(C) 2011 Adam Kwiatkowski. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
During a period of three years (2006–2008) the
carabid fauna in wet and humid forest habitats of different stages of
succession was studied at the Puszcza Knyszynska (north-east part of
Poland). The aim of this study was to determine how the assemblages of
the carabid fauna change in relation to the ongoing process of
succession. Using pitfall traps, 24 plots were sampled. The plots were
located in stands of different age, from two year old plantations to
more than 100 year old forests. Additionally, the stands were ordered
in three moisture classes (wet, humid and very humid) and two classes
of soil richness. As indicators for change in the carabid fauna in
relation to age of the stands Mean Individual Biomass (MIB), species
diversity and share of forest species were used. By applying
multivariate statistics the relation of the different habitat
characteristics to changes in the carabid fauna was examined. During the
study 8903 individuals belonging to 57 species were collected. Pterostichus niger represented 28% of the total catches and therefore the most common species. Another common species, Pterostichus melanarius,
contributed to 13% of the total catch. This species was caught at
every plot, even in the old forests. In contrast to the results
obtained by
Carabidae, Puszcza Knyszyńska, process of succession, wet and humid, forest habitats, Mean Individual Biomass
Carabid beetles represent one of the largest groups of
animals, occurring in almost every type of terrestrial habitat. In
Poland over 500 species of these insects are recorded (
The aim of the study was to analyse how the assemblages
of carabid beetles occurring in wet and humid forest habitats change
with an ongoing process of succession (age of the forest stands). These
changes were expected to be analogous to those observed in other forest
types as pine forests (
However, besides age of the forest stands, other factors like environmental conditions or habitat fragmentation (e.g.
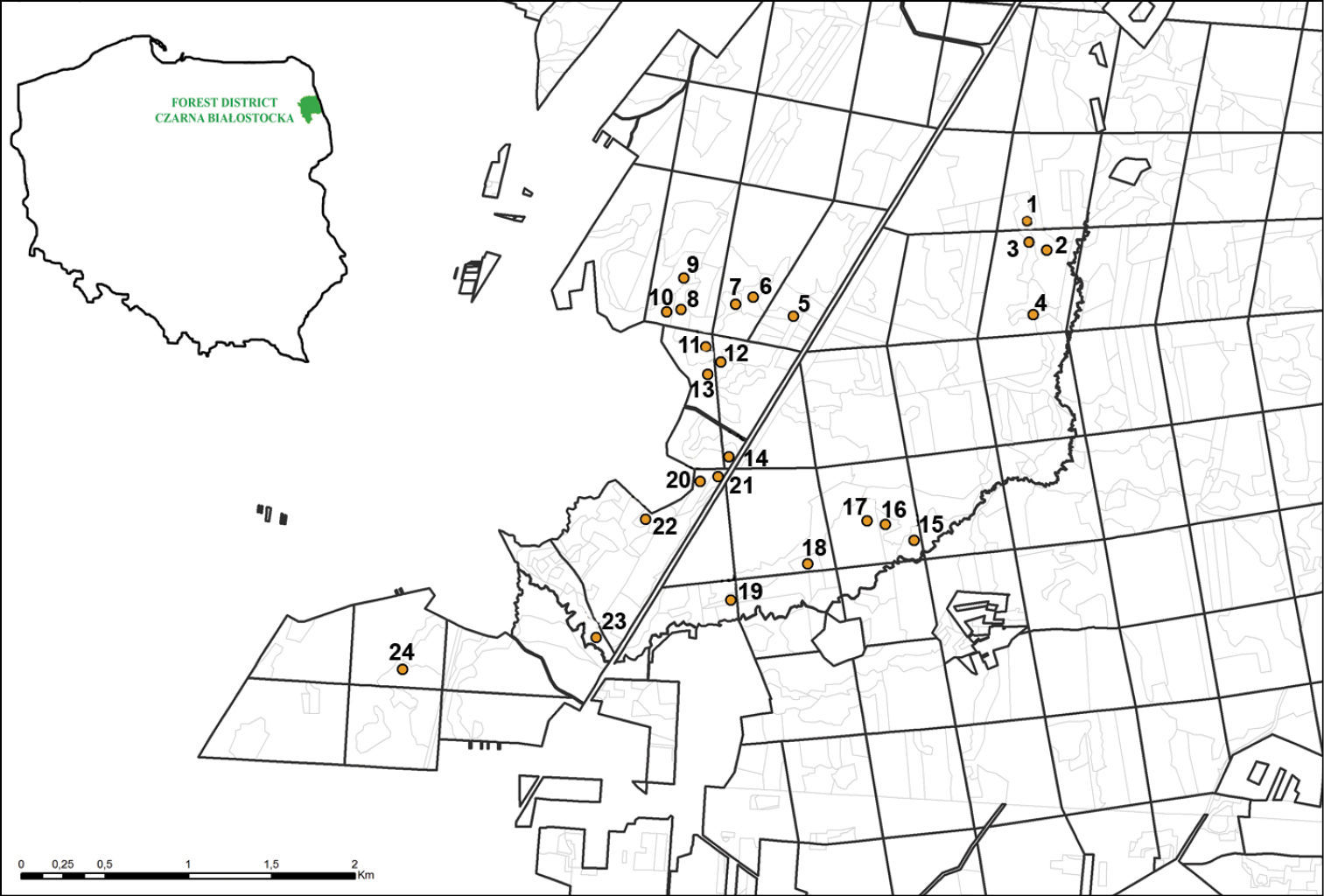
The study was carried out in 24 sampling plots (Fig. 1),
in the Puszcza Knyszyńska forest, located in the forest district
Czarna Białostocka (north-eastern Poland). The sampling plots were
located in independently treated units of the forest of different age
and size, at least 50 m apart. The age of the units ranges from a 2
year old plantation after clear cut to over 100 year old forests and
size ranges from about 1 ha to almost 11 ha. Additionally, the units
were classified with respect to moisture conditions and soil richness (Table 1)
accordingly to the periodical inventories of forest habitats and forest
resources done for the forest district Czarna Białostocka (
Location of the sampling plots in the Puszcza Knyszyńska forest. For specifications, see Table 1.
Characterisation of the studied forest units with respect to size, age, moisture and soil richness. See text for explanations.
| Number of sampling plot | Size (ha) | Age (years) | Moisture | Soil richness |
|---|---|---|---|---|
| 1 | 2.09 | 68 | 1 | 1 |
| 2 | 7.16 | 73 | 2 | 1 |
| 3 | 1.87 | 91 | 1 | 1 |
| 4 | 1.59 | 4 | 1 | 2 |
| 5 | 1.11 | 13 | 1 | 2 |
| 6 | 2.82 | 7 | 3 | 2 |
| 7 | 1.91 | 127 | 3 | 2 |
| 8 | 2.39 | 3 | 3 | 2 |
| 9 | 4.35 | 46 | 1 | 2 |
| 10 | 2.02 | 93 | 3 | 2 |
| 11 | 3.35 | 28 | 3 | 2 |
| 12 | 1.64 | 29 | 1 | 2 |
| 13 | 7.46 | 81 | 1 | 2 |
| 14 | 1.05 | 22 | 2 | 1 |
| 15 | 2.09 | 8 | 2 | 1 |
| 16 | 2.35 | 3 | 1 | 1 |
| 17 | 10.72 | 83 | 2 | 1 |
| 18 | 2.9 | 26 | 3 | 2 |
| 19 | 1.86 | 46 | 1 | 1 |
| 20 | 2.2 | 3 | 2 | 1 |
| 21 | 1.06 | 103 | 2 | 1 |
| 22 | 2.07 | 93 | 2 | 1 |
| 23 | 1.72 | 78 | 3 | 2 |
| 24 | 6.51 | 101 | 1 | 1 |
Carabid beetles were collected using pitfall traps (
At each sampling plot three pitfall traps were situated 5
m apart. Collection of carabids was carried out from 2006-2008, from
mid-May to the end of September and traps were emptied three times
during this period. Carabid beetles were identified using the keys of
For each sampling plot the following parameters of the
carabid fauna were calculated: number of collected species, share of
individuals of typical forest species, share of individuals of big
zoophages, share of individuals of autumn breeding species (
ln y = -8.92804283 + 2.55549621 ´ ln x (eq. 1)
Correlations between these parameters and the age of the
forest sites were tested using the Spearman rank correlation coefficient
(
To analyse the impact of the factors size, age, soil
richness and moisture of the sampling plots, a multivariate analysis
(RDA) was carried out using the CANOCO for Windows software package, v.
4.5 (
During the three years of investigation 8903 individuals, belonging to 57 species of carabid beetles, were collected (Table 2). Seven of these protected by law in Poland (
List of collected species and their ecological
characteristics concerning habitat preference, breeding type and
feeding type. Species are sorted by total abundance and dominance (%)
(nomenclature according to
| Species | Habitat preference | Breeding type | Feeding type | Total abundance | Dominance (%) |
|---|---|---|---|---|---|
| Pterostichus niger | F | A | B | 2472 | 27.78 |
| Pterostichus melanarius | O | A | B | 1219 | 13.70 |
| Pterostichus oblongopunctatus | F | S | S | 599 | 6.73 |
| Oxypselaphus obscurus | F | S | S | 589 | 6.62 |
| Patrobus atrorufus | F | A | S | 486 | 5.46 |
| Carabus granulatus * | E | S | B | 414 | 4.65 |
| Carabus hortensis * | F | A | B | 389 | 4.37 |
| Epaphius secalis | E | A | S | 308 | 3.46 |
| Pterostichus aethiops | F | S | S | 285 | 3.20 |
| Carabus coriaceus * | F | A | B | 269 | 3.02 |
| Cychrus caraboides | F | A | B | 246 | 2.76 |
| Europhilus fuliginosus | F | S | S | 198 | 2.22 |
| Pterostichus strenuus | F | S | S | 164 | 1.84 |
| Carabus glabratus * | F | A | B | 159 | 1.79 |
| Agonum afrum | F | S | S | 142 | 1.60 |
| Platynus assimilis | F | S | S | 92 | 1.03 |
| Calathus micropterus | F | A | S | 86 | 0.97 |
| Carabus nemoralis * | F | S | B | 80 | 0.90 |
| Carabus arvensis * | F | S | B | 67 | 0.75 |
| Pterostichus anthracinus | O | S | S | 60 | 0.67 |
| Poecilus versicolor | O | S | S | 54 | 0.61 |
| Pterostichus nigrita/Pterostichus rhaeticus | E | S | S | 51 | 0.57 |
| Badister lacertosus | F | S | H | 44 | 0.49 |
| Bembidion mannerheimi | O | S | S | 44 | 0.49 |
| Notiophilus palustris | F | S | S | 37 | 0.42 |
| Harpalus latus | E | A | H | 35 | 0.39 |
| Pterostichus vernalis | E | S | S | 31 | 0.35 |
| Stomis pumicatus | E | A | S | 31 | 0.35 |
| Clivina fossor | O | S | S | 29 | 0.33 |
| Loricera pilicornis | E | S | S | 29 | 0.33 |
| Agonum sexpunctatum | E | S | S | 24 | 0.27 |
| Harpalus quadripunctatus | F | S | H | 24 | 0.27 |
| Carabus cancellatus * | O | S | B | 22 | 0.25 |
| Pterostichus minor | E | S | S | 17 | 0.19 |
| Badister sodalis | O | S | H | 16 | 0.18 |
| Bembidion lampros | O | S | S | 14 | 0.16 |
| Leistus terminatus | F | A | S | 12 | 0.13 |
| Amara familiaris | E | S | H | 9 | 0.10 |
| Pseudoophonus rufipes | O | A | H | 9 | 0.10 |
| Synuchus vivalis | E | A | S | 8 | 0.09 |
| Amara lunicollis | E | S | H | 5 | 0.06 |
| Leistus piceus | F | A | S | 5 | 0.06 |
| Amara communis | O | S | H | 3 | 0.03 |
| Amara plebeja | E | S | H | 3 | 0.03 |
| Pterostichus diligens | F | S | S | 3 | 0.03 |
| Dyschirius globosus | E | S | S | 3 | 0.03 |
| Amara curta | O | S | H | 2 | 0.02 |
| Harpalus luteicornis | E | S | H | 2 | 0.02 |
| Nebria brevicollis | F | A | S | 2 | 0.02 |
| Notiophilus biguttatus | F | S | S | 2 | 0.02 |
| Amara similata | O | S | H | 2 | 0.02 |
| Agonum viduum | E | S | S | 2 | 0.02 |
| Amara brunnea | F | A | H | 1 | 0.01 |
| Harpalus anxius | O | S | H | 1 | 0.01 |
| Anisodactylus binotatus | E | S | H | 1 | 0.01 |
| Amara nitida | O | S | H | 1 | 0.01 |
| Platynus krynickii | F | S | S | 1 | 0.01 |
Habitat: F – typical forest species. E – eurytopic species. O – open habitat species
Breed: S – spring breeder. A – autumn breeder
Food: B – large zoophage. S – small zoophage. H – hemizoophage
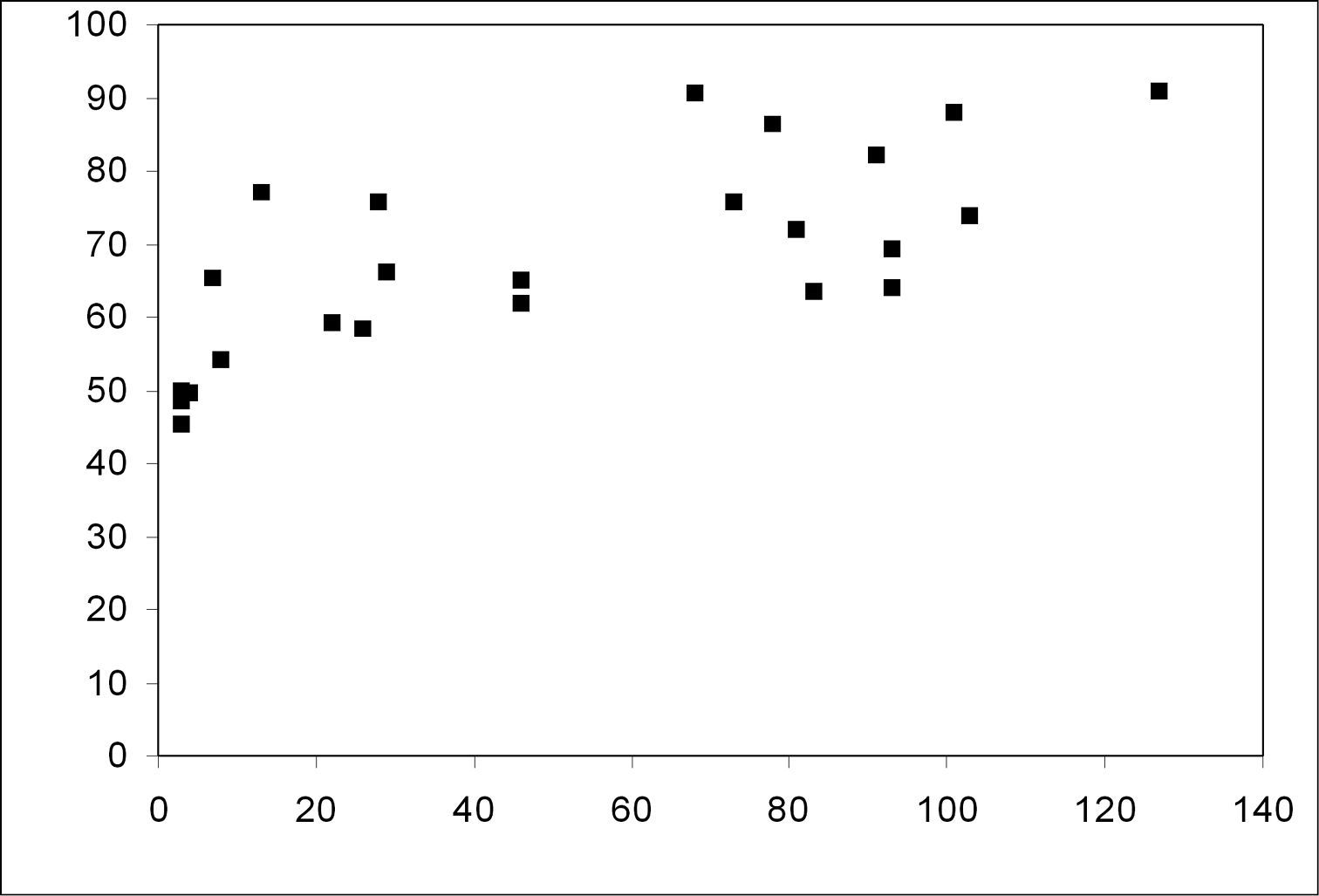
With increasing age of the forest stands the share of forest species increased significantly from below 50% in the young stands to almost 90 % in the older stands (Fig. 2; Spearman rank correlation coefficient rs=0.696, p<0.001). The share of large zoophages also increased significantly (Fig. 3; Spearman rank correlation coefficient rs=0.485, p<0.05), whereas the share of autumn breeders did not show a significant increase with the age of the stands (Spearman rank correlation coefficient rs=0, 371, n.s.). Species numbers remained rather constant with increasing age of the forest stands (Spearman rank correlation coefficient rs=0.18, n.s.). No significant correlation between MIB values and the age of the stands was observed (Spearman rank correlation coefficient rs=0.111, n.s.).
Relationship between the contribution of forest species and the age of sampled forest stands (Spearman rank correlation coefficient rs=0.696; p=0.001)
Relationship between the contribution of large zoophages and age of the sampled forest stands (Spearman rank correlation coefficient rs=0.485; p<0.05)
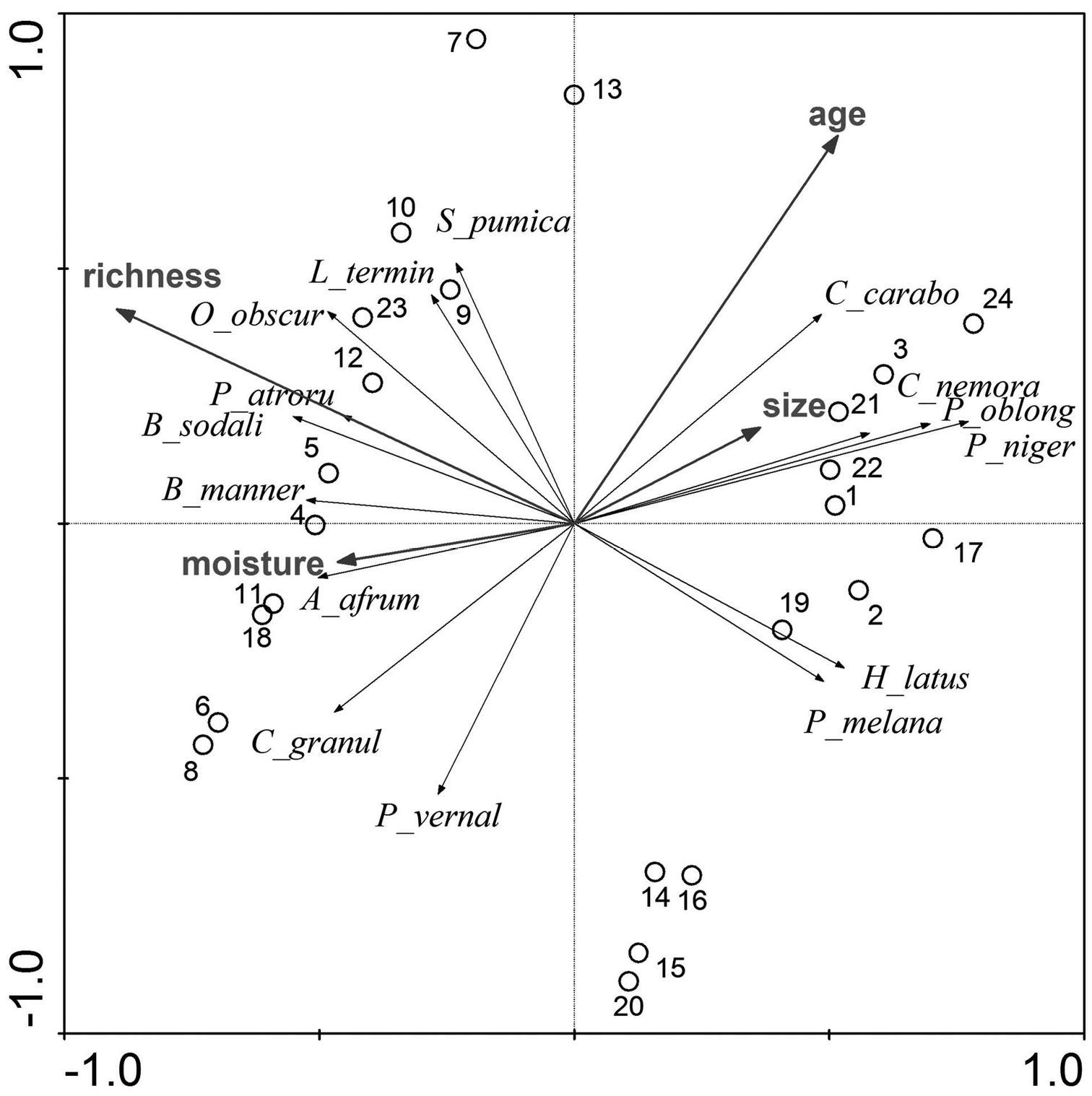
The first two axes of the RDA explain 36.2% of the variance in the species data and 91.5% of the variance in the fitted species data. The diagram (Fig. 4) indicates a stronger impact of age of the stands and soil richness, whereas the impact of moisture and size of the forest stands is less strong. The latter has the lowest impact on the structure of the carabid assemblages among the four studies factors. Age of the forest stand and soil richness are not correlated. Accordingly, the sampling plots are located along the first and second ordination axes concerning their soil richness and age. Sampling plots with very rich soil are located to the left of the diagram, whereas sampling plots of less rich soil are located to the right of the diagram. Younger sampling plots are located in the lower part and the older sampling plots are located on the upper part of the diagram.
Multivariate analysis (RDA) carried out with the dataset (number of the sampling plots as in Table 1). See text for explanations.
The main hypothesis of the study was only partly confirmed. The contribution of forest species and large zoophages to the carabid fauna increased significantly with age of the forest stands, but that of autumn breeders, number of species and MIB values did not. Age of the forest stands and soil richness were more important regarding the structure of carabid assemblages, whereas moisture and size of the forest stands appeared to be of less importance.
This increase of the contribution of forest species and
large zoophages was expected, since it was observed in other studies as
well, for example pine forests and boreal mixed-wood forests (
However, the contribution of autumn breeders and species
numbers did not change significantly, the latter contradicts the
results found for the succession in pine forests (
The results of the study indicate that the degree of
degradation and the process of regeneration of the carabid fauna in wet
and humid forest habitats on organic soil is somewhat different compared
to habitats of dry pine stands. This might be due to the fact that the
forests of the present study grow on very rich, organic soil. After a
clear cut in this type of forests, the method of soil preparation
before planting young trees is different from forests on dry and mineral
soil. The soil of the forests studied in the presented paper was never
ploughed. However, ploughing destroys the litter layer of the habitat,
which is necessary for the occurrence of many species characteristic of
forests (
In the Puszcza Knyszyńska forest, clear cuts in wet
habitats are usually no larger than 3 ha. Even if the size of the forest
seems to have a comparatively low impact on the structure of the
carabid assemblages in the present study, the size and shape of clear
cut areas may be important factors. In order to support species
diversity, it seems to be necessary to avoid creating large-sized open
areas by clear cutting in large forest complexes. In this context the
dispersal power of the respective species is important. For example,
according to
Despite some areas of clear cuts, the Puszcza Knyszyńska
forest as a whole can be regarded as an ancient woodland. However,
these clear cuts may contribute to fragmentation of the ancient forest,
thus being a threat for forest species diversity.
I would like to express my thanks to Dr Axel Schwerk for his friendly help with recognizing species and preparing the manuscript. I also thank two anonymous referees for their comments. This publication is communication no 276 of the Laboratory of Evaluation and Assessment of Natural Resources, Warsaw University of Life Sciences – SGGW.