(C) 2011 Nikolai N. Yunakov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Arostropsis groehni gen. et sp. n. is described from Baltic amber and temporarily placed in the tribe Naupactini. It differs from all recent Naupactini genera with open corbels by very short and flattened scape, distinct lateral carina of the pronotum and elytra, and the rostrum distinctly narrower than the head capsule. The shape of head in the extinct genus is somewhat similar to that of the extant Naupactini genera with enclosed corbels (Platyomus Sahlberg, 1823 and Aptolemus Schoenherr, 1842), but differs in the slender body, open corbels, very short antennal scape and epifrons without a median sulcus (only a longitudinal depression is slightly visible). It is also similar to the Tanymecine genus Pandeleteius Schoenherr, 1834 in general appearance, but distinct by the straight anterior edge of the pronotum, lack of postocular spurs, lobes, and vibrissae, a slightly sloping elytral declivity, lateral ridges on the pronotum, subflattened antennal scape, elongate rostrum, and sparsely setose epistome. A new synonymy of the generic names Protonaupactus Zherikhin, 1971 and Sucinophyllobius Wanat & Borowiec, 1986, syn. n., is established. The Madagascan genus Corecaulus Fairmaire, 1903 is transferred from the tribe Naupactini to the Brachyderini because of its connate claws and the similarity in chaetotaxy of the epistomal area with African and Madagascar Brachyderini genera. A key to the identification of known Baltic amber genera of Entiminae is proposed. A checklist of the prepleistocene fossil Entiminae, based on V.V. Zherikhin’s data, with remarks and corrections, is presented.
new genus and species, new synonymy, fossil Entiminae, Naupactini, Baltic amber, key, checklist
Having examined the Baltic amber weevils from the collection of Mr. Carsten Gröhn, a new genus and species belonging to the subfamily Entiminae is described. However its tribal attribution was questioned because of the aberrant shape of the head capsule and inaccessibility of structures normally used for diagnostics (e.g. genitalia). In studying the various characters of Entiminae weevils, it became readily apparent that one of the most useful diagnostic features at the suprageneric level is the structure of the mandibular processes. They show some variability within Entiminae in general, but within certain tribes is usually rather stable. Besides, the mandibular process of fossils can be used, if available, as an additional character for identification of tribes.
In the course of the current study, mandibular processes of 35 extant genera in 17 tribes, including 6 genera of the tribe Naupactini Gistel, 1848, were examined (results of this comparison will be published in a forthcoming paper).
In addition, non-American genera treated in the Naupactini were also examined. As a result, the Madagascar genus Corecaulus Fairmaire, 1903 (type, female examined, MNHN) was transferred from the tribe Naupactini to Brachyderini, as its claws are connate in the basal third and similar to those in the genera Podionops Schoenherr, 1847 (South Africa) and Lagocaulus Fairmaire, 1903 (Madagascar) with the long median sulcus at the vertex and chaetotaxy of the epistomal area.
The type specimens of some poorly known taxa were re-examined. Phyllobius sobrinus Voss, 1972 was placed in the genus Sucinophyllobius (
The earliest descriptions of fossils from the subfamily Entiminae Schoenherr, 1823 were published by
The Baltic amber weevils apparently share more similarity with recent groups occurring mostly in the Indo-Malayan (Oriental) and Neotropical Regions (
The genus Paonaupactus Voss, 1953 is monotypic and it is considered as a member of the tribe Anypotactini Champion, 1911 (
The usual optic equipment was used for descriptions, including a Leica MZ 16.0 microscope provided with a CCD camera and camera lucida. Morphological terms mostly follow
Measurements. All measurements were taken with an ocular-micrometer. Body length was measured from the anterior margin of the eyes to the apex of the elytra, and rostrum length from the rostrum apex to the anterior margin of the eyes. Width of the rostrum is the maximum distance between the lateral edges of the pterygia.
Imaging. All outline illustrations were drawn using a camera-lucida and modified with a Wacom Graphire 4 Classic XL A5 tablet in Corel Draw (version 11.633) Corel®. Merging of layers was done with Helicon Focus (version 5.0) HeliconSoft®. Amber samples were photographed as under normal conditions as well as in sugar syrup to provide more suitable light refraction.
Abbreviations of depositories. GPIH Institute of Geology and Palaeontology and Museum (Geologo-Paläontologisches Institut u. Museum), University of Hamburg; MNHN National Museum of Natural History (Museum National d’Histoire Naturelle), Paris; ZMUC Zoological Museum (Zoologisk Museum), University of Copenhagen.
Abbreviations of morphological terms. cb corbel, es epistomal setae, ed elytral declivity, fr frons, hp humeral prominence, ibt intero-basal tooth, ma mandibular process, lr lateral ridge of pronotum, pep parepistome.
Abbreviations in table. Agri Agriento (=Girgenti), Sicilia, Italy, Upper Miocene; Aix Aix-en-Province, France, Lower Oligocene; BalJ Baltic Amber, Baltic and North Sea coast, Upper Eocene; Boet Böttinger Marmors, Germany, Miocene; Cere Céreste, west to Apt, Alpes-de-Haute, Basses Alp Department, Provence, France, Lower Oligocene; Cela Célas, railway Uzés - Saint-Julien-de-Casignac, Fumades, Corents, Bassein Ales, Gard Dept., France, Upper Eocene, previously Lower Oligocene (Sannosien); Dece Lava Camp Mine, Jumachuk River Valley, Seward peninsula, Alaska, Pleocene-Pleistocene (5.7 mln - 27 000 - Deceit Formation); DomJ Dominican amber, Dominican Republic and Haiti; Lower Miocene; CerG Cerro Guido, Ultima Esperanza, Magallanes, Upper Cretaceous; Core Corent, Gergovia Plateu, south of Clermon-Ferran, Puy-de-Dom Department, France, Upper Oligocene; Flor Florissant, south fork of Twin Creek, Front Range near Pike’s Peak, Colorado, U.S.A., Lower Oligocene. GreR Green-River, 3-4 km western rail-way crossing of Green River; Utah, U.S.A, Middle Eocene; N1 Neogene, Miocene; N11 Neogene, Lower Miocene; N13 Neogene, Upper Miocene; N2 Neogene, Pliocene; K2 Upper Cretaceous; Dece Lava Camp Mine, Jumachuk River Valley, Seward peninsula, Alaska, Pleocene-Pleistocene site (5.7 mln - 27 000 - Deceit Formation); Oeni Oeningen, near Baden lake, Baden-Württemberg, Germany, Upper Miocene; Pg12 Paleogene, Middle Paleocene; Pg2 Paleogene, Eocene; Pg22 Paleogene, Middle Eocene; Pg23 Paleogene, Upper Eocene; Pg31 Paleogene, Lower Oligocene; Pg33 Paleogene, Upper Oligocene; RoaM Roan Mountain, Colorado, USA, Middle Eocene; Rott Siebengebirge, Germany; Lower Miocene, Aquitanian or Upper Oligocene; Sunc Sunchal, La Mendieta, Jujuy Prov., northern Argentina; Upper Paleocene (Lower Eocene); WhiR White River Badlands, South Dakota, boundary Eocene and Oligocene.
Taxonomic treatmentOrder Coleoptera Linnaeus, 1758
Family Curculionidae Latreille, 1802
Subfamily Entiminae Schoenherr, 1823
urn:lsid:zoobank.org:act:24BF76B1-8ADA-44BA-B111-FEED72EB7A05
Arostropsis groehni Yunakov & Kirejtshuk, sp. n.
The name of the new genus is formed from the Greek negative prefix “a”, “rōstron” (beak, bill, snout) and “opsis” (resembling a (specified) thing). Gender feminine.
Body elongate, in general appearance similar to Pandeleteius Schoenherr, 1834. Antenna with scape short, as long as pedicel (first funicular article; term after
urn:lsid:zoobank.org:act:25FBCC0C-8376-41C7-A86E-F024B1541EEC
http://species-id.net/wiki/Arostropsis_groehni
Figs 1–16Holotype “C 7968, GPIH 4516”, male (GPIH); the complete beetle with a clear integument is included in an irregular parallelepiped with the largest plane about 18.0 × 14.0 mm and the smallest one 11.0 × 7.0 mm; amber matter on right side from the beetle is rather homogeneous, but that from the left side of the inclusion consists of some layers, in which between the borders is a fine net of dark (almost blackish) organic matter.
The epithet of the new species is formed from the name of Carsten Gröhn, collector of its holotype.
Baltic Amber; Upper Eocene, Prussian Formation.
Baltic Sea coast and Amber quarry Jantarny near Kaliningrad (formerly Koenigsberg), Kaliningrad region, Russia.
Length 6.4, width 1.8, height 1.3 mm. Body slender, distinctly depressed from above. Pronotum and elytra strongly carinate at sides. Integument densely covered with small, apparently metallic, lanceolate (apparently green) scales at both sides of body and legs.
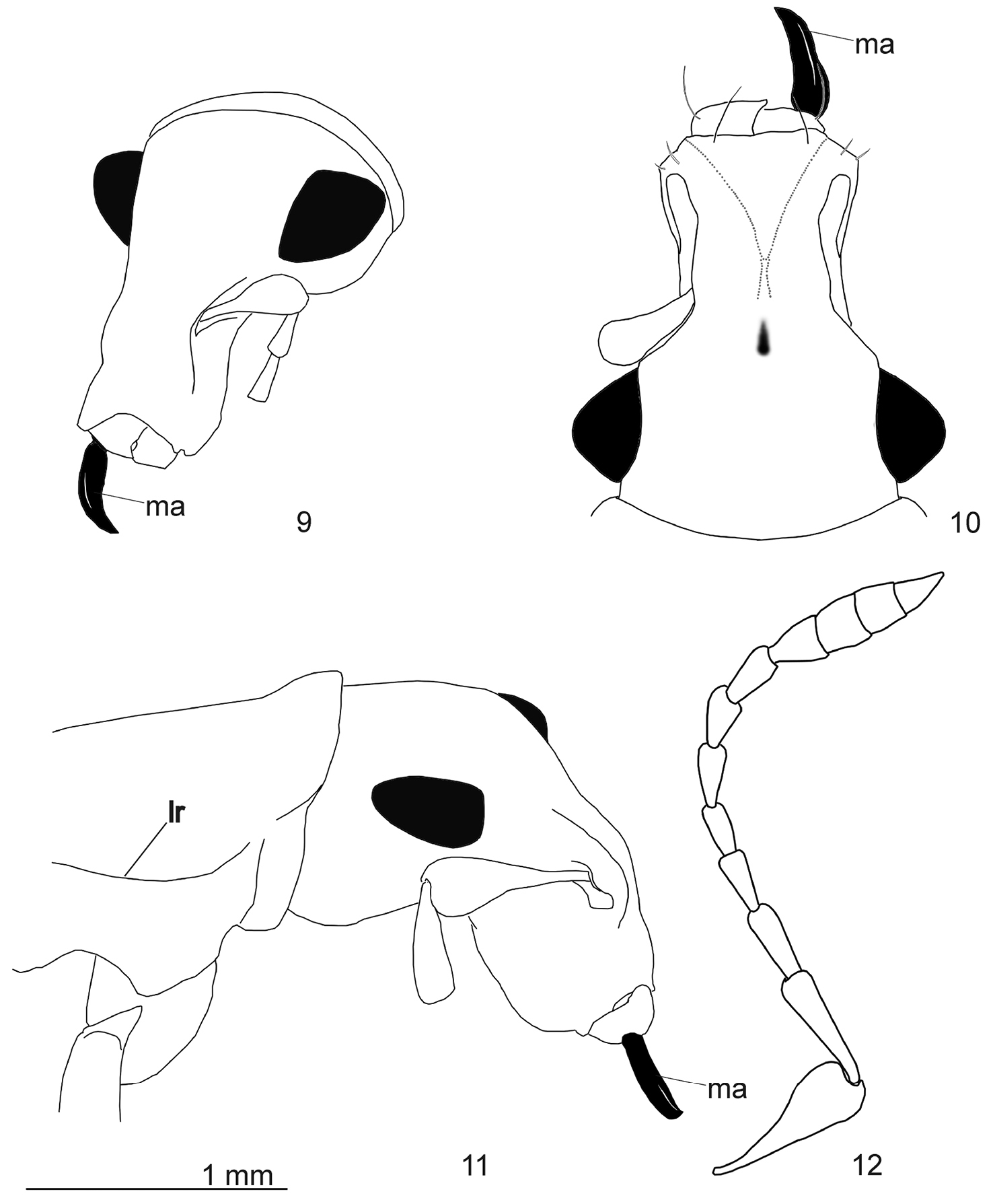
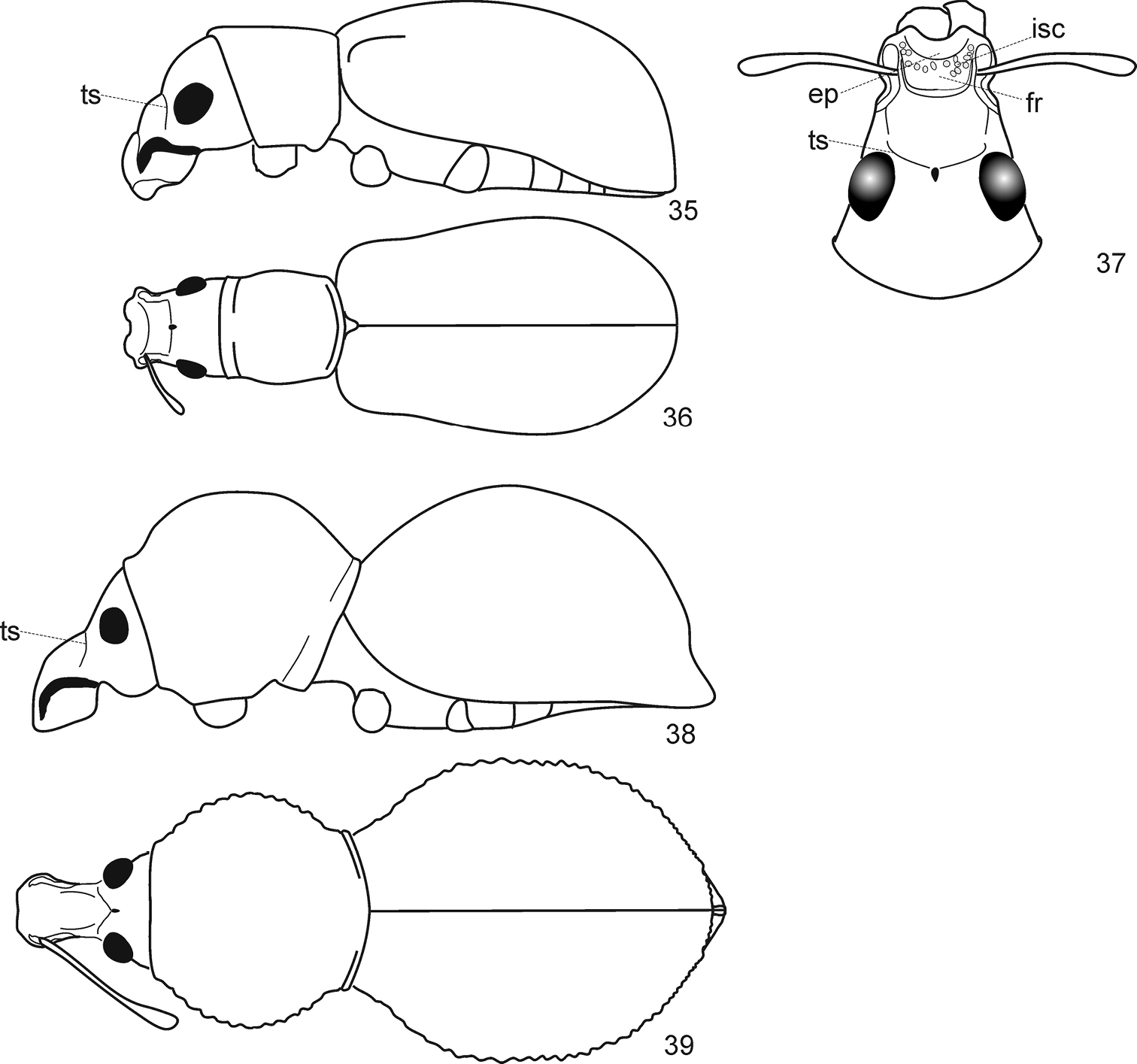
Head. Rostrum 1.5 times as long as wide, narrow, distinctly narrower than head capsule, laterally depressed. Pterygia weakly extending beyond lateral contour of rostrum. Epistomal area not depressed. Lateral edges of epifrons at middle convex, narrowed towards middle, then parallel-sided, without basal, transverse depression or sulcus. Median sulcus shallow, extending towards pit between eyes, not continuing towards vertex. Head capsule not constricted beyond eyes. Frons distinctly convex, almost twice as wide as epifrons at level of antennal insertions. Maximum width of head at posterior part of eyes. Scape comparatively short, about 1/4 as long as funicle, strongly expanded apically, somewhat compressed dorsoventrally, not extending beyond anterior third of eyes, directed obliquely downwards in folded state. Funicle slender; all segments elongate, funicular segment 1 about three times as long as wide, 2nd about two times as long as wide, 3rd about 1.5 times as long as wide; 4th-5th about two times as long as wide, segments 6-7 about 1.5 times as long as wide. Club spindle-shaped and comparatively thin, with distinctly separated segments, about as long as funicular segments 1-7 together. Mandibles entirely bare (without scales), moderately extended beyond buccal cavity. The only remaining left mandibular process knife-shaped, in apical third slightly curved inward, without internal prominences, about 1.5 times as long as protruding part of mandibles.
Pronotum elongate, almost parallel-sided, with anterior and posterior constrictions widely expressed, weakly and evenly convex at sides, with disc weakly convex transversely and anterior edge curving upward; posterior edge slightly bisinuate; posterior angles widely rounded and somewhat projecting posteriorly; anterior edge nearly straight.
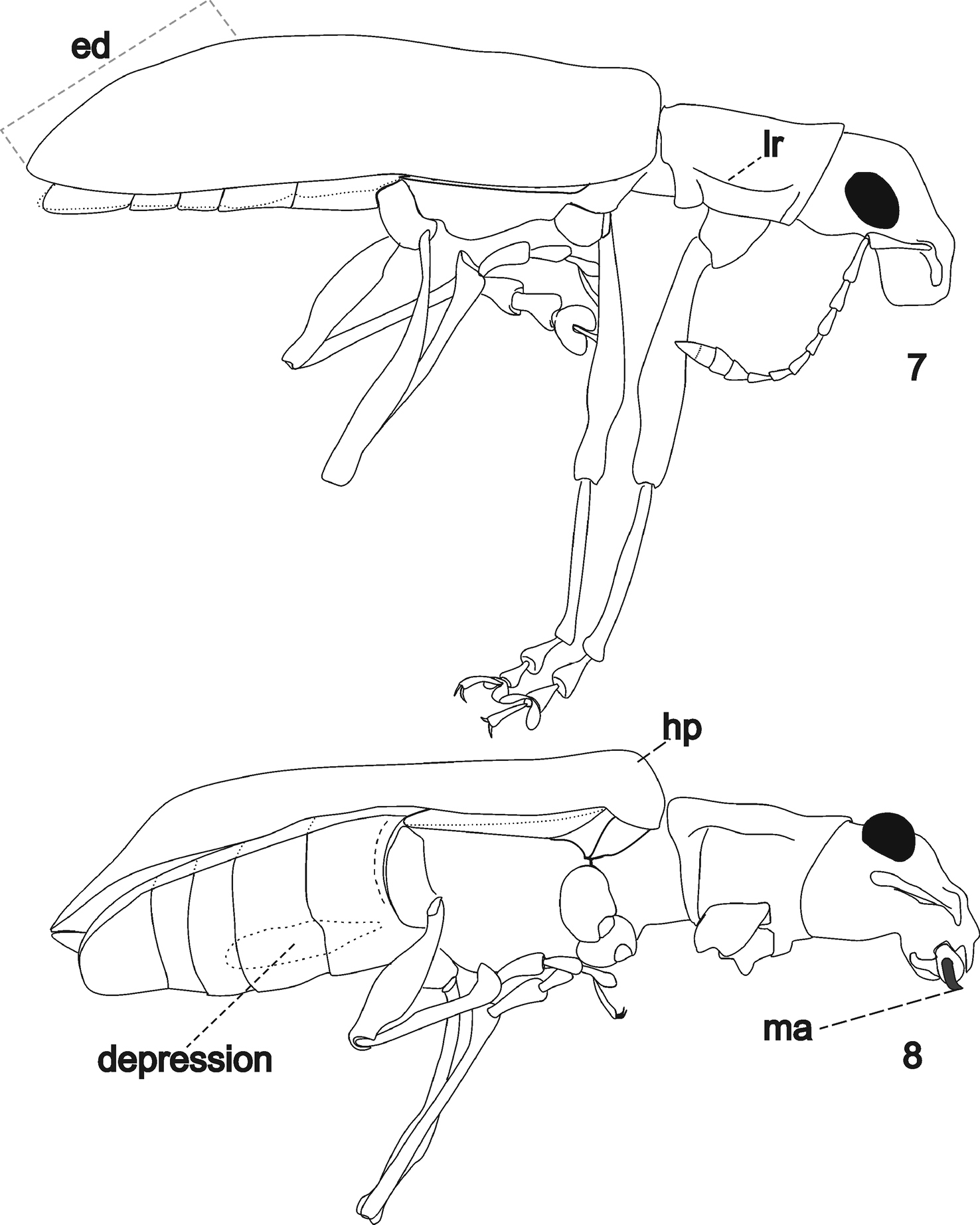
Elytra elongate, about 4.5 times as long as wide, parallel-sided, humeral prominences distinct (Fig. 8, hp), basal edge of elytra biconvex opposite the posterior pronotal depressions. Elytral declivity gently sloping (Fig. 7).
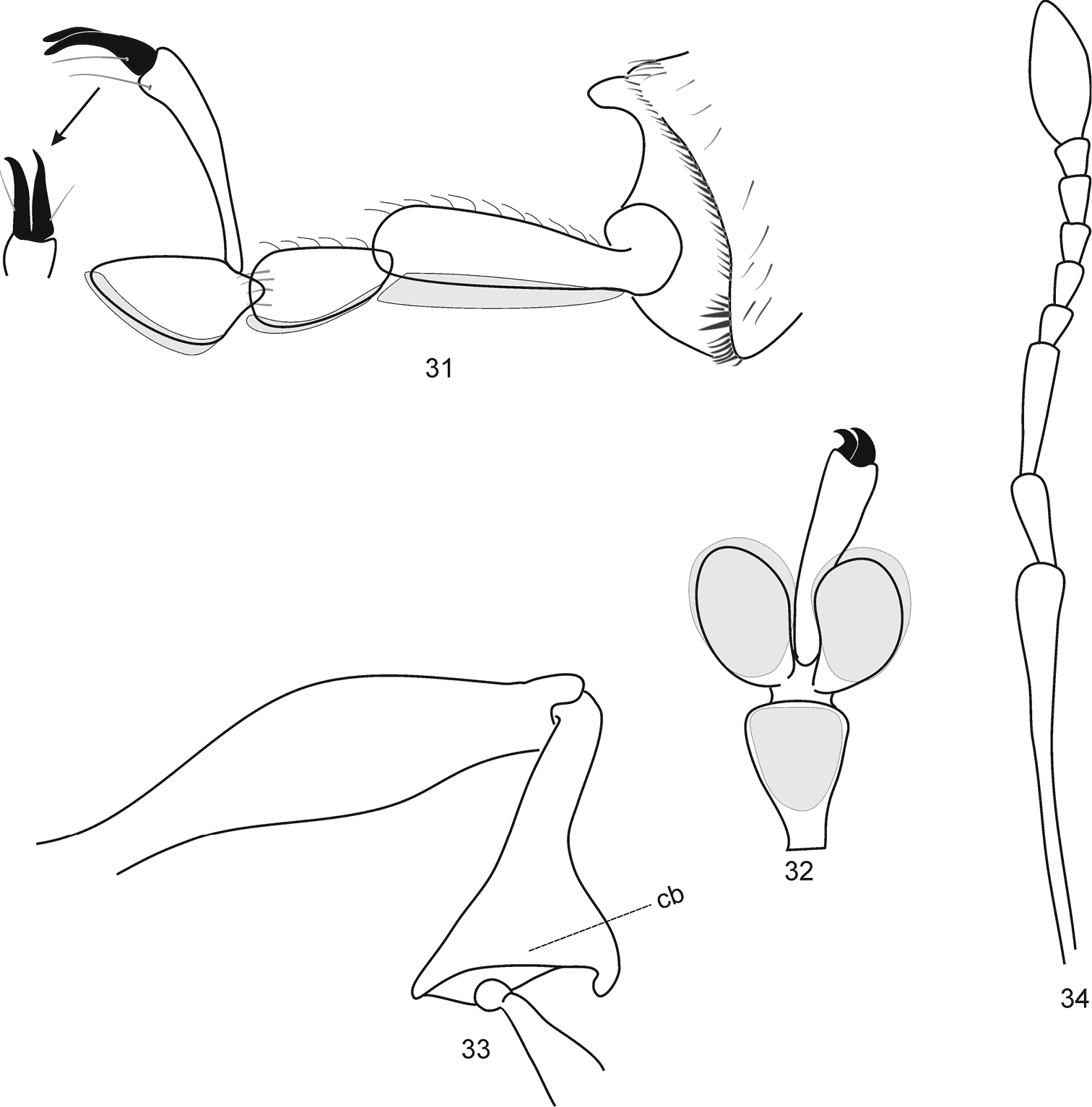
Legs slender. Femora slender, elongate, obtuse, weakly swollen in apical third. Tibiae slender and elongate. Protibiae gently curved inwards in apical third; with anterior row of thin spines. Metatibiae subflattened and with inner edge sinuate at apical third. Corbels open (Figs 6, cb; 8), without additional row of spines. Tarsi slender, setaceous, pelma well-developed (term after
Arostropsis groehni gen. et sp.n. 1 body, dorsal view 2 body, lateral view 3 body, ventro-lateral view 4 head, dorsal view 5 protarsi 6 metatibia apex. Abbreviations: cb – corbel, ma – mandibular process. Body length: 6.4 mm.
Arostropsis groehni gen. et sp.n. 7 body outline, lateral view 8 idem, ventro-lateral view (anterior and middle limbs removed). Abbreviations: ed elytral declivity, hp humeral prominence, lr lateral ridge, ma mandibular process. Body length: 6.4 mm.
Arostropsis groehni gen. et sp.n. 9 head, antero-lateral view 10 idem, antero-dorsal view 11 head and prothorax, lateral view 12 antenna, lateral view. Abbreviations: lr lateral ridge, ma mandibular process. Scale bar: 1 mm.
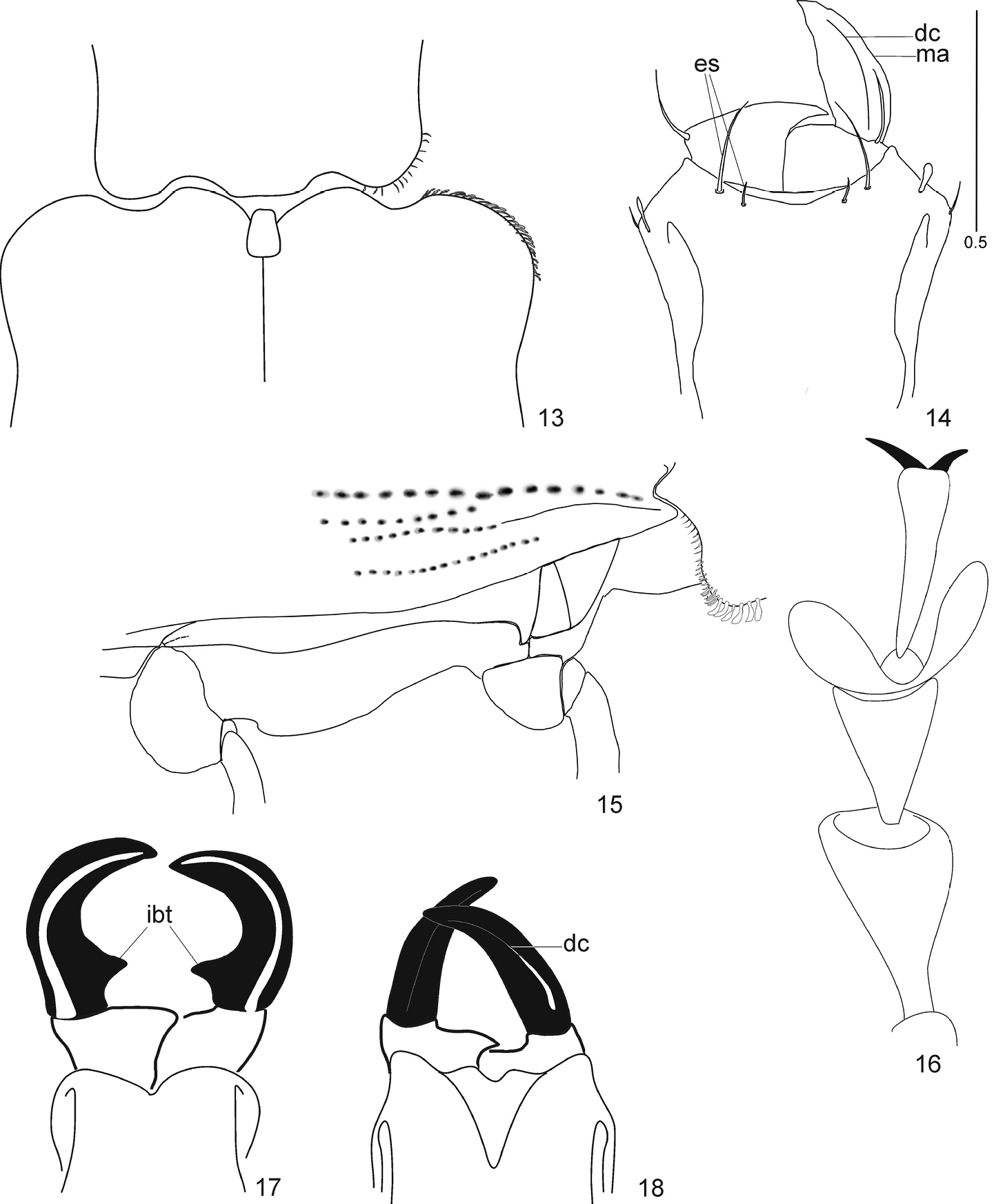
Species ofArostropsis, Lepropus, and Naupactus, details. Figs 17 and 18 (modified from
The new genus differs from all recent genera of Naupactini with open corbels and procoxae not completely separated from the prosternum (Mesagroicus Schoenherr, 1840, Eurymetopus Schoenherr, 1840, Melanocyphus Jekel, 1875, Trichonaupactus Hustache, 1939 ) in the following characters: short and depressed scape, rostrum narrow, epistomal area weakly setose, epifrons with a weakly developed median depression and vertex with very small fossa, not continuing to occiput.
Since Arostropsis gen. n. has free claws, only two other fossil genera, Paonaupactus Voss, 1953 and Protonaupactus Zherikhin, 1971, share the same character state and can be compared with the new genus. Arostropsis gen. n. strongly differs from both Paonaupactus and Protonaupactus in the following characters given in the key below.
Key to Baltic amber genera of Entiminae with free claws
| 1 | Scape short, reaching middle of eyes, strongly thickened apically; pedicel 1.5 times as long as or 2nd funicular article; 1st article of club similar in shape and size with 7th funicular article. Eyes irregularly convex, lateral, located significantly below level of frons (in lateral view). Epifrons without transverse sulcus before eyes. Lateral carina of prothorax developed. Pronotal disc depressed. Procoxae situated in middle of prosternum. Body length 6.4 mm | Arostropsis gen. n. |
| – | Scape long, reaching anterior edge of prothorax, not strongly thickened at apex; pedicel 0.7 time as long as 2nd funicular article; 1st article of club significantly different in shape from 7th funicular article. Eyes evenly convex, dorso-laterally, located almost at level of frons and somewhat extended above level of frons (in lateral view). Epifrons with more or less developed, transverse sulcus before eyes. Sides of prothorax evenly swollen, without carina. Pronotal disc moderately convex. Procoxae are closer to the anterior than to the posterior edge of prosternum. Body length 4.0-4.8 mm | 2 |
| 2 | Antennal club oval. 4.5-4.8 mm. | Protonaupactus Zherikhin, 1971 |
| – | Antennal club spindle-shaped. 4 mm. | Paonaupactus Voss, 1953 |
This new genus is undoubtedly a member of Entiminae due to the presence of mandibular processes. Due to structural characters: mandibular processes long, knife-shaped (ibt not developed) - claws free, vertex and epifrons combined in uniform structure without transverse sulcus before eyes, the new genus could be assigned to the tribes Naupactini or Geonemini Gistel, 1848. Emden separated these groups from other ‘brachyderoid’ tribes of Entiminae with free claws (Tanymecini and Anypotactini) by the following characters (Table 1).
Basic morphological characters of ‘brachyderoid’ tribes of Entiminae with free claws in comparison with the genus Arostropsis gen.n.
| Geonemini | Naupactini | Tanymecini | Anypotactini | Arostropsis | |
| 1. Postocular vibrissae | absent | absent | present | absent | absent |
| 2. Claws | free | free | free/connate | free | free |
| 3. Transverse sulcus | absent | present/absent | present/ absent |
present/ obsolete |
absent |
| 4. Eyes position | dorso-lateral | lateral | dorso-lateral | dorso-lateral | lateral |
| 5. Mentum covers maxillae | yes | yes | yes | yes/no | unknown |
| 6. Metatibial corbels | open/enclosed | open/enclosed | open/enclosed | open | open |
| 7. Mandibular processes | without ibt | without ibt | with ibt | unknown | without ibt |
| 8. Procoxae position | anterior | anterior/median | median | median | median |
The position of Arostropsis is tested following the table:
Presumption 1 (Geonemini). Arostropsis gen.n. lacks the key characters of Geonemini such as: evenly sloping lateral edges of epifrons, very narrow vertex and anterior position of procoxae. Consequently this genus cannot be assigned to this tribe..
Presumption 2 (Tanymecini). Arostropsis gen. n. could not be considered in the tribe Tanymecini, due to absence of postocular vibrissae at the prothorax. The amount of vibrissae varies from genus to genus within Tanymecini but at least a few vibrissae are present (some Pandeleteius). We do not know any case in which vibrissae are completely absent, so it is unlikely that Arostropsis belongs to Tanymecini.
Presumption 3 (Anypotactini). Due to absence of transverse sulcus between vertex and epifrons and U-shaped epistome in Arostropsis it is impossible to consider this genus within Anypotactini.
Presumption 4 (Naupactini). The strictly lateral position of the eyes, flattened epifrons and very broad vertex resembles that of Arostropsis within Naupactini, however, the shape of the rostrum is very different from that of any known member of this tribe. This transformation of rostrum probably resulted in reduction of the frontal fossa (Fig. 10) that is normally (in genera with broad rostrum) deep, long and continuing from the anterior portion of epifrons to the occiput. Such head shape together with the unusual slender body makes it difficult to recognize Arostropsis as a member of the tribe Naupactini.
Probable bionomy. The long legs and well developed tarsal pelma (term after
Protonaupactus microphthalmus Zherikhin, 1971
Protonaupactus microphthalmus Zherikhin, 1971, Protonaupactus viridis (Wanat & Boroviec, 1986), comb. n., Protonaupactus sobrinus (Voss, 1972), comb. n.
http://species-id.net/wiki/Protonaupactus_sobrinus
Figs 19–34Holotype: ZMUC903847, male (ZMUC); the complete beetle is included in a nearly regular amber piece, parallelepiped, with facets 14.0, 10.0 and 5.0 mm; one thin layer with wavy, light organic matter, small gas bubbles and small cracks located beneath the beetle.
Baltic Amber; Upper Eocene, Prussian Formation.
Denmark “Bernschteinschluss, Versterhavet bei Thisted, 17.xii.1895, Madsen leg.”.
Length 4.5, width 1.75, and height 1.65 mm. Beetle densely covered with metallic, shining scales (apparently green).
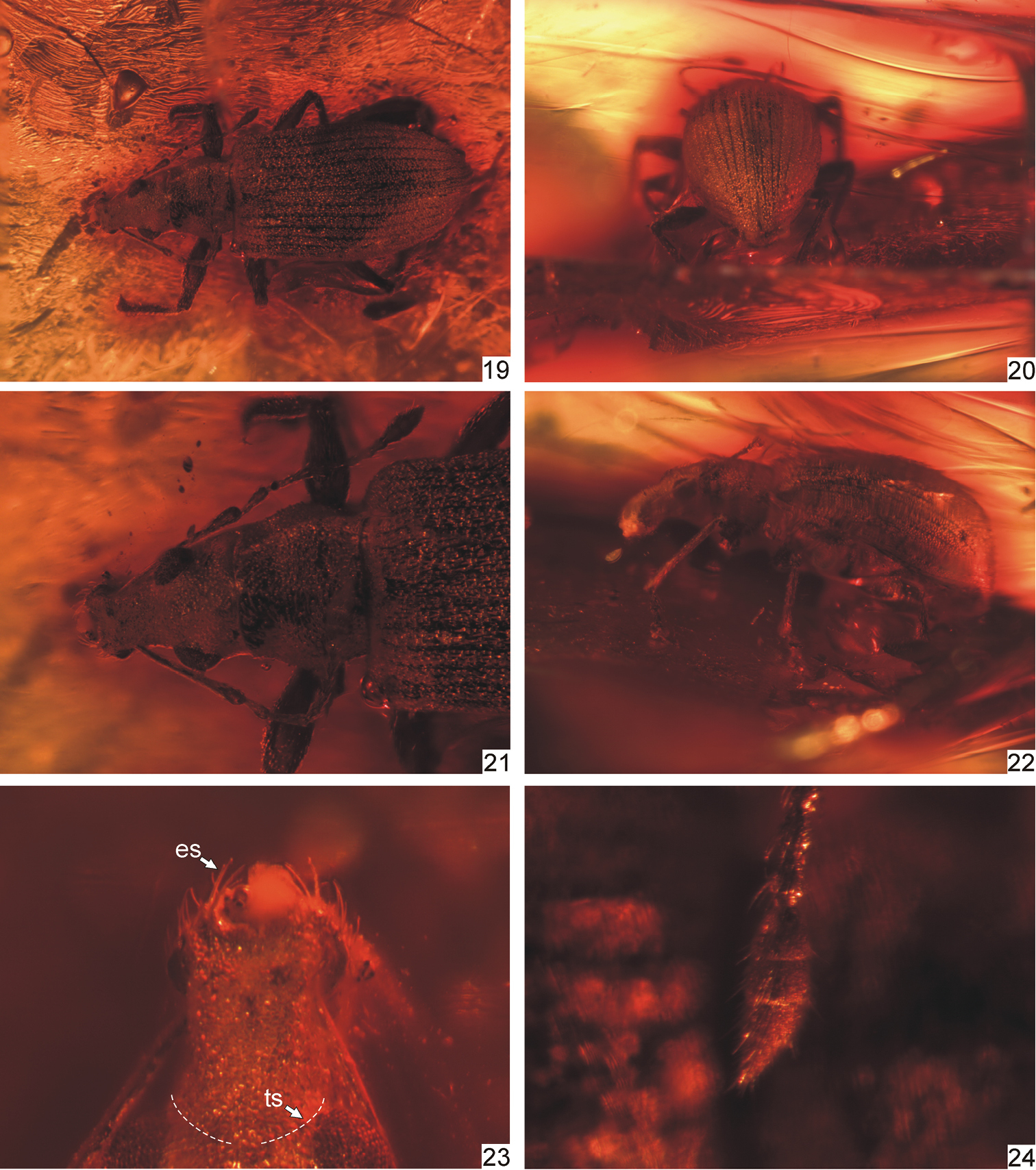
Head. Rostrum 1.5 times as long as wide at level of pterygia. Epifrons subparallel-sided, at level of antennal insertions abruptly widened anteriorly, weakly convex longitudinally, separated from frons by a distinct depression indicating a transverse sulcus hidden by dense scales. Pterygia strongly extending beyond lateral contour of rostrum. Epistome well-defined by U-shaped keel. Epistomal setae grouped in dense bunches at anterior epistomal angles. Each bunch bearing at least 5 setae. Epistomal angles pronounced apically. Anterior edge of pterygia densely setose. Antennal furrows distinct only in their basal half and not continuing obliquely to underside of rostrum, their dorsal and ventral edges somewhat divergent posteriorly. Eyes dorso-lateral, round and evenly convex. Frons slightly convex, as wide as epifrons between antennal insertions. Frontal fossa not visible because masking by scales. Antennae long. Scape straight, evenly thickened apically, reaching anterior constriction of pronotum. Funicle thin: pedicel about 0.7 time as long as 2nd funicular article; 2nd article about 3.5 times as long as wide; 3rd –7th articles weakly oblong, about 1.5 times as long as wide. Club ovoid, about 2.3 times as long as wide, its 1st article significantly different in shape from 7th funicular article.
Pronotum transverse; evenly convex at sides and disc, strongly constricted anteriorly and posteriorly. Its base slightly bisinuate. Anterior edge of pronotum straight, without postocular lobes, spurs or vibrissae. Scutellum significantly pronounced, subquadrate.
Elytra subparallel, hardly widened in apical half, with pronounced humeral prominences. Epipleural edge weekly but distinctly S-shaped. Wings well-developed, beetle apparently capable of flying. Elytral declivity abruptly sloping. Anterior edge of anal ventrite (hypopygidium) emarginate (Fig. 30).
Legs thin. Femora obtuse, weakly swollen in middle part. Protibiae straight at external edge, not widened at external apical angle. Metatibiae spatulate apically (Fig. 33), with open corbel but without additional setal row. Setal comb of all tibiae weakly-developed. Tarsi with well-developed setaceous pelma (term after
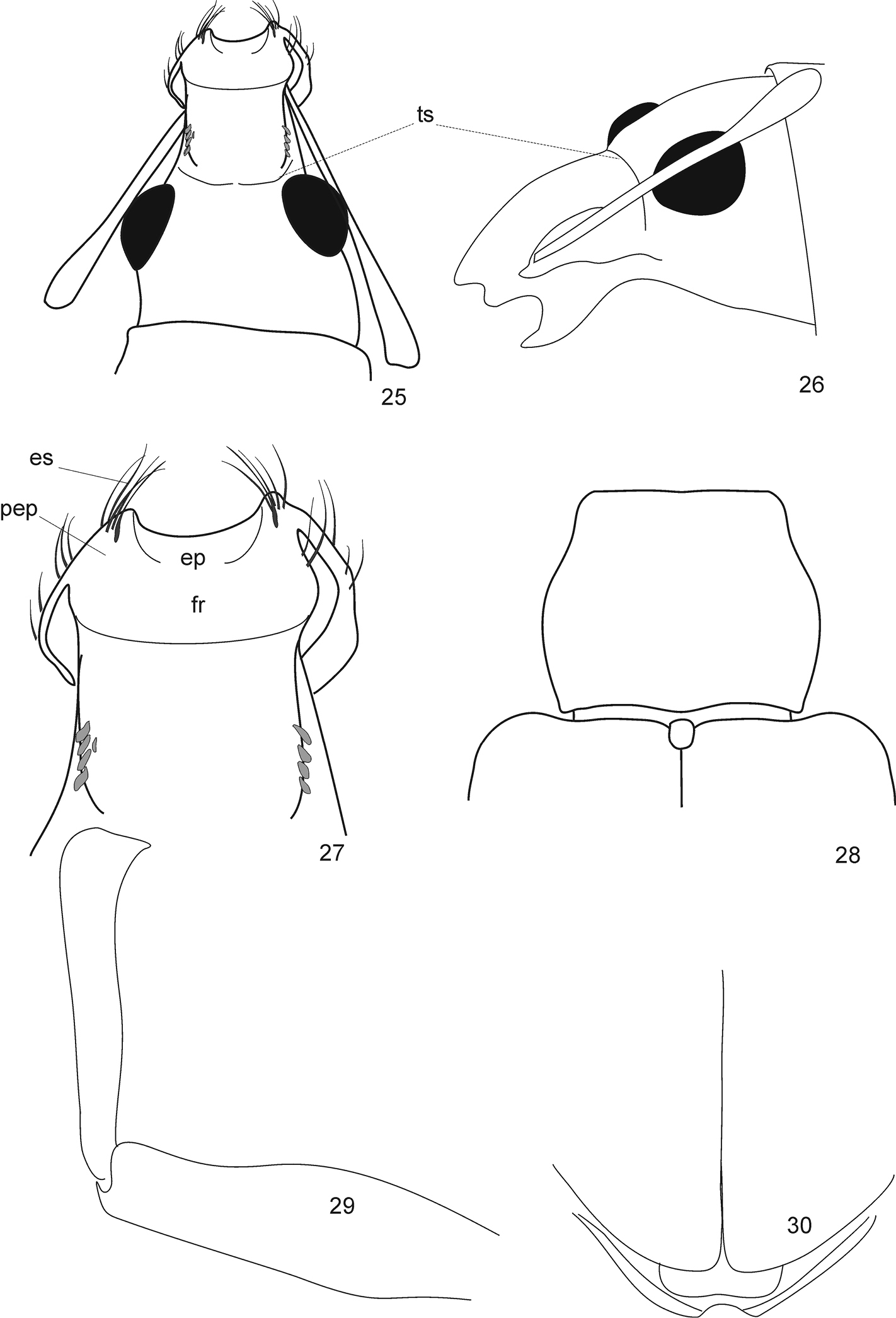
Protonaupactus sobrinus (Voss, 1972), holotype, male. 19 body, dorsal view 20 elytral declivity, posterior view 21 head and pronotum, dorsal view 22 body, lateral view 23 rostrum, dorsal view (transverse sulcus, indicated by dashed line, hidden by dense scale vestiture) 24 antennal club. Abbreviations: es epistomal setae, ts transverse sulcus. Body length: 4.5 mm.
Protonaupactus sobrinus (Voss, 1972), holotype, male. 25 head with antennal scape, dorsal view 26 idem, lateral dorsal view 27 epistomal area of rostrum 28 pronotum and elytral base, dorsal view 29 anterior right leg, dorsal view 30 elytral apex with pygidium and anal ventrite. Abbreviations: ts transverse sulcus, es epistomal serae, ep epistome, fr frons, pep parepistome. Scale bar: 1 mm (Figs 25, 26, 28, 30); 0.5 mm (Figs 27, 29).
Protonaupactus sobrinus (Voss, 1972), holotype, male. 31 metatibial apex and tarsus lateral view, and claws dorsal view 32 mesotarsus ventral view 33 posterior leg 34 antenna. Remark. Setose pelma (term after
This species is distinguished from Protonaupactus microphthalmus and Protonaupactus viridis by the metatibiae being strongly spatulate apically. The re-examination of the holotype of Phyllobius sobrinus Voss, 1972 demonstrates the particular structure of the head, which is very similar to some genera of Anypotactini (mostly from tropical America) and Cyphicerini (mostly from Old World tropics, distinctly separated groups): epistome U-shaped, convex, weakly sinuate anteriorly, and claws free (Figs 27, 31). This species also shares a well-developed, transverse sulcus on the rostrum (Fig. 25, 26, 35–39) with other groups of Anypotactini.
Anypotactini, details. 35, 38 body, lateral view; 36, 39 body, dorsal view; 37 head, dorsal view. 35–37 Anypotactus exilis Boheman, 1840, female (Venezuela); length 3, 3 mm 38, 39 Hyphantus buccifer Germar, 1824, female (Brazil); length 8, 2 mm. Abbreviations. isc incrustation scales, ep epistome, fr frons, ts transverse sulcus.
The comparison of Sucinophyllobius sobrinus, Sucinophyllobius viridis and Protonaupactus microphthalmus shows they are closely related species of the same genus. They share almost the same characters of the rostrum, with moderately defined U-shaped epistome having apically pronounced angles, long and slender antennae with the scape extending behind the anterior margin of the pronotum, funicular article 1 about 0.7 × as long as article 2, and article 2 about 3.5 × as long as wide.
Systematic position of ProtonaupactusAnypotactini and Cyphicerini are the only tribes of Entiminae whose relationships with Protonaupactus still need to be tested. Both tribes contain genera with the following characters: (1) claws free, (2) epistome deeply sinuate, parepistome distinctly protruding and bearing dense bunches of long setae, (3) swinging fossae long, fully visible in dorsal view, (4) pterygia strongly extended beyond rostrum.
The tribe Cyphicerini consists of rather diverse genera which may be divided into two subgroups by the structure of the prothorax and underside of the head: (1) a prosternum subflattened with straight anterior edge; b pronotum with straight anterior edge; c rostrum and head united in a consolidated structure; d underside of rostrum densely covered with scales, its sculpture uniform; e rostrum directed forwards (Mylacorrhinina and Myllocerina) or (2) a prosternum with anterior edge sinuate; b pronotum with distinct postocular lobes; c head and rostrum distinctly separated by transverse constriction; d basal area of underside of rostrum with glabrous integument which has a bare stripe reaching the eyes; e rostrum directed downwards (Acanthotrachelina, Cyphicerina, and Phytoscaphina).
Anypotactini include a group of genera that share a character set characteristic of the subgroup I of Cyphicerini and distinct from this subgroup in the transverse sulcus on the rostrum. However, this tribe displays an enormous variety in rostrum shape, and also shows both dorsal and lateral positions of the antennal scape which is normally folded above the eyes, along the rostrum, or folded obliquely downwards in the scrobe if developed. Both the antennal scape and funicle are quite slender, hence the antennae resemble a brachyderoid rather than otiorhynchoid type, like in Cyphicerini.
Anypotactus Schoenherr 1840, Polydacrys Schoenherr, 1833 and other genera with short rostrum and large pterygia belong to Anypotactini subgroup 1, and Prepodellus Kirsch, 1867, Hyphantus Germar, 1824 and other genera with long rostrum with very small pterygia belong to Anypotactini subgroup 2.
Protonaupactus combines both character sets, Anypotactini and Cyphicerini. Nevertheless, it can be easily distinguished from Cyphicerini subgr. 2 in sharing the basic character set of Cyphicerini subgr. 1 and Anypotactini subgr. 1. From Cyphicerini subgr.1 Protonaupactus differs particularly in the transverse sulcus on the rostrum (Fig. 25, 26). The new data on this fossil genus show that the distinctness between these tribes is not very clear and needs a further investigation.
The authors are pleased to express their gratitude to Carsten Gröhn (GPIH), who provided material for study. They also express sincere thanks to Lars Vilhelmsen and Alexey Solodovnikov (ZMUC) for the opportunity to examine the Baltic amber collection. Wolfgang Weitschat (GPIH) assisted to the authors in their attempts to seek the types of E. Voss which should be deposited in GPIH but now are missing. Steven R. Davis (University of Kansas, Lawrence), George Poinar Jr. (Oregon State University, Corvallis), and two anonymous referees made an essential contribution in improving the English and the content of the manuscript. The use of the data after Vladimir V. Zherikhin (published in
Checklist of prepleistocene fossil weevils of the subfamily Entiminae
(according to the taxonomic interpretation in
V.V. Zherikhin analyzed all available sources mentioning fossil Curculionoidea (Jurassic-Paleogene). Later the results of his analysis were published in the catalogue by
Checklist of prepleistocene fossil Entiminae.
Subfamilia Entiminae Schoenherr, 1823
| NN | Species | age and site of finding |
|---|---|---|
| Tribe Aterpini Lacordaire, 1863 (see note 1 below this table) | ||
| 1 | Genus incertus fossicius Scudder, 1893:75 (Geralophus) | Pg31, Flor |
| Tribe Tropiphorini Marseul, 1863 | ||
| 2 | Vitavitus thulius Kissinger, 1973 | N2, Dece |
| 3 | Rhytideres sexsulcatus (Heer, 1856) (Cleonus) (= Hipporhinus reynesii Oustalet, 1874; Brachycerus annosus Oustalet, 1874) | Pg31st, Aix N13, Agri |
| Tribe Cylydrorhinini Lacordaire, 1863 | ||
| 4 | Dorotheus guidensis Kuschel, 1959 | K2, CerG |
| Tribe Sitonini Gistel, 1848 | ||
| 5 | Sitona (subgenus incertus) lata Théobald, 1937 | ?Pg31, unknown |
| 6 |
Sitona (subgenus incertus) sp. ( |
Pg23, BalJ |
| Tribe Entimini Schoenherr, 1823 | ||
| 7 | Geralophus antiquarius Scudder, 1893 | Pg31, Flor |
| 8 | Geralophus occultus (Scudder, 1876) (Eurhinus) | Pg31, Flor |
| 9 | Geralophus lassatus Scudder, 1893 | Pg31, Flor |
| 10 | Geralophus scudderi Wickham, 1911 | Pg31, Flor |
| 11 | Eudomus robustus Scudder, 1893 | Pg31, Flor |
| 12 | Eudomus pinguis Scudder, 1893 | Pg31, Flor |
| 13 | Hipporhinops sternbergi Cockerell, 1926 | Pg12, Sunc |
| 14 | Genus incertus saxatilis Scudder, 1876 (Eudiagogus) | Pg22, GreR |
| 15 | Genus incertus effossus Scudder, 1876 (Eudiagogus) | Pg22, GreR |
| 16 | Genus incertus compactus Scudder, 1878: 765 (Ophryastes) | Pg22, GreR |
| 17 | Genus incertus heeri Germar, 1849 (Hipporhinus) (= matheroni Nicolas, 1891; similis Nicolas, 1891; intermedius Nicolas, 1891; marioni Nicolas, 1891; pertonii Nicolas, 1891 (Hipporhinus)) | Pg31, Aix |
| 18 | Genus incertus brevis Giebel, 1856 (Hipporhinus) | Pg31, Aix |
| 19 | Genus incertus schaumi Heer, 1856 (Hipporhinus) | Pg31, Aix |
| 20 | Genus incertus pumiceus Scudder, 1893 (Geralophus) | Pg31, Flor |
| 21 | Genus incertus repositus Scudder, 1893 (Geralophus) | Pg31, Flor |
| 22 | Genus incertus atavus Oustalet, 1870 (Bagous) | Pg33, Core |
| Tribe Tanymecini Lacordaire, 1863 (see note 2 below this table) | ||
| 23 | Genus incertus rugosus Deichmüller, 1881 (Thylacites) | Pg3, Kucl |
| 24 | Protainophthalmus asperulus (Heer, 1856) (Cleonus) (including Théobald, 1937) (= Brachyderes aquisextanus Oustalet, 1874, Brachyderes longipes Heer in Oustalet, 1874, Cleonus marcelli Oustalet, 1874) | Pg31, Aix, Cere |
| 25 |
Protainophthalmus margarum (Germar, 1849) (Sitona) (=antiqua Giebel, 1856) (Sitona) |
Pg31, Aix |
| 26 | Protainophthalmus punctulatus (Nicolas, 1891) (Desmidophorus) | Pg31, Aix |
| 27 | Protainophthalmus regularis (Nicolas, 1891) (Hipporhinus) | Pg31, Aix |
| 28 | Protainophthalmus rugicollis (Heer, 1847) (Lixus) | N13, Oeni |
| 29 | Protainophthalmus thaisi Nicolas, 1891 (Desmidophorus) | Pg31, Aix |
| 30 | Protainophthalmus tuberculatus Nicolas, 1891 (Desmidophorus) | Pg31, Aix |
| 31 | Lepropus sp. (Zherikhin, 1992) | N13, Agri |
| 32 | Genus incertus nudus Wickham, 1912 (Pandeleteius) | Pg31, Flor |
| 33 | Genus incertus secunda Wickham, 1912 (Paussopsis) | Pg31, Flor |
| Tribe Phyllobiini Schoenherr, 1826 | ||
| 34 |
Phyllobius sp. ( |
Pg23, BalJ |
| Tribe Polydrusini Schoenherr, 1823 (see note 3 below this table) | ||
| 35 | Polydrusus (Palaeodrosus) archetypus Zherikhin, 1971 | Pg23, BalJ |
| 36 |
Polydrusus (Subgenus incertus) sp. ( |
Pg23, BalJ |
| Tribe Anypotactini Champion, 1911 | ||
| 37 | Paonaupactus sitonitoides Voss, 1953 (=Polydrusus scheelei Voss, 1953; Otiorhynchus pellucidipes Voss, 1972) | Pg23, BalJ |
| 38 | Paonaupactus cephalotes (Voss, 1972) (Phyllobius) | Pg23, BalJ |
| 39 | Protonaupactus microphthalmus Zherikhin, 1971 | Pg23, BalJ |
| 40 | Protonaupactus sobrinus (Voss, 1972) (Phyllobius sbg. Subphyllobius); Wanat et Borowiec, 1986 (Sucinophyllobius) | Pg23, BalJ |
| 41 | Protonaupactus viridis (Wanat et Borowiec, 1986) (Sucinophyllobius) | Pg23, BalJ |
| Tribe Hormorini Horn, 1876 (see note 4 below this table) | ||
| 42 |
Hormorus undulatus (Uhler, 1855) (Chlorophanus) ( |
N1, USA |
| Tribe Pristorhynchini Heer, 1847 (Pristorhynchiden) | ||
| 43 | Pristorhynchus ellipticus Heer, 1847 | N13, Oeni |
| Tribe Naupactini Gistel, 1856 | ||
| 44 | Genus incertus florissantensis Wickham, 1914 (Cyphus) | Pg31, Flor |
| 45 | Genus incertus subterraneus Wickham, 1911 (Cyphus) | Pg31, Flor |
| Tribe Otiorhynchini Schoenherr, 1826 | ||
| 46 | Otiorhynchus boettingensis van Emden in Zeuner, 1931 | N13, Boet |
| Tribe Trachyphloeini Lacordaire, 1863 | ||
| 47 | Trachyphloeus sp. (Klebs, 1910) | Pg23, BalJ |
| Tribe Eudiagogini LeConte, 1874 | ||
| 48 | Promecops tumidirostris Poinar & Brown, 2011 | N11, DomJ |
| Tribe uncertain | ||
| 49 | Laccopygus nilesii Scudder, 1893 | Pg31, Flor |
| 50 | Evopes veneratus Scudder, 1893 | Pg31, Flor |
| 51 | Oligocryptus sectus (Scudder, 1893) (Eucryptus) | Pg31, Flor |
| 52 | Genus incertus atavina Heer, 1847 (Sitona) | N13, Oeni |
| 53 | Genus incertus crassirostris Heer, 1864 (Naupactus) | N13, Oeni |
| 54 | Genus incertus dilapsus Scudder, 1893 (Phyxelis) | Pg22, GreR |
| 55 | Genus incertus durus Scudder, 1893 (Cryptorhynchus) | Pg22, RoaM |
| 56 | Genus incertus eradicatus Scudder, 1893 (Phyxelis) | Pg22, RoaM |
| 57 | Genus incertus evanidus Scudder, 1893 (Omileus) | Pg31, Flor |
| 58 | Genus incertus evigoratus Scudder, 1893 (Phyxelis) | Pg22, WhiR |
| 59 | Genus incertus exanimis Scudder, 1876 (Eudiagogus, Epicaerus) | Pg22, GreR |
| 60 | Genus incertus fortis Cockerell, 1909 (?Syntomostylus) | Pg22, GreR |
| 61 | Genus incertus manderstjernai Heyden et Heyden, 1866 (Tychius) | N11, Rott |
| 62 | Genus incertus margarum Theobald, 1937 (Sitona) [HN] | Pg23, Cela |
| 63 | Genus incertus obdurefactus Scudder, 1893 (Exomias) | Pg22, RoaM |
| 64 | Genus incertus occubatus Scudder, 1893 (Evopes) | Pg31, Flor |
| 65 | Genus incertus perditus Scudder, 1876 (Otiorhynchus) | Pg22, GreR |
| 66 | Genus incertus petrarum Scudder, 1893 (Ophryastes) | Pg22, RoaM |
| 67 | Genus incertus recuperatus Scudder, 1893 (Lachnopus) | Pg31, Flor |
| 68 | Genus incertus rhenanus Meuner, 1923 (Laccopygus) | N11, Rott |
| 69 | Genus incertus subteractus Scudder, 1893 (Otiorhynchus) | Pg22, RoaM |
| 70 | Genus incertus subterraneus Scudder, 1893 (Scythropus) | Pg22, GreR, WhiR |
| 71 | Genus incertus terrosus Scudder, 1878 (Eudiagogus) | Pg22, RoaM, WhiR |
| 72 | Genus incertus venustulus Heyden et Heyden, 1866 (Sitona) | N11, Rott |
| 73 | Genus incertus sp. (Serres, 1829) (Naupactus) | Pg31, Aix |
Notes to table 2.
1. In contrast to the interpretations of Zherikhin, the tribe Aterpini is traditionally treated as belonging to the subfamily Cyclominae (
2. According to V.V. Zherikhin’s unpublished data, genus Protainophthalmus Zherikhin, 1992 comprises 7 species originally described within ‘Cleonus’, ‘Sitona’, ‘Desmidophorus’, ‘Hipporhinus’ and ‘Lixus’ from the Late Paleogene of France and Germany. Protainophthalmus asperulus (Heer, 1856) known by three type prints (see Oustalet 1874: 259–263 + plate 3: Figs 20 and 21), two of them (from Aix) are types. We consider they belong to different species or even different genera: since the first print (see Oustalet 1874: Fig. 20) has a transverse sulcus behind the epifrons, but the second print (Oustalet 1874: Fig. 20) does not have such a transverse sulcus. The original description of Protainophthalmus (
3. The genus Phyllobius Germar, 1824 could not be placed in Polydrusini because of significant differences in head structure: long and closed swinging fossae, antennal scrobes not developed (foveiform) and scape not folding while in rest. V.V. Zherikhin, proposed that the tribes Polydrusini and Phyllobiini are synonyms. He considered that the number of mandibular setae is constant and may be used for discrimination of suprageneric groups (
4. According to the current interpretation, the tribe Hormorini is assigned to Entiminae (