(C) 2012 Ze-hong Meng. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Seasogonia Young, 1986 is a sharpshooter genus with 13 species, four of them recorded from China. In this paper, Seasogonia sandaracata (Distant, 1908) is recorded as new for China and Seasogonia rufipenna Li & Wang, 1992 is regarded as a junior synonym of Seasogonia nigromaculata Kuoh, 1991. The morphological diversity of the female genitalia of Seasogonia is still poorly known. We provide herein detailed descriptions and illustrations of three Chinese Seasogonia species. Notes on the female genitalia of Seasogonia, including intraspecific and interspecific variation, and comparisons between the female genitalia of Seasogonia and of other related genera from China are provided. The preliminary results indicate that the female genitalia may provide useful features for the taxonomy of Seasogonia and other members of the Old World Cicadellini.
Membracoidea, Auchenorrhyncha, Cicadellinae, sharpshooter, morphology, taxonomy
The sharpshooter genus Seasogonia was established by
The female genitalia have yielded useful characters for the taxonomy of sharpshooters (
The male and female genital structures were prepared according to the techniques described by
http://species-id.net/wiki/Seasogonia_indosinica
Figs 1–3, 26–341 male, China, Guangxi Province, Huaping, 5 June 1997, coll. Yang Mao-fa; 3 males, 2 females, China, Guangxi Province, Jinxiu County, Dayaoshan, Alt. 500m, 28 April 2008, coll. Meng Ze-hong; 2 males, China, Hainan Province, Jianfengling, 14–15 May 1997, coll. Yang Mao-fa; 1 male, China, Hainan Province, Jianfengling, 17 April 2009, coll. Yang Zai-hua; 14 males, China, Hainan Province, Diaoluoshan, 10–12 April 2009, coll. Yang Zai-hua; 1 male, China, Hainan Province, Bawangling, 24 April 2009, coll. Yang Zai-hua; 1 male, China, Sichuan Province, Emeishan, 14 July 1995, coll. Yang Mao-fa; 3 males, China, Guizhou Province, Guiyang City, 5 June 1981, coll. Li Zi-zhong and Ma Gui-yan; 1 male, China, Guizhou Province, Guiyang City, 2 July 1986, coll. Li Zi-zhong; 4 males, 1 female, China, Guizhou Province, Guiyang City, 15 June 1992, coll. Zhang Yu-qiong; 10 males, China, Guizhou Province, Taijiang County, 9–17 May 1985, coll. Li Zi-zhong; 17 males, China, Guizhou Province, Libo County, 19–24 May 1995, coll. Chen Xiang-sheng; 8 males, China, Guizhou Province, Libo County, 24–30 May 1998, coll. Li Zi-zhong and Song Qiong-zhang; 2 males, China, Guizhou Province, Libo County, 14–17 June 2006, coll. Zhou Zhong-hui and Zhang Bin; 5 males, 3 females, China, Guizhou Province, Chishui County, 28 May 2000, Li Zi-zhong and Chen Xiang-sheng; 2 males, 3 females, China, Guizhou Province, Xishui City, 3 June, coll. Li Zi-zhong and Chen Xiang-sheng; 2 males, China, Guizhou Province, Chishui City, 28–30 May 2006, coll. Tang Yi and Yang Zai-hua; 1 male, China, Guizhou Province, Fanjingshan, 27 July 2001, coll. Li Zi-zhong; 2 males, 2 females, China, Guizhou Province, Fanjingshan, 2–3 June 2002, coll. Li Zi-zhong and Yang Mao-fa; 12 males, 7 females, China, Guizhou Province, Daozhen County, Dashahe, 22–27 May 2004, coll. Zhang Bin, Song Qiong-zhang, Xu Fang-ling and Chen Xiang-sheng; 39 males, 4 females, China, Guizhou Province, Leigongshan, 31 May to 5 June 2005, coll. Tang Yi, Li Zi-zhong, Zhang Bin, Song Qiong-zhang, Zhang Zheng-guang, Ge De-yan, Yang Zai-hua and Xu Fang-ling; 1 male, China, Guizhou Province, Anshun City, 20 July 2005, coll. Zhou Zhong-hui; 3 males, China, Guizhou Province, Duyun City, 5 May 2006, coll. Yang Zai-hua and Zhou Zhong-hui; 4 males, China, Guizhou Province, ShiBing County, Yuntaishan, 20–21 May 2009, coll. Yang Zai-hua; 43 males, 10 females, China, Guizhou Province, Suiyang County, Kuankuoshui, 2–9 June 2010, coll. Dai Ren-huai, Song Qiong-zhang, Li Hu, Li Yu-jian, Zhang Bin, Zheng Yan-li and Xing Ji-chun.
Myanmar, Vietnam, India, China (Fujian, Guangxi, Hainan, Sichuan, Guizhou, Yunnan).
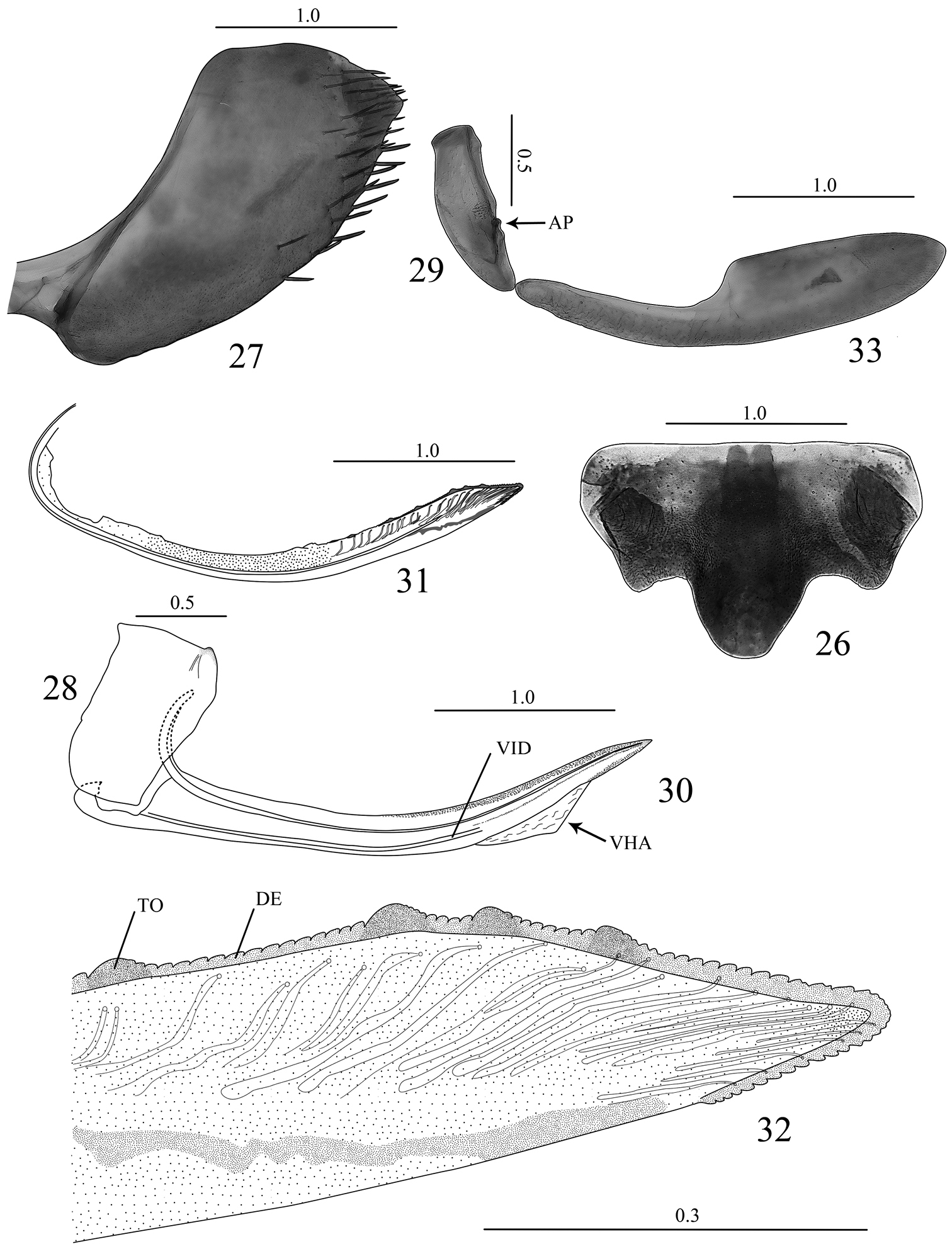
Abdominal sternite VII (Fig. 26), in ventral view, broader than long; anterior margin straight; posterior margin well produced medially, sometimes forming two distinct lateral lobes; lateral margins convergent posteriorly; surface with few small setae mostly on basal half. Internal sternite VIII not forming sclerites. Pygofer (Fig. 27), in lateral view, slightly produced posteriorly; posterior margin with subacute apex; surface with macrosetae on posterior portion and extending anteriorly along ventral margin, attaining midlength. Valvifers I, in lateral view (Fig. 28), nearly rectangular, slightly expanded posteriorly, posteroventral margin angulate; in ventral view (Fig. 34), forming lobes (LB) articulating with valvulae I. Valvifers II (Fig. 29), in lateral view, nearly fusiform, with small group of clustered setae near articulation point (AP). Valvulae I of ovipositor, in ventral view (Fig. 34), with base gradually broadening posteriorly; in lateral view (Fig. 30), shaft distinctly curved dorsally and with dorsal margin concave, with ventral hyaline area (VHA) near apex; dorsal sculptured area located on apical half, broadening to near apex and gradually narrowing to apex, formed by dense scale-like processes; ventral sculptured area restricted to apical portion, formed by dense imbricate processes; ventral interlocking device (VID) distinct on basal 2/3 of shaft; apex of shaft acute. Valvulae II of ovipositor (Figs 31 and 32), in lateral view, slightly expanded beyond basal curvature, distinctly curved dorsally and with dorsal margin concave; apex narrowly rounded; preapical prominence (PP) indistinct; shaft bearing approximately 4–6 teeth (TO) distributed on apical half behind basal curvature; each tooth semiround and bearing few denticles (DE) or not bearing denticles; denticles mostly distributed on dorsal margin of shaft between teeth and on dorsal and ventral margins of apical portion, dentate dorsal margin longer than ventral margin; ducts extending toward dorsal margin and toward apical portion of shaft. Gonoplacs (Fig. 33), in lateral view, with basal half narrow and apical half distinctly expanded; apex rounded.
Seasogonia indosinica (Jacobi), body of male (9.0 mm): 1 dorsal view 2 lateral view 3 ventral view. Seasogonia nigromaculata Kuoh, body of male (11.5 mm): 4 dorsal view 5 lateral view 6 ventral view. Seasogonia rosea Kuoh, body of male (10.9 mm): 7 dorsal view 8 lateral view 9 ventral view. Seasogonia sandaracata (Distant), body of male (11.2 mm): 10 dorsal view 11 lateral view 12 ventral view.
http://species-id.net/wiki/Seasogonia_nigromaculata
Figs 4–6, 13–19 We checked the holotype of Seasogonia rufipenna Li & Wang. Unfortunately, we failed to check the holotype of Seasogonia nigromaculata Kuoh, but based on its detailed Chinese descriptions and illustrations of external feature and male genitalia provided by
Male, holotype of Seasogonia rufipenna, Guizhou Province, Taijiang County, Shihuihe, 16 May 1985, coll. Li Zi-zhong.
China (Guizhou, Yunnan).
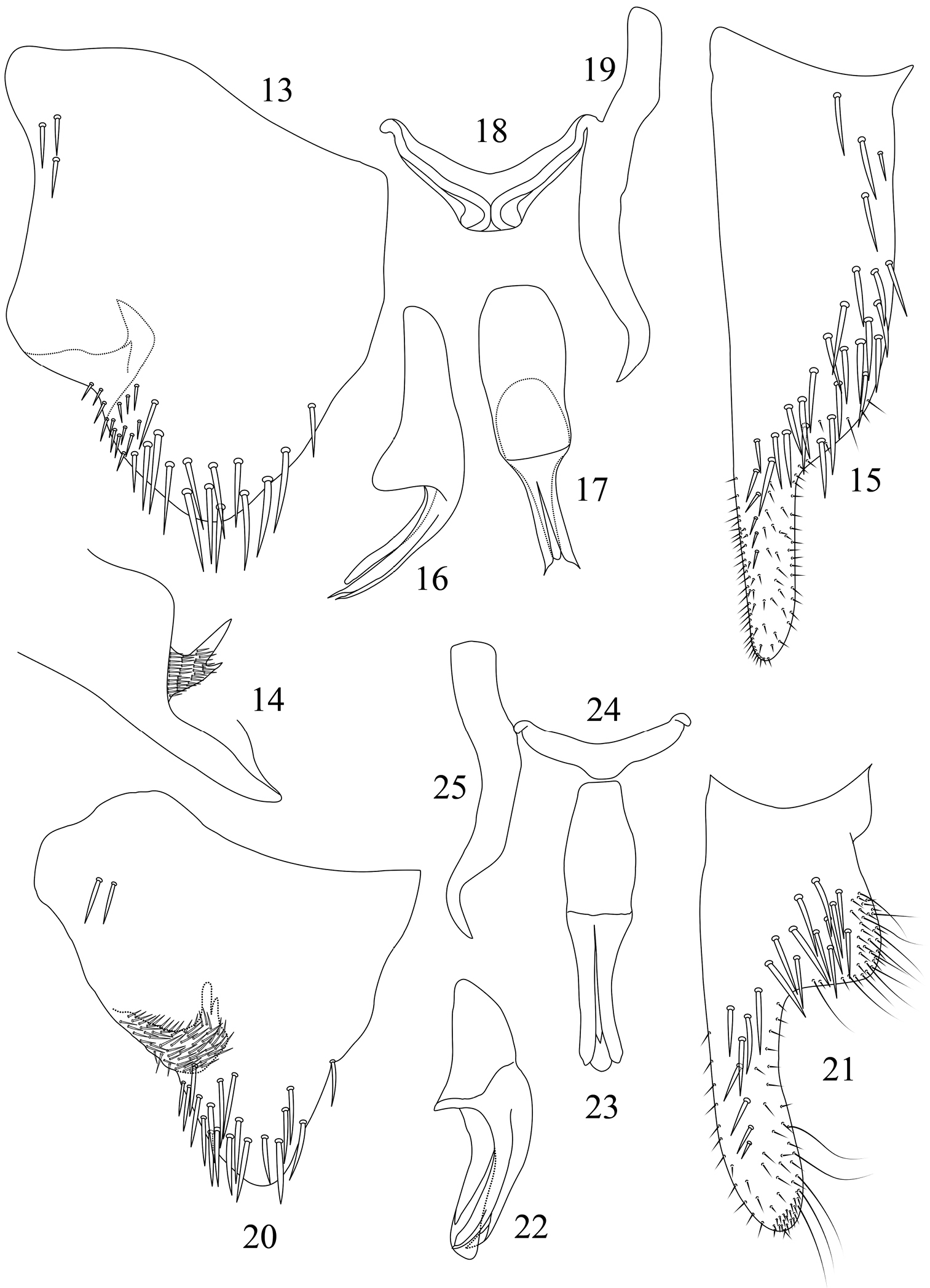
Pygofer in lateral view (Fig. 13), with broad base and gradually narrowed posteriorly, posterodorsal margin concave; apex narrowly rounded; with two or three macrosetae on basiventral portion and many macrosetae on posterior portion; pygofer process (Figs 13 and 14) arising near median-ventral margin, extending dorsally, bifurcate apically and divided into two processes and with small process as 1/3 long as the other one; with dense setae on basal and median portion. Subgenital plate (Fig. 15) with multiseriate macrosetae on broad basal two-third portion, with uniseriate macrosetae and some short microsetae on narrowed apical one-third portion. Aedeagus (Figs 16 and 17) broad at basal half and with subrounded median-dorsal process; shaft slender and with paired ventral processes diverging from base of shaft, processes with apex acute and exceeding apex of shaft. Connective (Fig. 18) Y-shaped, stalk short. Style (Fig. 19) slightly unciform apically.
Unknown.
Seasogonia nigromaculata Kuoh, male genitalia: 13 pygofer, lateral view 14 pygofer process, caudal view 15 subgenital plate, ventral view 16 aedeagus, lateral view 17 aedeagus, ventral view 18 connective, dorsal view 19 style, dorsal view. Seasogonia rosea Kuoh, male genitalia: 20 pygofer, lateral view 21 subgenital plate, ventral view 22 aedeagus, lateral view 23 aedeagus, ventral view 24 connective, dorsal view 25 style, dorsal view.
Seasogonia indosinica (Jacobi), female genitalia: 26 sternite VII, ventral view 27 Pygofer, lateral view 28 valvifer I, lateral view 29 valvifer II, lateral view 30 valvula I, lateral view 31 valvula II, lateral view 32 apex of valvula II, lateral view 33 gonoplac, lateral view. AP = articulation point, DE = denticles, TO = tooth, VHA = ventral hyaline area, VID = ventral interlocking device. Scale bars in millimeters.
http://species-id.net/wiki/Seasogonia_rosea
Figs 7–9, 20–25 and 35–371 female, China, Yunnan Province, Tengchong County, Shangyun Village, Alt. 1700–1900m, 15 July 2002, coll. Yang Mao-fa; 1 male, 1 female, China, Yunnan Province, Tengchong County, Gaoligongshan, Alt. 1900–2000m, 17 July 2002, coll. Yang Mao-fa and Song Hong-yan; 21 males, 7 females, China, Yunnan Province, Tengchong County, Gaoligongshan, Baihualing, Alt. 1800–2400m, 28 May to 3 June 2009, coll. Yang Zai-hua and Li Bin; 10 males, 9 females, China, Yunnan Province, Tengchong County, Gaoligongshan, 13–15 June 2011, coll. Yang Zai-hua and Li Yu-jian; 1 male, 1 female, China, Yunnan Province, Yingjiang County, Tongbiguan, Alt. 1400–1500m, 20 July 2002, coll. Yang Mao-fa and Song Hong-yan; 19 males, China, Yunnan Province, Yingjiang County, Tongbiguan, 1–3 June 2011, coll. Yang Zai-hua and Li Yu-jian; 7 males, China, Yunnan Province, Fugong County, Shangpa Town, 17–18 May 2010, coll. Ni Jun-qiang, Li Hu and Zhang Pei.
China (Yunnan).
Pygofer (Fig. 20) in lateral view, broad and triangular, gradually narrowed posteriorly; with several macrosetae on basiventral portion and many macrosetae on posterior portion; pygofer process arising near median-ventral margin, extending dorsally, bifurcate apically and divided into two processes, short process acute and nearly as 2/3 long as the other one; surface with dense setae except apex. Subgenital plate (Fig. 21) with anterior half broad, surface with multiseriate macrosetae on basal one-half and with uniseriate macrosetae and some short microsetae on posterior half. Aedeagus (Figs 22 and 23) broad at basal half and with angulate median-dorsal process; shaft with acute internal process and with paired ventral processes diverging from base of shaft, ventral processes with apex acute and extending as long as apex of shaft. Connective (Fig. 24) broad, V-shaped. Style (Fig. 25) slightly unciform apically.
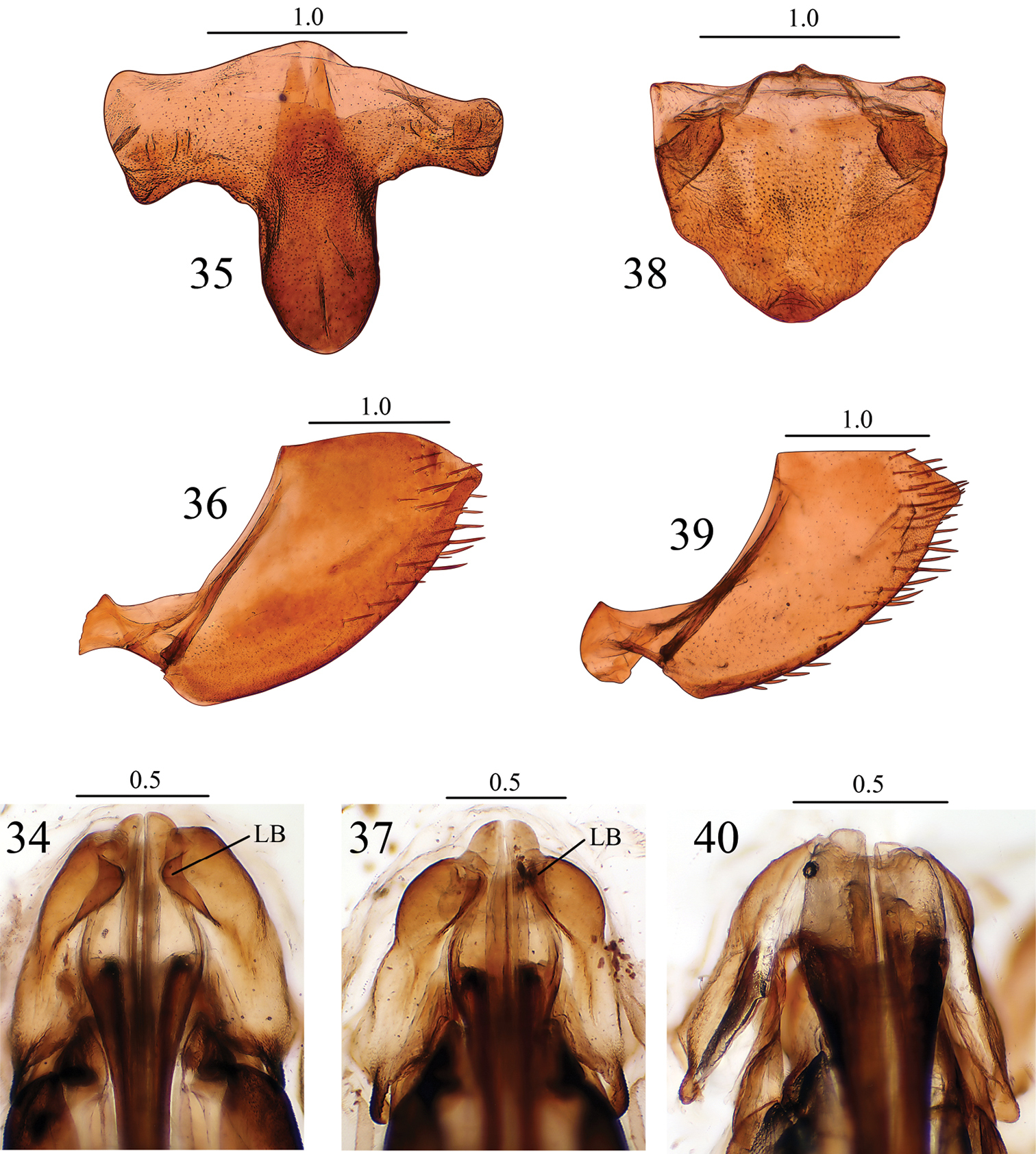
Abdominal sternite VII (Fig. 35), in ventral view, with posterior margin conspicuously more produced medially than in Seasogonia indosinica. Other characteristics as in Seasogonia indosinica.
Seasogonia indosinica (Jacobi): 34 valvifers I and bases of valvulae I, ventral view Seasogonia rosea Kuoh: 35 Sternite VII, ventral view 36 pygofer, lateral view 37 valvifers I and bases of valvulae I, ventral view Seasogonia sandaracata (Distant): 38 Sternite VII, ventral view 39 pygofer, lateral view 40 valvifers I and bases of valvulae I, ventral view. LB = lobe. Scale bars in millimeters.
http://species-id.net/wiki/Seasogonia_sandaracata
Figs 10–12, 38–40, 41–42, and 53–545 males, 1 female, China, Yunnan Province, Yingjiang County, Tongbiguan, Alt. 1200m, 15 June 2001, coll. Tian Ming-yi; 10 females, China, Yunnan Province, Yingjiang County, Tongbiguan, Alt. 1400–1500m, 20 July 2002, coll. Yang Mao-fa, Li Zi-zhong, Song Hong-yan and Dai Ren-huai; 32males, 7 females, China, Yunnan Province, Yingjiang County, 29 May to 3 June 2011, coll. Yang Zai-hua and Li Yu-jian; 13 males, 2 females, China, Yunnan Province, Yingjiang County, Xima Town, Alt. 1700m, 8–10 June 2009, coll. Yang Zai-hua and Li Bin; 2 males, 1 female, China, Yunnan Province, Yingjiang County, Tongbiguan, Alt. 270m, 13 June 2009, coll. Yang Zai-hua and Li Bin; 1 male, 11 females, China, Yunnan Province, Tengchong County, Shangyun Village, Alt. 1400m, 14 July 2002, coll. Li Zi-zhong and Yang Mao-fa; 1 male, 3 females, China, Yunnan Province, Tengchong County, Gaoligongshan, Alt. 1900–2000m, 17 July 2002, coll. Yang Mao-fa, Li Zi-zhong, and Song Hong-yan; 5 females, China, Yunnan Province, Longling County, Longxin, Alt. 1800m, 24 July 2002, coll. Yang Mao-fa, Li Zi-zhong, and Song Hong-yan; 1 male, 1 female, China, Yunnan Province, Tengchong County, Gaoligongshan, Alt. 1800–2400m, 28 May to 5 June 2009, coll. Yang Zai-hua and Li Bin; 3 males, China, Yunnan Province, Ruili County, Moli, Alt. 770m, 15 June 2009, coll. Yang Zai-hua and Li Bin; 7 males, 5 females, China, Yunnan Province, Ruili County, 5–7 June 2011, coll. Li Yu-jian and Yang Zai-hua; 1 male, China, Yunnan Province, Pianma, 10 May 2010, coll. Zhang Bin; 18 males, 4 females, China, Yunnan Province, Pianma, 17–19 June 2011, coll. Li Yu-jian and Yang Zai-hua; 47 males, 30 females, China, Xizang Province, Muotuo County, 6 May to 4 June 1980, coll. Jin Gen-tao and Wu Jian-yi (Specimens are deposited in Shanghai Entomological Museum, Chinese Academy of Sciences (SEMCAS)).
India, Myanmar, China (Yunnan, Xizang). New Record for China.
Abdominal sternite VII (Fig. 38), in ventral view, nearly as broad as long; anterior margin straight; posterior margin broadly convex. Pygofer (Fig. 39), in lateral view, slightly produced; posterior margin with subacute apex; surface with macrosetae on posterior portion and extending anteriorly along nearly whole of ventral margin. Valvifers I, in ventral view (Fig. 40), not forming lobes articulating with valvulae I. Valvulae I, in ventral view (Fig. 40), much broader than in Seasogonia indosinica. Other characteristics as in Seasogonia indosinica.
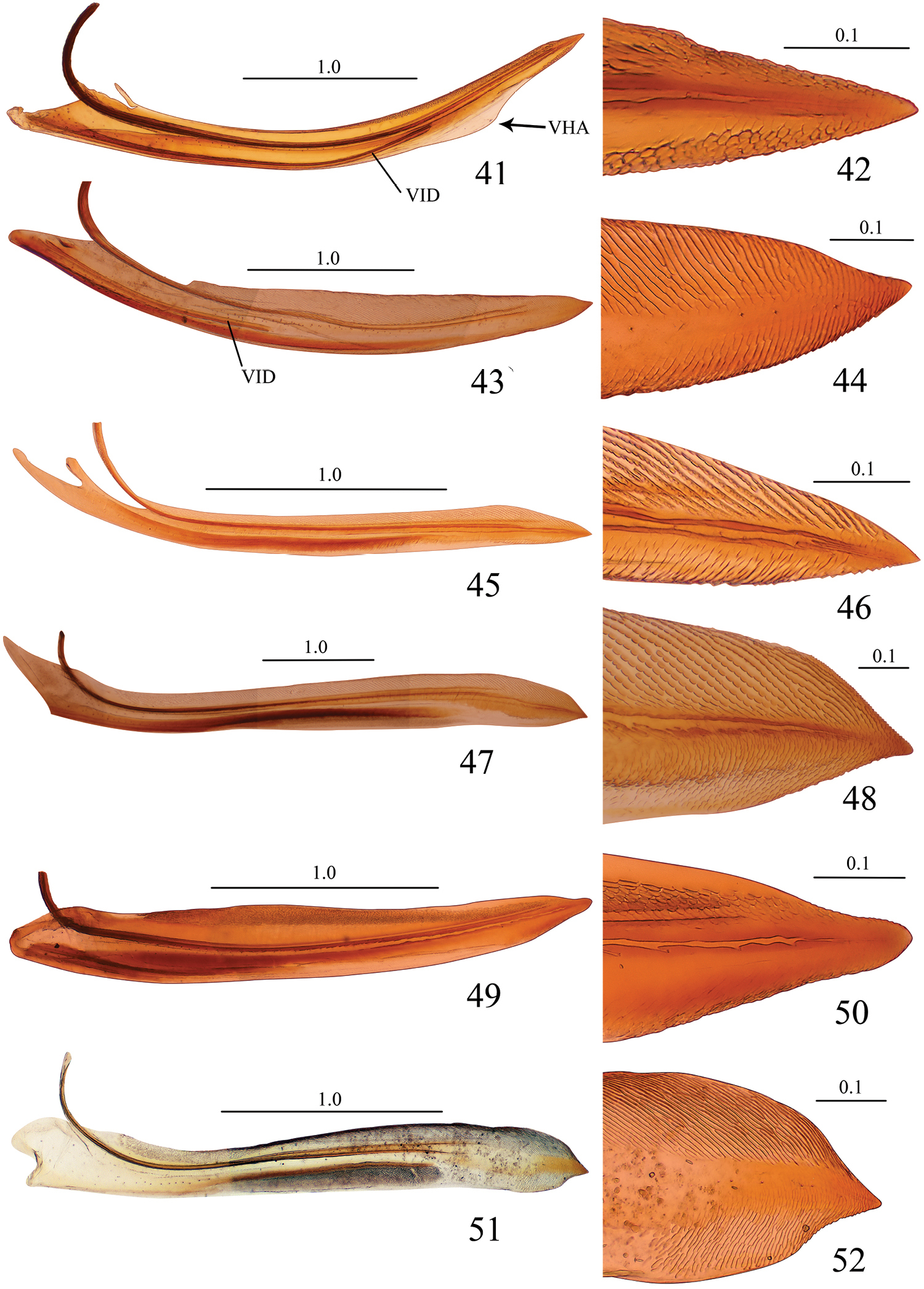
Valvulae I and their apical portions, lateral view. 41, 42 Seasogonia sandaracata (Distant, 1908) 43, 44 Gununga yoshimotoi Young, 1986 45, 46 Anagonalia melichari (Distant, 1908) 47, 48 Sphinctogonia lacta Zhang & Kuoh, 1993 49, 50 Cicadella viridis (Linnaeus, 1758) 51, 52 Stenatkina albopennis Yang, 2007. VHA = ventral hyaline area, VID = ventral interlocking device. Scale bars in millimeters.
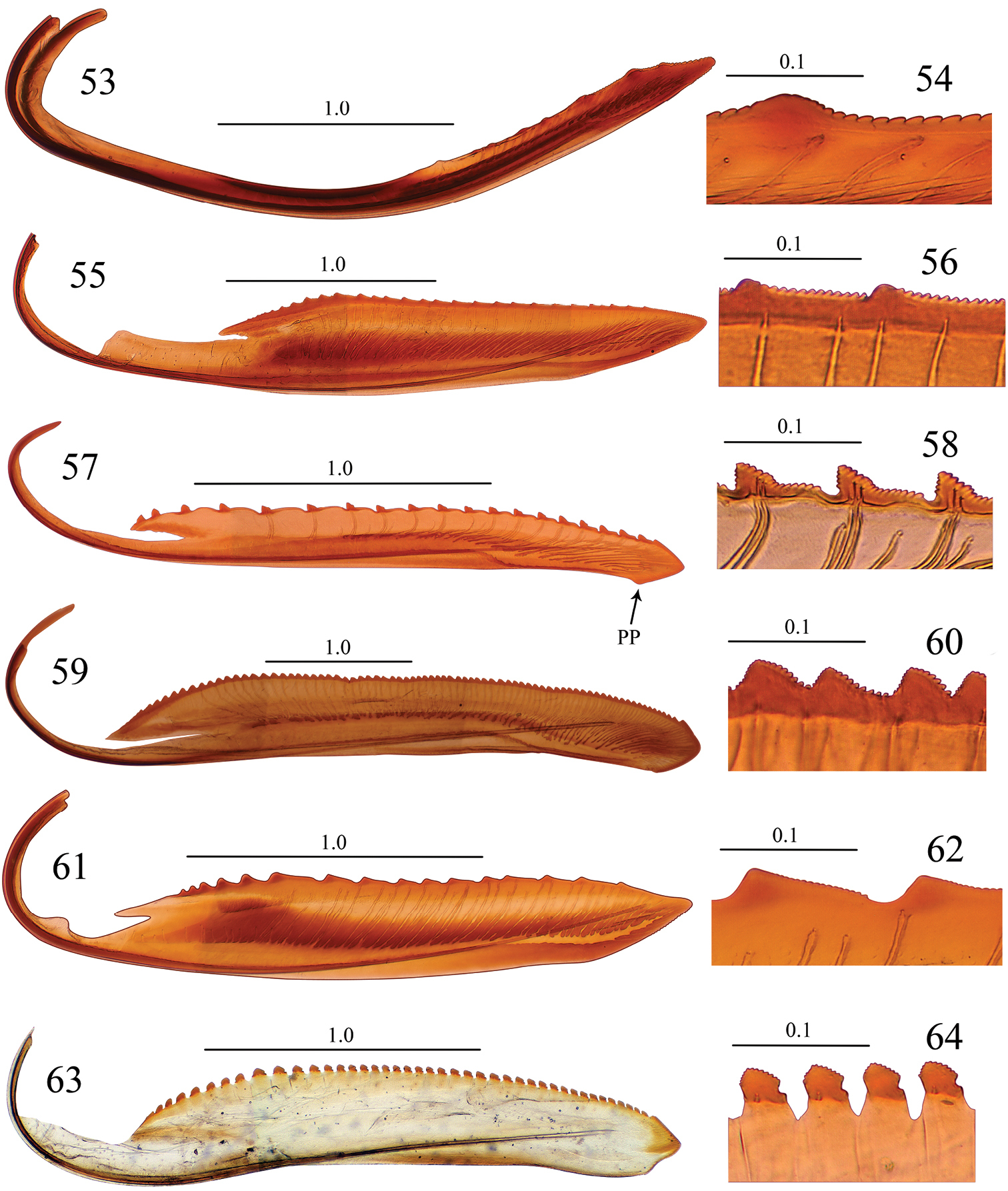
Valvulae II and their teeth, lateral view. 53, 54 Seasogonia sandaracata (Distant, 1908) 55, 56 Gununga yoshimotoi Young, 1986 57, 58 Anagonalia melichari (Distant, 1908) 59, 60 Sphinctogonia lacta Zhang & Kuoh, 1993 61, 62 Cicadella viridis (Linnaeus, 1758) 63, 64 Stenatkina albopennis Yang, 2007. PP = preapical prominence. Scale bars in millimeters.
Intraspecific variation. The number of teeth on valvulae II often varied from 4–7 teeth. In addition, the location of each tooth varied among different specimens, or between each valvula of a single specimen.
Interspecific variation. Females of Chinese Seasogonia species can be distinguished from each other mainly by the following characters: (1) the posterior margin of sternite VII is well produced medially and forms a median lobe in Seasogonia indosinica (Fig. 26) and Seasogonia rosea (Fig. 35), and the latter species has the projection conspicuously more elongated; in Seasogonia sandaracata (Fig. 38), the sternite VII is broadly convex and lacks a distinct median lobe; (2) in Seasogonia sandaracata, the macrosetae on the pygofer surface extend anteriorly distinctly farther than in the other two species (Fig. 39); (3) the valvifers I, in ventral view, form lobes (LB) articulating with valvulae I in Seasogonia indosinica and Seasogonia rosea (Figs 34 and 37), and the former species has the lobes slightly larger; Seasogonia sandaracata lacks the lobes (Fig. 40); (4) in Seasogonia indosinica and Seasogonia rosea (Figs 34 and 37), the bases of valvulae I, in ventral view, are more slender than in Seasogonia sandaracata (Fig. 40). Other characteristics are little changed.
The female abdominal sternite VII overlaps the bases of the ovipositor and usually has much interspecific variation.
The ventral view of basal region of female genitalia has been used to discriminate species in a genus of leafhoppers by some other workers (
Comparative notes on Seasogonia and other related genera from China. In the present paper, the previously unknown female genitalia of three species of Seasogonia from China were described and illustrated for the first time. Seasogonia is apparently closely related to the genus Sochinsogonia Young, 1986 in appearance, but in Sochinsogonia the posterior margin of the sternite VII is concave, whereas it is convex in Seasogonia; in addition, the valvulae I and valvulae II are distinctly curved dorsally in Seasogonia and not so in Sochinsogonia (
Unfortunately, we did not have at hand specimens of Sochinsogonia. Thus, we compared the female genitalia of Seasogonia with those of some other related genera from China (Figs 41–64). Based on the female genitalia, Seasogonia can be distinguished from other Old World Cicadellini by the following combination of characters: (1) the posterior margin of the sternite VII is distinctly convex (26, 35 and 38); (2) the valvulae I and II are distinctly curved dorsally (Figs 30, 31, 41 and 53); (3) the dorsal sculptured area of valvulae I and the teeth of valvulae II are distributed only on the apical half of shaft; the valvulae I have a ventral hyaline area (VHA) near apex (Figs 30 and 41); only a small number of teeth is present on valvulae II (Figs 31, 32 and 53); (4) the dorsal and ventral sculptured areas of valvulae I are formed by dense scale-like processes that are not arranged in oblique lines (Figs 30, 41 and 42). It is important to mention that a considerable amount of morphological diversity is observed in the valvifers I, the base of valvulae I and the shape of teeth of valvulae II (Figs 54, 56, 58, 60, 62 and 64). The structure of the valvulae II of Seasogonia seems very unusual for Cicadellini in general. We compared the shape and teeth with other cicadellines, but found little similarities on our studied genera of Old world Cicadellini.
The sclerites of the genital chamber described by
Sincere thanks to Gabriel Mejdalani (Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, Rio de Janeiro, RJ, Brazil), the two reviewers and the editor of Zookeys for reading the manuscript and making some corrections, various suggestions, and comments. We also thank Zhang Wei-nian, Liu Xian-wei, Yin Hai-sheng, Zhou Shun, Wu Jie and Zhu Weibing, section for Shanghai Entomological Museum, Chinese Academy of Sciences (SEMCAS) for making specimens available for this study. This research was supported by the National Natural Science Foundation of China (No. 30770253), the National Specialized Research Fund for Basic Science of the Ministry of Science and Technology of China (2006FY120100), Program for New Century Excellent Talents in University (NCET-07-0221), and the Provincial Foundation for Excellent Youth in Science and Technology Field of Guizhou (20050517), all awarded to YANG Mao-fa.