(C) 2011 Tanja Lessel. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Within the scope of the Integrated Rhine Program an ecological flood gate and channel was inserted into the polder “Ingelheim” to enhance animal and plant diversity. In 2008, carabid beetles and springtails were collected, using pitfall traps, to measure the effects of ecological flooding and a strong precipitation event at a flood-disturbed and a dry location in this area. At both localities, xerophilic and mesophilic carabid beetle species were dominant throughout the study period. The total number of individuals of hygrophilic species was comparatively constant, while species number increased, partly due to the changed moisture conditions caused by ecological flooding and strong precipitation. Carabid beetle diversity and evenness decreased marginally when ecological flooding was absent. Springtails represent a less mobile arthropod order, and as such the impact of ecological flooding was stronger. An increase in both numbers of species and individuals of hygrophilic and hygrotolerant species occurred in the flood-disturbed location after ecological flooding. After the sites at both locations had dried, the number of individuals belonging to these species declined rapidly. In contrast to carabid species, the strong precipitation event showed no influence on hygrophilic springtail species. Thus, collembolan diversity and evenness decreased markedly in the absence of flooding. We showed that ecological flooding has an influence on the spatial and temporal dynamics of different arthropod groups that inhabit the polder “Ingelheim”. These findings demonstrate the importance of using different arthropod groups as bioindicators in determining the ecological value of a particular polder design.
bioindication, community dynamics, drought, flooding, Integrated Rhine Program (IRP)
During the last three decades flood protection has become
one of the most important goals of countries along the entire course of
the river Rhine. Therefore, in 1982 the Integrated Rhine Program (IRP)
was established to reduce the economic and ecological impacts of a
200-year flood (an extraordinary flood event, which hypothetically
occurs only once in 200 years). The program includes the specific use of
hydroelectric power plants, the construction of several polder sites
(floodwater retention basins) and the relocation of dikes to enlarge the
flooding area of the river Rhine. An essential aim of the IRP is to
combine economic (flood protection) and ecological protective measures (
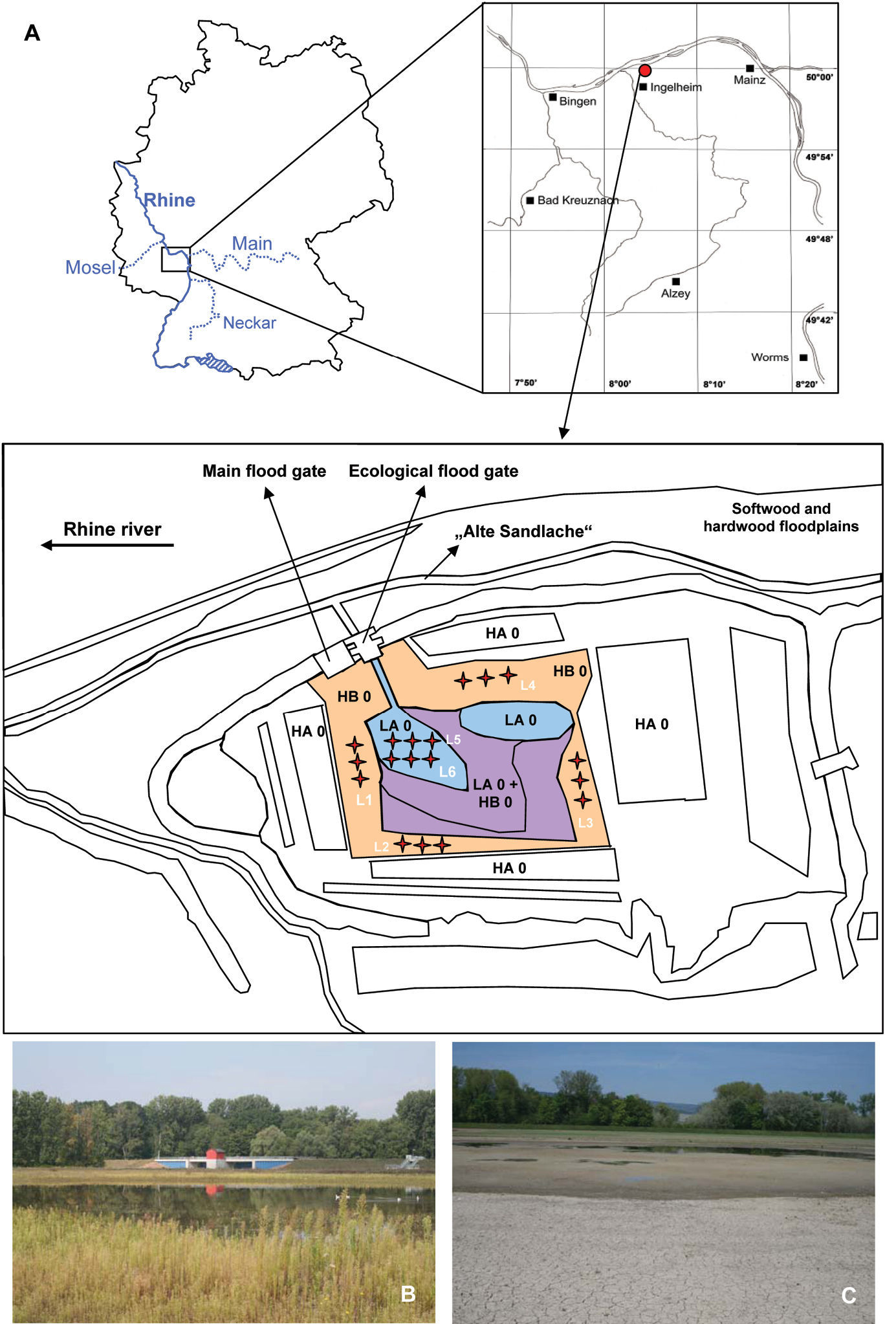
Location of the polder „Ingelheim“ in Germany and location of the different areas and pitfall trap localities (L1–L6) within this polder (A). Abbreviations: LA 0: ruderal area; HB 0: fallow area; LA 0 + HB 0: transition area between LA 0 and HB 0; HA 0: agricultural fields; L1–6: locations of the six pitfall trap groups (three pitfall traps per locality). The pictures show the main flood gate (left) and the ecological flood gate (right), and an ecological flooding in March 2007 (B) and the fast drying event in the ruderal area after ecological flooding in April 2007 (C).
For this reason the mobile carabid beetles (Coleoptera; Carabidae)
and the less mobile springtails (Collembola) were chosen to detect the
effects of ecological flooding on these arthropod groups. The ecology
and taxonomy of most Middle European species belonging to these two
groups have been well researched, making them particularly suitable for
such a study. As they can be sampled easily and cost-efficiently, they
are also potentially suitable bioindicators (
The main aims of this investigation were to determine the effects of ecological flooding on ground beetles and springtails, and to determine their bioindication value. Therefore, results of the 2008 vegetation period are presented, during which both an ecological flood event caused by high Rhine water levels and a flood caused by a strong precipitation event occurred. Between these two flooding events a short but severe drought period occurred at the study site. This vegetation period was of particular importance in answering the main questions posed here because of the fast sequence of the different flood and drought events.
Material and methodsThe Polder “Ingelheim” (49°59'N; 8°03'E, 81–82m a.s.l.) is located in a nature protection area called “Sandlache” near Mainz in the Northern Upper Rhine region. The feed stream of the polder flows through a natural backwater of the river Rhine, the “Alte Sandlache” (Fig. 1A). The central part of the study site was formerly characterised as a ruderal seepage area (now ruderal area) because of seepage water. Ecological flooding, through the ecological flood gate, should prevent the succession of this area from ruderal to fallow. The remainder of the study site is an active agricultural area. After the polder had been built between the agricultural land (HA 0) and the ruderal area (LA 0), an unused fallow area (HB 0) with a dense shrub layer developed (Fig. 1A). This area is dominated by Limosella aquatica (L.), Gnaphalium uliginosum (L.), Juncus bufonius (L.), Cyperus fuscus (L.), Potentilla supina (L.) and Lythrum hyssopifolia (L.) and serves as the riverbank during the ecological flooding of the ruderal area. The ruderal area mainly consists of Cirsium arvense (L.), Conyza canadensis (L.), Lactuca serricola (L.) and Sinapis arvensis (L.) and is usually completely flooded during an ecological flood event caused by high Rhine river water levels. During the vegetation period of 2008 the fallow area had a flood disturbance of less than 5% and the ruderal area of more than 30% (flood disturbance was calculated as the percentage of days that sampling could not be performed due to flooding). The soil of the polder is secondary loess with a high sand and loam content, typical of the region. Because of these soil conditions, strong precipitation events are sufficient to flood the ruderal area in particular.
For the study a total of 18 pitfall traps at six
locations (three traps per location, distance between the traps: 5m)
were used. Two locations were in the ruderal area (L5, L6) and the
remaining four locations were situated in the fallow area (L1–L4, Fig. 1A).
The pitfall traps had a diameter of 10 cm at ground level and were
protected from direct rainwater infiltration by a transparent cover (10 x
10 cm; plexiglas). The traps were filled with a saturated NaCl-solution
and detergent as killing agent (
Because a number of pitfall traps failed, mainly in the
ruderal area (due to flood disturbance), the total number of
individuals collected was transformed to the mean number of individuals
per trap and day (± Standard error; SE). Diversity (
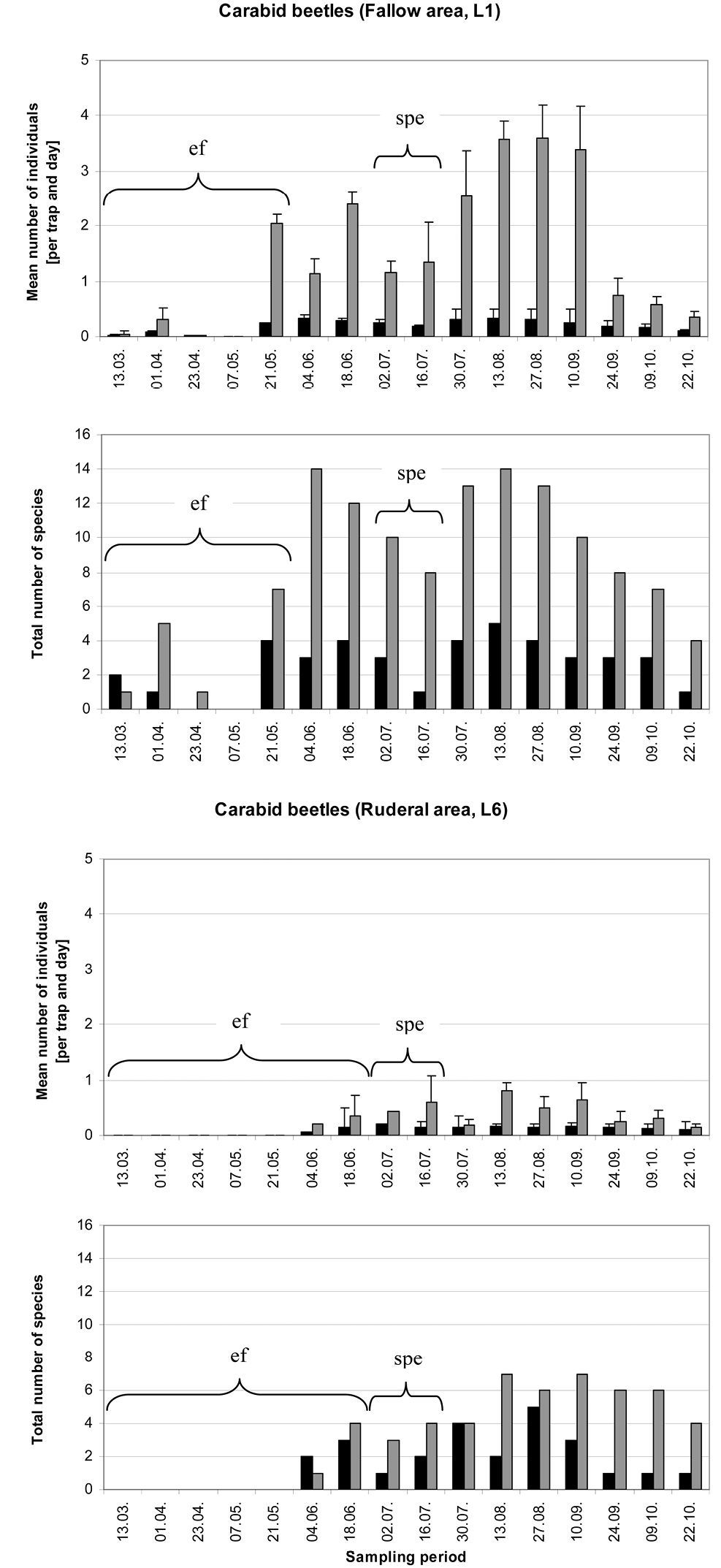
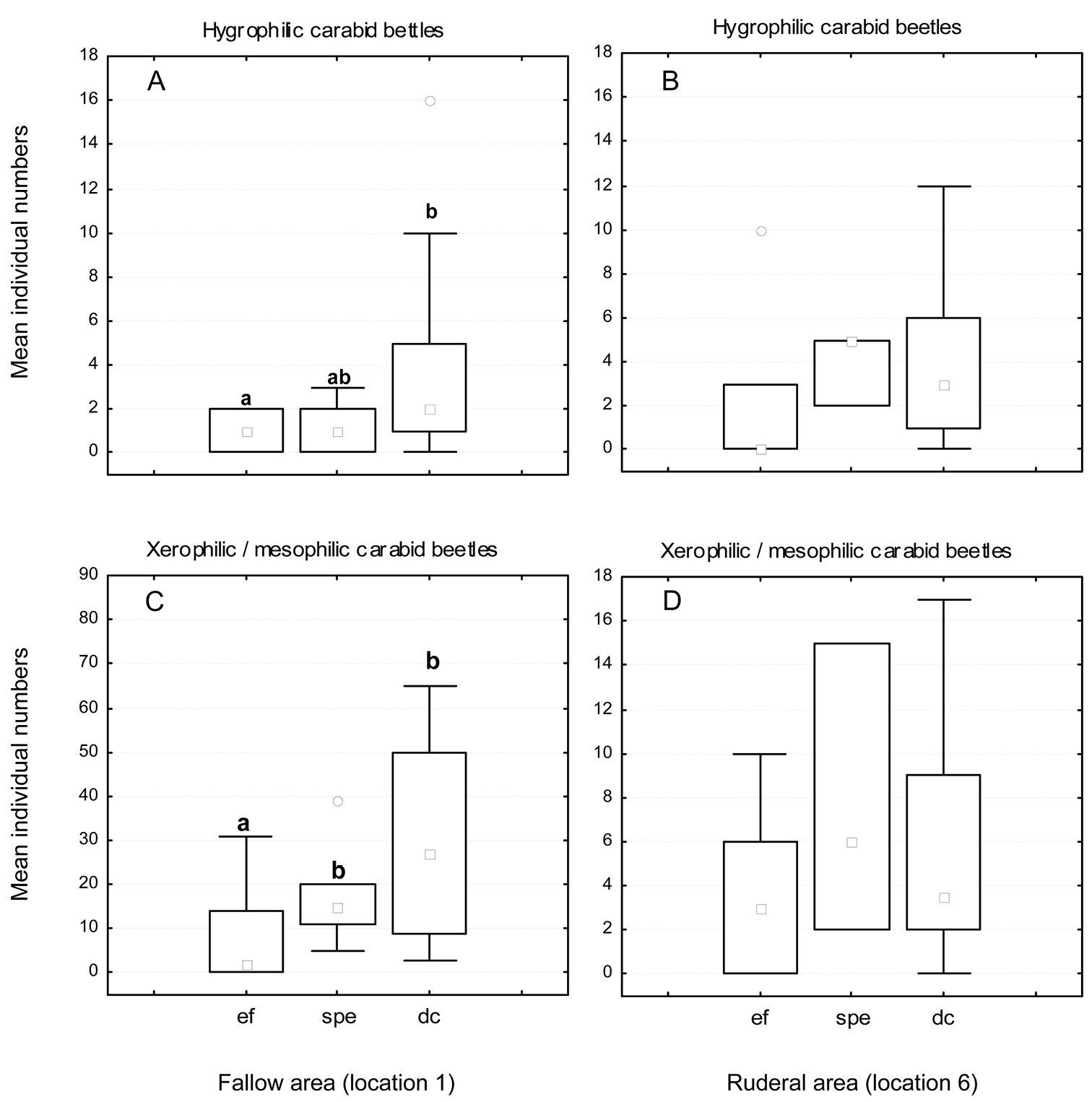
In the fallow area, 46 carabid species of 1490 individuals were collected, while 33 species of 514 individuals were collected in the ruderal area. In the fallow area, 26 xerophilic and two mesophilic species dominated, representing more than 64% of all individuals collected, while five eurytopic species comprised more than 28% of the catch. The 12 hygrophilic species only made up 8% of the catch (see Appendix 1). Harpalus luteicornis (Duftschmid, 1812) was the only species that could not be clearly classified using the literature and is thus marked uc (unclassified) in Table 1 and in Appendices 1 and 2. In the ruderal area, 12 hygrophilic species dominated the catch (20% of all individuals collected), while 12 xerophilic and one mesophilic species comprised nearly 30% of all individuals. This area was dominated by six eurytopic species, representing almost 50% of the catch, while Bembidion species are predominantly limited to the ruderal area (Fig. 2). There were only two species without clear classification (see Appendices 1 and 2). Table 1 shows the classification of the species and individuals with and without the impact of ecological flooding. When the ecological flooding period (and data) was excluded, only a small decrease in abundance and a disappearance of four species were detected in both localities. In the fallow area, the hygrophilic species Bembidion biguttatum (Fabricius, 1779), Ocys harpaloides (Audinet-Serville, 1821) and Stenolophus mixtus (Herbst, 1784) as well as the xerophilic species Microlestes maurus (Sturm, 1827) disappeared (Appendix 2). In the ruderal area, in addition to Demetrias atricapillus (L.), the hygrophilic species Anisodactylus binotatus (Fabricius, 1787), Bembidion biguttatum and Stomis pumicatus (Panzer, 1796) disappeared (Appendix 2). However, all species that disappeared comprised only small numbers of individuals. This is also confirmed by the comparison of diversity and evenness values with and without the ecological flood data. In both locations without the ecological flood data, Shannon-diversity showed only a small decrease, whereas evenness values remained almost unchanged (Table 1). Furthermore, both areas showed a constant dominance of xerophilic and mesophilic species in terms of species number and abundance over hygrophilic species throughout the vegetation period (Fig. 3). Especially Pterostichus melanarius (Illiger, 1798), Poecilus cupreus (L.), Harpalus rufipes (De Geer, 1774) and Harpalus affinis (Schrank, 1781) occurred asdominant and subdominant species (Appendix 2). Thus, ecological flooding appeared not to cause species or dominance shifts. This is also confirmed by the dominance of hygrophilic and xerophilic/mesophilic species during the different moisture periods (Fig. 4). In the fallow area the drought period showed significantly higher abundances of hygrophilic (Fig. 4A: U-test: p ≤ 0.01) as well as xerophilic/mesophilic species (Fig. 4C; U-test: p ≤ 0.01). Higher abundances after the strong precipitation event in the fallow area were only detected for xerophilic/mesophilic species (Fig. 4C; U-test: p = 0.044). In contrast to the fallow area, there were no clear differences between the mean carabid beetle abundances during the flooding and drought periods at the ruderal area (Location 6; Fig. 4B and 4D).
Total number of carabid beetle and springtail individuals and species at the fallow area (location 1) and the ruderal area (location 6) with and without data from the ecological flooding period. Shannon diversity, maximum diversity and evenness (Pielou) values are also given with and without data from the ecological flooding period. Abbreviations: e=eurytopic; hp=hygrophilic; ht=hygrotolerant; mp=mesophilic; uc=unclassified; xp=xerophilic; xt=xerotolerant.
| Carabid beetles | Springtails | |||||||||||||||||
| Individual number | Species number | Shannon-diversity (Hs) | Maximum diversity (Hmax) | Evenness (Pielou) | Individual number | Species number | Shannon-diversity (Hs) | Maximum diversity (Hmax) | Evenness (Pielou) | |||||||||
| hp | xp, mp | uc, e | hp | xp, mp | uc, e | hp, ht | xt, mp | uc | hp, ht | xt, mp | uc | |||||||
| Fallow area | ||||||||||||||||||
| With data of ecological flooding | 113 | 955 | 422 | 12 | 28 | 6 | 2.35 | 3.83 | 0.61 | 652 | 6333 | 16 | 3 | 7 | 5 | 1.07 | 2.71 | 0.39 |
| Without data of ecological flooding | 104 | 877 | 411 | 9 | 27 | 6 | 2.25 | 3.74 | 0.60 | 2 | 6169 | 5 | 1 | 6 | 3 | 0.73 | 2.30 | 0.32 |
| Ruderal area | ||||||||||||||||||
| With data of ecological flooding | 101 | 153 | 260 | 12 | 13 | 8 | 2.60 | 3.50 | 0.74 | 3040 | 2363 | 2 | 3 | 4 | 2 | 1.26 | 2.20 | 0.57 |
| Without data of ecological flooding | 87 | 140 | 251 | 9 | 13 | 7 | 2.54 | 3.37 | 0.75 | 3 | 2322 | 1 | 1 | 4 | 1 | 0.61 | 1.79 | 0.34 |
PCA of carabid beetle communities in the fallow area (location 1) and the ruderal area (location 6) during ecological flooding, the flood caused by a strong precipitation event and drought conditions. Only species with more than 1% dominance value in at least one area are included. Abbreviations of the species: A.mar=Agonum marginatum; A.bif=Amara bifrons; A.sim=Amara similata; B.lam=Bembidion lampros; B.pro=Bembidion properans; B.qua=Bembidion quadrimaculatum; C.pur=Carabus purpurascens; H.aff=Harpalus affinis; H.ruf=Harpalus rufipes; H.sma=Harpalus smaragdinus; N.bre=Nebria brevicollis; O.ard=Ophonus ardosiacus; P.cup=Poecilus cupreus; P.ant=Pterostichus anthracinus; P.mel=Pterostichus melanarius; P.nig=Pterostichus nigrita. Percentage variation explained by the two PCA axes is included.
Mean number of individuals per trap and day (± SE) and total carabid beetle species number at location 1 (fallow area) and location 6 (ruderal area) (n=3) during the vegetation period of 2008. Hygrophilic species (black bars) and xerophilic as well as mesophilic species (grey bars) are shown. Abbreviations: ef = ecological flooding; spe = strong precipitation event.
Mean number of individuals of hygrophilic (A/B) and xerophilic/mesophilic (C/D) carabid beetle species at the fallow (A/C) and ruderal area (B/D) during different moisture conditions. Abbreviations: ef = ecological flooding (higher Rhine water levels); spe = flood caused by a strong precipitation event; dc = drought conditions; ° outliers. Different letters represent statistically significant differences (Mann-Whitney U-test).
The different species- (Jaccard) and dominance- (Renkonen) based similarity indices confirmed this stable community structure (Table 2). However, during the entire vegetation period some hygrophilic species with similar numbers of individuals occurred in both areas, although the dominance of the most dominant hygrophilic species varied markedly. In the fallow area, Pterostichus anthracinus (Illiger, 1798) and Pterostichus nigrita were the most dominant hygrophilic species, whereas in the ruderal area Nebria brevicollis and Agonum marginatum dominated (Appendix 2). Species with a dominant or subdominant occurrence in only one location were Carabus purpurascens Fabricius, 1787, Pterostichus nigrita (Paykull, 1790) and Amara bifrons (Gyllenhal, 1810) in the fallow area and Bembidion quadrimaculatum (L. 1761), Bembidion lampros (Herbst, 1784), Nebria brevicollis (Fabricius, 1792) and Agonum marginatum (L.) in the ruderal area (Appendix 2). As such, the species- and dominance-based Wainstein-similarity index values were only about 25%, which is very low given the proximity of the two locations.
Comparison of the carabid beetle and springtail communities of the fallow area (location 1) and the ruderal area (location 6) using different species based and dominance based similarity indices (Jaccard, Renkonen and Wainstein) with and without data from the ecological flooding period. The percentages show the degree of similarity of the carabid beetle and springtail communities between the fallow and ruderal area. Values higher than 50% represent higher similarity of the communities between the two areas.
| Comparison location 1 with location 6 | Carabid beetles | Springtails | ||||
|---|---|---|---|---|---|---|
| Jaccard | Renkonen | Wainstein | Jaccard | Renkonen | Wainstein | |
| With data of ecological flooding | 46.3 | 52.5 | 24.3 | 60.0 | 53.1 | 31.7 |
| Without data of ecological flooding | 51.1 | 51.5 | 26.3 | 60.0 | 95.7 | 57.4 |
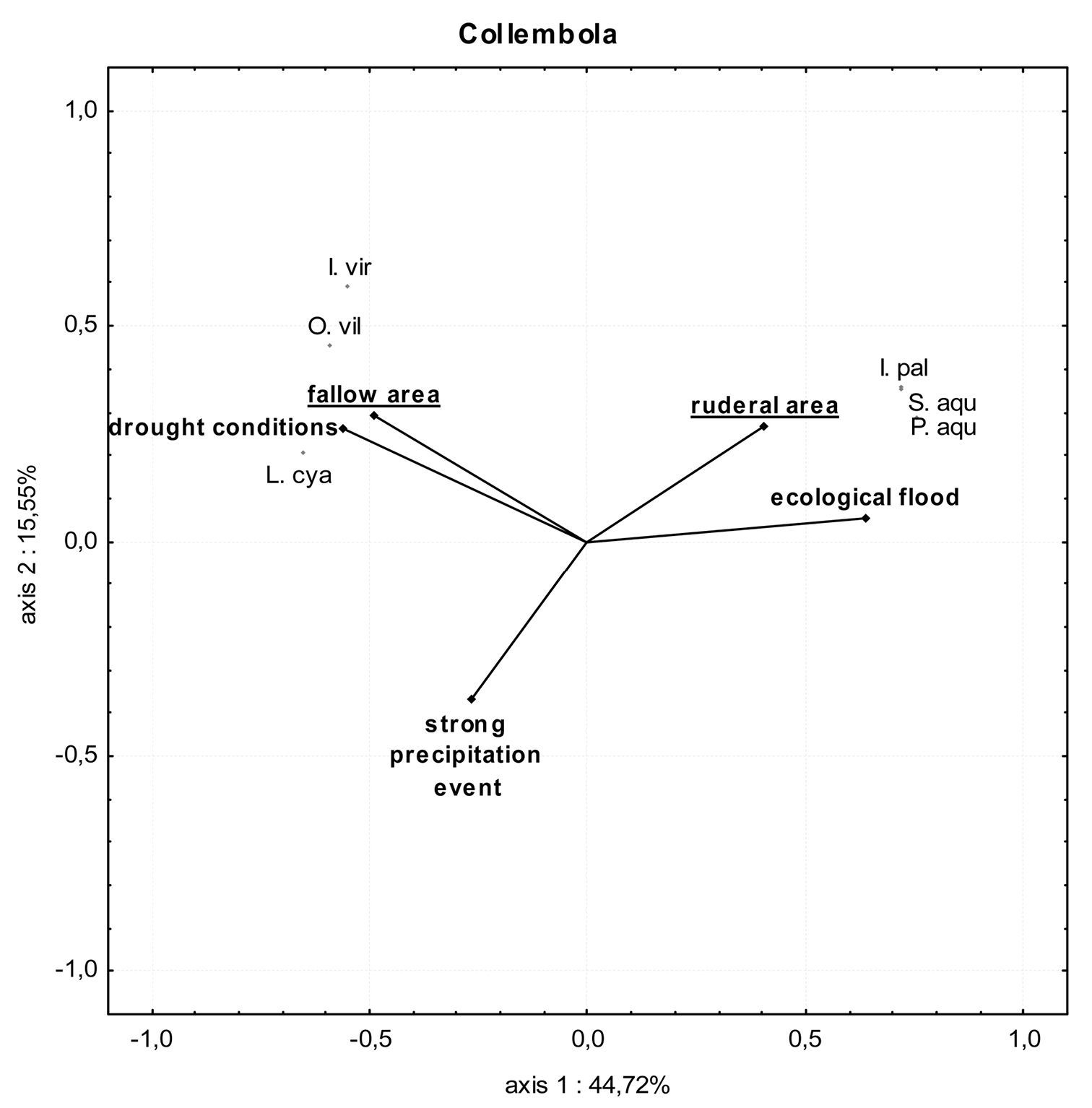
In the fallow area, 15 collembolan species and 7001 individuals were caught. With the ecological flooding data included, seven xerotolerant and mesophilic species dominated the catch (90% of all individuals collected), while the three hygrophilic and hygrotolerant species comprised less than 10% of all individuals collected (Table 1). Mainly xerotolerant and mesophilic species were detected when data from the ecological flood period were excluded. In the ruderal area, nine collembolan species with 5405 individuals were captured. Here, however, the ecological flooding data showed that three hygrophilic and hygrotolerant species made up 56% of the catch, while four xerotolerant and mesophilic species made up almost 44% of all individuals collected (Fig. 5). Without the ecological flooding data, hygrophilic and hygrotolerant individuals were almost absent, which resulted in a dominance value of almost 100% for xerotolerant and mesophilic species. This result was also reflected in the lower diversity and evenness values (Table 1). In the ruderal area in particular, these indices decreased markedly. During and shortly after ecological flooding caused by a higher Rhine water level, three hygrophilic and hygrotolerant species Podura aquatica (L.), Isotomurus palustris (Müller, 1776) and Sminthurides aquaticus (Bourlet, 1842) were highly abundant compared to all other species in both locations (Figs. 5 and 6, Appendix 3). The mean number of individuals of these species caught in the pitfall traps during the ecological flood was significantly higher than the mean number of individuals caught during the flood caused by the strong precipitation event (Fig. 7A and 7B; U-test: fallow area (L1): p ≤ 0.01; ruderal area (L6): p = 0.025). After the ecological flood event, these species completely disappeared from both areas. Furthermore, compared to the dry period, the strong precipitation event at the end of June had no effect on hygrophilic and hygrotolerant species (U-test: fallow area (L1): p = 0.89; ruderal area (L6): p = 0.36). Compared to the ecological flood event, mean numbers of individuals belonging to the mesophilic species Isotoma viridis Bourlet, 1839 and the xerotolerant species Orchesella villosa (Geoffroy, 1762) increased significantly during the flood caused by the strong precipitation event and under drought conditions (Fig. 7C and 7D; U-test: fallow area (L1): p ≤ 0.01; ruderal area (L6): p = 0.022). During the sampling period, many collembolan species show a spring and autumn peak with very high individual numbers. In the polder this autumn maximum was also dominated by these two species (Isotoma viridis and Orchesella villosa). The species-based Jaccard similarity index showed a value of 60% for both areas with and without the impact of ecological flooding, which indicates stable collembolan communities (Table 2). However, differences were obvious concerning the dominance-based Renkonen index and the combined Wainstein index. Without the ecological flooding data, the values of these indices were remarkably high at almost 96% (Renkonen) and 58% (Wainstein), due to the eudominance of Orchesella villosa and the dominance of Isotoma viridis in both locations (Table 2). However, with the inclusion of the ecological flooding data, these values were lower, mainly because of the influence of the eudominant species Isotomurus palustris in the ruderal area. As such, without data from the ecological flooding event, the collembolan communities of both locations were highly similar, while ecological flooding increased the heterogeneity of the collembolan communities of both locations.
Mean individual numbers per trap and day (± SE) and total species numbers of springtails of the pitfall traps of location 1 and location 6 (n=3) over the vegetation period 2008. Hygrophilic and hygrotolerant species (black bars) and xerotolerant as well as mesophilic species (grey bars) are shown. Abbreviations: ef = ecological flooding; spe = strong precipitation event.
PCA of springtail communities in the fallow area (location 1) and the ruderal area (location 6) during ecological flooding, the flood caused by a strong precipitation event and drought conditions. Only species with more than 1% dominance value in at least one area are included. Abbreviations of the species: I.pal=Isotomurus palustris; I.vir=Isotoma viridis; L.cya=Lepidocyrtus cyaneus; O.vil=Orchesella villosa; P.aqu=Podura aquatica; S.aqu=Sminthurides aquaticus. Percentage variation explained by the two PCA axes are included.
Mean number of individuals of hygrophilic/hygrotolerant (A/B) and xerotolerant/mesophilic (C/D) collembolan species at the fallow (A/C) and ruderal area (B/D) during different moisture conditions. Abbreviations: ef = ecological flooding (higher Rhine water levels); spe = flood caused by a strong precipitation event; dc = drought conditions; ° outliers. Different letters represent statistically significant differences (Mann-Whitney U-test).
The carabid beetle community structure showed clear
differences between the two locations. The fallow area is characterized
by more vegetation with higher structural diversity and plant
heterogeneity, while the ruderal area is characterized by a high level
of flood disturbance and less vegetation. The largest number of carabid
beetles was collected from the fallow area. The dominance of xerophilic
species such as Harpalus rufipes or Harpalus affinis
was expected. A comparatively high number of hygrophilic species were
also collected from this area, but with only a small number of
individuals. Interestingly, Agonum marginatum was found in the fallow area even though this species prefers riverbanks with less vegetation (
Because of the prolonged flood disturbance from March to
May and the strong precipitation event at the end of June 2008 fewer
carabid beetles were collected from the ruderal area. Flood events in
this area could favour the higher dispersal capacity of pioneer species (
Furthermore, the dominance of small carabid beetles such as Bembidion quadrimaculatum or Bembidion lampros can be explained by the work of
In contrast to the mobile carabid beetles, ecological
flooding had a considerable impact on the collembolan community at both
areas. Hygrophilic and hygrotolerant species occurred only during and
shortly after this flood event. The adaptation of these species to
coping with floods is passive drifting (
This investigation showed that, in addition to ecological flooding, other flooding events, such as strong precipitation or seepage water, are important factors for the spatial and temporal dynamics of different arthropod groups in such ruderal and seepage areas. These findings emphasize the value of using different taxa in the designing of future polder constructions. If only one arthropod group had been studied this might have led to the erroneous conclusion that ecological flooding has no effect or that it only affects this one bioindicator group. The data collected from several arthropod groups, however, provide more reliable and comprehensive information on the real ecological value of the polder structures.
The authors are much obliged to Carlos Mora-Ferrer, Johan Kotze and two reviewers for very valuable comments on and linguistic revision of the manuscript. This study received financial support from the Centre for Environmental Research and the Feldbausch foundation of the Johannes Gutenberg-University of Mainz.
Dominance [%] of hygrophilic (h), xerophilic/mesophilic (x/m), eurytopic (e) and unclassified (uc) carabid beetle species and individuals at location 1 (fallow area) and location 6 (ruderal area):
| Location 1 (fallow area) | ec | 13.03 | 01.04 | 23.04 | 07.05 | 21.05 | 04.06 | 18.06 | 02.07 | 16.07 | 30.07 | 13.08 | 27.08 | 10.09 | 24.09 | 09.10 | 22.10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. of pitfall traps | 3 | 3 | 2 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | total | |
| trapping days | 14 | 19 | 22 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 15 | 13 | ||
| Acupalpus meridianus | x | 2 | 1 | 3 | ||||||||||||||
| Agonum marginatum | h | 3 | 1 | 1 | 1 | 1 | 7 | |||||||||||
| Agonum muelleri | h | 1 | 2 | 1 | 4 | |||||||||||||
| Agonum sexpunctatum | h | 1 | 1 | |||||||||||||||
| Amara aenea | x | 4 | 4 | |||||||||||||||
| Amara apricaria | x | 1 | 1 | 2 | ||||||||||||||
| Amara aulica | x | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||
| Amara bifrons | x | 7 | 16 | 4 | 5 | 2 | 2 | 3 | 3 | 6 | 3 | 51 | ||||||
| Amara consularis | x | 1 | 1 | |||||||||||||||
| Amara similata | x | 1 | 2 | 1 | 11 | 8 | 2 | 1 | 1 | 27 | ||||||||
| Anchonemus dorsalis | x | 2 | 2 | |||||||||||||||
| Badister unipustulatus | h | 1 | 2 | 1 | 1 | 5 | ||||||||||||
| Bembidion biguttatum | h | 1 | 1 | |||||||||||||||
| Bembidion lampros | e | 1 | 1 | 1 | 3 | |||||||||||||
| Bembidion obtusum | x | 2 | 9 | 1 | 12 | |||||||||||||
| Bembidion properans | e | 2 | 2 | 2 | 3 | 9 | ||||||||||||
| Bembidion quadrimaculatum | e | 3 | 2 | 3 | 1 | 3 | 1 | 1 | 1 | 15 | ||||||||
| Brachinus explodens | x | 1 | 1 | |||||||||||||||
| Bradycellus harpalinus | x | 1 | 1 | |||||||||||||||
| Calathus ambiguus | x | 1 | 1 | 1 | 3 | 1 | 7 | |||||||||||
| Calathus fuscipes | x | 1 | 1 | 4 | 1 | 7 | ||||||||||||
| Calathus melanocephalus | x | 1 | 2 | 2 | 2 | 7 | ||||||||||||
| Carabus purpurascens | m | 1 | 7 | 9 | 6 | 25 | 64 | 87 | 98 | 18 | 4 | 319 | ||||||
| Chlaenius nigricornis | h | 1 | 1 | 1 | 1 | 4 | ||||||||||||
| Harpalus affinis | x | 12 | 6 | 8 | 6 | 5 | 7 | 8 | 2 | 2 | 2 | 58 | ||||||
| Harpalus distinguendes | x | 2 | 1 | 3 | 1 | 1 | 8 | |||||||||||
| Harpalus latus | e | 1 | 1 | 6 | 8 | |||||||||||||
| Harpalus luteicornis | uc | 2 | 1 | 1 | 4 | |||||||||||||
| Harpalus rubripes | x | 1 | 2 | 1 | 1 | 2 | 7 | |||||||||||
| Harpalus rufipes | x | 1 | 3 | 4 | 10 | 13 | 30 | 16 | 13 | 1 | 1 | 92 | ||||||
| Harpalus smaragdinus | x | 1 | 3 | 2 | 1 | 2 | 1 | 2 | 2 | 14 | ||||||||
| Leistus ferrugineus | x | 1 | 1 | |||||||||||||||
| Microlestes maurus | x | 3 | 3 | |||||||||||||||
| Microlestes minutulus | x | 1 | 1 | 2 | ||||||||||||||
| Nebria brevicollis | h | 1 | 2 | 1 | 4 | |||||||||||||
| Notiophilus palustris | h | 1 | 1 | 2 | ||||||||||||||
| Ocys harpaloides | h | 1 | 1 | |||||||||||||||
| Ophonus ardosiacus | x | 1 | 1 | 1 | 2 | 10 | 2 | 1 | 18 | |||||||||
| Ophonus azureus | x | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||
| Ophonus laticollis | x | 2 | 1 | 1 | 4 | |||||||||||||
| Poecilus cupreus | x | 3 | 39 | 16 | 57 | 21 | 19 | 32 | 33 | 32 | 17 | 4 | 10 | 8 | 291 | |||
| Pterostichus anthracinus | h | 2 | 4 | 2 | 3 | 6 | 1 | 3 | 21 | |||||||||
| Pterostichus melanarius | e | 9 | 28 | 16 | 7 | 27 | 74 | 94 | 98 | 10 | 17 | 3 | 383 | |||||
| Pterostichus nigrita | h | 4 | 2 | 2 | 6 | 11 | 9 | 19 | 4 | 5 | 62 | |||||||
| Pterostichus oblongopunctatus | m | 3 | 3 | |||||||||||||||
| Stenolophus mixtus | h | 1 | 1 |
Total number of carabid beetle individuals (Carabidae), number of flood-undisturbed pitfall traps and trapping days of location 1 (fallow area) and location 6 (ruderal area). Abbreviations: ec=ecological classification, h=hygrophilic, x=xerophilic, m=mesophilic, uc=unclassified, e=eurytopic.
| Location 1 | ec | 13.03 | 01.04 | 23.04 | 07.05 | 21.05 | 04.06 | 18.06 | 02.07 | 16.07 | 30.07 | 13.08 | 27.08 | 10.09 | 24.09 | 09.10 | 22.10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. of pitfall traps | 3 | 3 | 2 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | total | |
| trapping days | 14 | 19 | 22 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 15 | 13 | ||
| Ceratophysella bengtssoni | uc | 3 | 4 | 1 | 1 | 1 | 10 | |||||||||||
| Ceratophysella denticulata | uc | 1 | 1 | |||||||||||||||
| Neanura muscorum | m | 1 | 1 | |||||||||||||||
| Podura aquatica | h | 13 | 50 | 12 | 75 | |||||||||||||
| Isotomurus palustris | h | 437 | 106 | 10 | 4 | 15 | 2 | 574 | ||||||||||
| Isotoma viridis | m | 34 | 24 | 1 | 1 | 1 | 59 | 101 | 56 | 56 | 47 | 32 | 8 | 2 | 8 | 125 | 753 | 1308 |
| Entomobrya lanuginosa | m | 2 | 41 | 2 | 5 | 1 | 51 | |||||||||||
| Lepidocyrtus cyaneus | x | 3 | 4 | 2 | 27 | 32 | 42 | 33 | 23 | 6 | 1 | 7 | 39 | 77 | 296 | |||
| Lepidocyrtus paradoxus | uc | 1 | 1 | |||||||||||||||
| Orchesella villosa | x | 9 | 5 | 2 | 15 | 162 | 411 | 377 | 638 | 543 | 429 | 106 | 192 | 412 | 453 | 876 | 4630 | |
| Bourletiella hortensis | uc | 2 | 2 | |||||||||||||||
| Sminthurus viridis | x | 11 | 3 | 2 | 10 | 1 | 1 | 28 | ||||||||||
| Sminthurus nigromaculatus | m | 2 | 1 | 6 | 7 | 2 | 1 | 19 | ||||||||||
| Sminthurides aquaticus | h | 3 | 3 | |||||||||||||||
| Deuterosminthurus pallipes | uc | 1 | 1 | 2 |
Total number of springtail individuals (Collembola), number of flood-undisturbed pitfall traps and trapping days of location 1 (fallow area) and location 6 (ruderal area). Abbreviations: ec=ecological classification, h=hygrophilic/hygrotolerant, x=xerotolerant, m=mesophilic, uc=unclassified.
| Location 6 (ruderal area) | ec | 13.03 | 01.04 | 23.04 | 07.05 | 21.05 | 04.06 | 18.06 | 02.07 | 16.07 | 30.07 | 13.08 | 27.08 | 10.09 | 24.09 | 09.10 | 22.10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. of pitfall traps | 2 | - | - | - | - | 1 | 2 | 1 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | total | |
| trapping days | 14 | - | - | - | - | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 15 | 13 | ||
| Ceratophysella bengtssoni | uc | 1 | 1 | |||||||||||||||
| Podura aquatica | h | 192 | 65 | 67 | 324 | |||||||||||||
| Isotomurus palustris | h | 7 | 1318 | 823 | 388 | 3 | 2539 | |||||||||||
| Isotoma viridis | m | 3 | 1 | 5 | 4 | 66 | 19 | 6 | 98 | 188 | 177 | 567 | ||||||
| Lepidocyrtus cyaneus | x | 2 | 1 | 9 | 1 | 3 | 2 | 1 | 19 | |||||||||
| Orchesella villosa | x | 34 | 41 | 308 | 178 | 90 | 15 | 7 | 79 | 349 | 675 | 1776 | ||||||
| Sminthurus viridis | x | 1 | 1 | |||||||||||||||
| Sminthurides aquaticus | h | 9 | 75 | 76 | 17 | 177 | ||||||||||||
| Deuterosminthurus pallipes | uc | 1 | 1 |