(C) 2012 Nicolás Pérez Hidalgo. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The new species Rhopalosiphum chusqueae Pérez Hidalgo & Villalobos Muller, is described from apterous viviparous females caught on Chusquea tomentosa in Cerro de la Muerte (Costa Rica). The identity of the species is supported both by the morphological features and by a molecular phylogenetic analysis based on a fragment of the mitochondrial DNA containing the 5’ region of the cytochrome c oxidase 1 (COI) and on the nuclear gene coding for the Elongation factor-1 alpha (EF1α). The taxonomic position of the new species is discussed. An identification key to the Aphidinae species living on plants of Bambusoideae (Poaceae) is presented.
Rhopalosiphum, aphids, new species, molecular, Costa Rica
The high diversity of organisms in Costa Rica has been referred to as a product of diverse ecosystems resulting from the interaction between complex microclimates, soils, topography, and a variety of biological processes, as well as the position of the country in the land-bridge between North and South America. Costa Rica’s biodiversity comprises more than 500, 000 species of organisms, approximately 84% of which are yet to be described. This percentage is even higher (90%) if we take insects, fungi, bacteria and viruses into account (
During an expedition in 2008 in the area of Cerro de la Muerte (Cordillera de Talamanca), Costa Rica, three apterous viviparous females and several nymphs were collected on Chusquea tomentosa (Fig. 1). At first, they were assigned to the subtribe Rhopalosiphina Mordvilko, 1914 (Aphidini Latreille, 1802). This identification was confirmed in the laboratory when it was verified that the marginal papillae on abdominal segments I and VIII were in dorsal position to the corresponding stigmata. The morphological characters of the specimens resembled those of the genus Rhopalosiphum Koch, 1854, though the length of the setae were reminiscent of species in the subgenus Paraschizaphis Hille Ris Lambers, 1947 (Schizaphis Börner, 1931).
AView of the area where the types of Rhopalosipum chusqueae were captured, in Cerro de la Muerte (Costa Rica), with Chusquea tomentosa B view of the plant C and D details of the area where the aphids were located.
According to
A microscopic morphotaxonomic study of the specimens enabled the hypothesis that they could not be assigned to any known species, and strengthened the hypothesis that they be assigned to Rhopalosiphum. Molecular phylogenetic analysis based (1) on a fragment of the mitochondrial DNA containing the 5’ region of the cytochrome c oxidase 1 (COI) and (2) on the nuclear gene coding for the Elongation factor-1 alpha (EF1α) were used to verify both hypotheses.
Several species included in the subfamily Aphidinae are known living on plant species of the subfamily Bambusoideae (Poaceae), but only two belong to Rhopalosiphum (Aphidini): Rhopalosiphum arundinariae (Tissot, 1933) and Rhopalosiphum rufiabdominale (Schrank, 1899), and none to the genus Schizaphis.
Material and methods Material studiedThree apterous viviparous female and several nymphs (sample CRI-235) were recorded on Chusquea tomentosa Y. Widmer et L. G. Clark (Poaceae: Bambusoideae: Bambuseae: Chusquinae) in Ojo de Agua (Cerro de la Muerte, Cordillera de Talamanca, Costa Rica) (9°36'N, 83°47'W), 2968 m, 26.ii.2008.
Morphological studyThirty-three quantitative characteristics and the qualitative features of shape, sclerotization, pigmentation and cuticular ornamentation, were considered. The method used for measurements is that normally employed in our studies (
Total DNA was extracted separately from two samples, one of them containing a single nymph and the second the contents of the abdomen of 3 apterous adults, all kept in 96% ethanol. We followed the HotSHOT (Hot Sodium Hydroxide and Tris) method (
PCR amplification of the two gene fragments analyzed was carried out on 3 µl of the extracted DNA. A 710 bp fragment of the 5’ region of the mitochondrial cytochrome c oxidase subunit 1 (COI) was amplified using primers LCO1490 and HCO2198, described by
PCR products were purified by ammonium precipitation and reconstituted in 10 μL of LTE buffer (10mM Tris, 0, 1mM EDTA). Direct sequencing of amplified fragments was done in both directions using PCR primers (Efr2 was used as reverse primer for sequencing the EF1α fragment). Sequencing was conducted using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) following the manufacturer’s instructions, and samples were loaded onto an ABI 3700 automated sequencer.
Chromatograms were revised and sequences corresponding to each sample assembled using the Staden package v1.6.0 (
Phylogenetic analysis of COI sequences were done using MEGA version 4 (
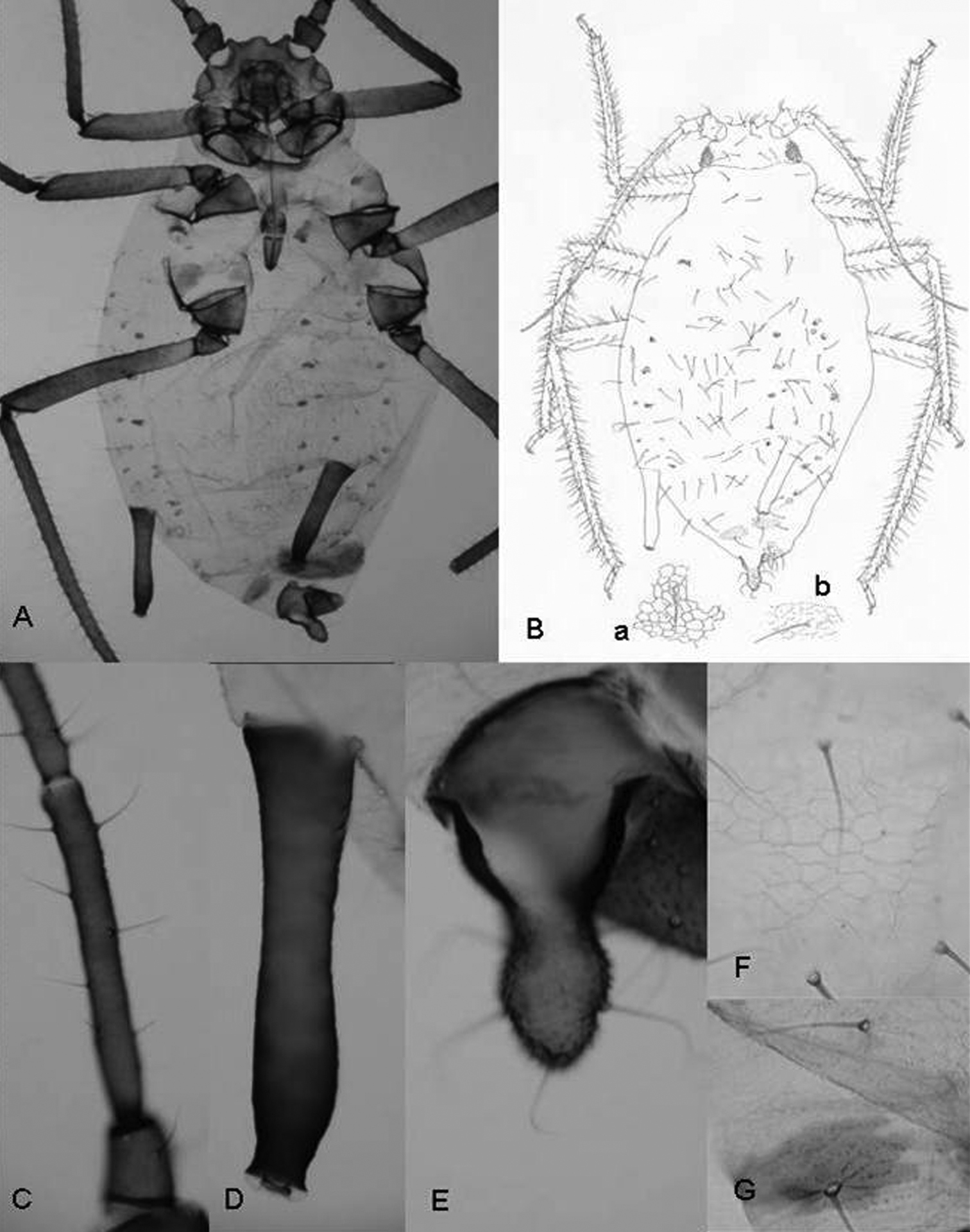
A study of the qualitative and quantitative (metric and meristic) characters of the specimens enabled us to establish the hypothesis that they belong to the genus Rhopalosiphum as, apart from the above-mentioned character of the marginal papillae on the abdomen, (1) when alive they are ovoid and when preserved the body is not very long and the margins are curved (Figs 2A, 2B), (2) the dorsal cuticle of the thorax and abdomen is membranous, except for the presence of intersegmental sclerites and a pair of large sclerites on abdominal segment VIII (Figs 2A, 2B), (3) the dorsal cuticle has a more or less regular reticulate area formed by coalescent spinules (Figs 2Ba, 2Bb, 2F, 2G), (4) the siphunculi are longer than the cauda and clearly constricted underneath the apical edge (Figs 2A, 2B, 2D), and (5) there are few setae on the cauda.
Rhopalosiphum chusqueae sp. n. A, B Habitus C antennal segment III D siphunculus E cauda. F (and B-a) detail of the cuticule of abdominal segment 3 G (and B-b) detail of cuticule of abdominal segment 8.
A comparison of the characters of these specimens with those of apterae in other species of Rhopalosiphum and Schizaphis also strengthened the hypothesis that they could not be assigned to any known species.
Molecular dataA 710 bp DNA fragment containing a portion of the mitochondrial COI gene was amplified through PCR from the two samples analysed. Useful sequences obtained from each sample consisted of 658 nucleotides. Identical sequences were obtained for both samples so that a single sequence was finally assigned and deposited in Genbank (accession number HE604204). The online identification engine available at the Barcode of Life Data Systems (BOLD) (
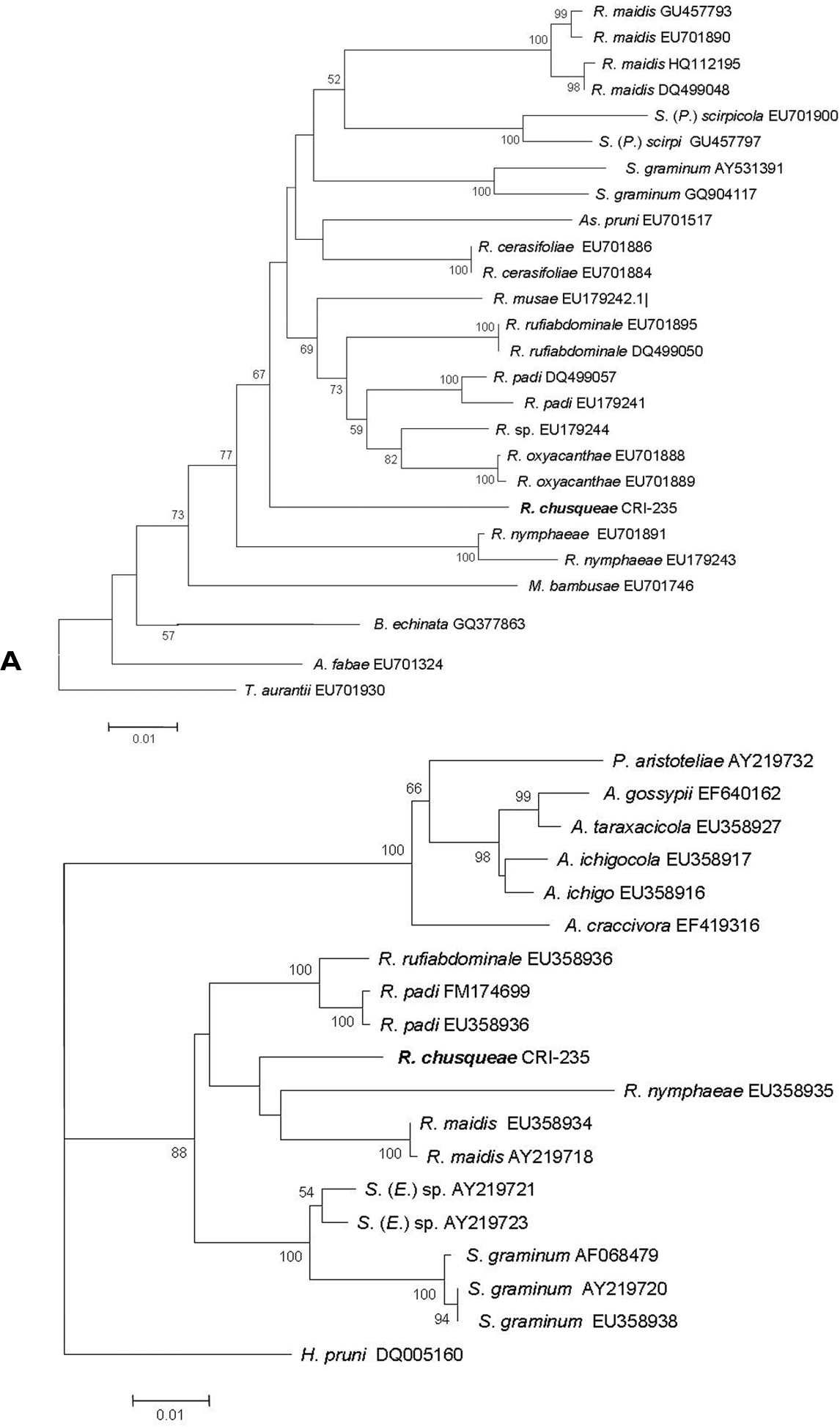
A Neighbour joining tree based on Kimura 2P distances obtained for the COI sequences from our new species (Rhopalosiphum chusqueae) and different Aphidini representatives obtained from the NCBI database. B Maximum Likelihood tree obtained for the EF1α sequences for our new species (Rhopalosiphum chusqueae) and different Aphidini representatives obtained from the NCBI database. Bootstrap support values obtained after 1000 replicates in A and 100 in B are indicated above branches if higher than 50%. Initials for genera are as follows: A, Aphis; As, Asiphonaphis; B, Braggia; H, Hyalopterus, M, Melanaphis; P, Paradoxaphis; R, Rhopalosiphum; S, Schizaphis (Schizaphis); S. (E.), Schizaphis (Euschizaphis); S. (P.), Schizaphis (Paraschizaphis); T, Toxoptera.
For the Elongation factor-1 alpha (EF1α) gene fragment, we obtained an identical sequence from the two analyzed samples of 987 bp which was deposited in the Genbank with accession number HE604205. Using sequences available for EF1α in NCBI for different Rhopalosiphum and closely related species within Aphidini, an ML tree was built that included the sequence obtained for our unknown species (Fig. 3B). As with the COI sequence, our unknown species grouped with strong support within a monophyletic clade that also included sequences from Schizaphis and Euschizaphis. However, unlike the COI tree, both Rhopalosiphum and Schizaphis-related sequences separated into two distinct clades, though with very low bootstrap support.
Discussion and conclusionMolecular data using both mitochondrial COI and nuclear EF1α gene sequences confirmed that the Rhopalosiphum chusqueae specimens belong to the same monophyletic clade as other Rhopalosiphum species occupying a rather basal position in the group likely closely related to other divergent Rhopalosiphum species such as Rhopalosiphum nymphaeae. Both trees revealed the close relationship between Rhopalosiphum and Schizaphis genera. Although COI sequences are widely used in taxonomy, their utility for phylogeny reconstructions seems rather limited as their phylogenetic signal is somewhat weak in comparison with other markers (
Approximately 15 species are classified in the genus Rhopalosiphum (
Species of Rhopalosiphum most resembling the new species due to their morphological characters are Rhopalosiphum rufiabdominale and Rhopalosiphum padiformis. The former originated from East Asia (
In view of the above, a new species can be established, the description of which follows.
urn:lsid:zoobank.org:act:D3A0466B-3858-46A8-A4BB-4618BDD956D3
Apterous viviparous female number 1 of measurement series, caught on Chusquea tomentosa, Pérez Hidalgo & Villalobos Muller leg., deposited in the Aphidological Collection of the University of León (CZULE), sample CRI-235.
2 apterous viviparous females (in separated slides) caught with the holotype.
The specific epithet, chusqueae is the genitive singular of the generic name of the aphid’s host plant.
(Figure 2). When alive globular oval and brown with white spots of wax on abdomen. Mounted 2.20–2.72 mm and pale in general with head, antennae, legs, siphunculi and cauda dark-brown.
Antennae 0.63–0.79 times body length. Antennal segment III (0.32–0.43 mm) shorter than segment IV (0.21–0.25 mm) plus V (0.20–0.26 mm); with setae 55–65 μm long and 1.8–2.6 times the articular diameter of the segment. Terminal process of segment VI (0.44–0.47 mm) 3.9–4.4 times the base (0.32–0.43 mm). Rostrum 0.52–0.61 mm long, reaching middle coxae, 0.19–0.27 times the body length. Ultimate rostral segment 0.13–0.15 mm long, approximately 1.7 times its basal width and 1.1 second segment of hind tarsus; it carries two accessory setae. Marginal papillae present on prothorax, on the abdominal segment 1 and 7, which are dorsally placed to the respective spiracles, and sometimes on segments 3 and 6. Dorsum of the abdomen with spinules forming reticulate ornamentation. Dorsal setae on abdominal segment 3 with delicate, pointed and 25–30 μm long and 3.0–3.9 times the articular diameter of antennal segment III and shorter than ventral ones, which are 90–110 μm long. Siphunculi slightly swollen with marked narrowing below the flange, 0.41–0.45 mm long, 0.16–0.20 times the body length and 2.1–2.2 times cauda. Abdominal segment 8 with two sclerites and four setae 90–110 μm long, delicate and pointed. Genital plate with 2 discal setae and near 26 posterior ones. Cauda finger-like, 0.19–0.21 mm long and carrying 5 setae.
Chusquea tomentosa (Poaceae, Bambusoideae) is the only known host of Rhopalosiphum chusqueae. This bamboo is endemic to the country and can be found in several areas of the Cordillera de Talamanca at an altitude of between 2450 and 3000 m (
On the plant, the aphids live close to the nodes well protected by the leaves (Figs 1C, 1D) and not easily detectable, as shown by fruitless efforts to locate other colonies.
So far, only one aphid species had been recorded on Chusquea: Hysteroneura setariae (Thomas) on Chusquea abietifolia Griseb, in Cuba (
This key has been prepared using the general structure and several couplets in all of those keys by Blackman and Eastop; thirteen Aphidinae species and subspecies have been included, and are: Hysteroneura setariae (Thomas, 1878), Melanaphis arundinariae (Takahashi, 1937), Melanaphis bambusae (Fullaway, 1910), Melanaphis meghalayensis bengalensis Raychaudhuri [D.N.] and Banerjee [C.], 1974, Melanaphis meghalayensis meghalayensis Raychaudhuri [D.N.] and Banerjee [C.], 1974, Melanaphis pahanensis (Takahashi, 1950), Melanaphis sacchari (Zehntner, 1897), Rhopalosiphum arundinariae (Tissot, 1933) and Rhopalosiphum rufiabdominale (Schrank, 1899) (Aphidinae Aphidini Rhopalosiphina), and Sitobion bambusicola (Ghosh [L.K.], 1986), Sitobion fragariae (Walker, 1848), Sitobion miscanthi (Takahashi, 1921) and Sitobion papillatum subnudum Remaudière, 1985 (Aphidinae Macrosiphini).
| 1 | Siphunculus without apical zone of polygonal reticulation. Abdominal segments I and VII with marginal tubercles (papillae) placed dorsally to the respective spiracular apertures. Cuticle of dorsum of the abdomen membranous, a sclerotized patch absent | 2 |
| – | Siphunculus with apical zone of polygonal reticulation (at least two rows of cells). Abdominal segments I and VII usually without marginal tubercles (papillae), but if they are present then spinal papillae present on head and several abdominal segments. Dorsum of the abdomen with a sclerotized patch more or less extended and pigmented | 12 |
| 2 | Aphids spindle-shaped, green when alive. Siphunculus very small (less than 0.7 times cauda), thin, cylindrical and narrow-based, flangeless, and with not functional aperture | Hyalopterus pruni [and other Hyalopterus spp.] |
| – | Aphids broad oval-shaped. Siphunculus 0.5–2.5 times cauda (if less than 0.6 times then less than 2 times longer than its basal width), shaped differently and with functional aperture | 3 |
| 3 | Siphunculus short, usually thick or rather thick, less than (often much less than) 2.4 times longer than its basal width, 0.4–1.2 times cauda, and usually with a well-developed, rather swollen flange | 4 |
| – | Siphunculus usually longer than cauda (if less than 1.2 times cauda then it is more than 2.4 times its basal width and/or has a small flange), tapering, cylindrical or swollen | 9 |
| 4 | Setae on antennal segment III at most 1.5 times the basal diameter of the segment. [Alatae viviparous females with wing veins dark bordered] | 5 |
| – | Setae on antennal segment III at least 2.0 times the basal diameter of the segment. [Alatae viviparous females with wing veins not dark bordered] | 6 |
| 5 | Cauda with only 4-6 setae. Coxae dark | Melanaphis bambusae |
| – | Cauda with 7-20 setae. Coxae pale | Melanaphis sacchari |
| 6 | Antennae five-segmented. Siphunculus 1.5 times its basal width at least | Melanaphis arundinariae |
| – | Antennae six-segmented. Siphunculus 1.4 times its basal width at most | 7 |
| 7 | Siphunculus 1.1–1.4 times its basal width. Terminal processus of antennal segment VI at most 2.3 times the base | Melanaphis pahanensis |
| – | Siphunculus 0.8–0.9 times its basal width. Terminal processus of antennal segment VI at least 2.2 times the base | [Melanaphis meghalayensis] 8 |
| 8 | Cauda with 4–6 setae and anterior half of the genital plate with 4–7 setae | Melanaphis meghalayensis meghalayensis |
| – | Cauda with 7–10 setae and anterior half of the genital plate with 2 setae | Melanaphis meghalayensis bengalensis |
| 9 | Setae on antennal segment III shorter than the basal width of the segment | 10 |
| – | Setae on antennal segment III longer than the basal width of the segment | 11 |
| 10 | Cauda at least 1.5 times its basal width, finger-shaped, with basal constriction, paler than cauda, and usually with 4 setae. [Alate viviparous females with only one oblique vein in hindwing] | Hysteroneura setariae |
| – | Cauda a little longer that its basal width, cone-shaped, without basal constriction, as dark as siphunculi, and with approximately 8 setae. [Alate viviparous females with two oblique veins in hindwing] | Rhopalosiphum arundinariae |
| 11 | Antennae usually five-segmented. Setae on antennal segment III 3.0–5.0 times the basal width of the segment. Abdominal segment VIII with 3–8 setae. Ultimate rostral segment 1.3–1.8 times second segment of the hind tarsus. Terminal processus of antennal segment VI 4.0–6.5 times the base | Rhopalosiphum rufiabdominale |
| – | Antennae six-segmented. Setae on antennal segment III 1.8–2.6 times the basal width of the segment. Abdominal segment VIII with 4 setae. Terminal processus of antennal segment VI 3.9-–4.4 times the base | Rhopalosiphum chusqueae sp. n. |
| 12 | Spinal tubercles (papillae) present on the head and abdominal segments (V)VI-VIII; marginal ones present on prothorax and abdominal segments (I)II–V and infrequently on VII | Sitobion papillatum subnudum |
| – | Spinal tubercles (papillae) absent; marginal ones on abdominal segment II–V usually absent, and always absent on abdominal segments I and VII | 13 |
| 13 | Cauda dusky (but not as dark as siphunculi) and with a rather pointed apex. Siphunculus 2.0–2.1 times cauda. Aphids yellowish when alive | Sitobion bambusicola |
| – | Cauda pale (very contrasted with siphunculi) with a variably shaped apex. Siphunculus 1.4–2.7 times cauda. Aphids variable in colour when alive | 14 |
| 14 | Siphunculi 1.75–2.25 times cauda, which has a rather rounded apex | Sitobion fragariae |
| – | Siphunculi 1.4–1.9 times cauda, which has a rather pointed apex | Sitobion miscanthi |
The authors thank the Agencia Española de Cooperación Internacional para el Desarrollo (AECID) [joint project of the universities of León and Costa Rica, number D/010523/07] for supporting this research and to the Universidad de Costa Rica for the scientific visit of W. Villalobos (July 2009) to Universidad de León and Institut Cavanilles de Biodiversitat i Biología Evolutiva. Work partially funded by MICINN project CGL2010-22043 to D. Martínez-Torres (Universitat de València). The authors also acknowledge the suggestions made by the academic editor and the reviewer S. Halbert.