(C) 2012 Javier Torréns. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Descriptions of the adults of the two species of Dicoelothorax Ashmead, Dicoelothorax parviceps and Dicoelothorax platycerus, and the eggs, planidia and pupae of Dicoelothorax platycerus Ashmead are provided. Females of Dicoelothorax platycerus deposit their eggs on the underside of leaves of Pseudabutilon virgatum (Cav.) Fryxell (Malvaceae). The host of Dicoelothorax platycerus is Ectatomma brunneum Smith (Formicidae: Ectatomminae).
Dicoelothorax, eggs , planidia, pupae, host ant, host plant
Eucharitidae are parasitoids of pupae of Formicidae (Hymenoptera: Aculeata) (
Dicoelothorax was established by
This genus includes two species distributed in the Neotropical region: Dicoelothorax parviceps Cameron (Argentina, Brazil, Colombia and Guyana) and Dicoelothorax platycerus Ashmead (Argentina, Bolivia and Brazil). The original descriptions of these species are vague and short, and there is no clear differentiation of species. Based on the collections examined and our new material, we were able to differentiate both species. Herein we provide new descriptions and diagnoses. Also, Dicoelothorax platycerus was collected in northwestern Argentina, and information on life history, immature stages, and a new host association are included.
Materials and methodsDicoelothorax platycerus were collected at San Vicente, Tucumán (26°25'36"S, 65°15'41"W; 740 m altitude) on March 12, 2009 on Pseudabutilon virgatum (Cav.) Fryxell (Malvaceae). Eggs were found on the underside of the leaves. Five females of Dicoelothorax platycerus were collected in the field and provided twigs with leaves, fruits, and flowers of different species of plants in 10 × 3.5 cm plastic tubes to monitor oviposition habits. Leaves of Pseudabutilon virgatum with eggs were placed into a cylindrical glass container of 10 × 10 cm with dampened cotton until emergence of the first instar (planidium). The planidia and some eggs were preserved in ethanol. Planidia were cleared in 10% KOH and both larvae and eggs slide-mounted in Hoyer’s medium.
Three nests of Ectatomma brunneum Smith (Formicidae: Ectatomminae) that were in close proximity to the adult collection and oviposition site were excavated. Adults, brood, and debris were collected into plastic containers. Adults and immature stages were then sorted from the debris, examined for parasitism, and subsequently returned to the containers to allow further development of immatures. The immature stages were examined once daily until all parasitoids or ants emerged from the cocoons.
Images were obtained using GT-VISION® ENTO-VISION software operating on a Leica M16 zoom lens linked to a JVC KY-F75U 3-CCD digital video camera; and LEICA APPLICATION SUIT (version 3.5.0) software operating on a Leica MZ12 linked to a Leica DFC295 digital video camera. Images were enhanced with COREL PHOTOPAINT and COREL DRAW (version 15); and some images processed with DEEP FOCUS (Stuart Ball).
Specimens studied are deposited in the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina (MACN); Instituto Fundación Miguel Lillo, Tucumán, Argentina (IFML); University of California, Riverside, California, USA (UCRC); and American Museum of Natural History, New York, USA (AMNH). Notes and detailed illustrations of the type material housed at the National Museum of Natural History, Washington (USNM) and the Natural History Museum, London (BMNH) were made available by J. Heraty (UCRC).
Morphological terms are from
http://species-id.net/wiki/Dicoelothorax_parviceps
Figs 1–5Distinguished from Dicoelothorax platycerus by the mesosoma and frenal processes having distinct, widely spaced longitudinal striae (Figs 2, 5); dorsal concavity of mesoscutum and scutellum smooth medially (Fig. 2); frenal processes in dorsal view distinctly tapered with apex narrowly rounded (Figs 2, 5); venation yellow with stigma pale brown; scutellar processes of male white and straight in lateral view, and 1.6× as long as scutellum (Figs 4, 5).
Female. Length3.8 mm. Head, mesosoma, coxae, petiole and Gt1 except distal part black; basal ¾ of femora, frenal processes, distal part of Gt1 and rest of terga brown; antenna yellowish to light brown; rest of legs and distal limits of terga yellowish. Wings slightly infuscate, venation yellow, stigmal vein pale brown (Fig. 3).
Head 1.7× as broad as high. Frons and face granulate, weakly strigose, with small and scattered setae (Fig. 1). Eyes separated by 2.3× their height. Malar space as long as height of eye. Antenna with 8 segments; scape 2.9× as long as broad, broader apically, smooth, with a few scattered setae. Length of flagellum 0.9× height of head, basal flagellomere (homologous to F1+F2;
Mesosoma. Midlobe of mesoscutum elevated anteriorly, with short, thin, decumbent and scattered setae; striate-rugose on anterior face of mesoscutum, sidelobes longitudinally striate, midlobe dorsally smooth and concave (Figs 2, 3). Axilla and scutellar disc smooth and concave dorsally, scutellar disc longitudinally striate laterally. Scutoscutellar sulcus (SSS) weakly crenulate dorsally; deeply invaginated and smooth laterally. In dorsal view, frenal processes tapering toward narrowly rounded apex, with longitudinal striae distinct and widely spaced; processes 3.8× as long as maximum width and 2.1× as long as scutellum (frp, Fig. 2), in profile curved over gaster. Upper half of mesepisternum and mesepimeron longitudinally striate. Hind coxa semiglobose, 1.9× as long as broad; with weak longitudinal striae and scattered, thin setae. Hind femur densely setose. Forewing 2.8× as long as broad; stigmal vein slender and perpendicular to wing margin, 3.3× as long as broad; postmarginal vein indistinct but present and about as long as stigmal vein (Fig. 2).
Metasoma. Petiole 4.1× as long as broad, 1.8× as long as hind coxa and 1.2× as long as hind femur; First gastral tergite (Gt1) smooth and without setae (Gt1, Fig. 3).
Male. Length 2.4 mm. Similar to female except for following. Antenna brown, frenal processes and venation completely white; Gt1 and following segments yellowish (Figs 4, 5); forewing hyaline. Head 1.3× as long as high. Eyes separated by 2.1× their height. Malar space 0.9× as height of eyes. Antenna pectinate; scape shorter than female, 2.4× as long as broad; branch of basal flagellomere 0.9× as long as height of head, following flagellomeres with branches progressively decreasing in length (Fig. 4). Mesoscutal depression rugose (Fig. 5); axilla and scutellar disc narrower than mesoscutum and with longitudinal striae. SSS deeply crenulate dorsally. Frenal processes smaller than female; frenal processes 4.3× as long as maximum width, 1.6× as long as scutellum; spines straight in lateral view (Figs 4, 5). Mesepisternum and mesepimeron with weak striae. Hind coxa 1.8× as long as broad. Petiole 3.4× as long as broad, 2.0× as long as hind coxa.
Dicoelothorax parviceps 1 head and antenna (female, sublateral) 2 mesosoma (female, dorsal) 3 habitus (female) 4 habitus (male) 5 mesosoma (male, dorsal).
Unknown.
COLOMBIA. Vichada, P. N. Tuparro, 16.vi.2000, Sharkey, UCRC_ENT 161564 (1 female, UCRC); same location and data, UCRC_ENT 92180 (1 male, UCRC).
http://species-id.net/wiki/Dicoelothorax_platycerus
Figs 6–31Distinguished from Dicoelothorax parviceps by the mesosoma and frenal processes having fine closely-spaced longitudinal striae, closer and more slightly raised in female (Figs 8, 9, 12, 14); dorsal concavity of mesoscutum and scutellum smooth or weakly striate medially (Figs 8, 9); frenal processes in dorsal view widened medially and narrowing only slightly to apex, which is almost the same width as their base and broadly rounded (Figs 8, 9); venation brown; scutellar processes of male yellowish with diffuse black longitudinal band medially and apex black, slightly curved in lateral view, and almost twice as long as scutellum (Figs 12, 14).
Dicoelothorax platycerus(female) 6 habitus 7 head and antenna (sublateral) 8 mesosoma (dorsal) 9 scutellum (dorsal) 10 mesosoma (lateral).
Female. Length 3.0–4.5 mm. Head, mesosoma, coxae, petiole and Gt1 except distal part black; flagellum, basal ¾ of femora, frenal process, distal part of Gt1 and rest of terga brown but with processes sometimes completely black; scape, pedicel and rest of legs and distal limits of terga yellowish (Figs 6, 8, 9). Wings slightly infuscate, venation brown.
Head 1.4–1.5× as broad as high. Frons and face granulate, weakly strigose, with small and scattered setae or without setae (Fig. 7). Eyes separated by 2.3–2.7× their height. Malar space 0.8–1.2× as long as height of eyes. Antenna with 8 segments; scape 2.4–2.8× as long as broad, slightly broader apically, smooth, with a few scattered setae. Length of flagellum 0.7–0.9× height of head, basal flagellomere 0.8–1.2× as long as scape, basal flagellomere ranging from serrate to clavate, following flagellomeres serrate, clava rounded (Fig. 7).
Mesosoma. Midlobe of mesoscutum elevated anteriorly, with short, thin, decumbent and scattered setae; striate-rugose on anterior face, sidelobes longitudinally striate, modlobe dorsally smooth or weakly striate and concave (Figs 6, 10). Axilla and scutellar disc smooth and concave dorsally, scutellar disc longitudinally striate laterally. SSS weakly crenulate dorsally and deeply invaginated and smooth laterally (Figs 8, 9). In dorsal view, frenal processes widened medially and tapering only slightly to apex, apically almost the same width as their base and broadly rounded, with longitudinal striae slightly marked and closely spaced; processes 3.1–3.5× as long as maximum width and 2.4–2.7× as long as scutellum (Figs 6, 8, 9); in profile, curved over gaster. Upper half of mesepisternum and mesepimeron longitudinally striate. Hind coxa semiglobose and elongate, 1.7–2.1× as long as broad; with weak longitudinal striae and scattered, thin setae (Fig. 10). Hind femur densely setose. Forewing 2.3–2.5× as long as broad; stigmal vein slender and perpendicular to wing margin, 1.9–2.2× as long as broad; postmarginal vein indistinct and less than half as long as stigmal vein (Fig. 8).
Metasoma. Petiole 3.6–4.1× as long as broad, 1.7–2.0× as long as hind coxa and 1.2–1.3× as long as hind femur; Gt1 smooth and without setae (Figs 6, 10).
Male. Length 3.0–3.8 mm. Similar to female except for following. Antenna brown, frenal processes yellowish with a diffuse black longitudinal band medially and apex black, this band can be extended laterally and covering almost entire surface, or it can be reduced to a narrow medial line (Figs 11, 12, 14); wing venation white, forewing hyaline. Head 1.5–1.6× as long as high. Eyes separated by 2.2–2.4× their height. Malar space 0.7–0.9× as height of eyes. Antenna pectinate; scape shorter than female, 1.8–1.9× as long as broad; basal flagellomere 0.9–1.0× as long as height of head, following flagellomeres with branches progressively decreasing in length (Fig. 13). Mesosoma with striae stronger than female, mesoscutal depression rugose (Fig. 12); axilla and scutellar disc narrower than mesoscutum and with longitudinal striae; scutellum with a small depression anterior to union of processes (Figs 12, 14). SSS deeply crenulate dorsally. Frenal processes narrowing toward apex; 3.7–4.4× as long as maximum width, 1.7–2.1× as long as scutellum (Figs 12, 14); in profile, uniformly and slightly curved over gaster. Hind coxa 1.8–2.1× as long as broad. Petiole 3.8–4.3× as long as broad, 1.7–2.1× as long as hind coxa. Gaster smaller than female.
Dicoelothorax platycerus (male) 11 habitus 12 mesosoma (dorsal) 13 head and mesoscutum (lateral) 14 scutellum (dorsal).
Eggs. Length of egg body 0.18 mm and caudal stalk 0.08 mm (Fig. 19). Undeveloped eggs are whitish and translucent with a smooth chorion, slightly flattened dorsally and convex ventrally, with a caudal stalk that is about half the length of the egg body. The egg is similar to other Eucharitinae as described by
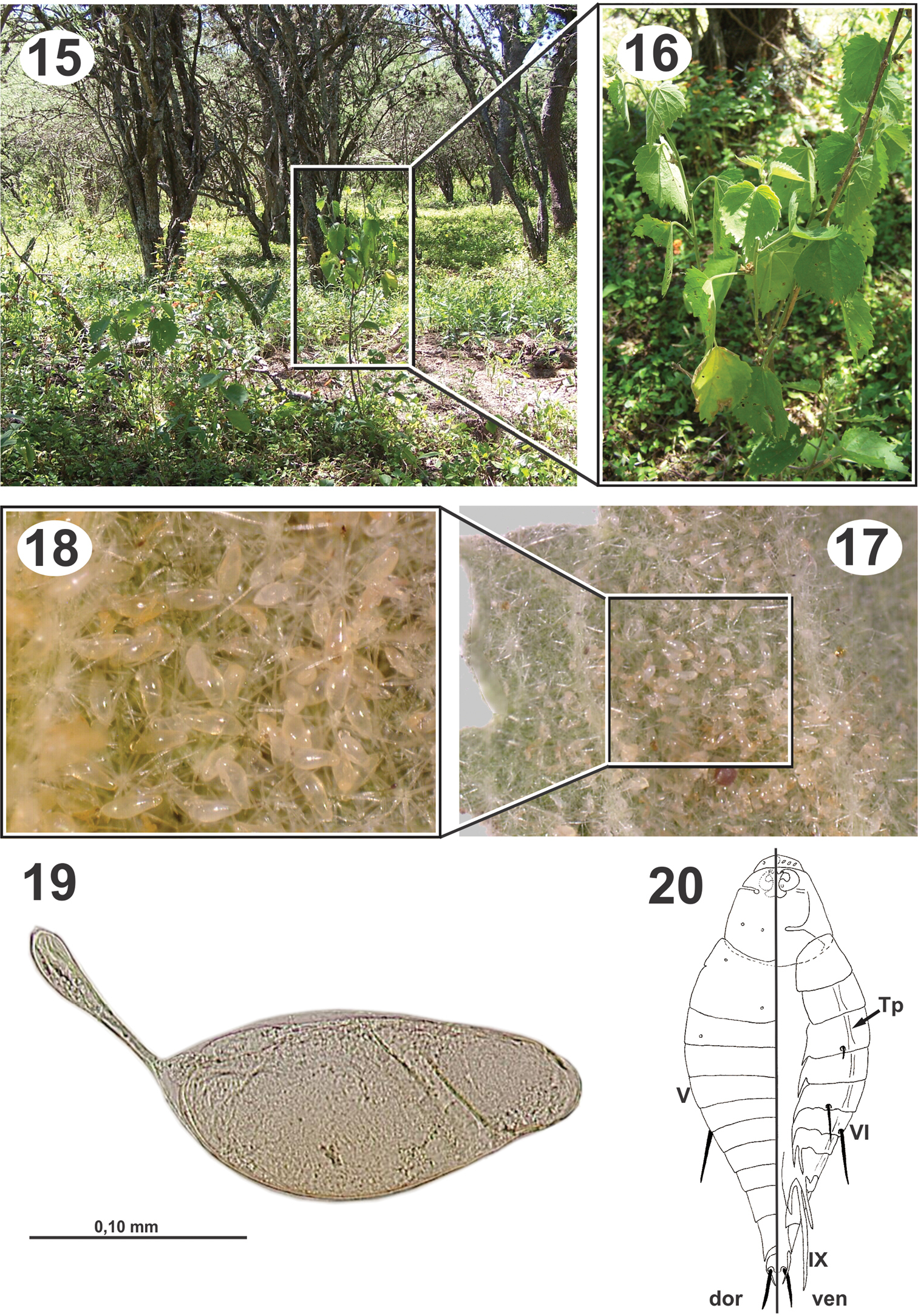
Biology and immature stages of Dicoelothorax platycerus 15 habitat 16 Pseudabutilon virgatum 17 underside leaf of Pseudabutilon virgatum with eggs 18 magnified area with eggs 19 egg 20 planidium.
As described for other Eucharitinae by
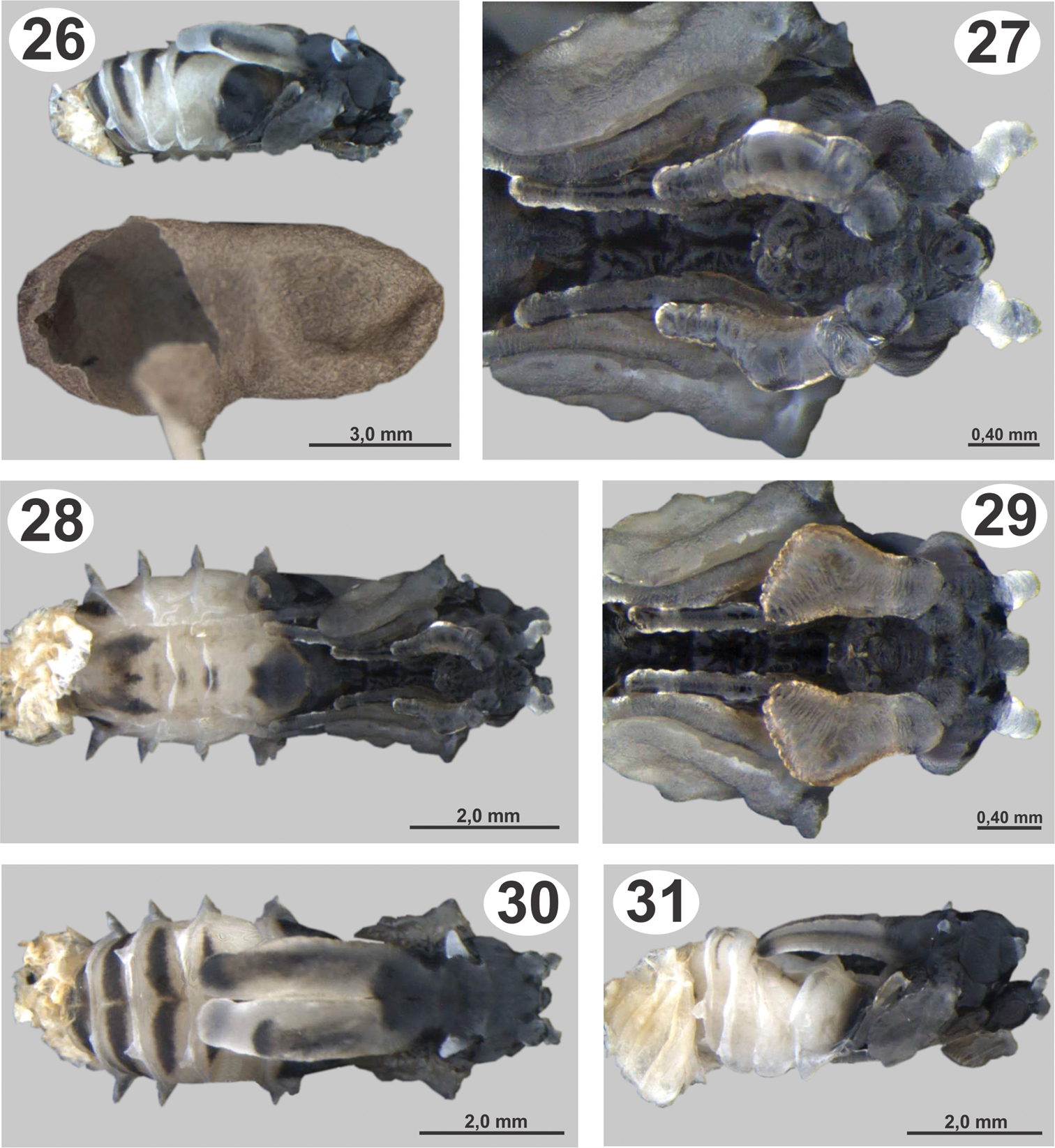
Length: 5.4–6.7 mm (Figs 26–31). The pupa are similar to the description by
Biology and immature stages of Dicoelothorax platycerus 21 nest entrace of Ectatomma brunneum (opening indicated) 22 brood chamber (indicated) 23 brood chamber magnified 24 prepupa parasitized (2nd instar larva indicated and magnified) 25 ant larva parasitized (attached planidium magnified).
Pupae of Dicoelothorax platycerus 26 pupa extracted with ant cocoon (female, lateral) 27 head (female, ventral) 28 pupa in ventral view (female) 29 head (male, ventral) 30 pupa in dorsal view (female) 31 pupa in lateral view (male).
Specimens were collected in San Vicente (Tucumán, Argentina). In this region it is common to find Aspidosperma quebracho-blanco Schlecht.(Quebracho blanco), Cassia, Cercidium sp. (Brea), Cereus validus Haworth, Harrisia pomanensis (F.A.C.Weber) Britton & Rose, Jodina rhombifolia Hooker et Arnott (Sombra de toro), Opuntia sp. (Tuna, Quimilo), and Prosopis sp. (Algarrobo). This vegetation corresponds to the chaco serrano ecoregion (sensu
Pseudabutilon virgatum is a ligneous shrub that grows not more than 1 m in height, persists year round, and blooms in the humid seasons (spring-summer); its leaves are ovate and marginally serrate and last to the beginning of the cold season (May-June) (Fig. 16).
Ectatomma brunneum workers were observed and sampled from under the plants with Dicoelothorax. In a radius of about 4m, we found three ant nests (H1–H3). The disposition of chambers and general structure of nests are similiar to those observed by
Collections of adults of Dicoelothorax platycerus, Pseudabutilon virgatum, and ant nests were made in 2009 (March 12) and 2010 (March 27 and April 3). Females placed in plastic tubes were observed ovipositing on the undersides of the leaves of Pseudabutilon virgatum (Figs 17, 18). A single gravid female oviposited about 40 eggs per 1 mm2 between the spicules forming the pubescence on the underside of leaves (Figs 17, 18). Numerous mites were observed on the leaves, and oviposition under the dense network of spicules appears to be a protection against egg predators. Eggs hatched within 10 days; however, many of the remaining eggs contained mature planidia that did not hatch. First instars (planidia) are very mobile and have a propensity to jump. Larvae presumably attach phoretically to foraging ants under the host plant and get carried back to the ant nest where they attack the ant larvae (
Ectatomma brunneum was reported as the ant host for an unidentified species of Kapala (Eucharitidae: Eucharitini) in French Guiana, (
ARGENTINA. Salta, Tartagal, xii.1971, UCRC_ENT 305490 and UCRC_ENT 305491 (2 males, AMNH). Salta, Güemes, 7.ii.1983, UCRC_ENT 305492 (1 female, AMNH); same location and data, UCRC_ENT 305493 (1 male, AMNH). Salta, Cabeza de Buey, 24°47'36"S, 64°01'57"W, 15–16.iii.2007, J.&J. Heraty & J. Torréns, UCRC_ENT 305494 (1 female, UCRC); same location and data, UCRC_ENT 305495 and UCRC_ENT 305496 (2 males, UCRC); same location and data, UCRC_ENT 305497, UCRC_ENT 305498 and UCRC_ENT 305499 (3 males, IFML). Salta, Cabeza de Buey, 24°47'36"S, 64°01'57"W, 19.ii.2008, P. Fidalgo, UCRC_ENT 305500 (1 female, MACN); same location and data, UCRC_ENT 305501 (1 male, MACN). Salta, Lumbreras, 25°12'19"S, 64°54'34"W, 14.iii.2009, J. Torréns, UCRC_ENT 305502 (1 female, IFML). Tucumán, San Vicente, 26°25'36"S, 65°15'41"W, 12.iii.2009, J. Torréns, UCRC_ENT 305503 and UCRC_ENT 305504 (2 females, IFML); same location, 27.iii.2010, J. Torréns, ex. Pupa of Ectatomma brunneum UCRC_ENT 305505 (1 female, IFML); same location and data, UCRC_ENT 305506 (1 male, IFML); same location, 03.iv.2010, J. Torréns, ex. pupa of Ectatomma brunneum, UCRC_ENT 305507 (1 male, IFML).
This investigation was made possible through funding by Project PICT 01238 from SECYT to JT and a National Science Foundation grant PEET DEB-0730616 to JMH. We thank Lic Alberto Slanis for the identifications of the plants, Adriana Aranda-Rickert, Joanne Heraty, Raúl Torréns, Romina Torréns and Sofía Clúa for their help in the field, and Dr Patricio Fidalgo and Elizabeth Murray for comments on the manuscript.