(C) 2012 Charlene Janion. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The first species of the genus Triacanthella to be recorded from Africa is described. Triacanthella madiba sp. n. belongs to the Southern Hemisphere group of the genus. It is morphologically closely related to Triacanthella vogeli Weiner & Najt, 1997 from Chile, and appears to be a gondwanian relict. The new species is also the first Triacanthella recorded from a guano habitat.
South Africa, Western Cape, gondwanian relict, cave, guano
The Cape Floristic Region in Western Cape Province of South Africa is the smallest Floral Kingdom in the world. Although its extraordinary rich flora has been well documented (
The genus Triacanthella is phyletically isolated among Hypogastruridae both from a morphological and a molecular point of view (
The terminology used in the text follows
| 0 | Sixth abdominal tergum with rosette-shaped tubercles, South America only | 1 |
| – | Sixth abdominal tergum without rosette-shaped tubercles | 5 |
| 1 | Empodium absent, claw without inner tooth | 2 |
| – | Empodium present (but rudimentary), claw with two inner teeth | 3 |
| 2 | Ommatidia G similar in size to the other ommatidia, Argentina | Triacanthella michaelseni Schäffer, 1897 |
| – | Ommatidia H and G reduced compared to the other ommatidia, Argentina | Triacanthella rosae Wahlgren, 1906 |
| 3 | Posterior anal spine less than half the size of the other two, dentes without apical lobe, Chile | Triacanthella vogeli Weiner & Najt, 1997 |
| – | Posterior anal spine at least half the size of the other two, dentes with distinct apical lobe | 4 |
| 4 | Macrochaetae long, half tergite macrochaetal chaetotaxy = 7 / 2, 3, 3 / 4, 4, 4, 4, Chile and Argentina | Triacanthella andina Cassagnau & Rapoport, 1962 |
| – | Macrochaetae short, half tergite macrochaetal chaetotaxy = 8 / 2, 4, 4 / 3, 3, 3, 3, Argentina | Triacanthella najtae de Izarra, 1971 |
| 5 | Posterior anal spine at least half the size of the other two | 6 |
| – | Posterior anal spine less than half the size of the other two | 12 |
| 6 | Mucro reduced to a small projection (i.e. almost absent) | 7 |
| – | Mucro well developed | 8 |
| 7 | Colour in alcohol pinkish, Campbell Island | Triacanthella sorenseni Salmon, 1949 |
| – | Colour in alcohol whitish-yellowish, Campbell Island | Triacanthella alba Carpenter, 1906 |
| 8 | Mucro simple and straight | 9 |
| – | Mucro more complex with two teeth | 10 |
| 9 | All ommatidia equally developed, tibiotarsi with clavate tenent hair, Argentina | Triacanthella massoudi Najt, 1973 |
| – | Two ommatidia (G and H) absent, tibiotarsi without clavate tenent hair, Australia | Triacanthella violacea Womersley, 1939 |
| 10 | Macrochaetae simple and smooth, New Zealand | Triacanthella rubra Salmon, 1941 |
| – | Macrochaetae serrated or brush-like | 11 |
| 11 | All ommatidia equally developed, apical lobe absent on dentes, New Zealand | Triacanthella purpurea Salmon, 1943 |
| – | Two ommatidia (G and H) reduced, apical lobe present on dentes, New Zealand | Triacanthella enderbyensis Salmon, 1949 |
| 12 | Two ommatidia (G and H) reduced | 13 |
| – | All ommatidia equally developed | 14 |
| 13 | Dentes reduced, empodium present (but rudimentary), Chile | Triacanthella clavata (Willem, 1902) |
| – | Dentes normally developed, empodium absent, New Zealand | Triacanthella terrasilvatica Salmon, 1943 |
| 14 | Mucro more complex with two teeth, colour whitish-yellowish in alcohol, claw without inner tooth, New Zealand | Triacanthella setacea Salmon, 1941 |
| – | Mucro with a distinct heel, colour pinkish in alcohol, claw with two inner teeth, South Africa | Triacanthella madiba sp. n. |
urn:lsid:zoobank.org:act:606016FB-A5C4-4B86-A9EC-E111EB7CCAEB
Holotype female and 17 paratypes (9 on slides and 8 in alcohol), South Africa: Western Cape, Cape Town, Table Mountain National Park, 10 March 2009, bat guano in Wynberg cave, extracted on Berlese-Tullgren funnel, (SAF-125, Louis Deharveng & Anne Bedos leg).
Holotype on slide and 9 paratypes (5 on slides and 4 in alcohol) in Iziko Museum (Cape Town, South Africa), 8 paratypes in Museum National d’Histoire Naturelle, Paris (4 on slides and 4 in alcohol).
Colour orange to pink alive, pinkish in ethanol even after one year (Fig. 1). Length 1.9 – 2.5 mm. Habitus of Southern Hemisphere Triacanthella (Figs 1, 6A).
Triacanthella madiba sp. n., aspect and colour after one year in 95% ethanol.
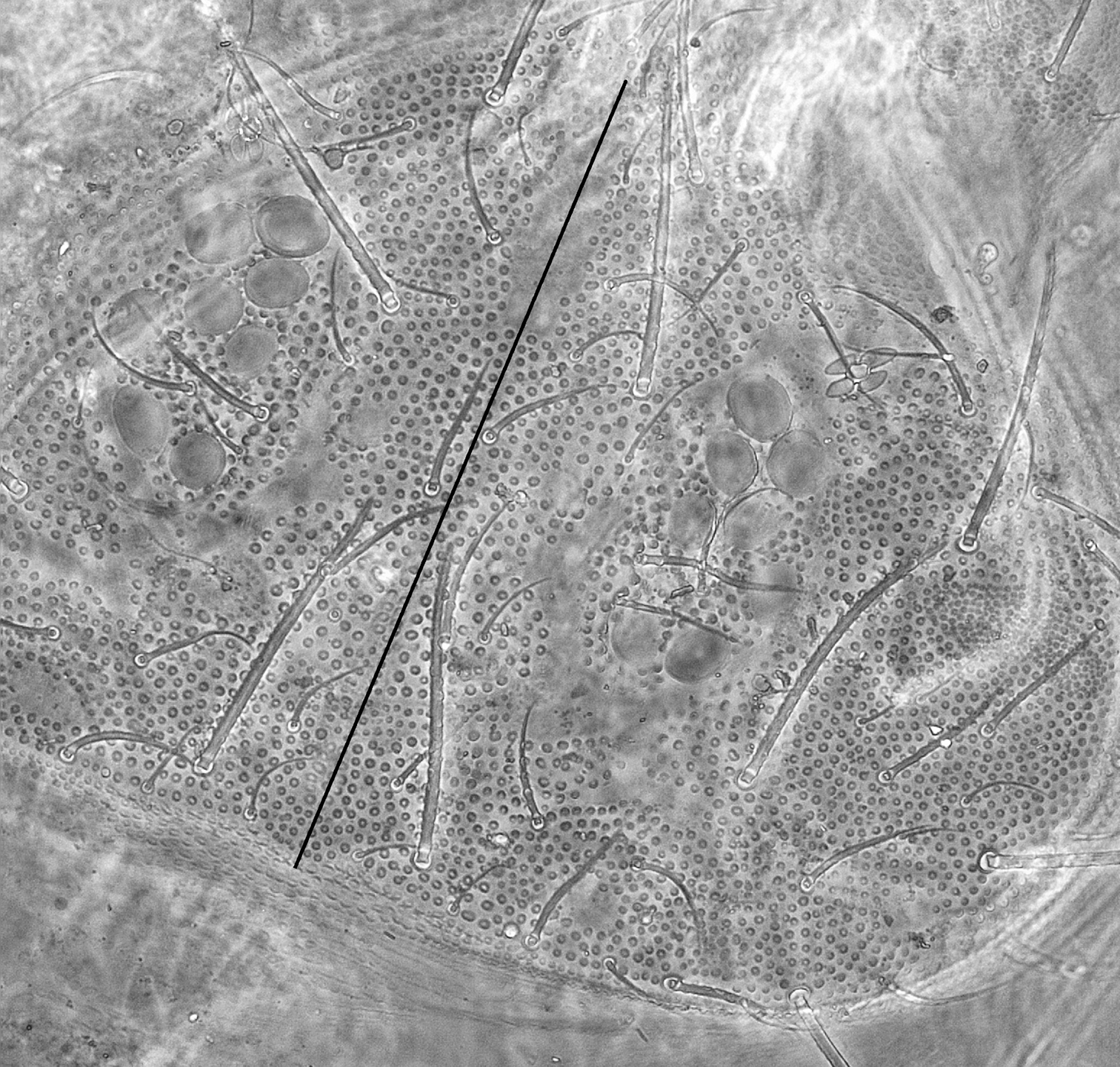
Dorsal integument ornamentation made of hemispherical and rather coarse secondary granules, with large areas devoid of secondary granules on head and tergites (Figs 2–3), symmetrically arranged; most noticeable are the long antero-axial one on head, those associated to classical suture zone of head (Fig. 2), the 1+1 amiboid ones on Th. II-III (Fig. 3A), and the triangular ones between Md and Mdl on Abd. I-III (Figs 3B-C); secondary granules smaller around these areas. Externally to ocular area is a large area where secondary granules are smaller and denser (Fig. 3D). Secondary granules larger along the axial zone (Fig. 3E). No rosette-like arrangement of secondary granules on Abd. VI. Ventral secondary granulation less coarse, more regular. Manubrium with secondary granules arranged in a characteristic linear pattern dorsally (Fig. 3F), and with large areas devoid of secondary granulation ventrally. Pseudopores not seen. Chaetotaxy characterized by a strong heterochaetosis dorsally and a moderate plurichaetosis on most body parts. Chaeta morphology described below, with macrochaetae, mesochaetae and S-chaetae on head and body, and various kinds of chaetae on antennae (Figs 4, 6C). No ordinary microchaetae except on praetarsus and genital plate.
Triacanthella madiba sp. n., dorsal side of head.
Triacanthella madiba sp. n., details of granulation types on dorsal side of the body. A amiboid primary granule area on Th. III B triangular primary granule area on Abd. III C triangular primary granule area on Abd. II, surrounded by smaller secondary granules D detail of the lateral plate of smaller secondary granules on head E axial area of Abd. V, with larger secondary granules between axial chaetae F linear arrangement of secondary granules on the manubrium. Scales: 30 µm.
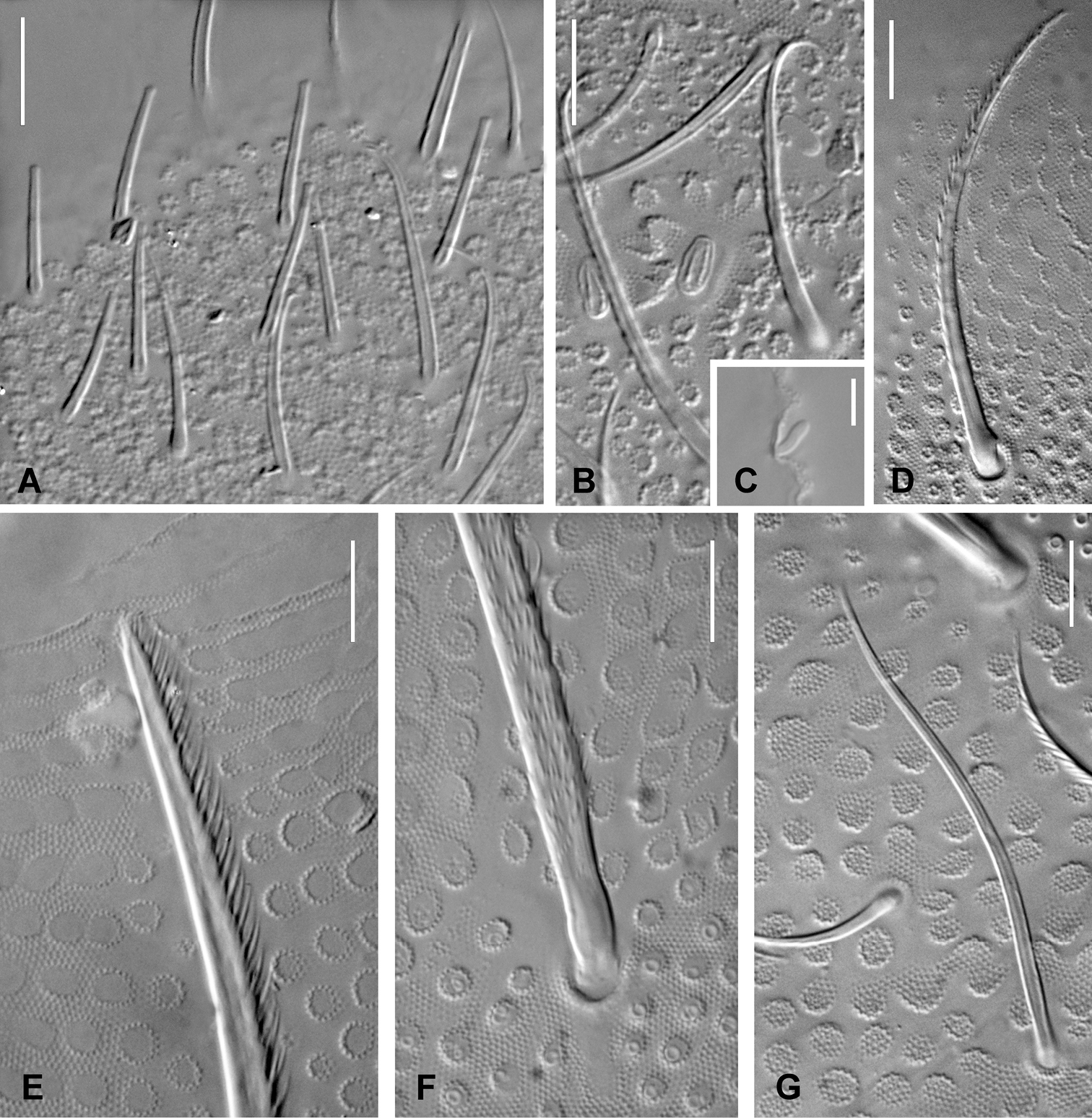
Antennae almost as long as head diagonal. Six kinds of antennal chaetae: i) thickened subcylindrical S-chaetae of medium size (2 on Ant. III and 6 on Ant. IV); (ii) S-microchaetae (3 on Ant. III and 1 on Ant. IV) (Figs 4B–C); (iii) blunt chaetae very similar to the S-chaetae, but longer and usually slightly thinner (on Ant. IV); (iv) acuminate ordinary chaetae of various length, smooth or weakly serrated, 11–12, 13–17 and 26–30 on Ant I-III, a few on Ant. IV (Fig. 4D); (v) thin, straight and smooth truncated chaetae numerous ventrally on Ant. IV (Fig. 4A); (vi) one ventro-distal papillate chaeta. Sensory organ of AIIIO with two short S-chaetae lying in ovoid sockets (S3 and S4, Fig. 4B), two longer guard S-chaetae shorter than nearest mesochaetae (S2 and S5) and one very small dorso-external S-microchaeta (S1); integument granulation significantly coarser between and above S3 and S4 (Fig. 4B). Antennal segment IV with most chaetae as subcylindrical, thickened, blunt S-chaetae, the shortest ones slightly thicker and more bent, including a central group of six; apical bulb trilobed; subapical organite rounded, very small; a short ovoid-elongate S-microchaeta present dorso-externally (Figs 4C, 6B).
Triacanthella madiba sp. n., types of chaetae. A truncated chaetae of the ventral side of Ant. IV B microchaetae S3 and S4 of AIIIO C S-microchaeta of Ant. IV D ciliated chaeta of Ant. III E distal part of a macrochaeta on Abd. I F basal part of a macrochaeta on Abd. III G S-chaeta on Abd. I. Scales: 10 µm (A, B, D, E, F, G); 5 µm (C).
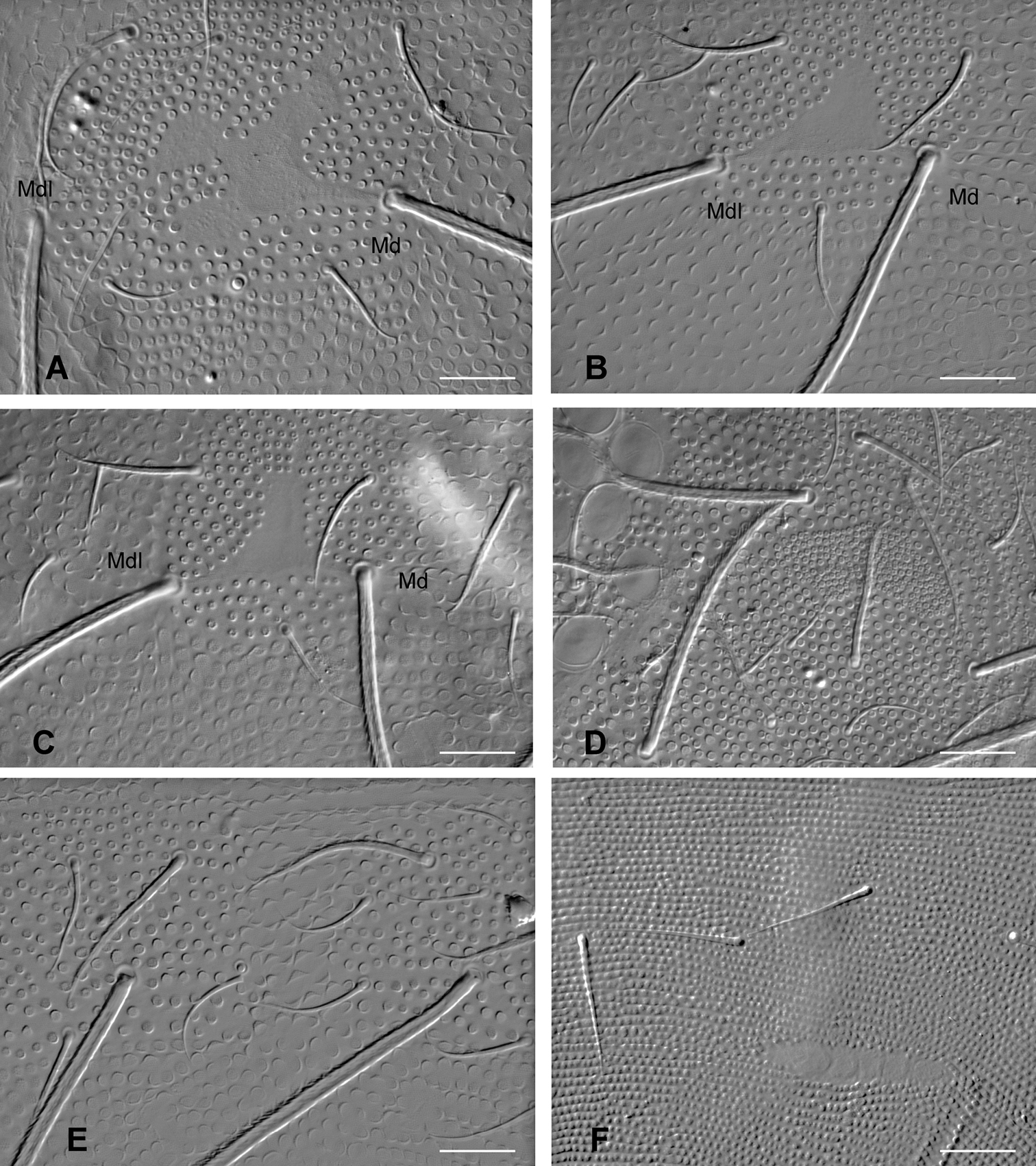
Eight ocelli on each side of the head, equal in size. Postantennal organ nearly equal in size to one ocellus, with 4 subequal vesicles (Fig. 2). Maxilla with a tridentate capitulum, a rounded basal flap and 6 variously fringed or ciliate lamellae (Figs 5C–D). Mandible head with 4 teeth on each side, the basal one slightly smaller on the left than on the right mandible (Figs 5A–B). Labrum chaetotaxy 4/4, 5, 4; labral chaetae distinctly longer than prelabral chaetae; labrum apical edge with a slight medial indentation; distal part with four irregular longitudinal ridges dorsally, and with subapical asymmetrical combs ventrally (Figs 5E–G); labral apical edge hemmed (Fig. 5G). Labium with 5–6 basomedian chaetae, 7 lateral chaetae, and a labial palp characterized by 7–8 proximal chaetae and a reduced number of distal chaetae (Fig. 5J): only 3 papillae, A, B, D; one ordinary chaeta (possibly e4, but with a socket) and 5 short, thickened, hyaline guards (a1, b1, b2, d2, Fig. 5I), with the fifth one probably the lateral process sensu
Triacanthella madiba sp. n., mouthparts. A mandible, right B mandible, left C maxilla head D proximal part of maxilla head, with basal flap bf E, F ventro-distal part of labrum with combs G dorso-distal part of labrum H clypeus I guards b2 and d2 of labial palp J labial palp (lp: lateral process). Scales: 10 µm.
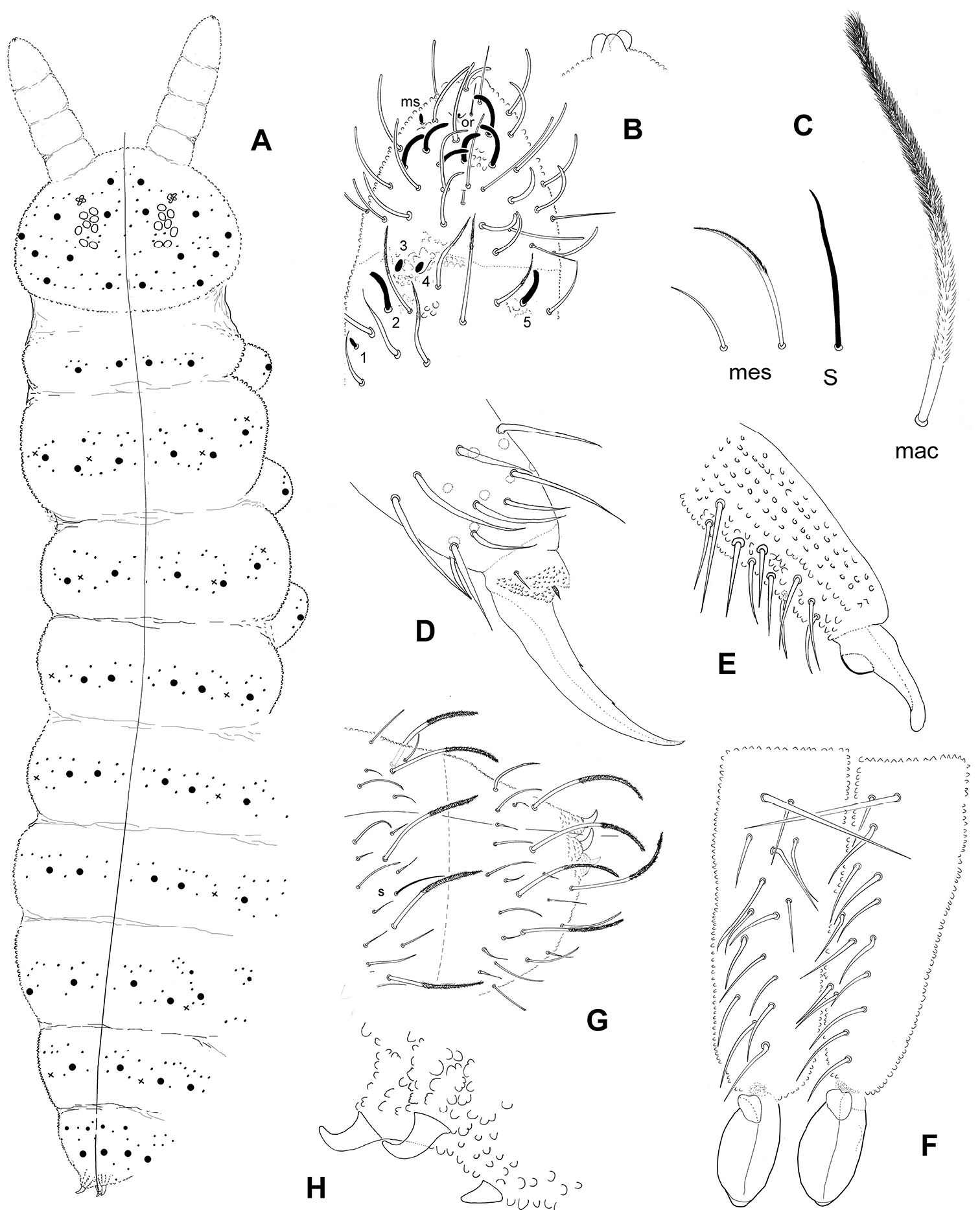
Chaetotaxy of tergites illustrated on Fig. 6A. Dorsal clothing plurichaetotic and heterochaetotic, with frequent asymmetries among shortest mesochaetae. Macrochaetae long, bent, and densely ciliated unilaterally on 2/3 to most of their length; mesochaetae, less bent, acuminate, less strongly ciliated to almost smooth; S-chaetae, thin and smooth, shorter than macrochaetae (Figs 4E–G, 6C). Macrochaetae formula per half-tergite: 8/2, 3, 3/3, 3, 3, 3(4), 3. Number of chaetae between macrochaetae Md per half-tergite: 1-2, 3-5, 3-4/2-3, 2-3, (1)-2, 3, 2-3 with many asymmetries. S-chaetae formula per half-tergite: 0, 2, 2/1, 1, 1, 1, 1; microchaeta ms absent. Abdomen VI chaetotaxy often asymmetrical, with one or two axial short mesochaetae; three anal spines on papillae, the posterior one less than half the length of the other two which are hook-like (Figs 6G–H).
No ventral chaetae on thoracic sternites. Number of ventral chaetae per half-tergite for Abd. II, III: 7, 13–17; anterior furcal subcoxa with 12–16 chaetae. All ventral chaetae are smooth ordinary chaetae. Lateral anal valves with 3 or 4 hr chaetae; upper anal valve with 7–9 hr chaetae.
Leg chaetotaxy slightly plurichaetotic. Trochanter with 7 chaetae. Tibiotarsi I, II, III with (proximal + distal): 8 + 11, 8 + 11, 7 + 11 acuminate chaetae. No clavate tenent hair. Claw with two inner teeth at about 40% and 65% of claw basis, and 1 + 1 latero-distal teeth, appressed on the integument and difficult to see at about 85% of claw basis (Fig. 6D). Empodial appendage short and pointed, internal to empodial apical tubercle according to Fig. 6D, 1+1 small praetarsal microchaetae. Ventral tube with 9–11 + 9–11 latero-distal chaetae, and 1–2 chaetae on each side of the sternite of Abd. I. Tenaculum with 3 + 3 teeth. Dens without ventro-apical lobe, bearing 10–15 chaetae dorsally with fine granulation (secondary granules smaller than chaetal sockets); the basal macrochaeta of the dens is about 2.3 the length of the nearest mesochaeta; well developed mucro with a large lamella and a very distinct dorso-basal heel (Figs 6E–F).
Triacanthella madiba sp. n. A habitus and chaetae distribution of the dorsal side (x: S-chaetae) B Ant. III distal and Ant. IV in dorsal view and detail of the apical bulb C morphology of dorsal chaetae: macrochaeta (mac), mesochaeta (mes), S-chaeta (S) D tibiotarsus and claw of leg III E mucrodens, lateral view F Mucrodens, dorsal view G Abd. V–VI tergites H anal spines.
Triacanthella madiba sp. n. shares numerous characters with Triacanthella vogeli Weiner & Najt, 1997, described from southern Chile. It differs mainly by the ocelli G and H being equal in size to the other ocelli and the absence of rosette-shape tubercles on Abd. VI. It is also morphologically close to Triacanthella andina Cassagnau & Rapoport, 1962 from Argentina, but macrochaetae are less numerous on Abd. I-III "(333 versus 444). In addition, the lamellae of the maxilla are shorter and the papillae bearing the anal spines are not as strong in Triacanthella madiba sp. n., as in Triacanthella andina. Overall, these three species are extremely similar morphologically in spite of being very remote geographically. Triacanthella madiba sp. n. differs from Australian and New-Zealand species by characters pointed out in the key. An additional important character is the chaetotaxy of the distal part of the labial palp, which is similar to that described by Fjellberg for an unidentified species of Australia (
Triacanthella madiba sp. n. is recorded in bat guano in a cave of Table Mountain National Park. This is the first record of the genus Triacanthella in a guano habitat and the first record of the genus for Africa. None of the Triacanthella species recorded so far are found in tropical regions. They are all restricted to temperate zones, where they occur in a large range of habitats in Europe (from xeric Mediterranean to permanently cold), while they are limited to humid and cool litter or surface soil layers in the southern hemisphere (Australia, New Zealand, Chile and Argentina,
The only subterranean records of the genus Triacanthella, include the record of Triacanthella copelandi in a cave in Tennessee (USA), without anymore detail, and a single specimen collected in a small shallow cave of oriental Pyrenees in France, that was described as Triacanthella proxima Delamare 1951, and later synonymised with Triacanthella perfecta. In the area around this last cave, Triacanthella perfecta is actually common in beech forest litter (unpublished observations), and its presence underground as a single specimen is obviously accidental. Conversely, Triacanthella madiba sp. n. occurs abundantly in the guano microhabitat of Wynberg cave and was not found outside in Table Mountain. The species can therefore be considered troglophilic in this area. Actually, Triacanthella madiba sp. n. may have been already recorded as Schaefferia (Typhlogastrura) sp. in
Although the labial palp of Triacanthella madiba sp. n. is similar to the unidentified Australian species (
We dedicate this species to Madiba, former President of South Africa, Nelson Rolihlahla Mandela, who celebrated his 20 years of freedom on 11 February 2010.
We thank the South African Speleological Association for assistance and SANParks for collecting permits, Steven Chown and three anonymous reviewers for comments, and Bettine Jansen van Vuuren for discussion. This work was supported by the France-South Africa grant no. 68652 to L. Deharveng and by DST-NRF Centre of Excellence for Invasion Biology.