(C) 2011 Federica Talarico. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In carabid beetles, physiological and behavioural characteristics reflect specific habitat demands and there is a strong correlation between body form and habit in species with different life style. In this study, we compared the morphometry and compound eye characteristics of three species of the genus Siagona: Siagona jenissoni, Siagona dejeani and Siagona europaea. These carabids have a stenotopic lifestyle in Mediterranean clayey soils, inhabiting the ground fissure system formed during the dry season. All species have a Mediterranean distribution and are nocturnal olfactory hunters, and are strict ant predators. For morphometric measurements, we considered body length (mm), wing length (mm), antenna length (mm), head width (mm), trochanter length (mm), number of ommatidia, eye surface area (mm2), ommatidia density (number of ommatidia/mm2 of eye surface area), head height (mm), thorax height (mm) and abdomen height (mm). The data revealed intersexual and interspecific differences. The three species differ in relative length of the antennae, density and number of ommatidia and relative trochanter length. Significant differences occurred in wing sizes, which are well developed in Siagona europaea, the only species capable of flight. When eye size is compared with other ground beetles of various lifestyles, Siagona shows pronounced “microphthalmy” an adaptation to subterranean life in clayey crevices of tropical and subtropical climates with a marked dry season.
Compound eyes, morphometric measurements, Siagona
Carabid beetles vary in body form and size, annual
rhythmicity and habitat choice. They also differ in many physiological
and behavioural characteristics that reflect specific habitat demands (
Moreover, most ground beetles with seemingly similar
body shapes have species-specific morphological peculiarities that
reflect the special demands of their niches (
The genus Siagona (tribe Siagonini) should be placed among the less derived Carabidae (Carabinae, Caraboidea Simplicia of
The biology of siagonines is poorly known.
Siagona europaea is exclusively myrmecophagous, both of adult ants and their brood (
Only fragmentary information is available for Siagona jenissoni and Siagona dejeani. They occur in southern Spain, between Cadiz and Malaga, in Portugal (
The sample consisted of 20 individuals (10 males and 10 females) for each species: Siagona jenissoni, Siagona dejeani and Siagona europaea. Specimens of Siagona europaea were collected in southern Italy (Calabria, Squillace, Catanzaro, 250 m a.s.l.) mostly by bait-traps in open fields and pastures during the spring of 2004, while Siagona jenissoni and Siagona dejeani were collected in southern Spain (Andalusia, between Algesiras and Cadiz) in March of 2005 (100–400 m a.s.l.).
Morphometric analysesThe animals were stored in alcohol (70%). Photographs were taken with a stereoscope (Zeiss Stemi SV 11Apo) and acquired by Matrox PC-VCR software (for Windows® 2000). For each individual, we measured body length (mm), wing length (mm), antenna length (mm), head width (mm), trochanter length (mm), number of ommatidia, eye surface area (mm2), ommatidia density (number of ommatidia/mm2 of eye surface area), head height (mm), thorax height (mm) and abdomen height (mm).
Relative measures of antennal lengths, number of ommatidia and eye surface area were weighted against head width, while trochanter length, head height, thorax height and abdomen height were weighted against body length. To determine the number of ommatidia and cornea size, we softened the specimens in hot potash lye for a few minutes. The cornea was removed and fixed through the following stations: distilled water, acetone, ethanol (70%), absolute ethanol and xylol. It was then spread on a microscope slide and photographed. Measurements were taken using Sigma Scan Pro 5 Software (SPSS® Inc.).
Statistical analysesSexual dimorphism in each species was tested using the Mann-Whitney U test (
The probability level was computed using a complete randomisation method (permutation or exact test; PExact) or by a Monte Carlo simulation based on 10 000 sampled tables (PMonteCarlo) when computation was not possible (
The multivariate general lineal model (GLM) with species and sex as main factors was applied to sensorial structures, eye asymmetry and main body size measures to verify previously performed univariate hypothesis testing. Multivariate differences between factors were tested by Pillai’s Trace, while univariate tests were computed using the type III sum of squares.
Means are reported with standard error of means (± SEM) throughout the text.
Statistical analyses were performed using the Statistical Package for Social Sciences 13.01 (SPSS® Inc.).
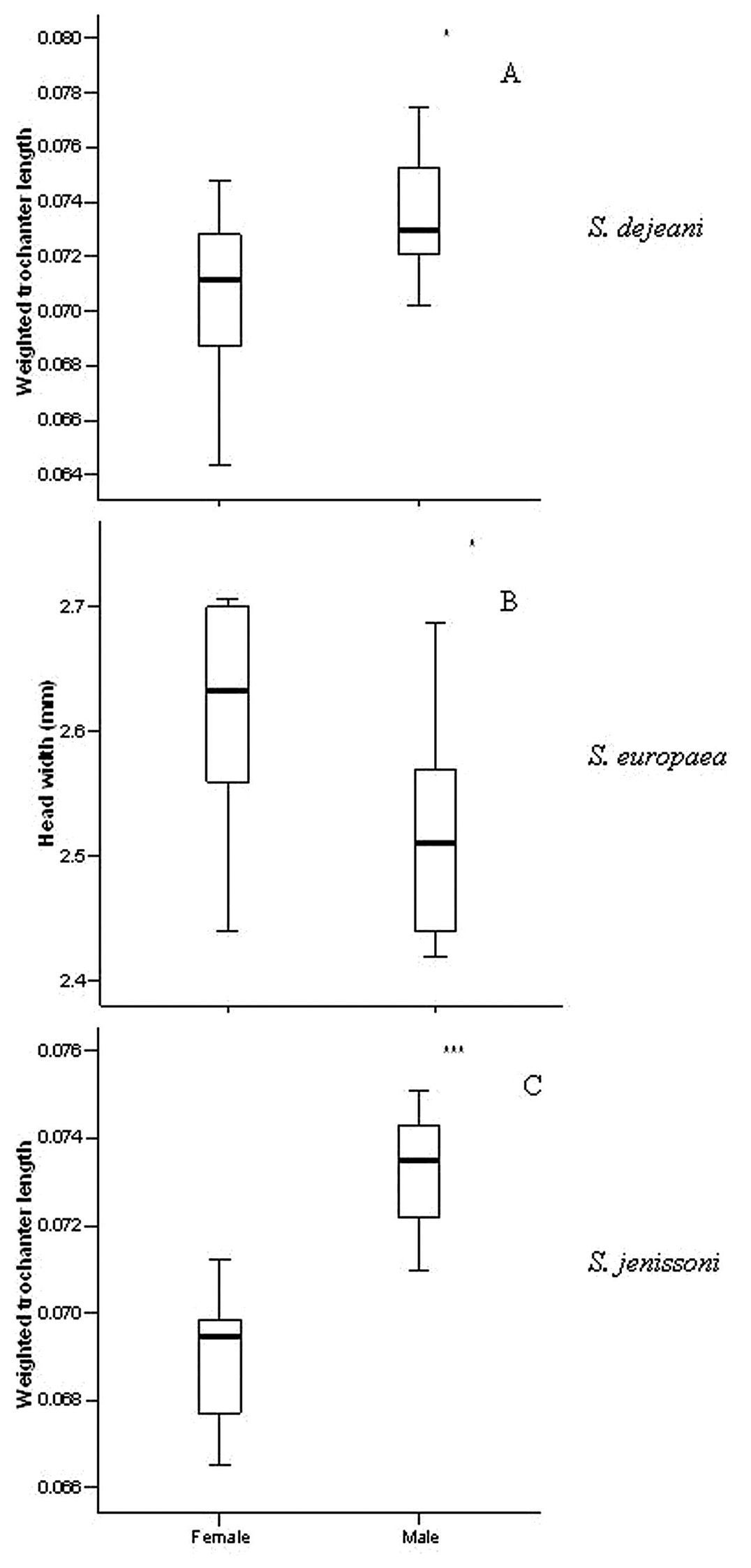
ResultsThe three species presented some sex differences related to size (Table 1, Fig. 1). Males of Siagona dejeani and Siagona jenissoni had significantly longer trochanters (relative to body length) compared to females (respectively U = 21.5, W = 76.5, PExact = 0.029 and U = 1.0, W = 56.0, PExact < 0.001), while in Siagona europaea females had wider heads than males (U = 23.0, W = 78.0, PExact = 0.043). Notably, there was no difference in the size of sensory structures (antennae and eyes) (PExact > 0.05); therefore, we evaluated specific differences in sensory structures with no concern for gender.
Measured traits between sexes in the three species: A weighted trochanter length (mm) in Siagona dejeani B head width (mm) in Siagona europaea C weighted trochanter length (mm) in Siagona jenissoni.
Sex differences in body and eye morphological characteristics (means and Standard Error of Means) in three species of Siagona. Mann-Whitney test results are shown, with significance levels estimated using a permutation procedure (PExact). Statistically significant results are in bold. L = left, R = right.
| Species | Gender | Mann-Whitney test | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | |||||||
| Mean | SEM | Mean | SEM | U | W | PExact | ||
| Siagona dejeani | Body length (mm) | 23.33 | 0.16 | 22.83 | 0.29 | 30.0 | 85 | 0.143 |
| Antenna length (mm) | 12.58 | 0.15 | 12.82 | 0.25 | 43.0 | 98 | 0.631 | |
| Head width (mm) | 5.06 | 0.06 | 5.09 | 0.09 | 46.5 | 101.5 | 0.796 | |
| Number of ommatidia L | 407.80 | 25.21 | 404.10 | 14.77 | 49.0 | 104 | 0.971 | |
| Number of ommatidia R | 404.30 | 12.47 | 356.40 | 15.87 | 22.0 | 77 | 0.035 | |
| Eye surface L (mm2) | 0.06 | 0.01 | 0.06 | 0.01 | 41.0 | 96 | 0.529 | |
| Eye surface R (mm2) | 0.08 | 0.00 | 0.07 | 0.00 | 33.0 | 88 | 0.218 | |
| Trochanter length (mm) | 1.65 | 0.02 | 1.71 | 0.04 | 39.0 | 94 | 0.436 | |
| Head height (mm) | 2.80 | 0.08 | 2.76 | 0.08 | 44.5 | 99.5 | 0.684 | |
| Thorax height (mm) | 3.37 | 0.06 | 3.33 | 0.06 | 44.5 | 99.5 | 0.684 | |
| Abdomen height (mm) | 3.22 | 0.13 | 3.17 | 0.11 | 44.5 | 99.5 | 0.684 | |
| Weighted antenna length | 2.49 | 0.04 | 2.52 | 0.05 | 42.0 | 97 | 0.579 | |
| Weighted trochanter length | 0.07 | 0.00 | 0.07 | 0.00 | 21.5 | 76.5 | 0.029 | |
| Weighted ommatidia L number | 80.86 | 5.30 | 79.51 | 2.89 | 49.0 | 104 | 0.971 | |
| Weighted ommatidia R number | 80.03 | 2.68 | 69.95 | 2.65 | 15.0 | 70 | 0.007 | |
| Weighted head height | 0.12 | 0.00 | 0.12 | 0.00 | 49.0 | 104 | 0.971 | |
| Weighted thorax height | 0.14 | 0.00 | 0.15 | 0.00 | 48.0 | 103 | 0.912 | |
| Weighted abdomen height | 0.14 | 0.01 | 0.14 | 0.00 | 49.0 | 104 | 0.971 | |
| Right ommatidia density | 5019.71 | 252.49 | 4857.98 | 162.19 | 48.0 | 103 | 0.912 | |
| Left ommatidia density | 19964.03 | 9939.31 | 20421.65 | 10096.35 | 43.0 | 98 | 0.631 | |
| Siagona europaea | Body length (mm) | 11.73 | 0.22 | 11.16 | 0.18 | 26.5 | 81.5 | 0.075 |
| Antenna length (mm) | 6.96 | 0.16 | 7.03 | 0.11 | 50 | 105 | 1.000 | |
| Head width (mm) | 2.64 | 0.04 | 2.52 | 0.03 | 23 | 78 | 0.043 | |
| Number of ommatidia L | 528.60 | 28.98 | 564.90 | 25.03 | 40 | 95 | 0.481 | |
| Number of ommatidia R | 494.10 | 31.81 | 536.10 | 17.72 | 36 | 91 | 0.315 | |
| Eye surface L (mm2) | 0.01 | 0.00 | 0.01 | 0.00 | 46 | 101 | 0.796 | |
| Eye surface R (mm2) | 0.01 | 0.00 | 0.01 | 0.00 | 48.5 | 103.5 | 0.912 | |
| Trochanter length (mm) | 0.85 | 0.02 | 0.84 | 0.02 | 39 | 94 | 0.436 | |
| Head height (mm) | 1.36 | 0.05 | 1.25 | 0.04 | 27 | 82 | 0.089 | |
| Thorax height (mm) | 1.71 | 0.05 | 1.71 | 0.03 | 46.5 | 101.5 | 0.796 | |
| Abdomen height (mm) | 1.84 | 0.05 | 1.76 | 0.07 | 35.5 | 90.5 | 0.280 | |
| Weighted antenna length | 2.65 | 0.06 | 2.79 | 0.04 | 31 | 86 | 0.165 | |
| Weighted trochanter length | 0.07 | 0.00 | 0.08 | 0.00 | 37 | 92 | 0.353 | |
| Weighted ommatidia L number | 200.85 | 10.92 | 223.42 | 8.45 | 32 | 87 | 0.190 | |
| Weighted ommatidia R number | 187.94 | 12.24 | 212.58 | 7.25 | 32 | 87 | 0.190 | |
| Weighted head height | 0.12 | 0.01 | 0.11 | 0.00 | 47 | 102 | 0.853 | |
| Weighted thorax height | 0.15 | 0.01 | 0.15 | 0.00 | 28 | 83 | 0.105 | |
| Weighted abdomen height | 0.16 | 0.01 | 0.16 | 0.01 | 49 | 104 | 0.971 | |
| Right ommatidia density | 64068.08 | 9474.82 | 71412.86 | 7665.16 | 44 | 99 | 0.684 | |
| Left ommatidia density | 68827.82 | 8122.49 | 68715.15 | 5377.99 | 40 | 95 | 0.481 | |
| Siagona jenissoni | Body length (mm) | 14.38 | 0.14 | 14.11 | 0.09 | 35 | 90 | 0.280 |
| Antenna length (mm) | 8.89 | 0.08 | 8.97 | 0.09 | 41.5 | 96.5 | 0.529 | |
| Head width (mm) | 3.20 | 0.04 | 3.31 | 0.03 | 25 | 80 | 0.063 | |
| Number of ommatidia L | 314.90 | 11.88 | 316.30 | 3.75 | 48 | 103 | 0.912 | |
| Number of ommatidia R | 346.50 | 9.23 | 363.00 | 15.99 | 42.5 | 97.5 | 0.579 | |
| Eye surface L (mm2) | 0.04 | 0.00 | 0.04 | 0.00 | 45.5 | 100.5 | 0.739 | |
| Eye surface R (mm2) | 0.04 | 0.00 | 0.03 | 0.00 | 41.5 | 96.5 | 0.529 | |
| Trochanter length (mm) | 0.99 | 0.01 | 1.03 | 0.01 | 23.5 | 78.5 | 0.043 | |
| Head height (mm) | 1.82 | 0.06 | 1.74 | 0.06 | 44 | 99 | 0.684 | |
| Thorax height (mm) | 2.09 | 0.06 | 2.20 | 0.07 | 38.5 | 93.5 | 0.393 | |
| Abdomen height (mm) | 2.24 | 0.13 | 2.28 | 0.12 | 48 | 103 | 0.912 | |
| Weighted antenna length | 2.79 | 0.03 | 2.71 | 0.03 | 24 | 79 | 0.052 | |
| Weighted trochanter length | 0.07 | 0.00 | 0.07 | 0.00 | 1 | 56 | <0.001 | |
| Weighted ommatidia L number | 98.82 | 4.35 | 95.57 | 1.12 | 36 | 91 | 0.315 | |
| Weighted ommatidia R number | 108.83 | 3.87 | 109.71 | 4.89 | 49 | 104 | 0.971 | |
| Weighted head height | 0.13 | 0.01 | 0.12 | 0.00 | 50 | 105 | 1.000 | |
| Weighted thorax height | 0.15 | 0.01 | 0.16 | 0.00 | 31 | 86 | 0.165 | |
| Weighted abdomen height | 0.16 | 0.01 | 0.16 | 0.01 | 45 | 100 | 0.739 | |
| Right ommatidia density | 10786.08 | 1392.59 | 11925.89 | 1320.63 | 42 | 97 | 0.579 | |
| Left ommatidia density | 8943.47 | 1037.65 | 9130.10 | 821.02 | 43 | 98 | 0.631 | |
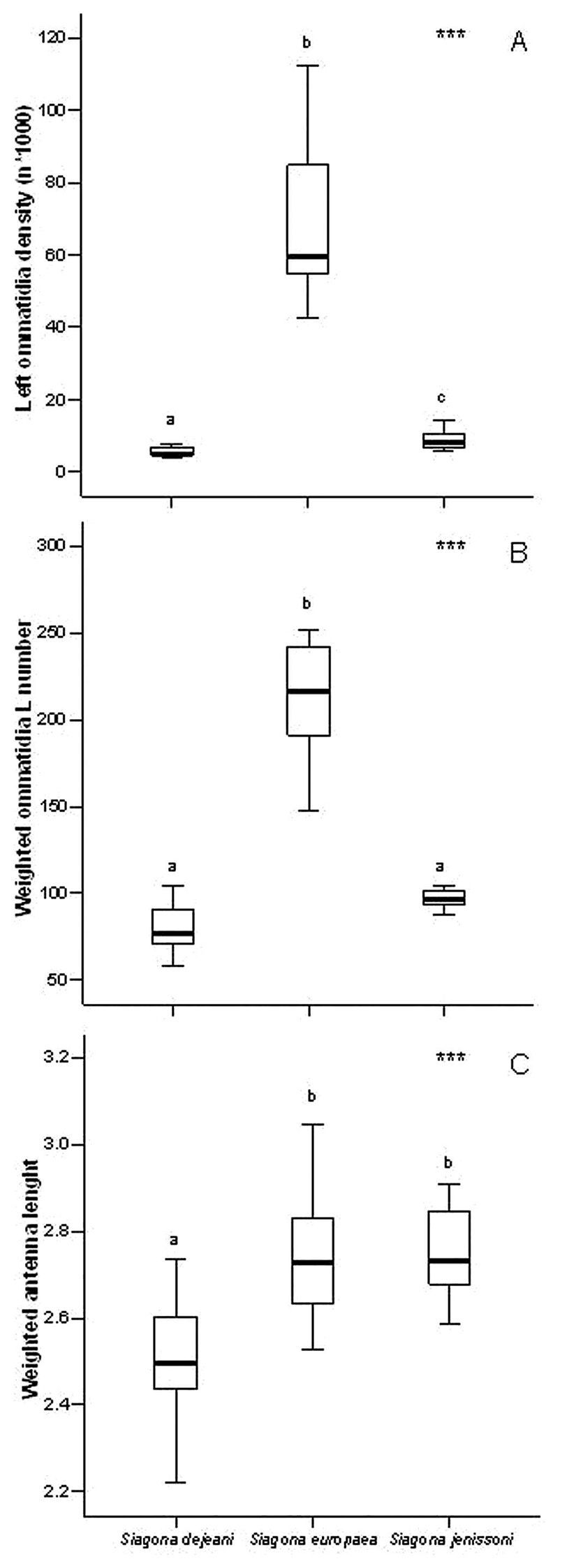
Ommatidia density differed significantly among species (X2 = 30.951, d.f. = 2, PExact < 0.001), being significantly higher (PExact < 0.05) in Siagona europaea and lower in Siagona dejeani (Table 2, Fig. 2A). The weighted number of ommatidia in Siagona europaea was higher (X2 = 45.057, d.f. = 2, PExact < 0.001), but there was no significant difference between Siagona dejeani and Siagona jenissoni (Fig. 2B). Siagona dejeani’s antennae were significantly shorter than those of the other two species (X2 = 24.521, d.f. = 2, PExact < 0.001) (Fig. 2C).
Measured traits: A ommatidia density (N/mm2) B weighted ommatidia number C weighted antenna length.
The GLM analysis confirmed these results, with the global morphological pattern differing among species (Pillai’s Trace = 1.609, F = 16.822, d.f. = 22, P < 0.001), but not between the sexes (Pillai’s Trace = 0.321, F = 1.894, d.f. = 22, P = 0.067).
Further investigation of the wing set showed that Siagona dejeani and Siagona jenissoni are brachypterous (respectively 1.93±0.03 mm and 0.94±0.03 mm wing lengths), while Siagona europaea has long wings (8.01±0.05 mm) folded under the elytra, and can thus be considered a macropterous species presumably capable of flight.
DiscussionThe three Siagona species investigated presented sex and inter-specific differences. The sexes differ only in size: males of Siagona dejeani and Siagona jenissoni had significantly longer trochanters (relative to body length) than females, while in Siagona europaea females had wider heads than males.
These Siagona species are olfactory hunters and belong to the third group of nocturnal species described by
Inter-specific differences in body and eye morphological characteristics (means and Standard Error of Means) in three species of Siagona. Kruskal-Wallis test results estimated with a permutation procedure (PMonte Carlo) are reported.<br/>
| Species | Kruskal-Wallis test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Siagona dejeani | Siagona europaea | Siagona jenissoni | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Chi-Square | df | PMonte Carlo | |
| Body length (mm) | 23.08 | 0.17 | 11.44 | 0.16 | 14.25 | 0.09 | 52.569 | 2 | <0.001 |
| Antenna length (mm) | 12.70 | 0.14 | 7.00 | 0.10 | 8.93 | 0.06 | 52.468 | 2 | <0.001 |
| Head width (mm) | 5.07 | 0.05 | 2.58 | 0.03 | 3.25 | 0.03 | 52.469 | 2 | <0.001 |
| Number of ommatidia | 405.95 | 14.23 | 546.75 | 19.10 | 315.60 | 6.07 | 44.612 | 2 | <0.001 |
| Eye surface (mm2) | 0.06 | 0.01 | 0.01 | 0.00 | 0.04 | <0.01 | 28.722 | 2 | <0.001 |
| Trochanter length (mm) | 1.68 | 0.03 | 0.85 | 0.01 | 1.01 | 0.01 | 51.865 | 2 | <0.001 |
| Head height (mm) | 2.78 | 0.05 | 1.31 | 0.03 | 1.78 | 0.04 | 50.937 | 2 | <0.001 |
| Thorax height (mm) | 3.35 | 0.04 | 1.71 | 0.03 | 2.14 | 0.05 | 49.799 | 2 | <0.001 |
| Abdomen height (mm) | 3.19 | 0.09 | 1.80 | 0.04 | 2.26 | 0.09 | 43.233 | 2 | <0.001 |
| Weighted antenna lenght | 2.51 | 0.03 | 2.72 | 0.04 | 2.75 | 0.02 | 24.521 | 2 | <0.001 |
| Weighted trochanter length | 0.07 | 0.00 | 0.07 | 0.00 | 0.07 | 0.00 | 9.586 | 2 | 0.007 |
| Weighted ommatidia number | 80.19 | 2.94 | 212.13 | 7.20 | 97.20 | 2.22 | 45.057 | 2 | <0.001 |
| Weighted head height | 0.12 | 0.00 | 0.11 | 0.00 | 0.12 | 0.00 | 5.979 | 2 | 0.049 |
| Weighted thorax height | 0.15 | 0.00 | 0.15 | 0.00 | 0.15 | 0.00 | 2.977 | 2 | 0.228 |
| Weighted abdomen height | 0.14 | 0.00 | 0.16 | 0.00 | 0.16 | 0.01 | 9.667 | 2 | 0.007 |
| Ommatidia density | 20192.84 | 6895.16 | 68771.49 | 4740.88 | 9036.79 | 644.29 | 30.951 | 2 | <0.001 |
Siagona europaea has a higher number of ommatidia, full-sized wings and a greater antenna vs. head ratio. These features are indicative of high dispersal powers, which is a good strategy for adapting to habitats such as pastures and fields, where natural or anthropogenic disturbances are frequent. Furthermore, it is likely that high powers of dispersal enhance the probability of finding new ant nests, which are often scattered rather than homogeneously distributed throughout the territory. Conversely, Siagona dejeani has shorter antennae, a lower number of ommatidia and smaller wings than the other two species. This is presumably related to a lesser need to search for a partner, as the beetles live in aggregation in which chemical cues easily allow males and females to meet.
In conclusion, the general morphometry of these three Mediterranean species of the genus Siagona
is typical of beetles living in narrow spaces, presumably in darkness,
for most of their life. As a consequence, eye morphology is well
adapted to their habitat demands and to olfactory/tactile predation.
Indeed, success in detecting ants or ant traces is assured by the
complex sensory structure of the labial palps, which has been described
in detail for Siagona europaea (
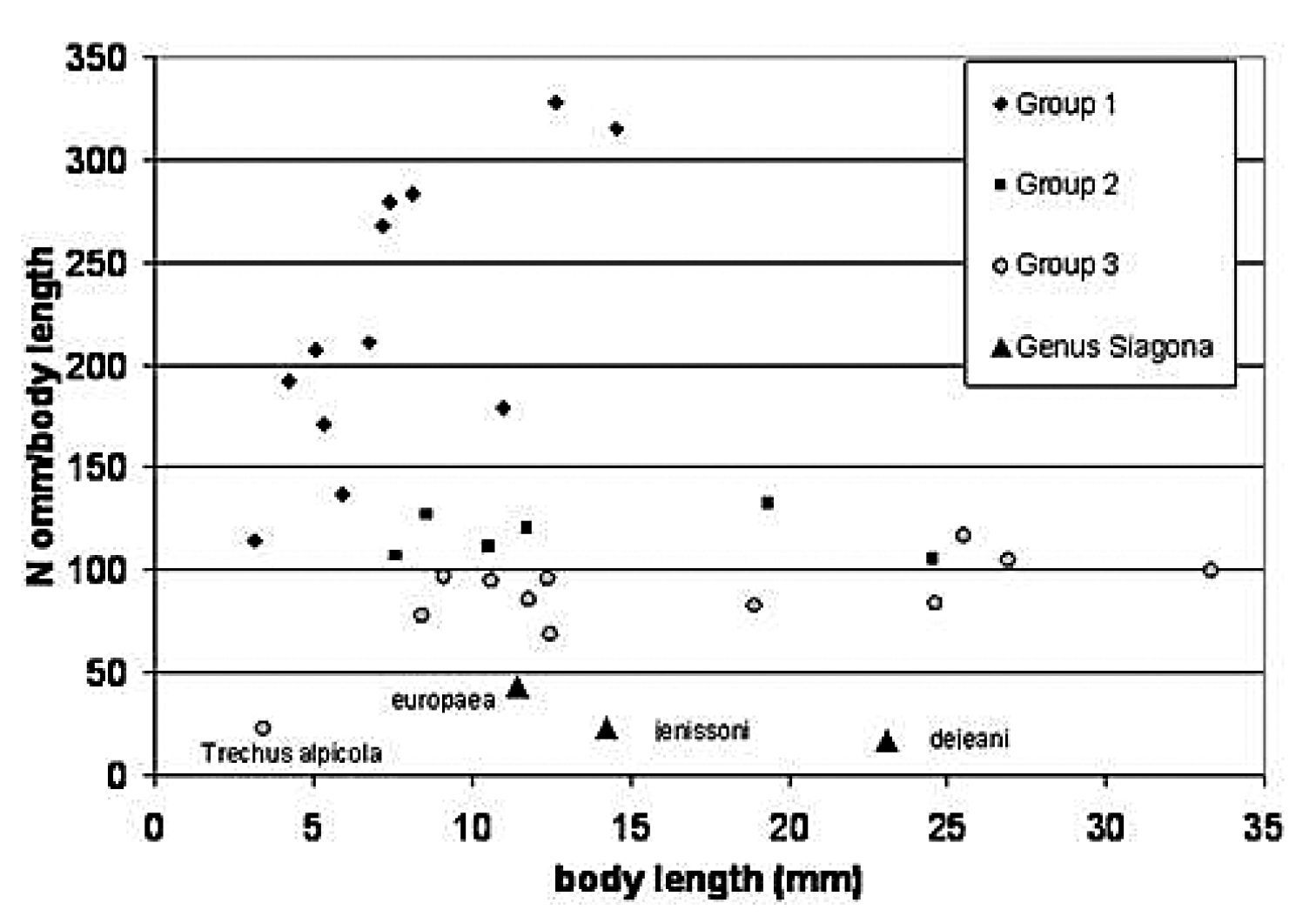
The carabid genus Siagona is a stenotopic ground dweller that preys on ants in the deep fissures of clayey soil. In Fig. 3 we compared the eye sizes of the three European species with the “ommatidial indices” of the three groups of
Ommatidial index versus body length in the three Bauer-Kredler groups of carabids and comparison with European species of Siagona (this study). Y-axis: mean number of ommatidia per body length in mm. In order of decreasing ommatidial index, Group 1: Cicindela campestris, Cicindela hybrida, Elaphrus cupreus, Elaphrus riparius, Elaphrus ullrichi, Elaphrus aureus, Notiophilus biguttatus, Asaphidion flavipes, Asaphidion pallipes, Asaphidion caraboides, Bembidion quadrimaculatum. Group 2: Carabus granulatus, Agonum sexpunctatum, Poecilus cupreus, Poecilus versicolor, Carabus auratus. Group 3: Carabus problematicus, Carabus lefebvrei, Carabus coriaceus, Leistus rufomarginatus, Nebria brevicollis, Pterostichus nigrita, Carabus preslii, Abax parallelepipedus, Patrobus atrorufus, Pterostichus burmeisteri, Trechus alpicola.
In conclusion, the European Siagona species exhibit a lifestyle thus far unknown in carabid beetles, i.e., a stenotopic adaptation to clayey soils of tropical and subtropical climates marked by a long dry season. The adults probably enter fissures in the clay at the beginning of the dry phase and are able to exploit the rich trophic resources (ant workers and perhaps ant brood) in this three-dimensional subterranean space.
This paper is dedicated to the dear memory of Prof. Tullia Zetto Brandmayr, who enthusiastically contributed to this study until her last days.