(C) 2011 Ron Felix. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The discovery of Calodromius bifasciatus in the nature reserve ‘De Kaaistoep’, the Netherlands, initiated research on this and related carabid beetles between 2000 and 2006. During this period we investigated the trunks of 26 Pedunculate oaks, mainly during nightly observations, to learn more about arboricolous carabid species. We observed more than 3000 specimens of 24 carabid species. The majority of these species were Dromius s.l., however Calodromius bifasciatus dominated the dataset. Our data on phenology clearly show that Calodromius bifasciatus is mainly active in winter; it even copulates just above freezing point. Other interesting observations were made as well; for instance the presence of a small sphere at the end of the abdomen and their hiding behaviour at low temperatures. Subsequently, we obtained similar information about other tree dwelling carabid species. In this article we present an overview of all species observed on the trunks, after which we shall focus on the observations made on Calodromius bifasciatus.
arboricolous, Dromius s.l., Laemostenus terricola, phenology, spheres
A decade ago, the discovery of Calodromius bifasciatus (Dejean) on the trunk of a small Pedunculate oak (Quercus robur)
near Tilburg (the Netherlands) was the start of a long term survey of
tree trunks in that area. Until its discovery in the Netherlands, Calodromius bifasciatus was considered a Western Mediterranean species (
A literature survey revealed that occurrence data from Northern France, Italy, Switzerland, Germany and Eastern Europe were based on very old records and could not be checked. Its presence and/or arrival in the Netherlands therefore seems difficult to relate to its currently known occurance. A source area for this species remains unknown. Although Calodromius bifasciatus is macropterous, flight observations are unknown. To obtain more insight into its dispersal behaviour, we placed flight interception traps (window traps) near and under trees where this carabid occurs, pitfall traps in the ground near the base of the trunk (but not beyond the outer range of the crown) but we never caught Calodromius bifasciatus. This was also the case with a frequently used light trap and a malaise trap in the vicinity.
Subsequently, we concentrated on the trunks themselves to learn more about the behaviour and life cycle of Calodromius bifasciatus. During this study we observed many more beetles and other tree-dwelling species (
In this paper we present information on the biology and ecology of Calodromius bifasciatus, as could be gathered by observing and collecting the species from 26 Pendunculate oak trees. Additionally, we provide some results on other observed carabid species of the same tree trunks.
Site description, material and methods De KaaistoepThe nature restoration area ‘De Kaaistoep’ lies
immediately west of Tilburg in the south of the Netherlands. It is a
former agricultural area, belonging to a waterworks company. The actual
research site consists of open arable grasslands on poor sandy soil.
This open area is bordered by woodland in the west, north and east. In
the area itself there are three large and two small artificial pools, a
brook and some patches or rows of deciduous trees and shrubs. Almost in
the middle of these grasslands there are two rows of Pedunculate oaks,
the trunks of which were investigated. One short row (A) runs from
north-north-west to south-south-east and numbers seven oaks. Another,
longer, row (B) runs from south-west to north-east and numbers 19 oaks.
Some of the trees in row B stand alone, this means that their crowns
do not touch other trees. Most of the oaks in row B are bigger and have
lower branches than the oaks in row A. The ground around row B is
covered with shoots of European elder (Sambucus nigra) and American black cherry (Prunus serotina),
grasses and dead twigs and branches of the trees. Many rabbit holes
surround row B. The trunks of row A are more exposed to the sun and wind
than those of row B. In row A the ground around the trunks is only
sparsely vegetated with short grasses, a few mosses and only few tree
branches lie on the ground. All trunks are covered by lichens and algae
and at the base of the trunks mosses are present. The oaks in row A are
much more densely covered by lichens than those of row B. All oaks are
healthy and undamaged. They stand at various distances from each other,
are 15–22 m tall, bear a crown of 10–20 m in diameter and have a trunk
girth of 90–230 cm. More details on ‘De Kaaistoep’ can be found in
We used a non-standard method to collect carabid beetles from the tree trunks: ‘wrapped paper bands’ (Fig. 1). The bands consisted of packing paper, longitudinally rumpled and wrapped around the trunk. They were put on two oak trees (on A5, i.e., the fifth tree from the north in row A; and on B6, i.e., the sixth oak from the west of row B). The bands were placed at about 1.6 m above ground level and were operational for four years. Later we installed additional bands around branches of tree A5 at various heights (4, 6 and 7 m high and close to the trunk, and one at 6 m height but at 4 m distance from the trunk). These additional bands were operational for three years. Every 6–8 weeks the bands were inspected by shedding the paper bit by bit over a white plastic tray. While removing the paper bands the bark underneath was carefully inspected and carabid beetles were identified and afterwards often released on the trunk they originated from.
Paper bands at several heights. Photo: Paul van Wielink
During more than six years, we monitored all 26 oaks
from the base up to about 3 m height, 144 times at night. For more
than two years within that period, the inspections were carried out
nearly every week always on the same day (104 times). We started 1 to 6
hours after sunset; early in summer and in relatively late winter. Each
visit took 35 to 90 minutes, depending on the number of beetles found.
The trunks were illuminated by torch and we counted and noted the
carabid beetles and their behaviour. For Dromius s.l.
species (which includes Dromius, Calodromius, Paradromius and
Philorhizus species), we noted the height and direction on the trunk,
observations on their mating and other behaviour, as well as the
presence of spheres on the tip of their abdomen (apex). Weather
conditions (temperature, direction and strength of the wind, humidity,
presence of fog, etc.) were noted as well. The light of the torches
used was too bright and disturbed the beetles. They immediately tried to
seek a hiding place or even dropped to the ground, so we switched to
using LED lights. Again, almost all carabid beetles were released after
identification. The circumference of each tree was measured at 1.60 m
height, as well as the depth of the clefts in the bark at that height. A
more detailed description of these methods can be found in
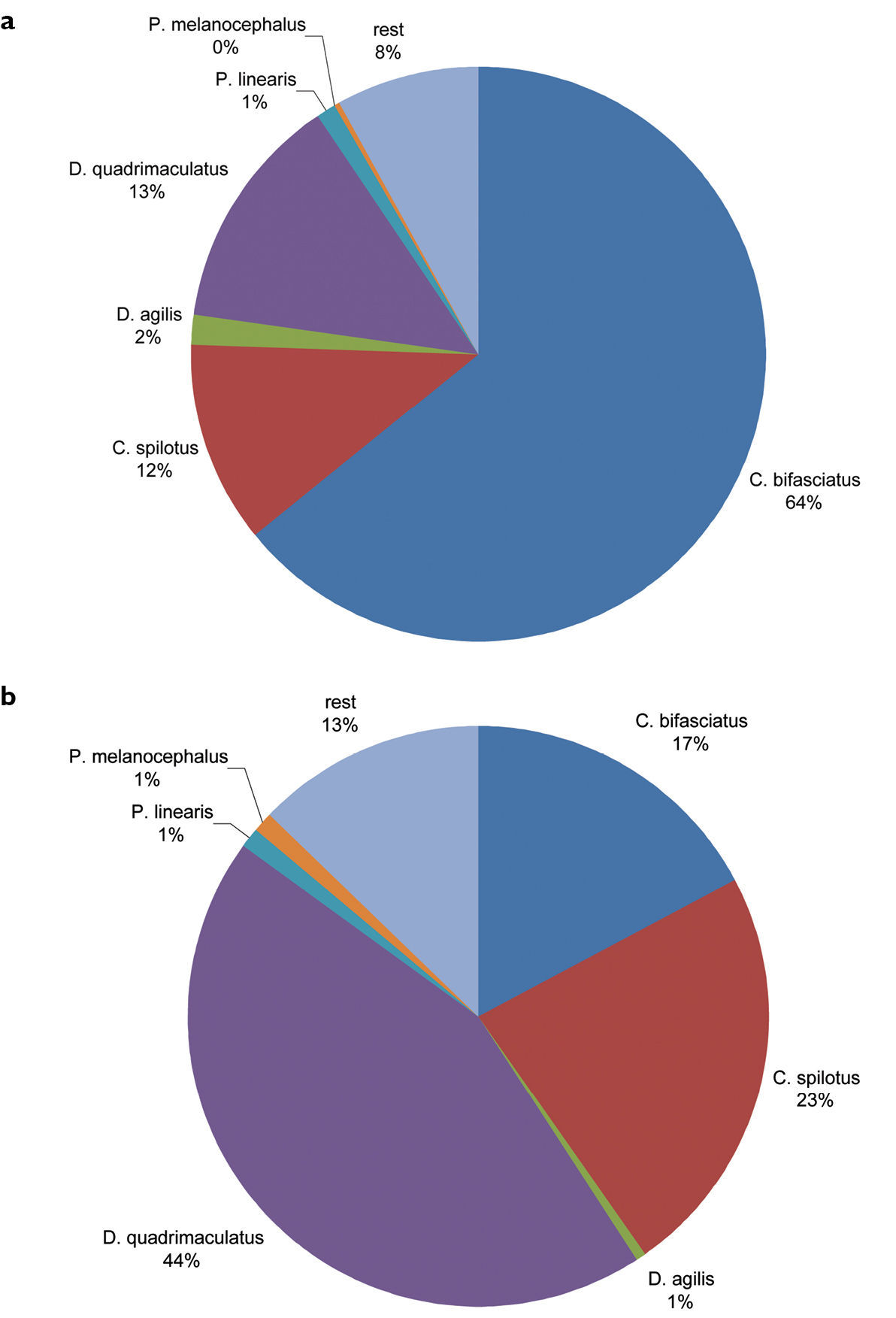
In total, we observed 3069 specimens of 24 carabids beetle species (Table 1). Of all carabid beetles found in the bands, 87% were Dromius s.l. and 17% Calodromius bifasciatus (Fig. 2). Of all carabid species noted during nightly observations, 92% were Dromius s.l. and 64% Calodromius bifasciatus (Fig. 2).
Survey of species and numbers of Carabidae observed at nightly inspections and in or behind bands on oak trees.
| Species | Nightly inspection | In/behind bands |
|---|---|---|
| Carabus problematicus Herbst, 1786 | 18 | 2 |
| Carabus nemoralis Müller, 1764 | 5 | - |
| Leistus rufomarginatus (Duftschmid, 1812) | 4 | - |
| Leistus spinibarbis (Fabricius, 1775) | 44 | 1 |
| Leistus ferrugineus (Linnaeus, 1758) | 8 | 1 |
| Nebria brevicollis (Fabricius, 1792) | 2 | 3 |
| Nebria salina Fairmaire & Laboulbene, 1854 | - | 9 |
| Nebria brevicollis/salina | 13 | - |
| Notiophilus rufipes Curtis, 1829 (larf) | 1 | - |
| Trechus obtusus Erichson, 1837 | 1 | - |
| Bembidion tetracolum Say, 1823 | 1 | - |
| Pterostichus niger (Schaller, 1783) | 1 | 2 |
| Calathus melanocephalus (Linnaeus, 1758) | - | 1 |
| Calathus rotundicollis Dejean, 1828 | 1 | - |
| Laemostenus terricola (Herbst, 1784) | 77 | 27 |
| Limodromus assimilis (Paykull, 1790) | 1 | - |
| Agonum thoreyi Dejean, 1828 | 1 | - |
| Bradycellus harpalinus (Serville, 1821) | 9 | - |
| Bradycellus verbasci (Duftschmid, 1812) | 1 | - |
| Dromius agilis (Fabricius, 1787) | 41 | 2 |
| Dromius quadrimaculatus (Linnaeus, 1758) | 377 | 165 |
| Paradromius linearis (Olivier, 1795) | 38 | 4 |
| Calodromius bifasciatus (Dejean, 1825) | 1654 | 64 |
| Calodromius spilotus (Illiger, 1798) | 378 | 86 |
| Philorhizus melanocephalus (Dejean, 1825) | 10 | 4 |
| larvae undet | 8 | 4 |
| Total number | 2694 | 375 |
Relative abundance ofCarabidaeduring weekly observations at night (a) and in the bands (b). (a) Of the 1903 Carabidaeobserved weekly at night, Dromius s.l. (the Dromius, Calodromius, Paradromius and Philorhizus species mentioned in the graph)accounts for 92% and Calodromius bifasciatus alone for 64%. (b) Of the 373Carabidaeobserved in the bands, Dromius s.l. accounts for 87% and Calodromius bifasciatus for 17%.
We observed six species of Dromius s.l. in and underneath the bands and during the nightly observations: 1718 Calodromius bifasciatus specimens (1654 at night and 64 in the bands), 542 Dromius quadrimaculatus (377 at night and 165 in the bands), 464 Calodromius spilotus (378 at night and 86 in the bands), 43 Dromius agilis (41 at night and 2 in the bands), 42 Paradromius linearis (38 at night and 4 in the bands) and 14 Philorhizus melanocephalus (10 at night and 4 in the bands). It must be noted that these numbers give no reliable indication of population size. Because we seldom collected specimens, many specimens were probably counted several times.
On some trees we always saw more Calodromius bifasciatus than on other trees. We counted the number of specimens we saw on every tree. We computed the amount of square meters of a trunk up to 2.5 m of the tree and the average number of specimens per square meter of that part of the trunk, and there was no relation between the circumference of the tree and the number of specimens. We also counted every Calodromius spilotus and Dromius quadrimaculatus observed. There was no correlation between the numbers of the three carabid species on the separate trees. For results concerning these observations we refer to a previous publication (Felix & Van Wielink 2008). On some trees we saw many of ants (Formicidae) or isopods (Isopoda), and some trees carried more algae, lichens or mosses than others. We never quantified these phenomena, but we have gained the impression that there is also no relation between the abundance of the mentioned species and Calodromius bifasciatus.
The four most abundant species Dromius s.l., Calodromius bifasciatus, Calodromius spilotus and Dromius quadrimaculatus were also present in the band on a branch at 6 m height and at 4 m from the main trunk but in far lower quantities than in the other bands. All specimens of Dromius agilis were found in row B. Perhaps the sheltered position of the trunks in this row explains this observation.
Phenology of Calodromius bifasciatus and Dromius s.l. speciesCalodromius bifasciatus
was active on the bark at an air temperature between -3.5 and 17°C,
and mostly in the range 4–8°C. The maximum number of specimens we
observed on one single evening was 85 on the 20th November and the 27th
of December 2003. The temperatures were 8°C and 6°C respectively, the
wind was southwest, strength 4 and 3. During both evenings the
atmosphere was humid without rain or wet trunks.
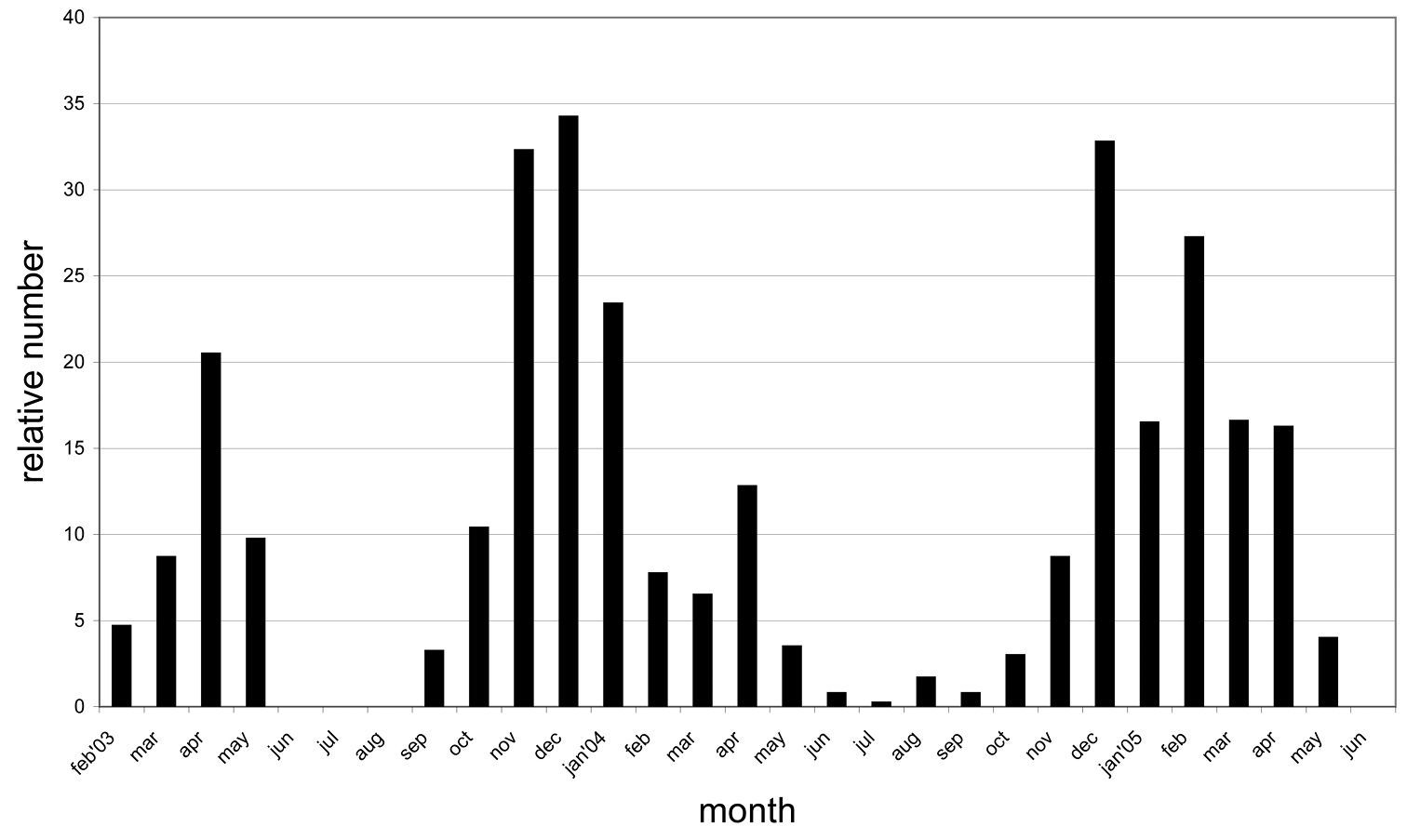
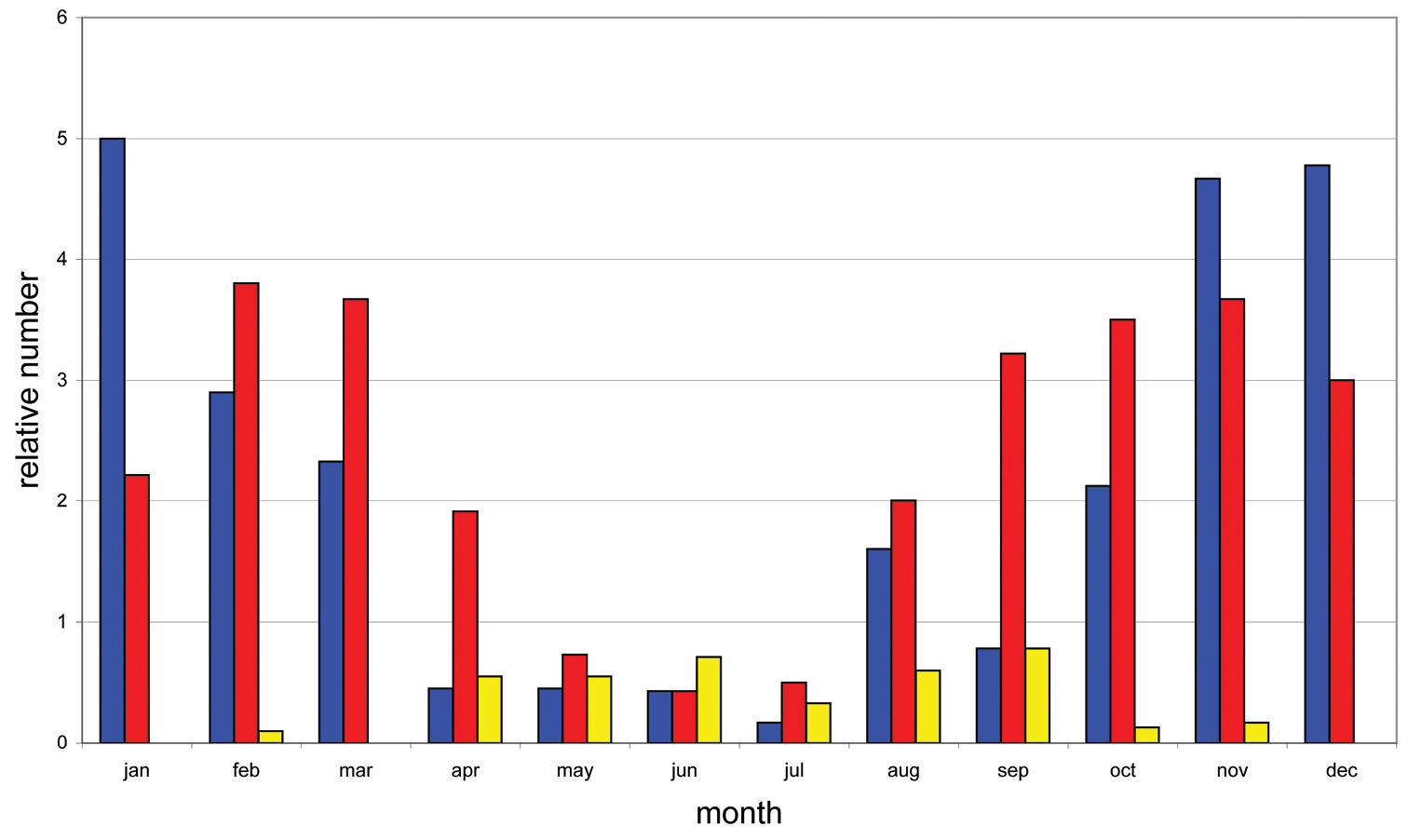
Based on weekly observations at night on the lower 3 m of the trunks during two years we can present the phenology of Calodromius bifasciatus (Fig. 3). Its main activity takes place in winter: observation periods November 2003 to January 2004 and December 2004 to February 2005. In summer, this species was almost absent. Dromius quadrimaculatus and Calodromius spilotus have their optimum from September to March (Fig. 4). Dromius agilis was seen in far lower numbers, and practically only from April to September (Fig. 4).
Phenology of Calodromius bifasciatus: relative presence in 29 consecutive months. Relative presence: the number of beetles per month divided by the number of nightly observations during that month (the number of nightly observations varied between 2 and 4 per month).
Phenology of Calodromius spilotus, Dromius quadrimaculatus and Dromius agilis: relative average presence per month. Relative presence: see Fig. 3. Blue: Calodromius spilotus, Red: Dromius quadrimaculatus, Yellow: Dromius agilis.
During the 144 nightly observations, we noted 63 copula in 1654 Calodromius bifasciatus of which there were 46 copula amongst 1219 specimens during the 104 weekly observations. Copula were seen during the whole activity period from October to April, and at temperature ranges of -1 to 17°C. During the evening, eight copula were seen; 21% of the observed specimens. The temperature that evening was 8°C and humidity was high. Also Calodromius spilotus was seen copulating in winter.
We never found larvae, neither on the trunks at night, nor in the bands. However we found two freshly emerged specimens of Calodromius bifasciatus on a trunk on the 24th August 2001, indicating recent pupation at this time and location.
Observing Calodromius bifasciatus, we noticed ‘spheres’ on the abdomen of females (Fig. 5). These spheres were different in size, from about 0.3 to 1.0 mm. Their outside is granulated with lichens or algae and the inner side is very smooth. These spheres are probably egg-cases (see discussion). We collected a few females with spheres and almost always they dropped these structures rapidly. We noted 69 females with a smaller or greater sphere during the 144 nightly observations. In the 104 weekly observations there were 60 of them, about 5% of the observed specimens. The spheres were almost exclusively seen from November to May. A few times we even saw matings while the female was bearing a sphere. The spheres were seen during nights when the temperature was between 3 and 15°C, so within the normal range of activity and copula. We also noted behaviour that indicated how Calodromius bifasciatus makes these spheres. We found Calodromius bifasciatus specimens biting algae or lichens, then stepping forward and rubbing the tip of the abdomen over the spot where they had just bitten. While biting, the abdomen was directed upwards and the hind legs were stretched. While rubbing their abdomen against the lichens, the posture was reverse. Sometimes the specimens had a sphere on their abdomen, sometimes they did not. When we saw this behaviour, the temperatures ranged from 3 to 13°C and air humidity was usually high.
Abdomen with sternites of Calodromius bifasciatus with a sphere. Note the granulate outside of the sphere. Photo: Ron Felix.
Dromius s.l. species were by far the most abundant Carabidae on the trunks. In addition, 23 other species of Carabidae were observed, most of them in very low numbers (Table 1). Some genera were often found on the trunk, whether in sight at night, or in/underneath the paper bands: Carabus s.l. (especially Carabus problematicus) Leistus s.str. (especially Leistus spinibarbis), Nebria s.str. and Laemostenus terricola. With some of these species, it was previously unknown that they climbed trees. For example, Paradromius linearis and Philorhizus melanocephalus were known as ‘strictly ground dwelling’ (
Dromius s.l.
species are flat (but relatively broad) and are built to hide in very
narrow places. It probably moves all over the trees when it is dark and
during daytime it stays in the lower parts, where hiding is easier.
Trunks with (at least partially) a structure of many fine and narrow
clefts (in the lower parts) would be more appreciated by Calodromius bifasciatus and other tree-living Dromius s.l. species than trunks with deep, but open and wide clefts.
Calodromius bifasciatus
seems to avoid contact with the ground. We hardly ever found them in
pitfall traps that were placed in the close surroundings of the
investigated trees and we did not find them in the soil after digging at
the foot of the tree either. Once we observed an agglomeration at the
base of a tree. This agglomeration was observed during a very cold,
freezing night, and was located about 1 cm below the soil surface,
but it was still on the bark. On the other hand,
The common opinion seems to be that Dromius s.l. species are not seen on the lower parts of the tree in summer, because they are high up in the crown (
The behaviour of biting algae and sphere building has not been recorded before in Europe.
During the nightly observations many copula of Calodromius bifasciatus were seen during the whole activity period from October to April, during nights with temperatures ranging between -1 to 17 °C. Nightly observations on tree trunks, especially in autumn, winter and spring, can thus be very rewarding in observing ecological phenomena of corticolous Carabidae.
We like to thank Henk Spijkers for his participation and creative suggestions at the beginning of the project.