(C) 2011 Anita Georges. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
As a result of an invasion by the native grass Elymus athericus (Link) Kerguélen (Poaceae) in the last 10 years, a major change in vegetation cover has occurred in salt marshes of the Mont Saint-Michel bay, Western France. The impact of such an invasion on carabid assemblages, a dominant group of terrestrial arthropods in these habitats and containing several stenotopic species, is investigated here. In our study site, carabid data are available from 1983 and 1984, allowing a comparison of species distribution ranges in salt marshes before (1983–1984) and after (2002) the Elymus athericus invasion. A total of 16, 867 adults belonging to 40 species were caught. By considering the presence-absence of species shared between studies, we show that the invasion by Elymus athericus promoted the progression of non-coastal species (mainly Pterostichus s.l. spp.). This did however not interfere with resident species distributions, finally resulting in higher carabid species richness in the entire area. The species composition and abundances of carabid assemblages were also compared between natural and invaded stations in 2002. The main result is that abundances of some halophilic species decreased in one invaded plot (in case of Pogonus chalceus (Marsham 1802)) whereas the opposite pattern was observed for other species (e.g., Bembidion minimum (Fabricius 1792)). Invaded habitats were characterized by lower percentages of halophilic species and higher total species richness.
Coleoptera, Carabidae, native invasive species, salt marsh, ecological indicators
Intertidal salt marshes are ecosystems located between
land and sea, undergoing periodical flooding during tides, occurring
around twice a month in West-Europe. This creates some special habitat
conditions, and marsh plants and animals often have special adaptations
to cope with these. Salt-marsh arthropods are able to withstand floods
and salinity by physiological, behavioural or morphological adaptations
(e.g.,
More recently, salt marshes have been invaded in many West-European sites by the nitrophilous grass Elymus athericus (Poaceae) (
According to
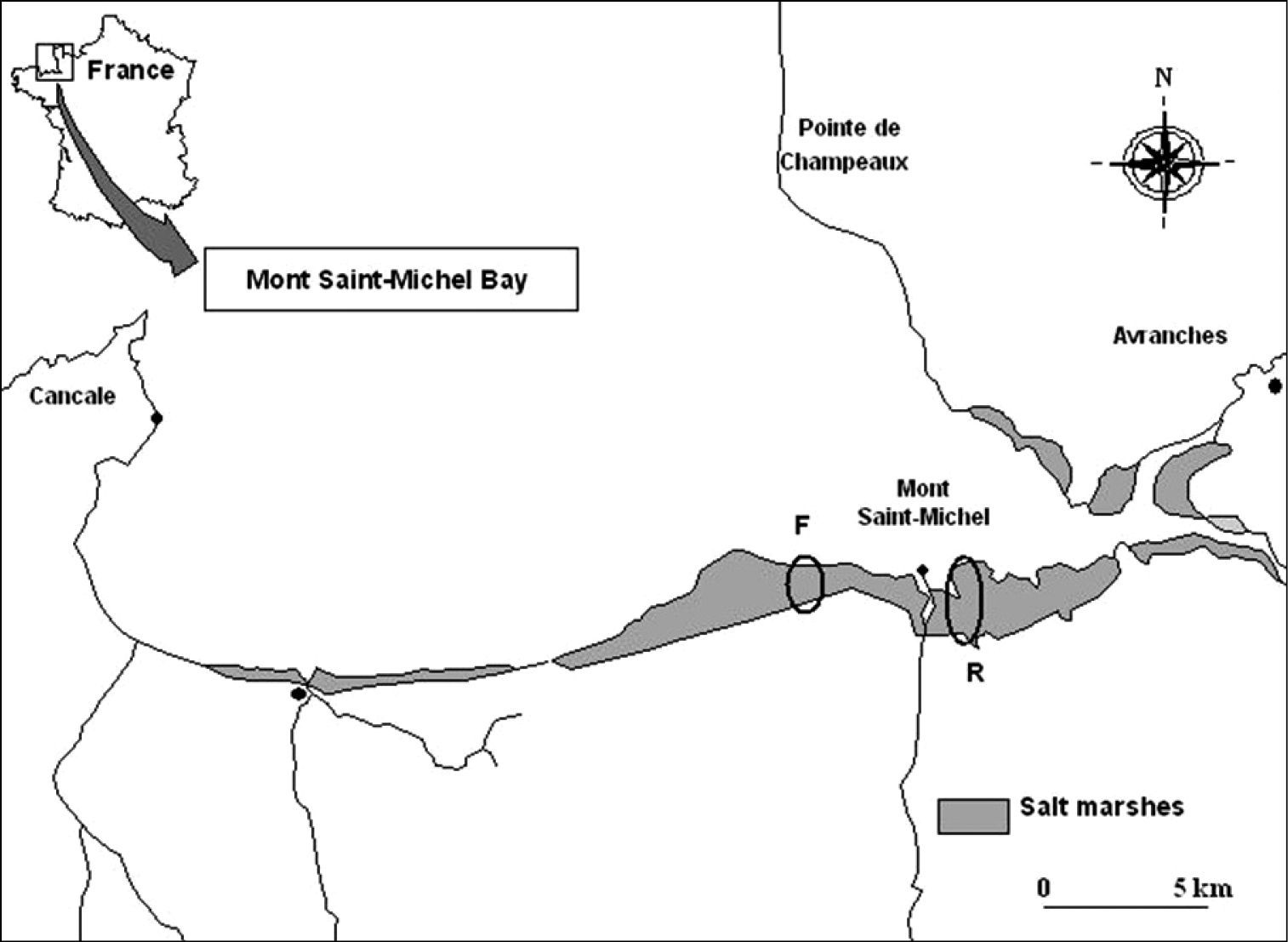
The Mont Saint-Michel bay (NW France) is an extensive littoral zone (500 km²) located between the regions Brittany and Normandy (48°40’N, 1°40’W). Two sites have been studied in salt marshes: “la Ferme Foucault”, on the western part of the Mont St.-Michel (coded F; 48°37’N, 1°32’W) and “la Rive” on the eastern part of the Mont St.-Michel (coded R; 48°37’N, 1°29’W) (Fig. 1).
For the diachronic approach, ground beetle populations were compared at seven stations (A to G) located along the same land-sea transect at the “Ferme Foucault” site between 1983–1984 and 2002. During the study of 1983–1984, Elymus athericus was restricted in this salt marsh to the dyke (station A) and to the upper marsh (station B), but absent from stations C-G. Invasion by Elymus athericus modified the plant cover of the sampling stations between 1984 and 2002. The middle marsh and lower marsh stations (station C till F), dominated in 1984 by Atriplex portulacoides (Chenopodiaceae), were dominated by Elymus athericus in 2002.
Secondly, natural (dominated by Atriplex portulacoides), and invaded (dominated by Elymus athericus)
stations were studied at different marsh levels in the synchronic
approach. Comparisons of paired stations (natural and invaded – coded N
and I, respectively) were spatially replicated three times for avoiding
pseudo-replication (
For both the synchronic and diachronic approaches,
ground beetles were sampled with pitfall traps, consisting of
polypropylene cups (10 cm diameter, 17 cm deep) with ethylene-glycol as
preservative. Traps were covered with a raised wooden roof to keep out
rain. They were emptied weekly when tides permitted (i.e., about three
weeks per month). Pitfall traps were grouped by four and spaced 10 m
apart, this being considered to be the minimum distance for avoiding
interference between traps (
Ground beetles were preserved in 70% ethanol and identified using
Statistics on the abundances of halophilic species
were performed only for species represented by at least 10 individuals
in couples of stations. Catches in pitfall traps were related to
trapping duration and pitfall trap perimeter, which calculates an
“activity trappability density” (number of individuals per day and per
meter –
Location of the study sites (Mont St-Michel Bay, France). Codes: F ‘Ferme Foucault’ R ‘la Rive’.
A total of 24 species (represented by 7, 774 individuals) and 35 species (represented by 8, 588 individuals) were caught in 1983–1984 and in 2002, respectively. Five species were exclusive to the first sampling period and 16 to the second one. All the species that were only recorded in 1983–1984 were caught in very low numbers (max. 2 individuals), four species on the dyke (Clivina colaris, Dromius linearis, Harpalus rufibarbis and Harpalus rufipes) and only one in the salt marsh (Dyschirius chalceus). As the sampling effort was quite different between 1983–1984 and 2002 (see Material and Methods), it cannot be concluded that the ‘appearance’ of species between the two studies can be related to the invasion by Elymus athericus. The comparison in distribution was thus restricted to the 19 shared species (Table 1).
In terms of distribution ranges, two groups of carabids were distinguished: species with constant distribution range in the salt marsh or on the dyke and species with an increased distribution range between 1983–1984 and 2002. Eight species were caught on the dyke in 1983–1984 and in 2002, and seemed not to have progressed with Elymus athericus in the salt marsh (Amara equestris, Anisodactylus binotatus, Bembidion tetracolum, Harpalus anxius, Leistus fulvibarbis, Nebria brevicollis, Pterostichus melanarius and Pterostichus niger: Table 1). Eight other species had a similar habitat range in the salt marsh, extending from the upper to lower marsh or from the dyke to the lower marsh (halophilic species: bold in Table 1), plus two high-marsh living species (Badister bipustulatus and Pterostichus vernalis), one low-marsh living species (Dyschirius salinus) and one species with a discontinuous range along the land-sea transect (Loricera pilicornis).Only three speciesshowed an extension of their distribution in the salt marsh, both to the upper and lower marsh (Bembidion iricolor, Bembidion lampros and Pterostichus cupreus).
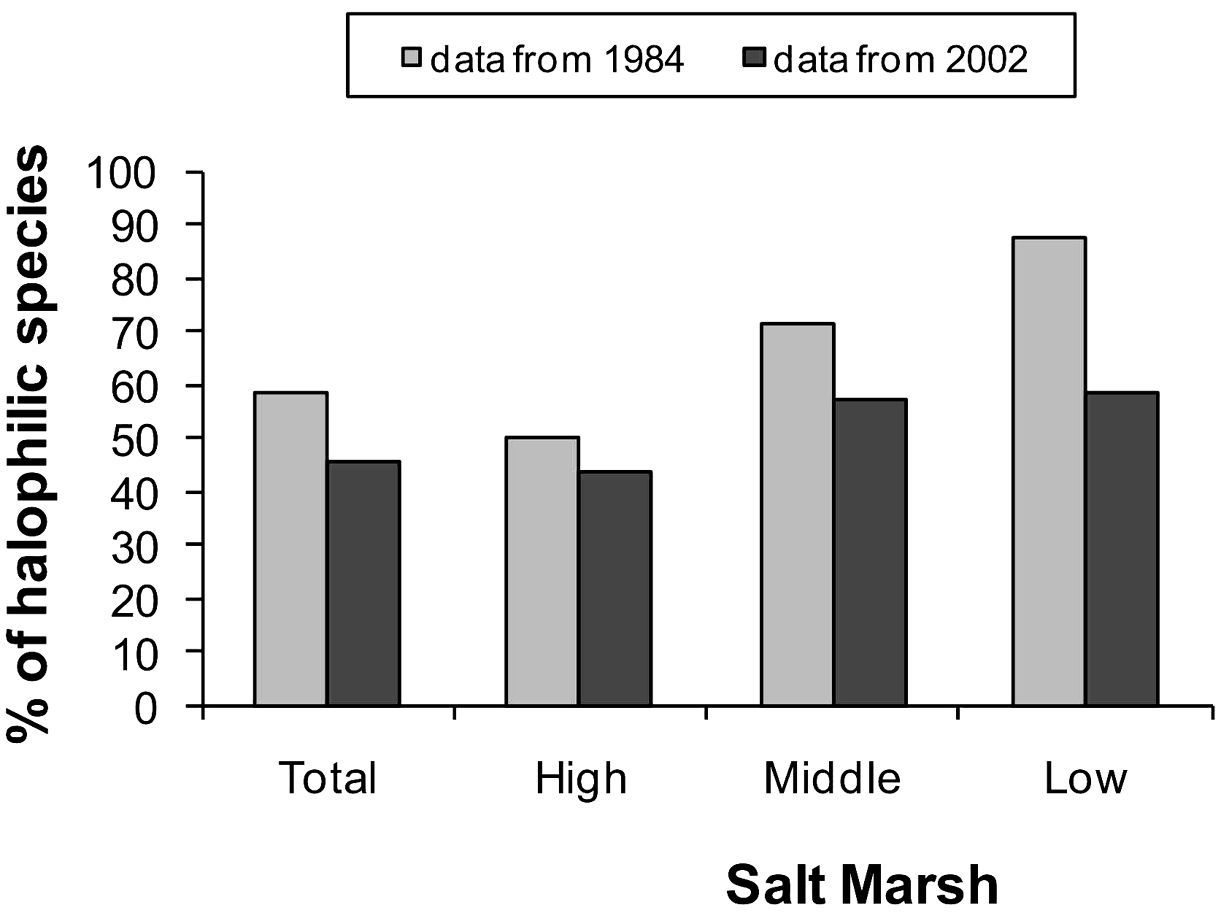
The Elymus athericus invasion led to a decrease in the percentage of halophilic species in invaded salt marshes (Fig. 2).
Changes in the percentage of halophilic species in the salt marsh after the invasion by Elymus athericus.
Comparison of total catches (number of individuals) between 1983–1984 and 2002 along a land-sea transect (Foucault site; bold: halophilic species). The letters A–G indicate different sampling stations. In 1983–1984, only stations A–B had a dominant Elymus athericus cover; in 2002 at all stations Elymus athericus was present (dominant cover for stations A to F).
| Period | A | B | C | D | E | F | G | Total | |
|---|---|---|---|---|---|---|---|---|---|
| SHARED SPECIES | |||||||||
| Amara equestris (Duftschmid 1812) | 1983–84 | 1 | 1 | ||||||
| 2002 | 1 | 1 | |||||||
| Anisodactylus binotatus (Fabricius 1787) | 1983–84 | 3 | 3 | ||||||
| 2002 | 6 | 6 | |||||||
| Badister bipustulatus (Fabricius 1792) | 1983–84 | 1 | 1 | ||||||
| 2002 | 2 | 2 | 4 | ||||||
| Bembidion iricolor Bedel 1879 | 1983–84 | 2 | 2 | 2 | 6 | ||||
| 2002 | 5 | 98 | 89 | 21 | 1 | 4 | 218 | ||
| Bembidion lampros (Herbst 1784) | 1983–84 | 1 | 2 | 3 | |||||
| 2002 | 1 | 12 | 18 | 3 | 5 | 4 | 1 | 44 | |
| Bembidion minimum (Fabricius 1792) | 1983–84 | 4 | 1 | 40 | 52 | 10 | 2 | 109 | |
| 2002 | 1 | 31 | 13 | 5 | 80 | 96 | 3 | 229 | |
| Bembidion normanum Dejean 1831 | 1983–84 | 1 | 24 | 39 | 244 | 149 | 53 | 510 | |
| 2002 | 2 | 6 | 8 | 13 | 212 | 131 | 24 | 396 | |
| Bembidion tetracolum (Say 1823) | 1983–84 | 1 | 1 | ||||||
| 2002 | 1 | 1 | |||||||
| Dicheirotrichus gustavii Crotch 1871 | 1983–84 | 2 | 11 | 83 | 2121 | 2622 | 393 | 5232 | |
| 2002 | 2 | 8 | 2 | 3 | 136 | 237 | 156 | 544 | |
| Dyschirius salinus Schaum 1843 | 1983–84 | 1 | 1 | 2 | |||||
| 2002 | 5 | 5 | |||||||
| Harpalus anxius (Duftschmid 1812) | 1983–84 | 2 | 2 | ||||||
| 2002 | 1 | 1 | |||||||
| Leistus fulvibarbis Dejean 1826 | 1983–84 | 3 | 3 | ||||||
| 2002 | 1 | 1 | |||||||
| Loricera pilicornis (Fabricius 1775) | 1983–84 | 1 | 1 | ||||||
| 2002 | 1 | 1 | 2 | ||||||
| Nebria brevicollis (Fabricius 1792) | 1983–84 | 3 | 3 | ||||||
| 2002 | 1 | 1 | |||||||
| Pogonus chalceus (Marsham 1802) | 1983–84 | 8 | 4 | 65 | 42 | 678 | 617 | 436 | 1850 |
| 2002 | 13 | 100 | 193 | 126 | 1628 | 1290 | 2243 | 5593 | |
| Pterostichus cupreus (Linnaeus 1758) | 1983–84 | 3 | 5 | 8 | |||||
| 2002 | 7 | 41 | 9 | 2 | 59 | ||||
| Pterostichus niger (Schaller 1783) | 1983–84 | 24 | 24 | ||||||
| 2002 | 1 | 1 | |||||||
| Pterostichus vernalis (Panzer 1795) | 1983–84 | 4 | 4 | ||||||
| 2002 | 2 | 1 | 3 | ||||||
| Pterostichus melanarius (Illiger 1798) | 1983–84 | 4 | 4 | ||||||
| 2002 | 12 | 12 | |||||||
| SPECIES NOT RECOLLECTED IN 2002 | |||||||||
| Clivina collaris (Herbst 1786) | 1983–84 | 2 | 2 | ||||||
| 2002 | 0 | ||||||||
| Dromius linearis (Olivier 1795) | 1983–84 | 1 | 1 | ||||||
| 2002 | 0 | ||||||||
| Dyschirius chalceus Erichson 1837 | 1983–84 | 1 | 1 | ||||||
| 2002 | 0 | ||||||||
| Harpalus rufibarbis (Fabricius 1792) | 1983–84 | 2 | 2 | ||||||
| 2002 | 0 | ||||||||
| Harpalus rufipes (Degeer 1774) | 1983–84 | 1 | 1 | ||||||
| 2002 | 0 | ||||||||
| NEW SPECIES FOUND IN 2002 | |||||||||
| Anchomenus dorsalis (Pontoppidan 1763) | 1983–84 | 0 | |||||||
| 2002 | 1 | 1 | |||||||
| Agonum muelleri (Herbst 1784) | 1983–84 | 0 | |||||||
| 2002 | 5 | 1 | 6 | ||||||
| Amara lunicollis Schiödte 1837 | 1983–84 | 0 | |||||||
| 2002 | 3 | 3 | |||||||
| Amara plebeja (Gyllenhal 1810) | 1983–84 | 0 | |||||||
| 2002 | 2 | 1 | 3 | ||||||
| Amara tibialis (Paykull 1798) | 1983–84 | 0 | |||||||
| 2002 | 2 | 2 | |||||||
| Anisodactylus poeciloides (Stephens 1828) | 1983–84 | 0 | |||||||
| 2002 | 2 | 2 | |||||||
| Bembidion obtusum Serville 1821 | 1983–84 | 0 | |||||||
| 2002 | 10 | 16 | 1 | 27 | |||||
| Calathus mollis (Marsham 1802) | 1983–84 | 0 | |||||||
| 2002 | 1 | 1 | |||||||
| Clivina fossor (Linnaeus 1758) | 1983–84 | 0 | |||||||
| 2002 | 1 | 1 | |||||||
| Dicheirotrichus obsoletus (Dejean 1829) | 1983–84 | 0 | |||||||
| 2002 | 2 | 12 | 5 | 478 | 572 | 301 | 1370 | ||
| Harpalus distinguendus (Duftschmid 1812) | 1983–84 | 0 | |||||||
| 2002 | 1 | 2 | 3 | ||||||
| Harpalus melancholichus Dejean 1829 | 1983–84 | 0 | |||||||
| 2002 | 1 | 1 | |||||||
| Microlestes minutulus (Goeze 1777) | 1983–84 | 0 | |||||||
| 2002 | 2 | 2 | |||||||
| Pogonus littoralis (Duftschmid 1812) | 1983–84 | 0 | |||||||
| 2002 | 1 | 13 | 1 | 15 | |||||
| Pterostichus versicolor (Sturm 1824) | 1983–84 | 0 | |||||||
| 2002 | 4 | 14 | 2 | 20 | |||||
| Tachys scutellaris Stephens 1828 | 1983–84 | 0 | |||||||
| 2002 | 10 | 10 | |||||||
| Total | 156 | 363 | 505 | 397 | 5594 | 5727 | 3620 | 16362 | |
A total of 505 individuals belonging to 17 species were sampled in the three pairs of natural and invaded stations. The synchronous comparison of natural and invaded habitats revealed the existence of eight shared species. Two species were exclusive to natural habitats (Pogonus littoralis and Pogonus luridipennis) and six to invaded habitats (Anisodactylus poeciloides, Bembidion obtusum, Harpalus anxius, Harpalus distinguendus, Pterostichus cupreus and Pterostichus versicolor). Total species richness was higher in invaded habitats than in the natural ones (Table 2). Significant interactions between habitat type and station were found for species richness and two species Pogonus chalceus and Dicheirotrichus gustavii. Mean species richness was significantly higher in an invaded station compared to its adjacent natural one (one-way Anova, F-ratio=22.04, p=0.003, d.f.=7). More Pterostichus chalceus were caught at a natural station than at the paired invaded one (one-way Anova, F-ratio=14.68, p=0.009, d.f.=7). Dicheirotrichus gustavii was significantly higher in an invaded station compared to the natural one (one-way Anova, F-ratio=6.89, p=0.039, d.f.=7) and the opposite pattern was found in another couple of stations (one-way Anova, F-ratio=11.94, p=0.014, d.f.=7). Bembidion minimum was significantly higher in invaded habitats compared to natural ones (Factorial Anova, F-ratio=5.91, p=0.025, d.f.=20). No difference between habitat types was found for Dicheirotrichus obsoletus and Bembidion normanum (Table 2).
Comparison of total species richness (total S), mean species richness (mean S) and means abundances (as expressed in number of individuals per day and per meter) of Pogonus chalceus, Dicheirotrichus obsoletus, Bembidion normanum, Dicheirotrichus gustavii and Bembidion minimum between natural (N) and invaded (I) habitats. Means in bold are significantly different (p<0.05) between habitat types (mean ± s.e., see text for details in statistics).
| Total S | N | I | N1 | I1 | N2 | I2 | N3 | I3 |
|---|---|---|---|---|---|---|---|---|
| 11 | 14 | |||||||
| Mean S | 6.17±0.35 | 6.92±0.51 | 5.50±0.50 | 8.75±0.48 | 6.25±0.63 | 6.50±0.65 | 6.75±0.63 | 5.50±0.65 |
| Pogonus chalceus | 7.66±1.90 | 4.75±1.91 | 1.11±0.32 | 1.68±0.24 | 13.50±3.35 | 10.76±4.63 | 8.37±1.55 | 1.80±0.74 |
| Dicheirotrichus obsoletus | 1.38±0.60 | 1.68±0.72 | ||||||
| Bembidion normanum | 0.78±0.35 | 0.98±0.40 | ||||||
| Dicheirotrichus gustavii | 0.45±0.15 | 0.68±0.29 | 0.03±0.02 | 0.02±0.01 | 1.14±0.15 | 1.99±0.29 | 0.19±0.04 | 0.05±0.02 |
| Bembidion minimum | 0.29±0.11 | 0.52±0.10 |
By comparing data from 1983–1984 to 2002, we could show
that only three species have extended their distribution range with the Elymus
invasion, despite the existence of several dyke-inhabiting species
(eight continental species with constant distribution). This result is
opposite to those obtained for spiders in the same study site, with
many range-expanding species (
Although the sampling effort was quite different between
1983–1984 and 2002, we assume that around 11 records of the 16 new
species during the second sampling period can also be due to the
invasion by Elymus.
In fact, several continental species were discovered after the
invasion in relatively high numbers (i.e., more than five individuals),
both on the dyke and in the salt marsh. Among them, most species are
linked to high contents of organic matter and a more pronounced litter
layer (e.g., Agonum muelleri, Bembidion obtusum and the polyphagous Pterostichus versicolor) or are even partly phytophagous (Amara spp. and Harpalusspp.:
The synchronic study revealed that almost half of the
species (8/19), both continental and halophilic ones, were shared
between natural and invaded habitats. Three species, all halophilic,
were exclusive to natural habitats. Conversely, six species were
exclusive to invaded habitats, among them some of the species that
colonized the marsh after the invasion by Elymus athericus (e.g., Bembidion lampros or Pterostichus cupreus). New conditions created by the grass Elymus
– mainly an enhanced litter layer and higher plant cover – thus lead to
the establishment of several continental species directly or indirectly
linked to organic matter or to the litter (as shown by
Although few deleterious impacts of invasion by Elymus athericus
on carabids were found, management could be necessary to reduce the
effects of invasion and decrease the rate of spread of the invasive
plant. Sheep grazing – despite being a good potential method for
biological control of invaders (
Long-term monitoring of population dynamics is thus recommended for halophilic species in invaded, natural and managed habitats. Special attention could be paid to less dominant species, as their small populations could be reduced faster than other, dominant, salt-marsh carabids. This study confirms the high value of carabids as bioindicators (as they present a high percentage of specialist species) and shows the possibility of using long-term surveys for ecological studies, if carefully interpreted.
We thank S. Le Gleut, E. Pétillon, S. Cobbold, G. Dubois and G. Montfort for field assistance, and François Rineau for help in identification. Sébastien Dugravot improved the English version of this manuscript. This study was supported by the French Ministry of Environment (Programme ‘Espèces invasives’) and the CNRS Zone Atelier ‘La Baie du Mont Saint-Michel et ses bassins versants’.