in the territories of modern taiga and tundra zones, being replaced by mezo- and xerophylous herbal vegetation.
That is, mammoths and other mega mammals could
rarefy forests and promote the distribution of zoogenic herbal
vegetation consisting of cereals (
Consequently, Pleistocene forests were rare, and meadow and steppe plants were significant in the Siberian ecosystems.
I therefore suggest that the species composition of insects was affected by two important factors:
1. Cool and dry climate which caused low winter temperatures and a wide distribution of permafrost.
2. Pasture of large herbivorous mammals (mammoth and accompanying species) which caused the formation of «pasture» savannas with an abundance of herbal vegetation and rare forests.
Do these factors define the composition of insect complexes as “mixed” faunas at 59°–56°N?Firstly, cool and dry climate may cause a southward advance of arctic and sub-arctic species (Diacheila polita, Curtonotus alpinus, Curtonotus torridus, Pterostichus (Cryobius) spp.). As such, the warming and drying of local habitats (such as slopes with a southern exposition) in dry and cold climatic conditions and their subsequent salinity may cause the occurrence of some halophilic beetles.
Secondly, the pasture of mammoths and other mega mammals may cause the distribution of grasslands with a dominance of cereals and an abundance of weevils of the genus Otiorhynchus. The presence of sub-boreal steppe and sub-alpine species (Poecilus ravus, Cymindis mannerheimi, Chrysolina perforata) may have been caused by both environmental conditions and pasturable load. Rarefaction of woods may explain the lack of species inhabiting forest litter (Calathus micropterus, Pterostichus oblongopunctatus etc.); presence of single trees - occurrences of xylophagous beetles (bark beetle Phoelotribus spinulosus etc.). The fertilization of the soil may have caused the occurrence of coprophagous beetles (dung beetles of the genera Aphodius).
A combination of these factors may have caused the distribution of several landscapes.
In the central and northern parts of the region north
of 59°N, the cold climate and corresponding mammoth pasture formed
communities similar to modern tundra and forest tundra. South of 59°N
and up to 57°N, specific landscapes and according insect faunas were
formed. These conclusions do not contradict literature data on the
palaeo-geography of that period (
It may be assumed, that ground beetles of the species Carabus sibiricus, Poecilus ravus, Pterostichus pinguedineus, Cymindis mannerheimi and others have been widely distributed in the territories of the central part of Northern Eurasia, so that these insects may form an integral part of the landscapes containing the “mammoth faunas”.
Factors leading to the disappearance of the “mammoth faunas”At the beginning of the Holocene (10, 000 yr. BP) in the Northern Hemisphere significant climatic changes took place, modifying all natural communities, and the final degradation of “mammoth faunas” took place. The largest mammals, mammoth (Mammuthus primigenius), woolly rhinoceros (Coelodonta antiquitatis), and giant deer (Megaloceros giganteus), having the greatest effect on terrestrial ecosystems, died out about 10–8, 000 years ago, and the ranges of other species, such as reindeer (Rangifer tarandus), musk ox (Ovibos moschatus)) shifted either northwards to the tundra and forest tundra, or southwards, to the steppes, such as the saiga antelope (Saiga tatarica).
The subsequent Early Holocene warming and humidification of climate, the extinction of mammoths and its consequent failing of the “pasture load” caused the reforestation and water logging of vast territories, the formation of the boreal belt, the transformation of the flora and fauna, and the wide distribution of conifer forests.
Therefore, climatic changes and the extinction of the large mammals happening between the Pleistocene and Holocene caused the disappearance of the “mixed” or non-analogue insect faunas. However, the insect species did not die out, but only changed the location of their ranges. At that time the “mixed” insect faunas disintegrated into groups of single species, shifting their ranges northwards (Curtonotus alpinus, Pterostichus (Cryobius) spp.), southwards (Pogonus spp., Cymindis mannerheimi) or eastwards (Poecilus (Derus) hanhaicus, Poecilus (Derus) ravus, Poecilus (Derus) major, Pseudotahoxenus dauricus, Amara minuta). These species only left the territories studied but could survive these environmental changes in other regions of northern Eurasia, such as Mongolia, Eastern Siberia, or the Pamir Mountains, where environmental conditions are more compatible to their ecological requirements.
Comparison with other regions of North EurasiaThe “mixed” or non-analogue faunas of the central
part of North Eurasia were compared with synchronous insect faunas as
described for East Siberia (
Sub-fossil insect assemblages from Northeastern
Siberia may reflect the existence of tundra steppe landscapes which have
no analogue among modern ecosystems (
Insect faunas at the end of the Middle Pleninglacial in Western Europe (
It is possible that these faunas, belonging to the “mixed” type, were distributed mainly in the Central part of North Eurasia (including West Siberia and the Ural Mountains) during the Late Pleistocene (MIS 4-MIS 2). So, similar faunas were found in the Gornova site, situated in the South Ural Mountains, near Ufa city (data given by F.G.Bidashko (Kazakhstan)). These assemblages are characterized by abundance of remains of the genus Otiorhynchus (similar to Otiorhynchus politus), the presence of Poecilus ravus, Pogonus spp., Belopus spp. and other species, with the presence of some endemic forms (Nedria uralensis).
Conclusions1. Sub-fossil insect assemblages allow us to reconstruct several elements of the natural zonality which existed in the central part of Northern Eurasia during the terminal phase of the Middle Pleninglacial (MIS 3). In the northern and central parts of the region north of 59°N, the cold climate and the corresponding mammoth pasture formed communities similar to modern tundra and forest tundra. In the southern part of the study area between 57° and 59°N, specific landscapes and corresponding insect faunas formed, known as “mammoth savannas”.
2. Insect faunas of a “mixed” type of the Ural Mountains and West Siberia differ from East Siberian sub-fossil insect assemblages found in synchronous layers with the presence of numerous fragments of weevils Otiorhynchus which are morphologically similar to Otiorhynchus politus, as well as the halophilic beetles of the genera Pogonus and Belopus. Steppe beetles, such as weevils of the genus Stepanocleonus did not establish assemblages in West Siberia. Significant differences between insect assemblages from the central part of northern Eurasia and Western Europe were marked too. These faunas cannot be identified both as forest tundra nor tundra steppe and differ even from modern insect communities of East Siberia relict tundra steppes.
3. The species composition of insect complexes was determined not only by climate, but by pasture pressure of mammoths and other herbivorous mammals as well. A pasture load occurred in all territories of the Ural Mountains and West Siberia, but is defined differently in different parts of the study area. In the central and northern parts of the region north of 59°N, a combination of these factors formed communities similar to modern tundra and forest tundra in accordance to the southward advance of arctic and sub-arctic insect complexes relative to contemporary faunas. In those territories lying during the terminal phase of MIS 3 between 59° and 57°N insect faunas existed without any analogues among modern insect complexes and included sub-arctic, sub-boreal steppe species, halophilic insects and weevils of the genus Otiorhynchus and similar to Otiorhynchus politus.
The author thanks his colleagues at the Laboratory of Historical Ecology of IPAE – Dr. P. Kosintsev, Dr. A. Borodin, Dr. E. Kuzmina for discussions during the preparation of this article, Dr. A. Borodin, Dr. P. Kosintsev, Dr. V. Stefanovsky, Mr. S. Zykov - for their help with collecting fossil data, Mrs. O. Korona and Mrs. S. Trofimova for providing palaeo-botanical data, Dr. P. Kositsev and Mr. N. Erokhin – for their help in obtaining radiocarbon data. Special thanks to Dr Tijs van Kolfschoten (Leiden Univ., the Netherlands) and Hans Turin for improving and reviewing this manuscript. This work was supported by the Russian Foundation for Basic Research (project 10–04-96102-r_Ural_a).