(C) 2011 Gil Wizen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Epomis is represented in Israel by two species: Epomis dejeani and Epomis circumscriptus. In the central coastal plain these species are sympatric but do not occur in the same sites. The objective of this study was to record and describe trophic interactions between the adult beetles and amphibian species occurring in the central coastal plain of Israel. Day and night surveys at three sites, as well as controlled laboratory experiments were conducted for studying beetle-amphibian trophic interaction. In the field we recorded three cases of Epomis dejeani preying upon amphibian metamorphs and also found that Epomis adults share shelters with amphibians. Laboratory experiments supported the observations that both Epomis species can prey on amphibians. Predation of the three anuran species (Bufo viridis, Hyla savignyi and Rana bedriagae) and two urodele species (Triturus vittatus and Salamandra salamandra infraimmaculata) is described. Only Epomis dejeani consumed Triturus vittatus. Therefore, we conclude that the two species display a partial overlap in food habit.
Epomis, Carabidae, amphibians, predation, feeding behavior, congeneric difference in food habit
Invertebrates are known predators of juvenile and adult amphibians. The majority of reports list arachnids (e.g.
Following Brandmayr et al. (2010) we rank Epomis as a separate genus and not as a subgenus of Chlaenius. The genus Epomis belongs to the Chlaeniini
tribe in which about 20 species are known, mainly from tropical Africa
and south and south-eastern Asia. Five species are known from the
Palaearctic region (
So far, to the best of our knowledge, predation of an amphibian by an adult Epomis beetle was reported in a single note, describing the predation of a juvenile Rana nigromaculata by Epomis nigricans Wiedemann 1821, in Japan (
During the period of 2007 – 2009 we conducted 103 daytime surveys at 26 sites along the central coastal plain (from south of Tel-Aviv to north of Hadera) in order to examine the presence of Epomis species close to freshwater bodies where amphibians are usually present. The specimens observed were identified and recorded. Selected specimens were deposited in the Natural History Collection, Tel-Aviv University.
Field observationsWe conducted daytime and night surveys at three sites in the central coastal plain (Table 1). The location of the study sites is shown in Figure 1. Outside this study, observations on Epomis circumscriptus life history dynamics were conducted in two additional sites in the central coastal plain (Qadima and Kfar Netter, Table 1).
Location and number of daytime and night surveys conducted in the study sites.
| Site name | Coordinates | daytime surveys | night surveys |
|---|---|---|---|
| Dora | 32°17'30"N, 34°50'48"E | 27 | 5 |
| Berekhat Ya’ar | 32°24'16"N, 34°54'61"E | 37 | 11 |
| Samar | 32°26'23"N, 34°53'01"E | 15 | 11 |
| Qadima | 32°27'25"N, 34°89'64"E | - | - |
| Kfar Netter | 32°28'65"N, 34°87'28"E | - | - |
During daytime surveys we searched for adult beetles under natural and artificial shelters. The former consisted of any local wooden debris or rocks of various sizes. For artificial shelters we used 40×40 cm cement tiles. At night we used white-light flashlights (Hyundai, Search Finder 1×106 candle power) to locate adult beetles and amphibians and to record their activity outside shelters. Each survey (day or night) lasted for two hours. When predation interaction was encountered, the entire event was recorded.
Laboratory observationsWe supplemented the field observations of predation interactions with controlled experiments in the laboratory, in which we exposed a known species of amphibian to one or other species of Epomis. The encounter experiments were conducted in one liter plastic containers (10.5cm high; 14.5cm diameter) with moist peat-moss as substrate in which an individual beetle was reared. A randomly selected metamorph of one out of five amphibian species occurring in the coastal plain was added to the container with the beetle. These metamorphs were measured (snout-vent for anurans; snout-end of tail for urodeles) with a caliper (± 0.05mm) and weighed using an analytical scale (± 0.001g). For each experiment we used a naive amphibian and beetle. Beetles presented with crushed house crickets (Acheta domestica) served as a control for feeding interaction. The beetles are used to this food because we routinely feed them with crushed crickets once a week. We fed the amphibian metamorphs daily with live house crickets. Food was not presented to the beetle or the amphibian on the day of the experiment. All observations were made under natural light. We documented the predation encounter using a Canon powershot SX10 video camera. The video recording started 10 seconds before releasing the amphibian into the beetle’s container, and was carried out in 10 minute clips until the interaction ended. In addition, we documented the interaction with still photographs (DSLR, Canon EOS 20D and Canon EOS 50D).Distribution records and observations of predation behavior did not require statistical analysis.
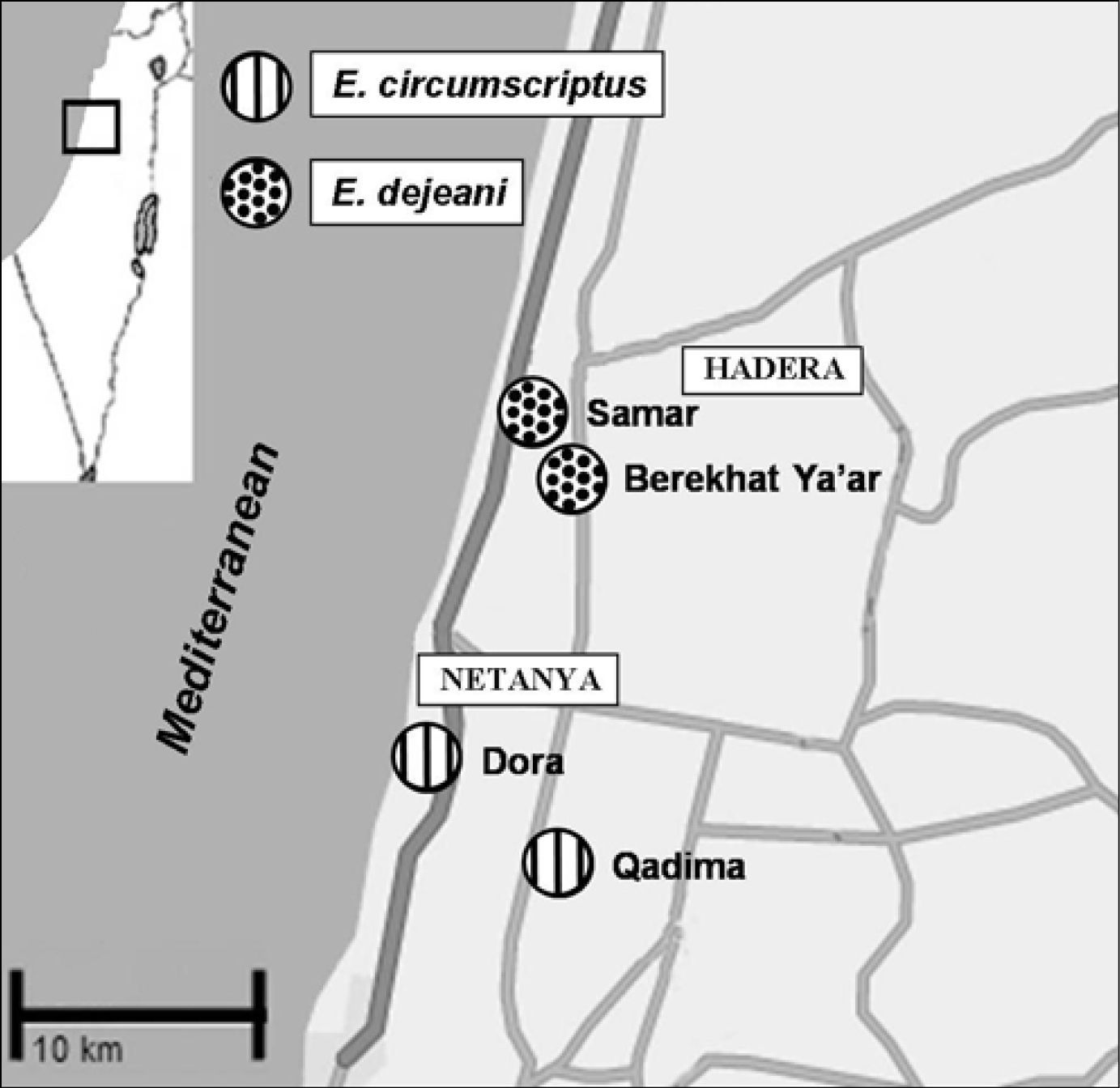
Results DistributionIn 103 surveys conducted in 26 sites in the coastal plain, Epomis beetles were recorded in four sites only, all within a radius of 18km (Table 2). The two species were never found in the same site (Fig. 1); Epomis dejeani was found in Berekhat Ya’ar and Samar, whereas Epomis circumscriptus was found in Dora, Qadima and Kefar Netter (west of Qadima).
Distances (in km) between the surveyed sites, central coastal plain, Israel.
| Dora | Qadima | Berekhat Ya’ar | Samar | |
|---|---|---|---|---|
| Dora | - | |||
| Qadima | 5.1 | - | ||
| Berekhat Ya’ar | 14.4 | 14.8 | - | |
| Samar | 16.3 | 17.4 | 2.8 | - |
We observed three events of adult beetles, Epomis dejeani only, preying on Bufo viridis metamorphs (two in March, one in July), all during night surveys. On seven out of 79 daytime surveys we recorded adult beetles co-occurring with amphibians (metamorphs, juveniles and an adult) under the same shelters (Table 3; URL: Amphibian - Adult Epomis interaction). In all these cases a single adult beetle (male or female) was sharing a shelter with amphibians. Co-occurrence with Epomis circumscriptus was recorded in March and April and with Epomis dejeani in February, March and May. Although we did not observe predation interaction in the above cases we did find in one case the remains of three devoured metamorphs of Bufo viridis (URL: Amphibian - Adult Epomis interaction). One of the authors observed similar remains of Bufo viridis under a shelter occupied by Epomis circumscriptus at another site (Qadima, Fig. 1).
Developmental stage and number of individuals of amphibians (Adl.= Adult; Juv.= Juvenile; Met.= Metamorph; in parentheses, number of records) recorded co-occurring with adult Epomis beetles in the field under the same shelter.
| Epomis circumscriptus | Epomis dejeani | |||||
|---|---|---|---|---|---|---|
| Amphibian species | Adl. | Juv. | Met. | Adl. | Juv. | Met. |
| Bufo viridis | 0 (45) | 1 (2) | 30 (1) | 0 (72) | 0 (72) | 0 (72) |
| Hyla savignyi | 0 (45) | 0 (45) | 0 (45) | 1 (1) | 1 (1) | 2 (1) |
| Rana bedriagae | 0 (45) | 0 (45) | 4 (1) | 0 (72) | 0 (72) | 0 (72) |
Distribution of Epomis species in the study area, central coastal plain, Israel, 2007–2009 (square in left corner shows location of study area).
In the laboratory we found that Epomis dejeani preyed on all five amphibian species presented to it in 38 experiments (100% predation occurrence, Table 4). In the case of Epomis circumscriptus predation occurred in 78% of 37 experiments. In all the experiments involving Triturus vittatus and Epomis circumscriptus, predation did not take place (Table 4).
Comparison of predation of juveniles of five amphibian species by adult beetles of two Epomis species. Weights and lengths (anurans – snout-vent; urodeles - snout-end of tail) of the amphibians and shown. n indicates number of experiments.
| Amphibian species | Mean weight ±SD (g) | Mean length ±SD (mm) | Epomis circumscriptus | Epomis dejeani | ||
|---|---|---|---|---|---|---|
| Predation (%) | n | Predation (%) | n | |||
| Bufo viridis | 0.38±0.11 | 16.3±1.5 | 100 | 17 | 100 | 18 |
| Hyla savignyi | 0.24±0.03 | 15.8±1.0 | 100 | 5 | 100 | 5 |
| Rana bedriagae | 1.24±0.32 | 23.4±1.4 | 100 | 5 | 100 | 5 |
| Triturus vittatus | 0.21±0.03 | 33.0±1.9 | 0 | 8 | 100 | 8 |
| Salamandra salamandra infraimmaculata | 1.19±0.36 | 54.7±4.1 | 100 | 2 | 100 | 2 |
On March 26th, 2008 at ca. 10 pm we observed at the Berekhat Ya’ar site, ca. 50m from the pond, an Epomis dejeani female biting a Bufo viridis metamorph on the lower back area and dragging it for a short distance (ca. 20cm). We then observed the female devouring the metamorph for a period of 27 minutes, starting at the back area, and leaving only the fore and hind limbs. Twenty minutes later, at a distance of ca. 250m from the pond, we observed a different Epomis dejeani female feeding on a Bufo viridis metamorph in a crevice in the ground. On July 6th, 2008 at 7 pm we observed on the pond bank at the Samar site a male Epomis dejeani feeding on a Bufo viridis metamorph. The beetle was chewing on the rear legs of the metamorph. Upon our approach it abandoned the site, leaving its prey behind.
In all of the laboratory experiments involving Bufo viridis, Hyla savignyi and Salamandra salamandra infraimmaculata metamorphs, adults of both Epomis species demonstrated a similar response of immediately jumping on the amphibian’s back, biting at the lower back area (Fig. 2a). This caused the amphibian metamorph to jump, trying unsuccessfully to shake the beetle off. Using its mandibles, the beetles made a horizontal incision in the lower back of the amphibian (Fig. 2b) causing it to cease moving within ca. 1–2 minutes. Subsequently the beetle started chewing on the back and sides of the metamorph (Fig. 2c). Within an hour (Hyla savignyi and Salamandra salamandra infraimmaculata) to an hour and a half (Bufo viridis), only the amphibian’s limbs and head remained (Fig. 2d). In all these cases the beetle’s abdomen swelled noticeably (Fig 2e). In some cases (Bufo viridis n=5; Hyla savignyi n=4; Salamandra salamandra infarimmaculata n=2) the beetle continued feeding, consuming the amphibian’s eyes as well. In all cases (n=5 for Epomis dejeani; n=5 for Epomis circumscriptus), predation of Rana bedriagae metamorphs started with the beetle biting at one of the rear limbs. Despite the vigorous jumping of the Rana metamorph the beetle hung on successfully. Within ca. 40 seconds the metamorph ceased to struggle and the beetle changed position to the posterior venter where it initiated chewing. Feeding continued for ca. two hours.
Predation of amphibians by adult Epomis: a Bufo viridis juvenile by Epomis circumscriptus b Hyla savignyi juvenileby Epomis circumscriptus c Bufo viridis juvenile by Epomis circumscriptus d Salamandra salamandra infraimmaculata metamorph by Epomis dejeani e Hyla savignyi juvenile by Epomis circumscriptus f Triturus vittatus metamorph by Epomis dejeani (photographs by Gil Wizen).
Four out of the five amphibian species were consumed by the two Epomis species, whereas Triturus vittatus was consumed only by Epomis dejeani. In all cases, predation of Triturus vittatus started by biting at the central venter (Fig. 2f). Feeding lasted for 27–34 minutes, and when it ended only a few bones remained. In contrast, most Epomis circumscriptus (n=5) completely avoided any encounter with Triturus vittatus. In two cases of Epomis circumscriptus the beetle jumped on the newt but did not initiate biting, and within ca. 10 seconds turned away from the amphibian. It then moved its forelegs and antennae through its mouth parts; this display appeared as cleaning behavior. In one case Epomis circumscriptus clasped Triturus vittatus by its neck using its mandibles and carried it for a short distance (ca. 10cm). The beetle then dropped the newt on the ground and ceased biting. The beetle was restless, repeatedly moving its forelegs and antennae through its mouth parts as described above.
The amphibian-Epomis predation interaction is demonstrated in photos and short videos (URL: Amphibian - Adult Epomis interaction).
DiscussionTwo Epomis
species occur in the central coastal plain of Israel. In the course of
this study, they were recorded in four sites only, within a radius of
<20 km, but never in the same site. Climate, soil type and
vegetation were similar in the four sites in which the beetles occur. In
the absence of neither a physical barrier nor an apparent habitat
difference the segregation of the species to different sites may be a
case of sympatric species that do not occur in the same sites (reviewed
in
Adults of the two Epomis
species share shelters with amphibians during the day. The encounter
between predator and prey is inevitable when the two become active at
night. The outcome of this interaction is invariably fatal for the
amphibian. Adult Carabidae are phytophagous, zoophagous and mixophagous (
In the field we have evidence for predation of Bufo viridis by the two Epomis species. In laboratory experiments we found that one of the Epomis species preyed upon three anurans and two urodeles while the other species avoided Triturus vittatus.
An in-depth investigation of predation of amphibians by Epomis
species in Israel has revealed that the diet of the two sympatric
congeners that do not occur at the same site overlaps only partially.
Most reported studies on food habits demonstrate diet partitioning as
well as overlap in congeneric sympatric species. These reports include
vertebrates such as fish (
We thank Prof. Leon Blaustein and Asaf Sadeh (Haifa University) for providing the specimens of Salamandra salamandra infraimmaculata. Liron Goren (Tel-Aviv University) assisted in capturing Triturus vittatus metamorphs. Prof. Thorsten Assmann and the reviewers provided constructive remarks. The permit for collecting amphibians was issued by the Nature and Parks Authority, Israel. We thank Naomi Paz (Tel-Aviv University) for editorial assistance and Rona Cohen (Tel-Aviv University) for web-site assistance.