(C) 2011 Robert Blakemore. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Current descriptions add natives Aporodrilus aotea sp. n., Aporodrilus ponga sp. n. and Notoscolex repanga sp. n., plus new exotic records to the numbers of megadrile earthworms known from New Zealand, which are now raised from 193 to 222 species in five families, viz: Acanthodrilidae, Octochaetidae and Megascolecidae, plus Lumbricidae and Glossoscolecidae for exotics. Overlooked spermathecal diverticula have been located for Notoscolex equestris Benham, 1942 and for Megascolex animae Lee, 1959 and non-tubular prostrates were misconstrued as tubular in Megascolides tasmani Lee, 1959. Of these latter three species, a lectotype is designated for Notoscolex equestris and holotypes of the other two are briefly redescribed. Whereas Megascolides tasmani now belongs in Notoscolex Fletcher, 1887 and Megascolides animae belongs in Anisochaeta Beddard, 1890, further lack of dorsal pores in Notoscolex equestris as with Notoscolex esculentus (Benham, 1904) and Notoscolex mortenseni (Michaelsen, 1924) newly qualifies all three as additional combs. novae in primarily Tasmanian genus Aporodrilus Blakemore, 2000.
Eco-taxonomy, Annelida: Oligochaeta, new taxa, ICZN, island biodiversity
The definitive earthworm study completed 50 years earlier (
For natives, few had subsequent reports and because of this approximately 77 were automatically listed as “Threatened” or “Endangered” in the Department of Conservation (DoC) threatened species list (
A study by
Changes invoked by
• Restatement of validity of Acanthodrilidae, Octochaetidae and Megascolecidae (plus Exxidae at one time thought from NZ) as separate families.
• Neoendemic Microscolex macquariensis (Beddard, 1896) is removed as Macquarie Island is now claimed by Australia (see
• Because Rhododrilus disparatus Lee is meroic it was transferred as a new combination in Leucodrilus Lee by Blakemore (
• Octochaetus was proven to have native Australian representatives too, e.g. the native Octochaetus ambrosensis (Blakemore, 1997) and similar species in Queensland where Adroitplema Blakemore, 2006 (nom. n. pro Neodiplotrema Dyne, 1997 non Yamaguchi, 1938) is now a junior synonym (see
• Sylvodrilus Lee is retained as the type is anisochaetine, i.e., classed as non-lumbricine (cf. Eudinodriloides).
• Plutellus Perrier species are transferred to Graliophilus Jamieson which is said to have tubular prostates (as “flattened tubes”) in its type species; those species having non-tubular prostates more appropriately belong in Zacharius Blakemore, 1997.
• Megascolides McCoy, 1878 is retained, although species with non-tubular prostates are returned or reallocated to Notoscolex Fletcher, 1886/7 for which its junior synonyms are: Tokea Benham, 1904; ?Nelloscolex Gates, 1939; ?Lennoscolex Gates, 1960; Pseudonotoscolex Jamieson, 1971; Pseudocryptodrilus : Jamieson, 1974, 2000 (part. cf. Megascolides); Oreoscolex Jamieson, 1973; Araucaridrilus, Jamieson, 2000;?Plutelloides Jamieson, 2000 (but cf. Megascolides) – synonyms from
• Endemic Perionyx Perrier, 1872 spp go into originally defined Perionychella Michaelsen, 1907 [syn. Terrisswalkerius – for its putative type Perichaeta canaliculata Fletcher, 1887 and similar species with non-tubular prostates – see
• Diporochaeta Beddard, 1890 is retained with its original definition [including the balance of Terrisswalkerius spp (part. – but not type or other species with non-tubular prostates – see
• Perionychella shoeana (Cognetti, 1912) position is rendered uncertain by its original description as: “Each prostate is a tongue-shaped body, not divided into lobes” being revised by Lee’s (1959: 325) inspection of new material (the type not being located) to “Prostates short tongue-shaped organs, projecting laterally through xviii [18], surrounded by thin sheath and each consisting of a number of distinct lobes”. Nevertheless, having non-tubular prostates qualify it for Perionychella; cf.
• Megascolex Templeton, 1844 species from Australia and New Zealand are now placed in Anisochaeta Beddard, 1890 for which Trichaeta Spencer, Spenceriella Michaelsen, Gemascolex Edmonds & Jamieson, Pericryptodrilus and Propheretima Jamieson are junior synonyms (see
• Species having tubular prostates and previously placed in Spenceriella (the neotype of which was stated to have racemose prostates, although this is possibly a mistake - see
• Monotypic Eudinodriloides Lee, 1959 was placed under Decachaetus Lee, 1959 in
•
• All “Michaelesen (1923)” species should be changed to
Specimens were sketched, dissected and described under low power microscope using the techniques and conventions noted in
urn:lsid:zoobank.org:act:F014AE5E-D434-4119-B949-E5AB86DB1E4E
http://species-id.net/wiki/Aporodrilus_aotea
Fig. 1Holotype Auckland Museum; AMNZ 5254. Single complete specimen, now dissected, from New Zealand, Great Barrier Island, Little Windy Hill (ca. 36°10'S, 175°23'E). Coll: 2.IX.2001, J.W. Early & R.F. Gilbert. “Under rock on forest floor. L11002”. “W-025” on lid. (Small tissue sample was taken for DNA analysis - code RJB09).
Etymology. After Maori name for Great Barrier Island; Aporodrilus is treated as masculine but this place name remains genderless as a noun in apposition.
Diagnosis. Aporodrilus having spermathecal pores paired segmentally in 6, 7 and 8; holandry with seminal vesicles in 9 and 12; oesophageal glands annular in 10–14; large genital markings paired in 17/18 and 18/19 on either side of male pores.
Body circular tapering at both ends. Dark, matt grayish pigment with iridescent cuticular sheen; paler intersegments and setal auriolae. Length 140 mm with 75 segments. Prostomium epilobous. Setae lumbricine, 8 per segment in rows becoming increasingly irregular further back. Clitellum not well marked. Dorsal pores absent. Nephropores not found (meroic). Spermathecal pores segmental, equatorial just below setae a on 6, 7 and 8. Female pores mid-ventral pair anteriormedian to setae a on 14. Male and prostatic pores combined on tiny mounds on 18 in position of deleted setae a. Penial setae not found. Genital markings large, longitudinally symmetrical pads, paired in 17/18 and smaller in 18/19.
Aporodrilus aotea sp. n. ventral view with dorsal view of epilobous prostomium, spermathecae, prostate and gizzard in 5 in situ; and lumbricine setal ratios on 12–14; plus lateral view of tail end. [Boxed spermatheca is for comparison of Aporodrilus esculentus (Benham, 1904) from Benham’s fig. 67 and from
Pharyngeal mass to 4. Septa 4/5-10/11 thin, only 11/12/13 with slight thickening and thereafter membranous. Gizzard strong and elongate apparently in 6-7 but discernable in 5 by tracing septum 5/6 to near base despite dorsal-wards displacement. Dorsal blood vessel single; hearts paired and increasingly large in 9-13; supra-oesophageal vessel in 10-13. Nephridia meroic with forests of avesiculate tubules on body wall. Spermathecae in 7, 8 and 9 each with elongate, flaccid ampulla and single, small, clavate diverticulum (inseminated) near base implicated with anterior septum which is transgressed. Holandric: minute funnels in 10 and 11 ventrally; seminal vesicles paired, racemose posteriorly in 9 and anteriorly in 12. Ovaries paired as free egg-string bunches ventrally in 13; ovisacs not found. Prostates tubuloracemose extending to ca. 22 from small flaccid ducts to male pores in 18. Oesophagus with oesophageal glands small in 10 and larger in 11-13 then small again in 14; glands more saccular than composite but dilated compared to extraneous oesophageal width. Intestinal origin in 16. Typhlosole and caeca not found (absent). Gut contains fine colloidal reddish soil.
Lack of dorsal pores is usually associated with aquatic habitat, but possibly also with high rainfall/soil-moisture, however, the strong gizzard suggests a loamy diet. Further ecological and/or behavioural information is wanting.
Aporodrilus aotea compares with Aporodrilus mortenseni (Michaelsen, 1924) that differs, not least, by having its three pairs of spermathecal pores intersegmental in 6/7/8/9 and by lacking genital markings. However, in the review by
urn:lsid:zoobank.org:act:C7CD2EDF-0B2A-4EEA-A34B-4CF06F927A04
http://species-id.net/wiki/Aporodrilus_ponga
Fig. 2a, 2bHolotype Auckland Museum; AMNZ 5255. Single mature, posterior amputee rather poorly preserved from Waitakere Ranges, Waiatarua. Coll: 9.V.1995, G. Ripley. “Nikau/Ponga forest L761”; “W-012” on lid. (Small tissue sample was taken for DNA analysis coded RJB10). [Two other specimens from the same jar are a posterior portion of a worm (AMNZ 5256) matching the dimensions and frayed edge of the current specimen is itself missing its tip; the other (AMNZ 5254) is a large mature, anterior amputee that is certainly different and probably a new species but which is inadequate for formal description here].
After Maori name for silver fern Cyathea dealbata (G. Forster) Swartz, 1801, from the habitat detail and also the symbol commemorating the All Blacks victory in 2011 Rugby World Cup; Aporodrilus is masculine, but a noun in apposition is genderless.
Aporodrilus having spermathecal pores paired intersegmentally in 7/8/9; metandric with seminal vesicles in 12; no oesophageal glands; genital marking as a distinct pad in 17/18 with male pores on lower rim replacing setae a.
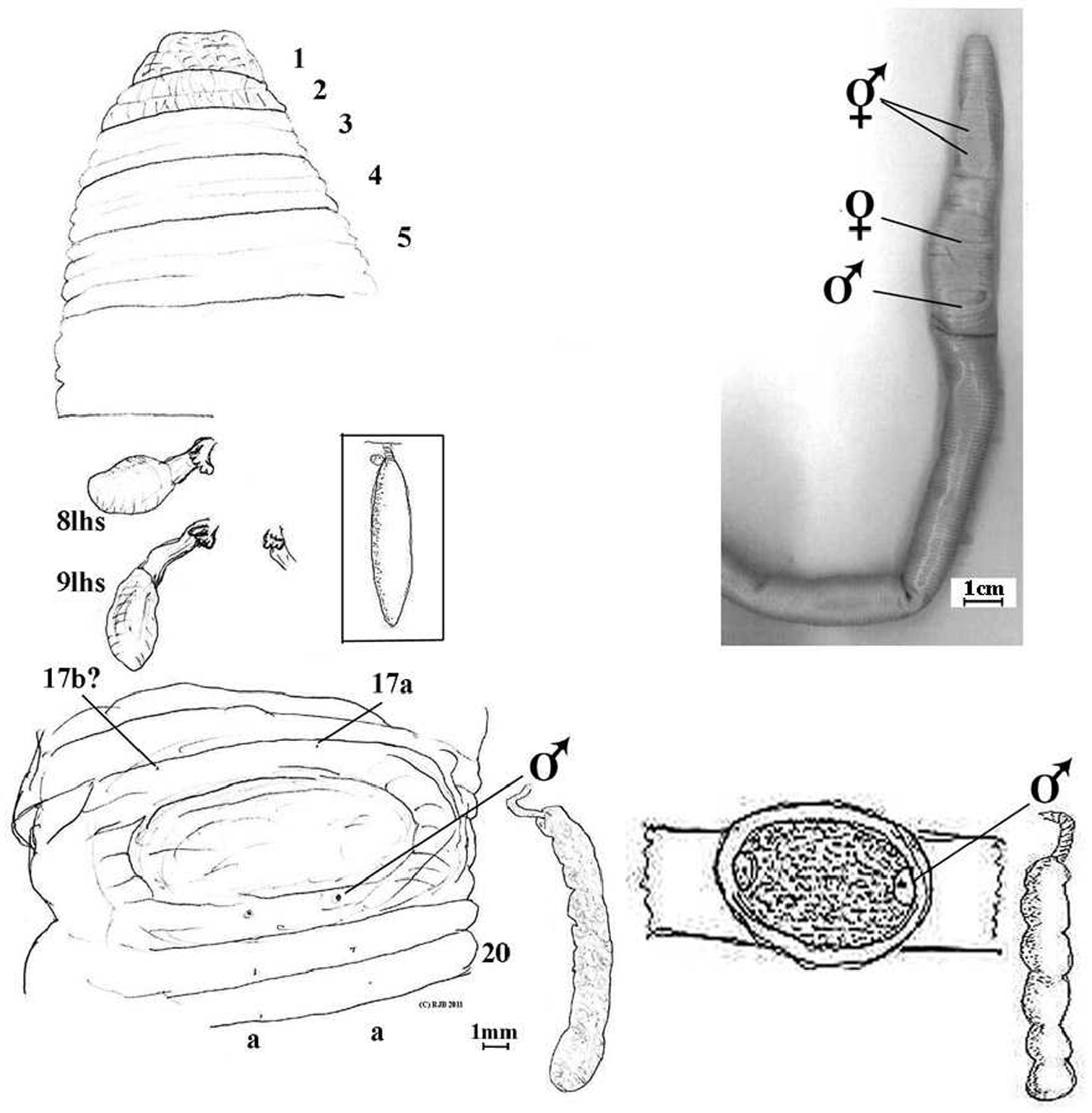
Body robust, dorsally canaliculated in parts before amputation. Pale putty coloured in alcohol. Length 220+ mm anterior portion (a posterior fragment in jar is also 220mm and if from same specimen would give length = 440 mm). Prostomium much wrinkled prolobous. Setae lumbricine, obscure in anterior and mostly occluded on clitellum apparently converging towards male pores; further back the rows except for setal a lines become progressively irregular. Clitellum slightly more tumid and yellowy in ½13–17 (or thereabouts). Dorsal pores absent. Nephropores absent (meroic). Spermathecal pores intersegmental, detected by probe from interior and approximately in setal a lines in 7/8/9. Female pores large paired on 14 (setae obscure) in line with setae a of 13. Male pores superficial on 18 in place of deleted setae a on bottom rim of pad (detected by probe internally). Penial setae not found. Genital marking as a large pad in 17/18 distending both adjacent segments.
Aporodrilus ponga ventral scan of Holotype (colour).
Aporodrilus ponga dorsal view of prolobous prostomium, spermathecae (8lhs and 9lhs and part of 9rhs) and prostate in 18lhs in situ. Male field is shown with setae 17b? and 17a marked (setae a occluded by male pores on 18). [Boxed spermatheca of Notoscolex hakeaphilus Benham, 1949, with Benham’s sketch of its male field and prostate shown for comparison].
Septa and pharyngeal mass absent before 5, septa 5/6–12/13 greatly thickened, thereafter membranous. Gizzard mucular barrel in 5. Dorsal blood vessel single; hearts sinuous in 9–13. Nephridia meroic forests on body wall. Spermathecae paired in 8 and 9 each with flask-shaped ampulla on equally long flat duct with multilocular diverticular frill (inseminated) near base. Probably metandric as paired seminal vesicle seen in 12 only. Testis and ovaries not located, probably minute and lost in musculature of septa and body wall. Prostates rounded but finely incised throughout so not as found in Acanthodrilidae and Octochaetidae (cf. Exxidae), i.e. tubuloracemose with small flaccid ducts in 18. Oesophagus without noticeable dilations (what I initially took as a hemispherical thickening of posterior of 9 was determined as a septum). Intestine substantial yet dilated and easily ruptured, origin appears in 15 or 16. Gut contains finely ground organic matter, organic soil plus coarse multi-coloured grits.
Anterior musculature and thickened septa are associated with strong burrowing, and lack of (anterior) dorsal pores may aid maintenance of hydroskeletal turgor pressure.
Aporodrilus ponga differs from Aporodrilus aotea on almost each specific point. According to
Without information to the contrary we must reluctantly accept the balance of Benham’s earlier diagnosis, in which case a new name for this specimen has merit. Confirmation of independence of either species now depends on reinspection of Benham’s type, apparently beyond the brief, budget and resources of successive workers for the last 62 years, including the present one.
urn:lsid:zoobank.org:act:796E44B7-47A8-4D10-B491-8BBA8B6A85C3
http://species-id.net/wiki/Notoscolex_repanga
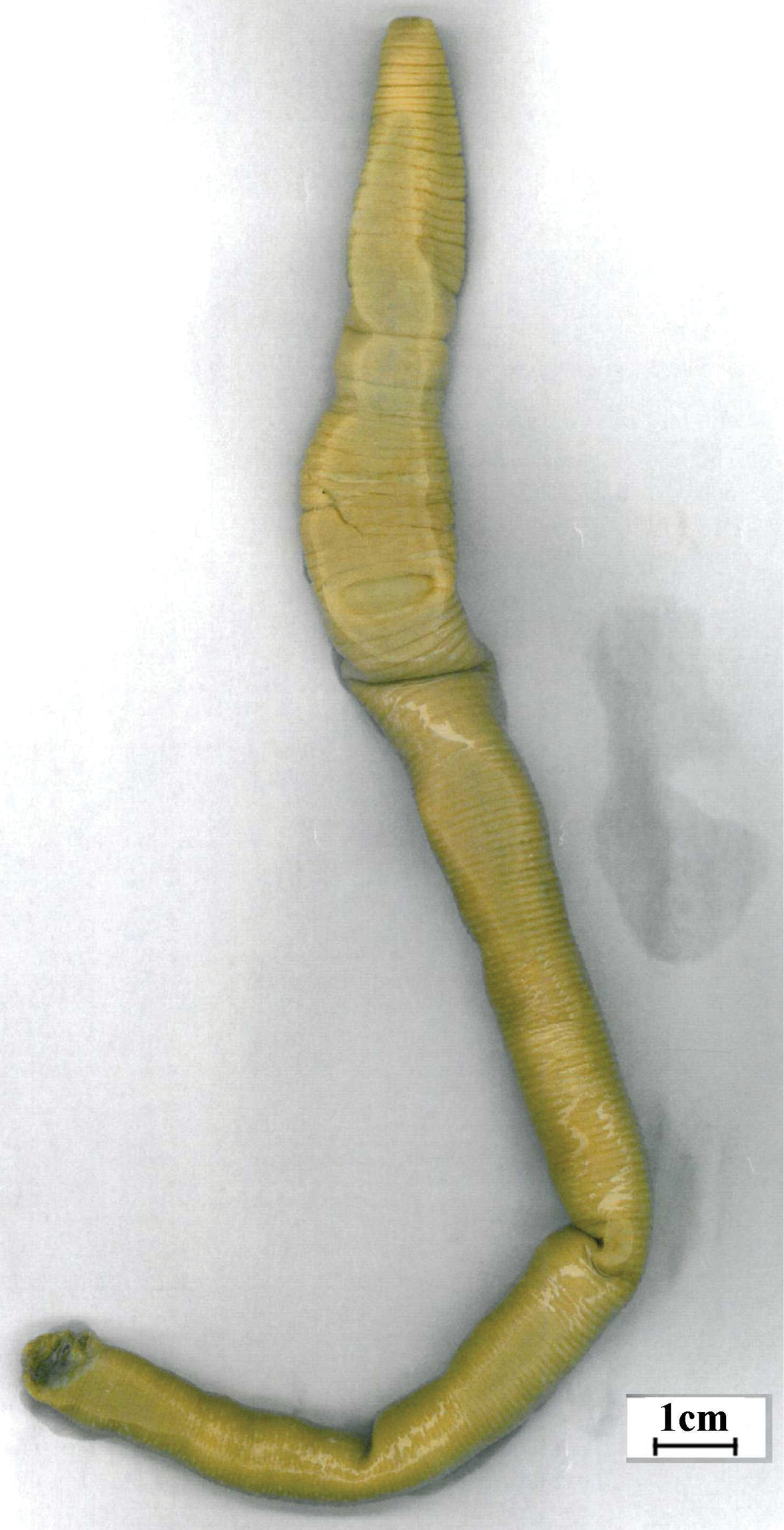
Fig. 3Holotype Auckland Museum; AMNZ 5253. Single complete specimen, now dissected, from New Zealand, Cuvier Island (36°26'S, 175°46'E) SE catchment 40–60 m. Coll: 3.IV.2000, J.W. Early & R.F. Gilbert. “Under rock in stream bed. L8229” “W-024” on lid. (Small tissue sample was taken for DNA analysis coded RJB07).
After Maori name for Cuvier Island; Notoscolex is treated as masculine but this place name remains genderless as a noun in apposition.
Notoscolex having spermathecal pores paired posteriorly in 7 and 8 but with clavate spermathecae anteriorly in 8 and 9; holandry with seminal vesicles in 9 and 12; oesophageal gland annular in 12; genital markings mid-ventral in 16/17/18/19.
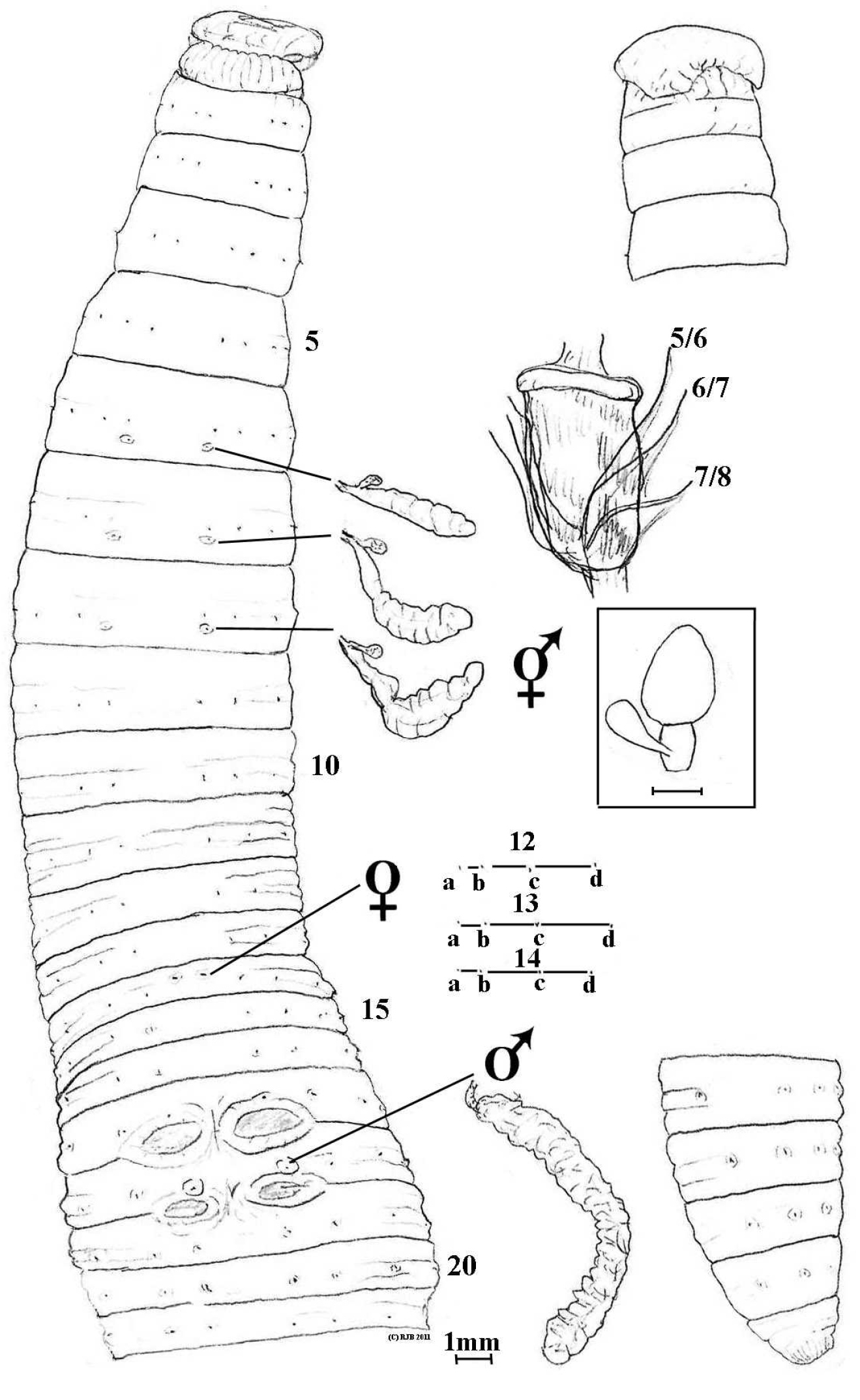
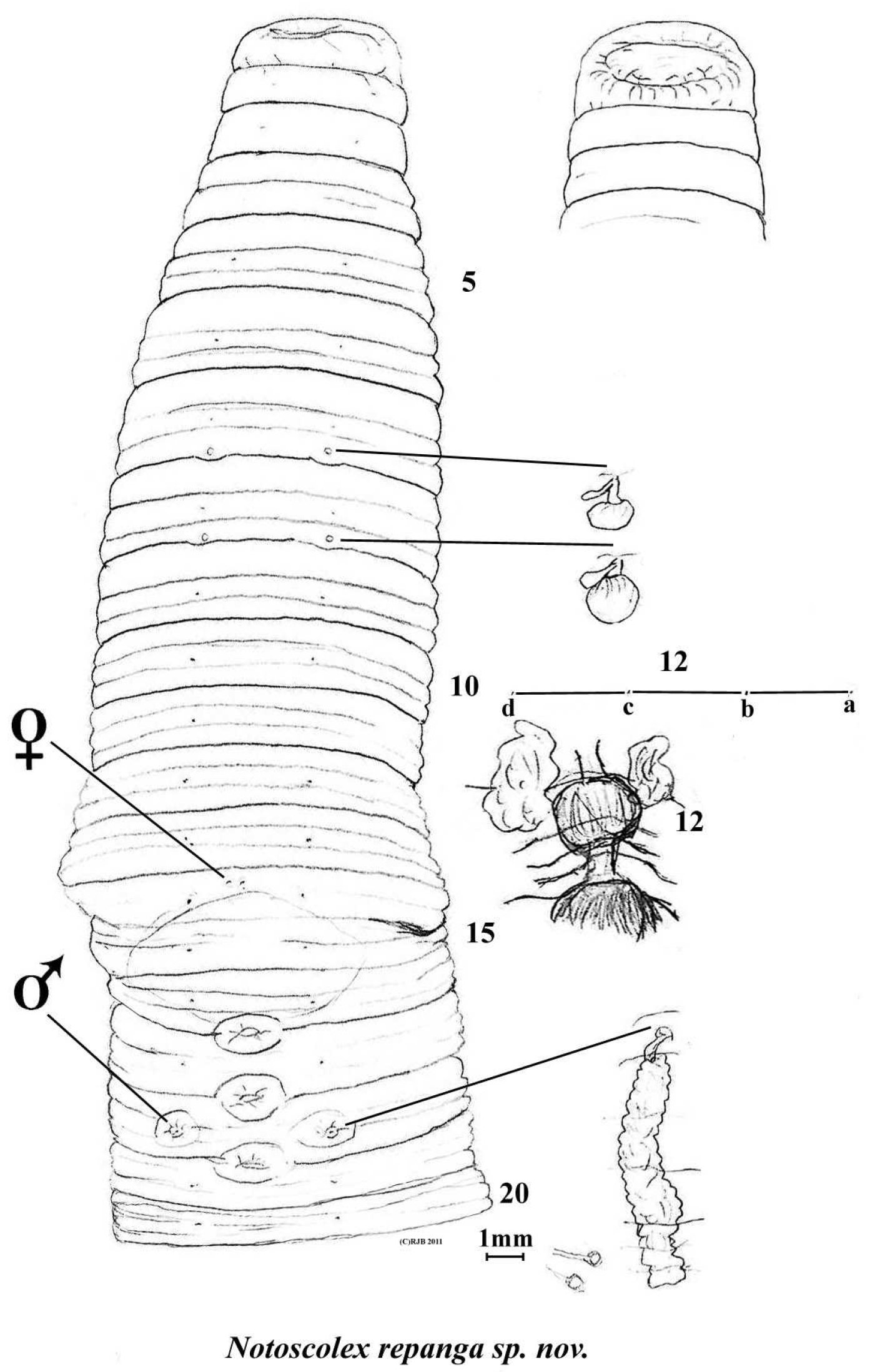
Body circular. Pale unpigmented in alcohol. Length 135 mm with 149 segments. Prostomium prolobous. Setae lumbricine, 8 per segment in mostly regular rows and almost equidistant throughout. Clitellum not marked. Dorsal pores present but minute and difficult to detect, possibly commencing from 9/10 or 10/11. Nephropores not found (meroic). Spermathecal pores segmental, posteriorly just above intersegments in setal a lines on 7 and 8. Female pores difficult to detect with certainty, possibly mid-ventral pair anterio-median to setae a on 14. Male and prostatic pores combined on small tumescences on 18 in position of deleted setae a. Penial setae not found. Genital markings mid-ventral eye-shaped sucker pads in 16/17, 17/18 and 18/19; a yellowy midventral patch from ½14-16/17 may be artefactual.
Notoscolex repanga ventral view with dorsal view of prolobous prostomium, spermathecae, prostate and oesophageal gland in 12 in situ; and lumbricine setal ratio in 12. (Small structures near scale bar are probable unidentified parasites, attached on intestine in region of 35–40).
Septa increasingly thickening from 4/5–10/11; 11/12 thin and thereafter membranous. Gizzard large but weak in 5. Dorsal blood vessel single; commissurals in 5–8; hearts paired and small in 9, much larger in 10–13; supra-oesophageal vessel not found. Nephridia meroic with several avesiculate tubules almost evenly spaced in several rows on body wall in each segment. Spermathecae in 8 and 9 each with saccular ampulla and single, small clavate diverticulum (non inseminated). Holandric: testes and funnels minute in 10 and 11 ventrally; seminal vesicles paired, racemose in 9 and, larger, in 12. Ovaries paired as fine string masses ventrally in 13; ovisacs not found. Prostates flattened, tubuloracemose extending to ca. 24 from small ducts to male pores in 18. Sessile tumidity associated with genital markings internally. Oesophagus large and folded in on itself in anterior in 6–9 at least; with annular dilated oesophageal gland in 12. Intestinal origin in 16. Typhlosole and caeca not found (absent). Gut contains fine silt with few organic fragments. Intestine paler and concertinaed between 35–40 where several (gregarine?) parasitic cysts on stalks attach to it (see figure).
Habitat location (under rocks in stream) would indicate an aquatic or semi-aquatic life style, a conclusion supported by the pale colouration plus reduced dorsal pores and gizzard; while the folded oesophagus in the anterior would allow considerable extension (for movement and feeding) and the gut contains silty (alluvial?) soil. Alternatively, this specimen may be an unintentional interloper washed into the stream from adjacent soil; more ecological information is needed to confirm or disconfirm this.
Notoscolex is primarily an Australian genus with representatives in Sri Lanka and southern India as well as NZ. The current specimen although large is possibly subadult (it has genital markings but lacks a distinct clitellum and spermathecae uninseminated) yet appears to be a distinct species. Its morphology is comparable to the nine previously known regionally compatriot Notoscolex species, all confined to the north of the North Island, many of which were at some time placed in the cohesive genus Tokea Benham, 1904. This latter genus was made junior synonym of Notoscolex following
Of the nine or so New Zealand species now known, Notoscolex repanga differs: from Notoscolex sapidus, Notoscolex urewerae, Notoscolex huttoni and Notoscolex suteri, all by
Superficially, Notoscolex repanga is somewhat similar to several Megascolides spp., such as Megascolides viridis, Megascolides raglani, Megascolides irregularis, Megascolides alba and Megascolides novaezealandiae, but it differs from all generically by its non-tubular prostates, and specifically by virtue of combination of segmental spermathecal pores and three mid-ventral genital markings, plus an oesophageal gland in 12 only and its last hearts in 13 rather than 12.
A further Megascolides species occurring in NZ and now possibly extinct was described by Schmarda in 1861 under the title of “Hypogaeon orthostichon, ” that is subjected to separate treatment in a forthcoming publication (Blakemore submitted.).
Types are redescribed for Anisochaeta animae (Lee, 1959) and Notoscolex tasmani (Lee, 1959) comb. n., and newly designated for Aporodrilus equestris (Benham, 1942) comb. n. As with Tasmanian Notoscolex tasmanianus Fletcher, 1887, the erstwhile representative of temporary genus Pinguidrilus Jamieson, 1974, that was found by
http://species-id.net/wiki/Anisochaeta_animae
Fig. 4From Unuwhao Mt., nr Spirits Bay, northern extremity of Northland, NZ.
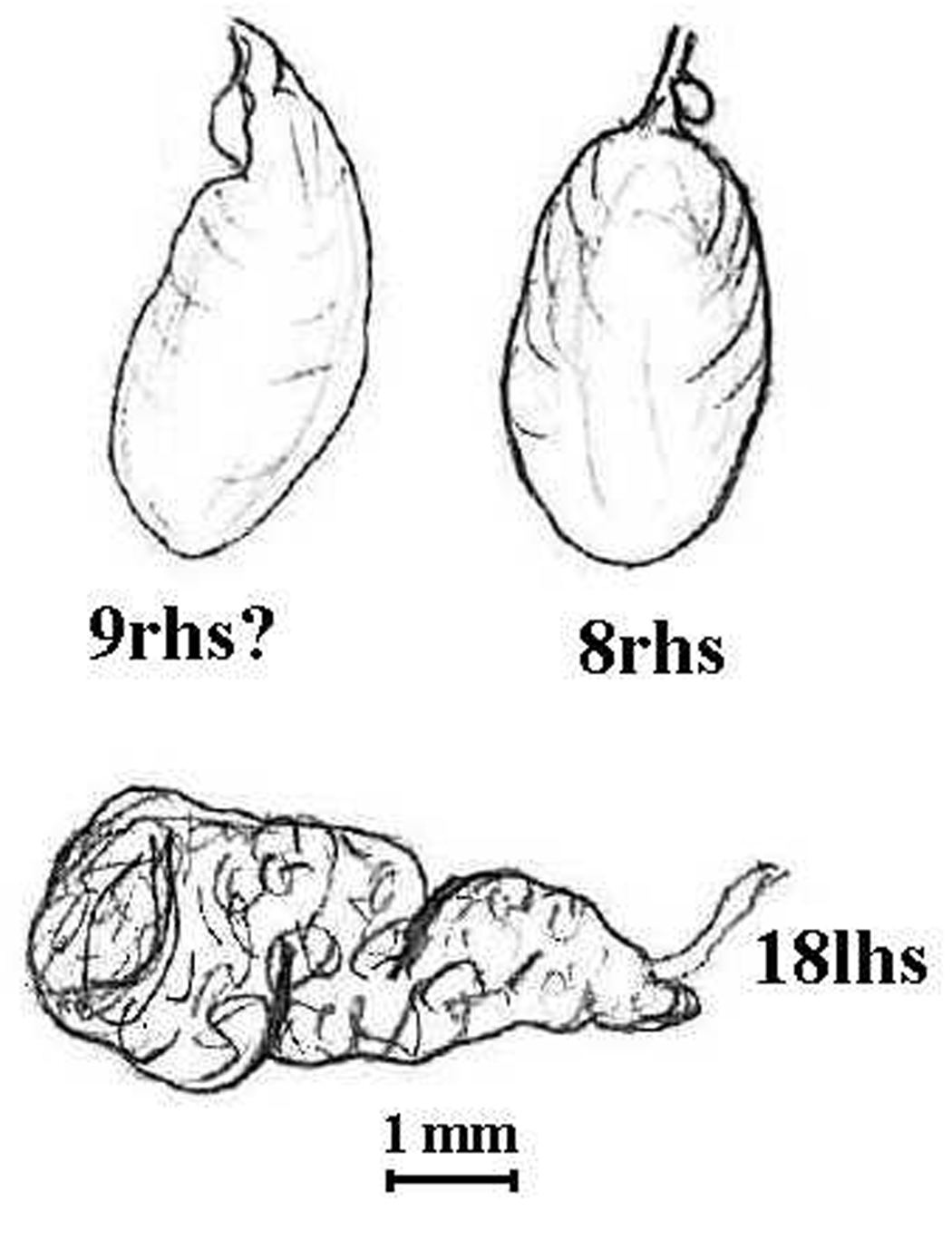
AMNZ 5038 for Megascolex animae has the following label in jar: “Unuwhao nr. Spirits Bay, N.Z. Coll: A.W.B.P. Feb 1946 AM8 1039”. It is a substantial specimen – 196 mm × 13.5 mm – collected by former director of Auckland Museum, A.W.B. Powell in Feb. 1946. Although the specimen in alcohol is now bleached of its earlier dark brown dorsal pigmentation and is wrinkled and hardened, it is yet well preserved. Reinspection of the type largely conforms to Lee’s original except that his figured spermathecae (from 9rhs that he included in a separate vial) is broken off after the diverticulum, and diverticula are newly found to be present in the remaining in situ spermathecae (see figure). The peristomium was not obviously cleft ventrally and genital markings, rarely described by Lee, were not found, but dorsal pores were: small in 4/5 and more obvious afterwards. Specimen AMNZ 5038 is the monotypic holotype fixed by original designation under (
Anisochaeta animae (Lee, 1959). Holotype (AMNZ 5038); spermatheca labelled 9rhs? is from separate vial and is a broken off ampulla; that labelled 8rhs is in situ showing small iridescent diverticulum (no parasites were found internally); the tubuloracemose prostate is from 18lhs.
http://species-id.net/wiki/Notoscolex_tasmani
Fig. 5Known only from Great Island, Three Kings Islands.
Specimen AMNZ 5039 has the following labels in its jar: “Auckland Museum Coll No. 13 Wet Greywacke gravel. Tasman Stm. Great Island. Three Kings 31.xii.52 J.S. Edwards”; “HOLOTYPE Megascolides tasmani Lee Tasman Stm, Great Is. Col. J.S. Edwards 30/12/52 1021. 3KI3” [note slightly different dates], a further label is blank. Somewhere are two other non-type specimens from the same locality according to Lee’s account (
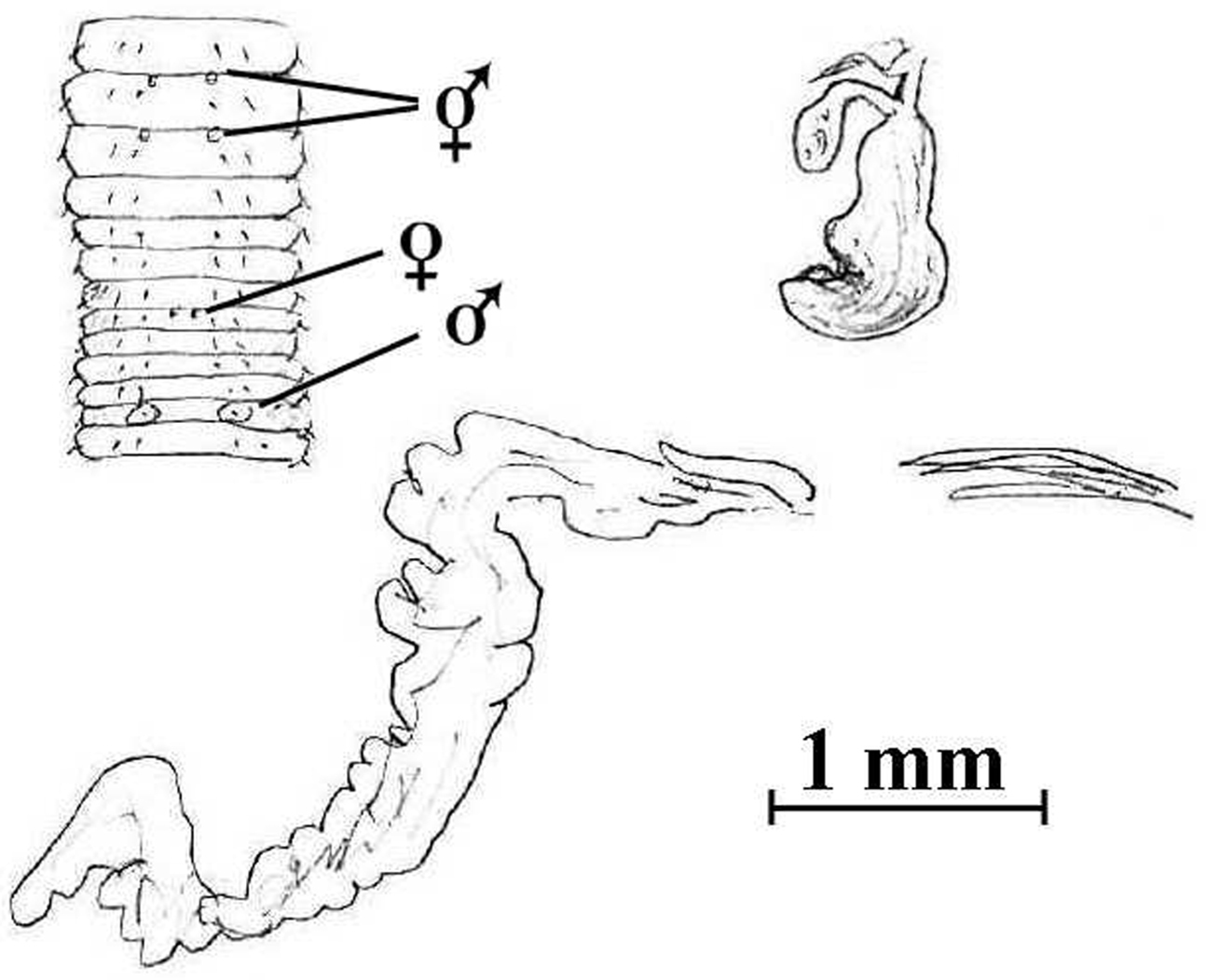
The type specimen is dark and brittle, shrunken to 53 mm long. Lee has 67.5 mm with 132 segments but it is a posterior amputee so must naturally be greater (there is also a slight possibility it acquires more setae posteriorly). Dorsal pores, not noted by Lee, are present from 10/11, at least. It appears lumbricine with widely spread setae that converge slightly towards male pores. Lee has overlooked the penial setae which are protruding (due to shrinkage?) from each of the male pores on 18. In 17 in ab lines is a reddish patch that may be a residual genital artefact. The female and spermathecal pores are no longer obvious. Vascularization is mostly as described by Lee, i.e., commissurals are in 6–9, hearts are in 10–12 from dorsal blood vessel that loops between septa in 11, 12, (not 13) 14, 15 and 16 thereafter single (
Notoscolex tasmani (Lee, 1959). Holotype (AMNZ 5039); sketch of anterior body (after
Having non-tubular prostates qualifies this taxon as a new combination in Notoscolex. The remote chance it acquires extra setae posteriorly after cut would permit it in Anisochaeta. Penial setae are unusual for New Zealand Megascolecidae, but it is interesting that they do not correspond well to the length of the spermathecal diverticula (see
http://species-id.net/wiki/Aporodrilus_equestris
Fig. 6Poor Knights Islands, New Zealand [an online report, along with several other New Zealand earthworms, as a Marine invertebrate from Mexico - http://mexinverts.lifedesks.org/pages/1545 (Oct. 2011) is clearly a mistake].
Two specimens in jar: AMNZ 5040 a larger ~200mm specimen dissected previously, and AMNZ 5280 a smaller complete mature 140 mm long. Labelled “TYPES Notoscolex equestris
There is some slight confusion with Notoscolex equestris Benham, 1942, in that
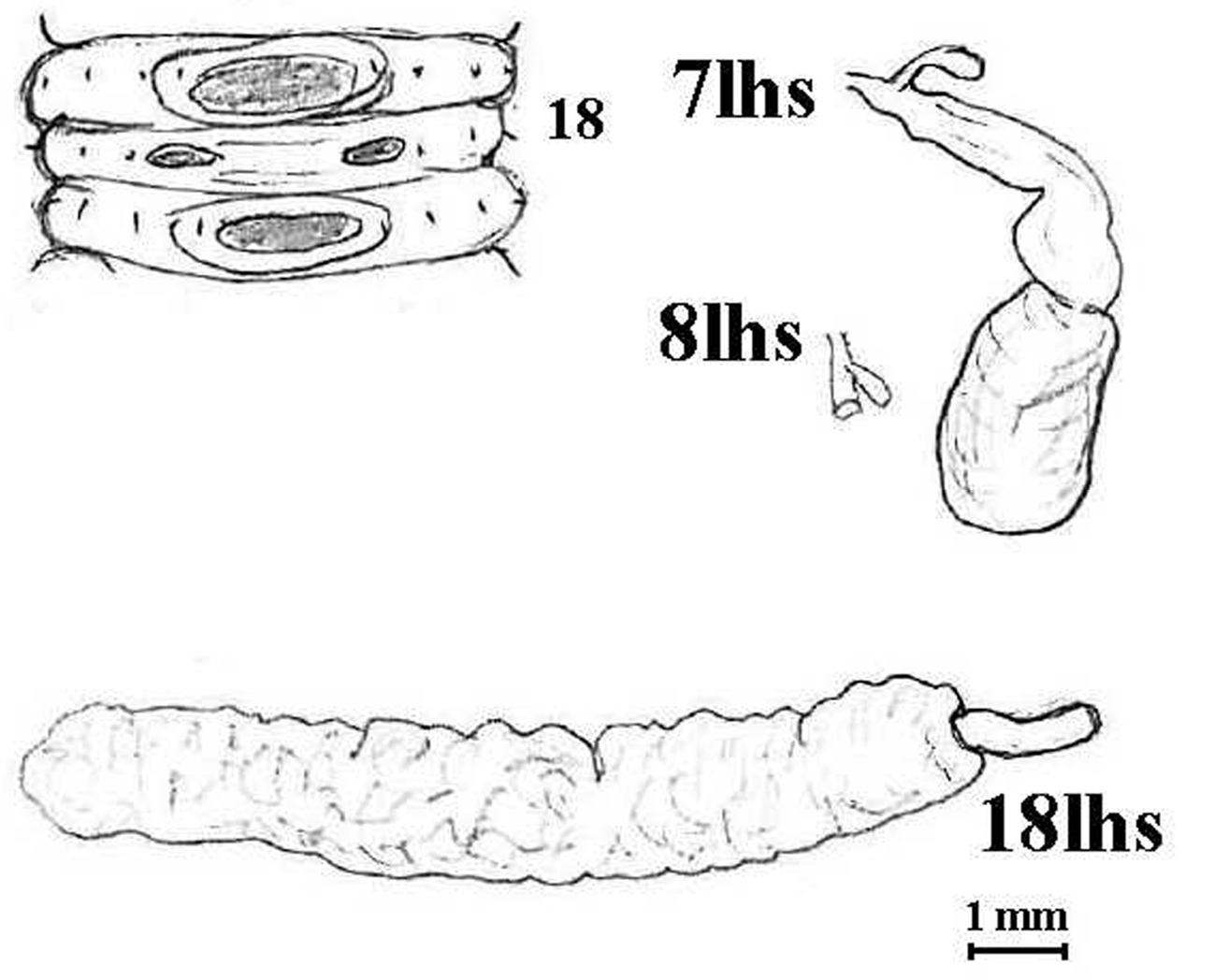
Both specimens are here inspected and described: the larger one – that entirely agrees superficially with Benham’s figures – had been previously dissected with the 8lhs spermatheca, 18rhs prostate, and the anterior of the intestine removed and missing from the jar. Additions to Benham’s and Lee’s earlier descriptions are that the highly wrinkled prostomium is construed as pro-epilobous rather than prolobous, and no ventral cleft is present on the peristomium. Benham was “unable to detect the dorsal pores owing to the strongly contracted state of the body” and, for some reason, Lee omitted mention of them entirely except for exotic Lumbricidae. They are here confirmed as being absent throughout the body in both specimens (i.e., qualifying for Aporodrilus). Setae c and d are increasingly irregular. Spermathecae are in 7–9 but for 8lhs only the stub remains with the small diverticulum still attached (hence overlooked by earlier workers who also mistook slight folds in the soft duct as “excrescences”); as for other spermathecae, the small diverticula are visible by their slight iridescence just above the body wall at the base of the duct [see Fig. 6 and cf.
Aporodrilus equestris (Benham, 1942). Lectotype (AMNZ 5040); sketch of male field of paralectotype for comparison with Benham’s figures; also spermathecae (7lhs, 8lhs as a stub with missed diverticulum, 9lhs not shown) and prostate in situ.
Both specimens are surely syntypes (one dissected agrees and key organs removed suggests they were figured by Benham, although Lee also dissected a prostate) and, under
Meroic Aporodrilus has lumbricine setae, tubuloracemose prostates and typically an intestinal origin in 16 (or 17) – as do many other species referable to Notoscolex – but it definitively lacks dorsal pores. Previously, only sixteen species and one sub-species were known, all from Tasmania (
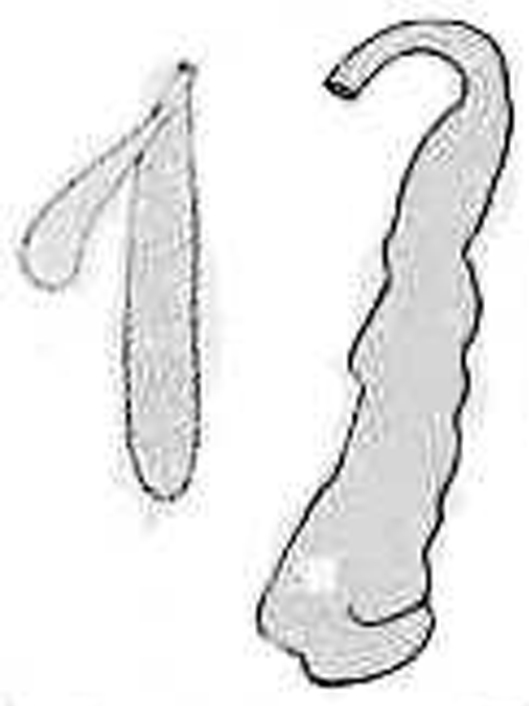
Spermatheca and prostate of Aporodrilus mortenseni from
While inexplicably ignoring the Australian genus Reflectodrilus Blakemore, 2005,
Australian Spenceriella were moved to Anisochaeta by
In actuality,
Proper generic resolution hinges on molecular testing of Diporochaeta type-species, Diporochaeta intermedia (Beddard, 1888) from New Zealand. Whereas the four “Diporochaeta” samples these authors did test (SB 3 = Diporochaeta chathamensis?; WM5 an identical species they call “Diporochaeta n. sp. 1”; SB6 = Diporochaeta brachysoma, and an unidentified specimen from Tasmania) were partitioned into two separate “clades”, with (WM20) what they call “Perionyx shoeanus” or “Perionychella shoeanus” (sic) and “Megascolides tasmani”(WM83) intervening (cf. its treatment within). The only other Perionychella in their analysis, “Perionychella kershawi” (AF406567/ AY048484), is not only a misidentification, it is also the wrong species in the wrong genus as its proper title is “Diporochaeta cf. kershawi”. This last is certain as these specimens were personally collected, preserved and identified from Tasmania by the present author. Thus, rather than clarity we get further confusion and, as with several previous molecular phylogenetic works, the only errors in their otherwise informative study are the names.
Discussion of biogeography and phylogeny of NZ species is also somewhat invalidated by inability to differentiate genera when
The first lineage (clade g) contains Megascolides and Spenceriella, the latter also labelled as Megascolides because they are intermingled with Megascolides proper.
Since the last decade, Spenceriella Michaelsen, 1907 species have been subsumed in prior Anisochaeta Beddard, 1890, as noted above, and most NZ Megascolides McCoy, 1878 have now also been shown to belong in Australian Notoscolex Fletcher, 1887.
Finally, Buckley et al.’s argument for phyogenetic relationships of off-shore island taxa representing geological history or ocean currents largely ignores the human component whereby earthworms are frequently transported to new areas, inadvertently or sometimes deliberately, especially when used by Māori for fishing bait or food source (Benham 1905,
Appreciation goes to Dr Tom Trnski, John Early and staff of Auckland Museum for facilitating the present study on their worm collection. ZooKeys editors and peers are thanked for their comments and suggestions that have greatly improved this contribution.