(C) 2011 Donald R. Davis. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The new genus Macrosaccus Davis & De Prins is proposed for three species formerly assigned to the genus Phyllonorycter: Macrosaccus robiniella (Clemens), Macrosaccus morrisella (Fitch), and Macrosaccus uhlerella (Fitch); two new, closely related species: Macrosaccus neomexicanus Davis and Macrosaccus gliricidius Davis, are also proposed. Descriptions of the adults, pupae, larvae, life histories, and distributions are supplemented with photographs, line drawings, and scanning electron micrographs. Larvae of all species are serpentine/blotch leaf miners on various genera of the plant family Fabaceae. The genus is endemic to the New World, with the invasive species Macrosaccus robiniella now widely established in Europe.
Biogeography, DNA barcodes, host plants, hypermetamorphosis, genital morphology, larval morphology, Lithocolletinae, pupal morphology, leaf mining, taxonomy
The gracillariid subfamily Lithocolletinae includes 503 species (
Characters defining genera within the Lithocolletinae are still being investigated. Most of these concern the life history and morphology of the preimaginal stages (
The purpose of this paper is to propose and diagnose a new lithocolletine genus, Macrosaccus, and to document the five species we recognize within this New World group. This study is long overdue because one species, Macrosaccus robiniella (Clemens), has become a serious invasive pest on the introduced Robinia pseudoacacia L. (Fabaceae) over much of Europe. With this contribution we also attempt to broaden the understanding of the generic definitions within Lithocolletinae. We transfer three previously known Phyllonorycter species to Macrosaccus, clarify the synonymy, and designate lectotypes whenever possible for the species-group taxa. Additionally, we provide DNA barcodes as identification aids and descriptions of two new congeneric species which also were reared from Fabaceae.
MethodsCollecting and rearing. Field investigations were carried out in Europe (Belgium), Canary Islands (La Palma), and in several states within the United States (Arizona, Illinois, Maryland, New Mexico). All specimens examined in this study were reared from species of Fabaceae which are summarized in Table 1.
Leaves containing mines with larvae were placed in plastic bags or rearing containers periodically moistening the lids protecting the specimens from drying out. Specimens were pinned, spread and mounted in the usual way for morphological examination. Some voucher samples of reared specimens were fixed in 100% ethanol for DNA analysis. Larvae and pupae collected on Robinia pseudoacacia L. were preserved in 75% ethanol.
Table 1. Macrosaccus and four Phyllonorycter species that feed on Fabaceae.
| Moth species | Host plant species | Country | Reference |
|---|---|---|---|
| Macrosaccus gliricidius Davis, sp. n. | Gliricidia sepium (Jacq.) | Guadeloupe, Honduras | Present study |
| Macrosaccus morrisella (Fitch, 1859) | Amphicarpa bracteata (L) Fernald | Canada | Present study |
| Macrosaccus morrisella (Fitch, 1859) | Amphicarpa bracteata (L) Fernald | U.S.A. |
|
| Macrosaccus morrisella (Fitch, 1859) | Amphicarpa sp. | Canada | Present study |
| Macrosaccus morrisella (Fitch, 1859) | Strophostyles leiosperma (Torrey & A. Gray) | Canada | Present study |
| Macrosaccus neomexicanus Davis, sp. n. | Robinia neomexicana Gray | U.S.A. | Present study |
| Macrosaccus robiniella (Clemens, 1859) | Robinia hispida L. | U.S.A. |
|
| Macrosaccus robiniella (Clemens, 1859) | Robinia pseudacacia L. | Belgium |
|
| Macrosaccus robiniella (Clemens, 1859) | Robinia pseudacacia L. | U.S.A. |
|
| Macrosaccus robiniella (Clemens, 1859) | Robinia viscosa Vent | U.S.A. |
|
| Macrosaccus uhlerella (Fitch, 1859) | Amorpha fruticosa L. | U.S.A |
|
| Phyllonorycter cytisifoliae (M. Hering, 1927) | Chamaecytisus proliferus (L.) Link | Canary Islands: La Palma |
|
| Phyllonorycter foliolosi Walsingham, 1908 | Adenocarpus foliolosus (Ait.) DC | Canary Islands: La Palma |
|
| Phyllonorycter medicaginella (Gerasimov, 1930) | Medicago sativa L. | Belgium |
|
| Phyllonorycter nigrescentella (Logan, 1851) | Vicia sepium L. | Belgium |
|
Adults were examined externally using either MZ12.5
or Nikon SMZ 1500 stereomicroscopes (maximum magnification 200×).
Genitalia were prepared following
For scanning electron microscopy, the immatures were
immobilized by moment freezing at -27°C. Pupae were sputtered-coated
with gold using a Bal-TEC/SCD 005 Sputter Coater. The images were taken
with a Jeol MP 35060 camera combined with a Jeol JSM-5400 LV Electron
Scanning Microscope and processed using the Orion 4 High Resolution
Image Grabbing System software. Larval terminology follows
The spellings of all species names were retained as originally proposed.
Molecular analysisSequences of the 658bp Cytochrome Oxidase I were
generated at the Biodiversity Institute of Ontario, University of
Guelph, Canada. DNA was extracted from legs or entire bodies of adult
moths using a QIAGEN DNeasy Extraction Kit (Qiagen, Inc., Valencia,
CA). Primers LepF1 and LepR1 (
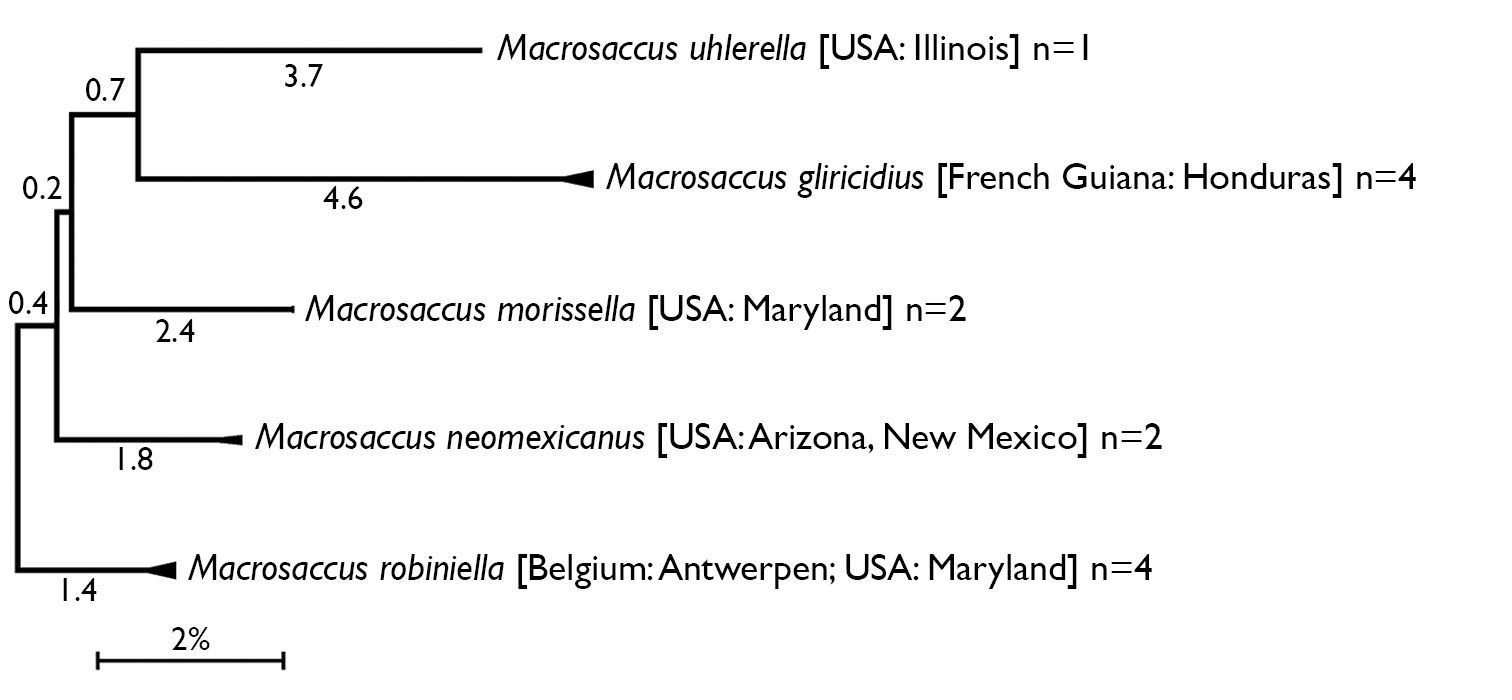
Compressed subtree sequenced data for cytochrome c oxidase I (COI) of Macrosaccus, derived from 13 samples among 5 species based upon neighbor-joining analysis with Kimura 2-parameter model. Numbers above branches indicate branch length. Sequence lengths obtained for all samples were 658bp each.
Mitochondrial DNA (COI) sequence divergence (%) among species of Macrosaccus. Uncorrected average pairwise distances are shown for the barcoding region of (COI). Shaded cells contain means within species distances. Cells below shaded diagonal contain mean between species distances. Species abbreviations in the heading refer to species listed in left column.
| gliri | morr | robi | neom | uhle | |
|---|---|---|---|---|---|
| Macrosaccus gliricidius | 0.4 | ||||
| Macrosaccus morrisella | 8.1 | 0.0 | |||
| Macrosaccus robiniella | 8.1 | 4.7 | 0.4 | ||
| Macrosaccus neomexicanus | 7.1 | 4.5 | 4.1 | 0.5 | |
| Macrosaccus uhlerella | 8.6 | 6.7 | 6.5 | 7.3 | 0.0 |
Whenever possible the primary types of every species were examined. Lectotypes were designated from the syntypic series whenever available.
Abbreviations of Institutions from which specimens were examined are:
ANSP Academy of Natural Sciences, Philadelphia, Pennsylvania, USA.
BMNH The Natural History Museum (formerly the British Museum (Natural History)), London, United Kingdom.
CCDB Canadian Centre for DNA Barcoding, University of Guelph, Canada
CNC Canadian National Collections of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Canada.
CU Cornell University, Ithaca, New York, USA.
INHS Illinois Natural History Survey, Champaign, Illinois, USA.
MCZ Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA.
RMCA Royal Museum for Central Africa, Tervuren, Belgium
USNM Collections of the former United States National Museum, now deposited in the National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA.
Systematic Accounturn:lsid:zoobank.org:act:2451DAED-FEB2-4E03-B86C-88F10584A067
Lithocolletis robiniella Clemens, 1859, by original designation.
Macrosaccus is assigned to the subfamily Lithocolletinae on the basis of the following putative morphological synapomorphies: hindwing vein Rs parallel to vein M and costal margin; adults rest with body parallel to surface; adult head with occipital tuft; and pupation occurring within the mine.
Superficially, Macrosaccus is similar to nearly all other genera of Lithocolletinae, sharing such characters as a well developed occipital tuft; a forewing pattern accentuated with oblique, whitish strigulae; and by the mode of pupation which occurs inside a silken cocoon within the whitish blotch mine usually on the underside of the host leaf without any prepared exit opening. However, in contrast to the typically solitary larvae and pupae of other Lithocolletinae genera, those of Macrosaccus are often gregarious inside a single, composite mine. The wing venation of Macrosaccus is similar to that of Cameraria and Phyllonorycter in possessing five apical veins, but it differs from the two latter genera in having Rs4 rising either from the base of Rs3 or stalked with Rs3. The hindwing venation is similar to Cameraria, Chrysaster, Leucanthiza, Neolithocolletis, and Phyllonorycter, but differs from Cremastobombycia, Hyloconis, Porphyrosela, and Protolithocolletis in the absence of vein M2. In the male genitalia, the sternum 8 is not produced caudally as in Chrysaster, Leucanthiza, and Protocolithocolletis. In Cameraria, Cremastobombycia, Hyloconis, Neolithocolletis, Phyllonorycter, and Porphyrosela, the sternum 8 forms a large flap underlying the valvae. The apex of the tegumen in Macrosaccus possesses a pair of tiny setae as in Cameraria, Chrysaster and Porphyrosela, but unlike Phyllonorycter which lacks apical setae. The transtilla of Macrosaccus is complete like that of other lithocolletine genera, but it differs from that of Cameraria and Hyloconis where it is incomplete. The female genitalia of Macrosaccus are characterized by numerous, microscopic spine-like signa which are scattered within the subcaudal part of corpus bursae (in other lithocolletine genera the corpus bursae bears other types of signa). Though the adult head of Macrosaccus is very similar to that of Protolithocolletis, the venation between these two genera differs with the forewing of Protolithocolletis more developed in possessing veins Rs1 and M2. The pupae provide perhaps the best characters for generic distinction, with that of Protolithocolletis lacking the spinose accessory cremaster ridge on sternum 7, which is characteristic for Macrosaccus.
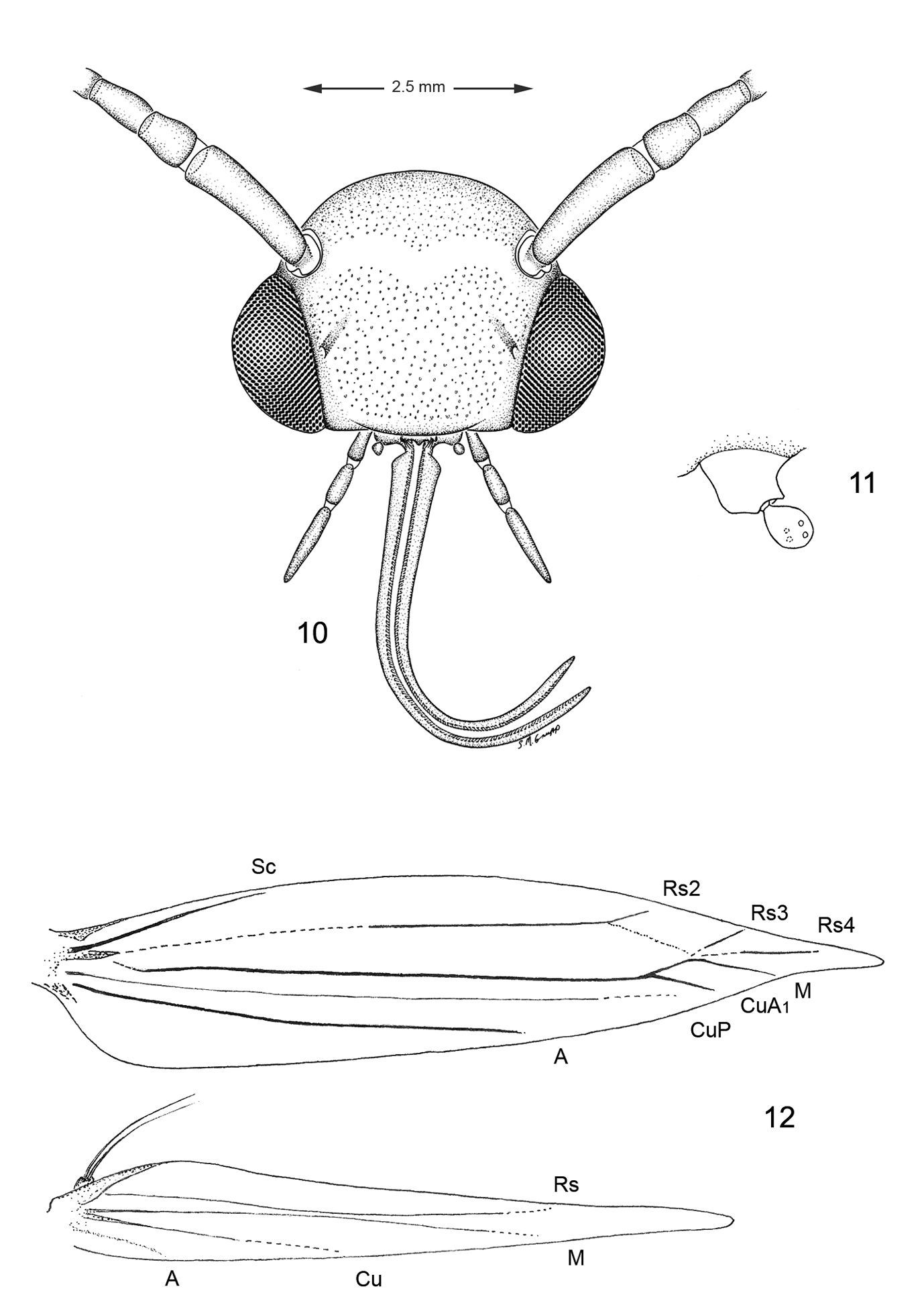
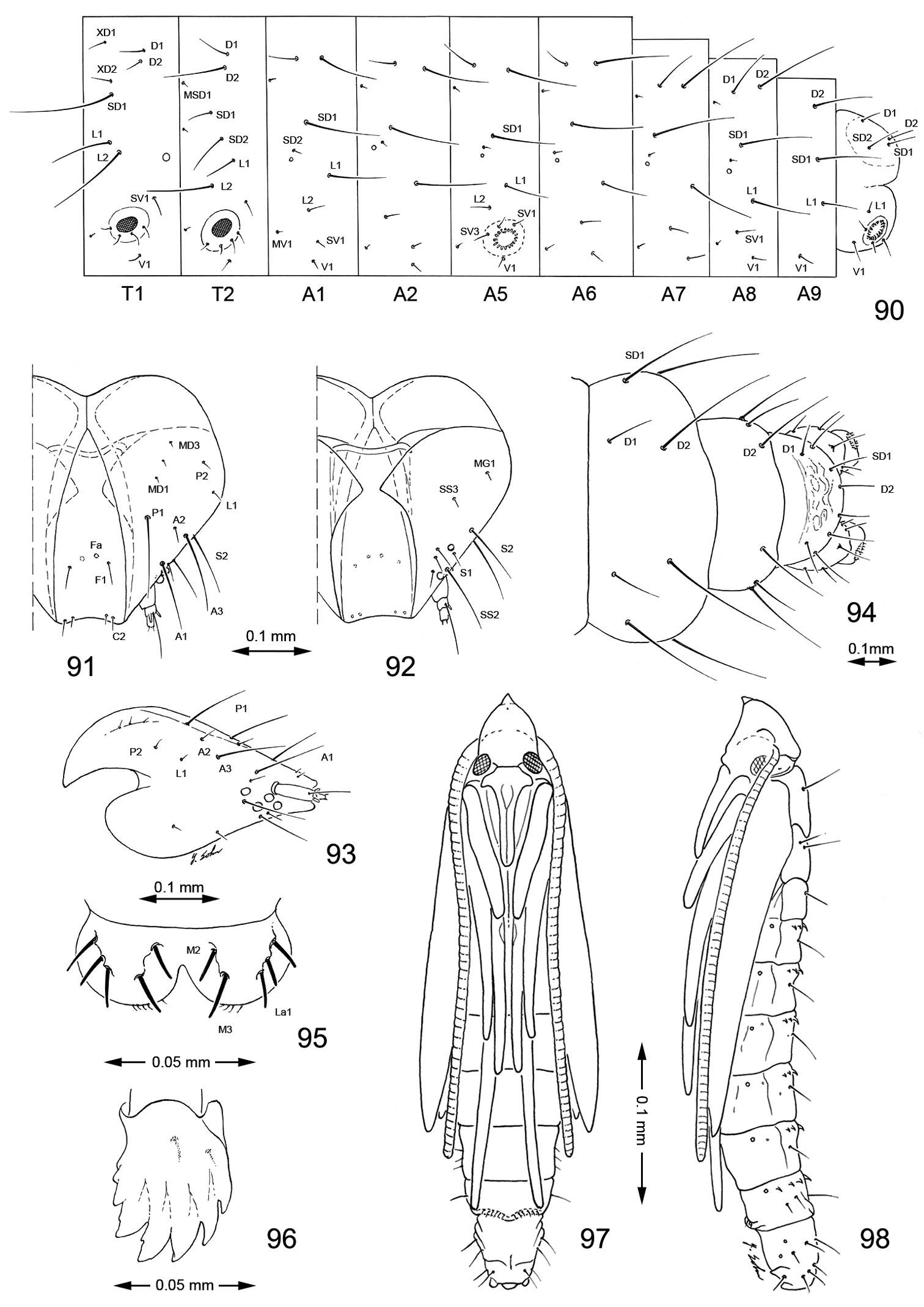
Head (Figs 10, 11). Vertex covered with long dense tuft of piliform scales; frons with smooth appressed scales; eyes of midsize; interocular index (= vertical eye diameter/interocular distance) ~ 0.75–0.96. Antenna about 0.7× the length of forewing (n=9), smooth scaled, with a single row of scales per segment; scape with dense pecten. Proboscis well developed, naked, ca. 1.8–2.5× length of labial palpus. Maxillary palpus very short, rudimentary, ~ 0.5× length of labial palpomere II, and directed laterally; consisting of 2 articulated segments; basal 2 segments fused; segment 3 free, spherical. Labial palpus slender, drooping, with ratio of segments from base 1.5: 1: 2.
Thorax (Fig. 12). Forewing slender, maximum width/length ratio ca. 0.2, narrow at apex. Venation with 8 veins, apical part with 5 veins; Sc strong, extending nearly to costa, basal half of R indistinct, Rs2 present, Rs3 arises from apex of the cell, position of Rs4 variable, arising either from base of Rs3 or stalked with Rs3, M and CuA1 separate, CuP indistinct (fold) for entire length, 1A strong, separate, discal cell either open (with absence of crossvein between Rs2 and Rs3) or closed, extending ~ 0.78 of wing length. Hindwing lanceolate, maximum width 0.12 that of length, venation reduced, similar to Phyllonorycter; Sc very short, Rs very long, extending almost to apex; basal 2/3 of M indistinct, parallel to Rs, distal part of M ends near distal 3/5: basal half of Cu strong, distal half indistinct, ending slightly before midway along dorsum; frenulum a single stout bristle in male, 2 tightly appressed bristles in female, retinaculum in male consisting of a broadly triangular curved fold from the ventral base of Sc and a few stiff, forward directed scales situated on the posterior part of Cu.
Abdomen. The margins of the abdominal opening strongly sclerotized and broad laterally, the sclerotized margination of abdomen opening unconnected on T2, S2 apodemes long, ~ half the length of S2, generally slender but more stout at basal 1/3 and very slender at distal 2/3; two pairs of tiny spinules on S2 sublaterally, and a pair of tiny spinules on S3–S6 sublaterally. Sternum 8 in male undeveloped.
Male genitalia. Tegumen relatively short, broad, moderately sclerotized laterally. Caudal portion covered with numerous tiny setae. A pair of long, slender setae present at apex of tegumen. Vinculum broad, U-shaped with very slender, elongate saccus which ranges from 1.1–1.7× the length of valva. Valvae symmetrical, moderately broad, costal margin nearly straight to slightly curved; ventral margin variable between species from slightly convex to slightly concave over distal half with apex varying from fully round to abruptly narrowing; median surface of valva with sparse setae of medium length; apex of valva densely covered with longer, more stout setae. Transtilla complete and well developed, laterally expanded into rounded lobes. Aedeagus very long, nearly as long as entire genital capsule (from apex of tegumen to anterior end of saccus), straight and slender, of uniform diameter along its length; caudal end of vesica usually with long, slender cornuti; phallobase ~ ¼ total length of aedeagus.
Female genitalia. Papillae anales flattened, strongly interconnected, covered with short setae mostly along apical margin; basal bar broad but weakly sclerotized. Posterior apophyses slightly longer than width of papillae anales, straight and slender. Segment 8 short, weakly sclerotized. Anterior apophyses as long or slightly shorter than posterior apophyses, with moderately broad bases, then slender extending to caudal 1/3 of segment 7. Ostium bursae opens medially, near caudal margin of segment 7; sterigma simple, without cuticle folds, antrum funnel-shaped, narrowing anteriorly. Subcaudal area of segment 7 mottled with numerous tubercles. Ductus bursae ~ 2× times longer than segment 7; a membranous accessory bursae ~ 2/3 the length of corpus bursae, arising from middle to anterior 1/3 of ductus bursae, with a smaller lateral pouch arising ~ midway along side of accessory bursae. Corpus bursae 1.0–2.0× the length of segment 7, subcaudal region of corpus bursae usually with scattered spicules or with spicules arranged in linear rows in Macrosaccus robiniella.
Hypermetamorphic with five larval instars. Earliest instars (1–3) highly modified sapfeeders with strongly depressed bodies and reduced chaetotaxy; 3 pairs of stemmata arranged in a lateral, anterior cluster on head; labrum short and broad, bilobed; anterior margin broadly concave, roughened, with minute dentations along inner margin of lateral lobes; maxillary and labial palpi absent. Later instars (4 and 5) tissue feeders, with cylindrical bodies. Head approximately round with full complement of mouthparts; 4 pairs of stemmata present; antenna 3-segmented with first segment moderately long; labrum strongly bilobed with raised median portion on each lobe; M1 absent; numerous secondary spines visible from inner, ventral perimeter of labrum. Thorax with SD1 elongate, immediately ventral to XD2; SD2 absent on T1, present on T2–3L group bisenose on T1–3. SV unisetose on T1–3. Legs relatively short but fully developed; coxae widely separated, with 4 coxal setae. Abdomen with D and SD groups bisetose on A1–8, 10; unisetose on A9; L group bisetose on A1–5, unisetose on A6–10; prolegs present on A3–5, 10; crochets of A3–5 arranged in a uniordinal circle; anal proleg with crochets arranged in a uniordinal semicircle opened caudally; anal plate with 4 pairs of setae.
Head with vertex terminating in a relatively short, broadly triangular, acute frontal process (cocoon cutter). Abdomen mostly covered dorsally and ventrally with dense, minute spines; dorsum of A2–7 with a single anterior row of short, stout spines; caudal half of sternum 7 with a transverse ridge (accessory cremaster) bearing ~ 18–21 mostly longitudinal rows of short, blunt spines; cremaster of A10 greatly reduced, nearly absent, consisting of 1–2 pairs of minute tergal spines.
The generic name is derived from the ancient Greek μακρο- (long) and σάκκος (bag) in reference to the elongate saccus in the male genitalia. Gender masculine.
Several morphological specializations closely associate Macrosaccus with the genera Chrysaster, Cremastobombycia, and Phyllonorycter. Some of these involve the moderately produced proboscis (~ 2× the length of the labial palpi) and the very reduced, two-segmented (with basal segment relatively enlarged), broad maxillary palpi (Figs 10, 11). The wing venation of all three genera is nearly identical and is among the most reduced within Gracillariidae. Only three branches of Rs are present in the forewing, accompanied by single branches of M and Cu (Fig. 12). Venation in the lanceolate hindwings is similarly reduced with only three major veins usually preserved (Rs, M, and Cu) in addition to the extremely basal Sc+R1. The position of Rs4 in Macrosaccus differs somewhat from that in the aforementioned three genera in arising either from the base of Rs3 or stalked with Rs3. Perhaps more significantly is that the discal cell is usually open in Macrosaccus due to the total or near absence of the Rs2-Rs3 crossvein. This crossvein is usually present in the other genera.
The most distinguishing feature in the male genitalia of Macrosaccus is the extremely long, rodlike saccus, whence the generic name is derived. The male saccus in Chrysaster, Cremastobombycia, and Phyllonorycter is either undeveloped or much shorter and stouter (except in two Afrotropical species Phyllonorycter farensis and Phyllonorycter obandai).
Likewise sternum 8 in all males of these three genera is extended
caudally as a variably lengthened plate beneath the genitalia, compared
to being unmodified in Macrosaccus. The female genitalia of Macrosaccus
typically possess a relatively large, variably shaped accessory bursa
arising approximately midway along the long, slender ductus bursae. The
corpus bursae contains dense patches or faint rows of minute spines.
The accessory bursae in Phyllonorycter originates more caudally near the ostium, and usually two, circular and variably sclerotized signa are present (
The pupa of Macrosaccus
is characterized by an accessory cremaster on abdominal sternum 7 that
is unlike that of any other known gracillarid genus. This consists of a
raised transverse ridge bearing ~ 18–21 mostly longitudinally oblique
rows of short, blunt spines (Figs 84, 85). The accessory cremaster when present in Phyllonorycter
differs greatly in consisting of a raised triangular area located
midventrally on sternum 7 with 1–2 pairs of stout spines projecting
laterally (
In addition to the foregoing morphological
characters, a preliminary molecular phylogeny based on ten genes also
strongly places Macrosaccus apart from Phyllonorycter (
Five species, all indigenous to the New World, are currently recognized in the new genus Macrosaccus. The high sequence divergence of the barcoding region of COI (> 7%) between species (Fig. 1, Table 2) further confirms the species concepts previously determined by morphological and larval host information. Sequence divergences within species for the 12 samples with multiple specimens were low and varied between 0–0.62% (Macrosaccus gliricidius), 0% (Macrosaccus morrisella), 0–0.46% (Macrosaccus neomexicanus), and 0–0.71% (Macrosaccus robiniella). The latter included specimens from Belgium and the United States.
Diagnostic comparisons between adults and pupae of Macrosaccus and Phyllonorycter.
| Character | Macrosaccus | Phyllonorycter |
|---|---|---|
| Sternum 8 | Unmodified (not extended) | Caudally extended |
| Male genitalia: apex of tegumen | With 2 setae | No setae |
| Male genitalia: saccus | Saccus longer than valva in all species | Saccus shorter than valva except in two Afrotropical species |
| Male genitalia: setation of valva | Only apex of valva densely covered with elongate, stout setae | Other types of setation |
| Male genitalia: aedeagus | ca. 2× as long as genital capsule from apex of tegumen to anterior end of vinculum | Significantly shorter except in three Afrotropical species |
| Female genitalia: signum | Consisting of numerous microscopic spicules scattered or in linear series on subcaudal part of corpus bursae | Signa not scattered, often confined to 1–2 moderately sclerotized, oval areas |
| Forewing venation | Rs4 arises either from base of Rs3 or is stalked with Rs3 | Rs3 and Rs4 separate |
| Pupa: accessory cremaster of sternum 7 | An elongate, transverse ridge bearing 18-21 oblique rows of minute spines | No transverse ridge; instead located midventrally, with 1-2 pairs of lateral spines |
| 1 | Valva of male of uniform width to broadly rounded apex (Fig. 32); host Gliricidia sepium | Macrosaccus gliricidius |
| – | Male valva narrowing before apex | 2 |
| 2 | Valva gradually tapering to narrow apex (Fig. 23); host Robinia neomexicana | Macrosaccus neomexicanus |
| – | Valva constricted before apex | 3 |
| 3 | Valva strongly constricted at middle (Fig. 28); distal half less than half the width of sacculus; host Amorpha fruticosa | Macrosaccus uhlerella |
| – | Valva slightly constricted near apex | 4 |
| 4 | Forewing with a short, oblique white streak from base of costa; median white fascia complete, slightly curved outward (Fig. 5). Valvaconstricted before apex; arms of transtilla reduced (Fig. 18); hosts Amphicarpa bracteata, Strophostyles leiosperma | Macrosaccus morrisella |
| – | Forewing without basal white costal streak; median white fascia usually broken, strongly oblique (Figs 2–4). Valva constricted closer to apex; arms of transtilla broader (Fig. 13); hosts Robinia pseudoacacia, Robinia hispida, Robinia viscosa | Macrosaccus robiniella |
http://species-id.net/wiki/Macrosaccus_robiniella
Figs 1–4, 10–17, 36–40, 59–98, Tables 1, 2, 4, 5The overall appearance of this widespread eastern North American (and now well established European) species most closely resembles that of the more southwestern US species, Macrosaccus neomexicanus. The more abruptly constricted apical region of the valvae and the minute, longitudinally oriented striae and spicules of the corpus bursae readily distinguish it from Macrosaccus neomexicanus.
(Figs 2–4). Forewing length 2.3–3.1 mm.
Head: Frons smooth, shiny white. Vertex extremely rough; vestiture consisting of a tuft of elongate, piliform, mostly dark brown, intermixed with white, scales. Labial palpus white. Antenna mostly dark fuscous dorsally for most its length, with dark area narrowing to a more slender dark streak toward basal 1/4–1/3 its length; antenna mostly white ventrally; apical segment entirely white.
Thorax: Dark brown dorsally, white ventrally; tegula dark brown, with pale grey to white suffusion anteriorly. Forewing pattern complex, costal half mostly light orange brown crossed by 4 equally spaced, white costal strigulae, each bordered basally, sometimes faintly, by black to dark grey and distally by light grey scales; basal 2 strigulae strongly oblique; a fifth, minute, white strigula sometimes arising from black apical spot before forewing apex. Basal third and dorsal half of forewing usually darker, mostly black to sometimes pale golden grey between strigulae; slender white streak from base of wing usually indistinct or absent; a greyish, oblique strigula often evident near base of wing which connects with a larger, more distinct greyish strigula from dorsal margin; dorsal margin also with 3, usually less distinct white strigulae approximately opposite to distal 3 white strigulae from costa; basal dorsal strigula usually contiguous with second costal strigula. Apex of forewing with a large black apical spot, which is rarely reduced; fringe mostly light grey. Hindwing, including fringe, uniformly grey. Foreleg mostly dark fuscous dorsally, white ventrally, with 2 white annuli around basal tarsomeres; midleg with 2 oblique bands of white dorsally over tibia; tarsomeres more broadly banded with white dorsally; hindleg mostly white with much of tibia pale fuscous dorsally, and with 3 broad, pale fuscous annuli dorsally over tarsomeres.
Abdomen: dark fuscous dorsally and white ventrally with greyish suffusion on anterior portion of segments 2–7 laterally and sometimes ventrally on A8.
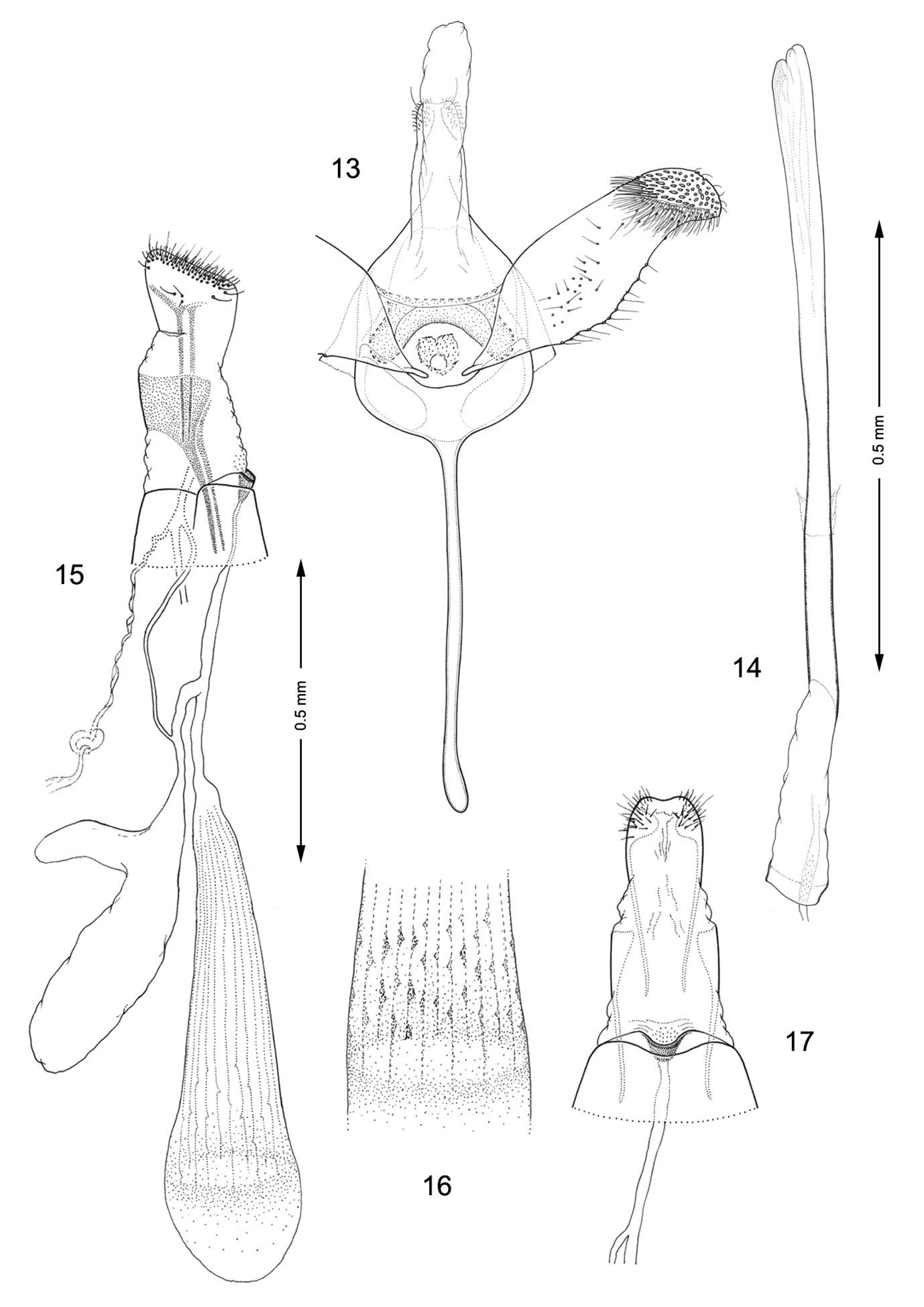
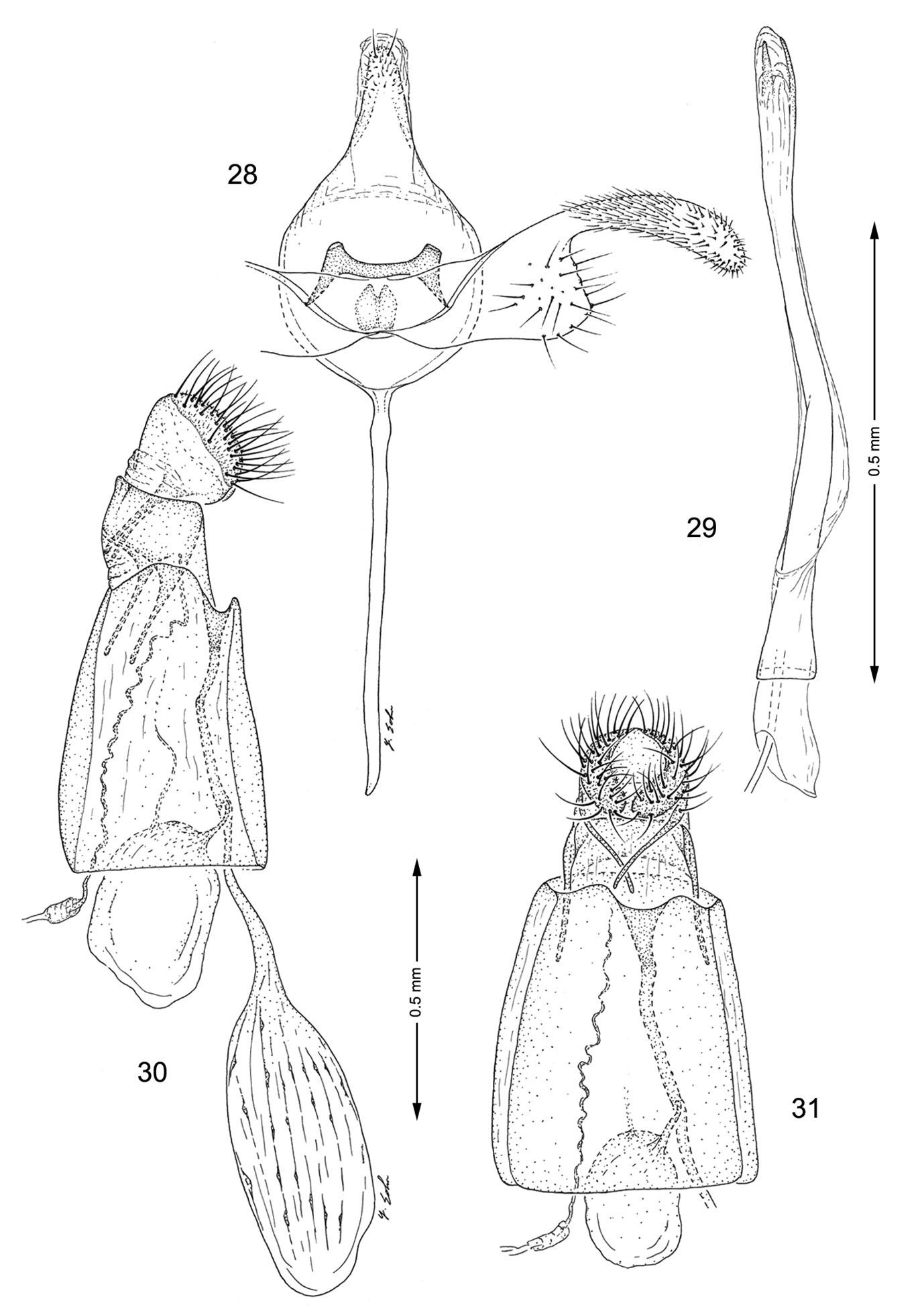
Male genitalia (Figs 13, 14): Valva relatively simple, similar to Macrosaccus morrisella in form, gradually constricted before apex; apex rounded, densely setose; base of costa fused to moderately thickened, arched transtilla; transtilla with rounded knoblike lateral projections that extend anteriorly in repose (more caudally when valvae are spread widely apart); saccus a slender, elongate rod ~ 1.2× length of valva. Aedeagus very long and uniformly slender, ~ 2.1× length of valva.
Female genitalia (Figs 15–17): Ductus bursae long and slender, nearly half the length of elongate corpus bursae. Accessory bursae ~ 2/3 the length of corpus bursae, arising from anterior 1/3 of ductus bursae; with a smaller lateral pouch arising ~ midway along side of accessory bursae. Corpus bursae gradually broadening anteriorly, with faint longitudinal striae in wall which bear longitudinal rows of low, dentate ridges around anterior third of corpus bursae; walls of anterior end (distal 1/5) of corpus bursae entirely membranous.
Adults 2–4. Macrosaccus robiniella. 2♂, USA: Maryland, (2.8 mm) 3 ♂, USA: Maryland, (3.0 mm) 4 BELGIUM: Antwerp, (3.0 mm) 5 Macrosaccus morrisella, ♂, USA: Maryland, (2.5 mm) 6 Macrosaccus neomexicanus, USA: Arizona, (3.2 mm) 7 Macrosaccus uhlerella, USA: Illinois, (2.5 mm) 8 Macrosaccus uhlerella, USA: Illinois, (3.0 mm) 9 Macrosaccus gliricidius, ♂, HONDURAS: Morazán, (2.4 mm). (Forewing lengths in parentheses).
Adult morphology, Macrosaccus robiniella. 10 Head 11 Detail of left maxilla 12 Wing venation.
Genitalia, Macrosaccus robiniella. 13–14 Male. 13 Genital capsule, ventral view 14 Aedeagus. 15–17 Female. 15 Lateral view 16 Detail of signa within corpus bursae 17 Segments 7–10, ventral view.
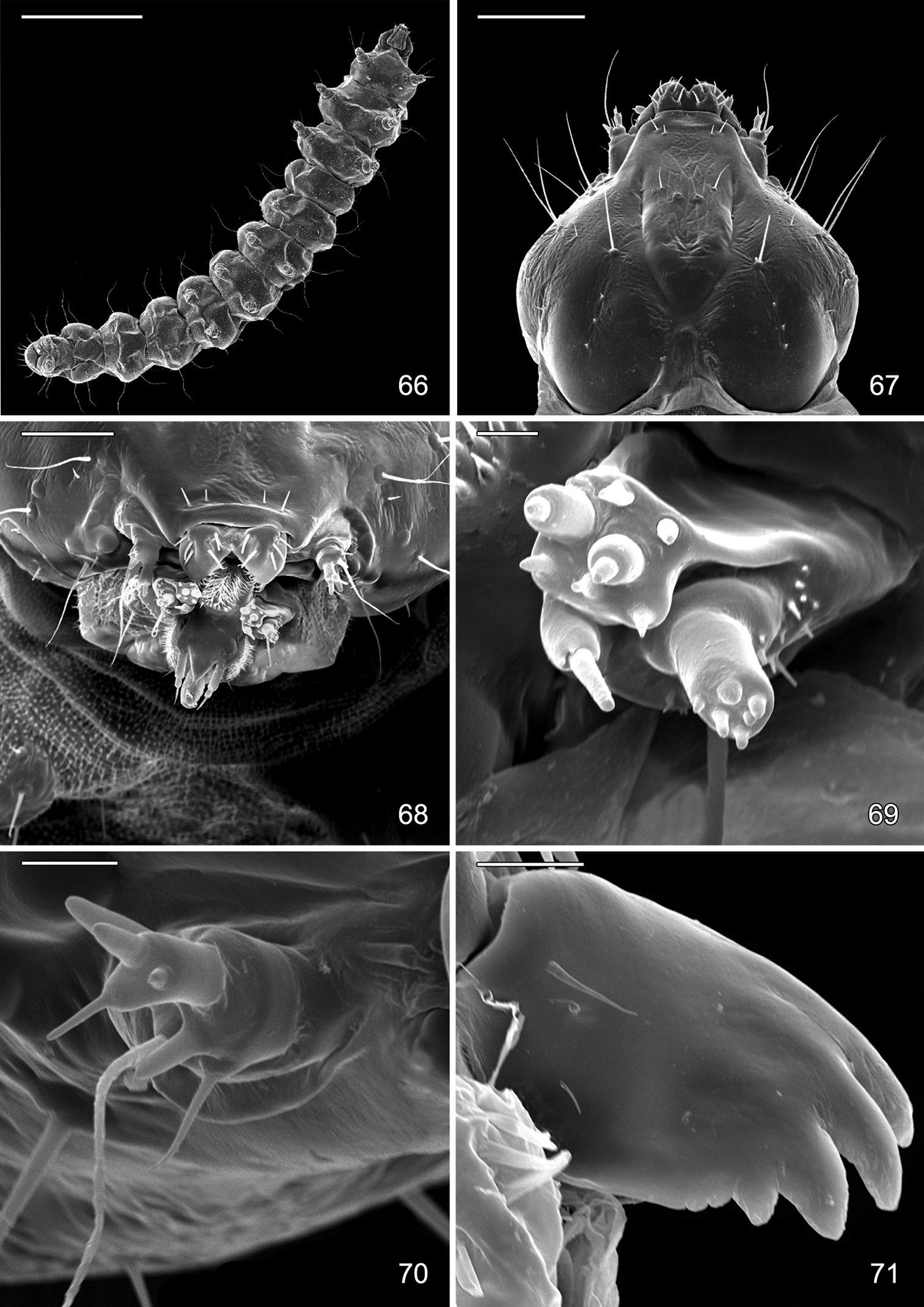
(Figs 59–80, 90–96). Hypermetamorphic; five larval instars. Earliest instars (1–3) highly modified sapfeeders with strongly depressed bodies and reduced chaetotaxy; maximum length 3.7 mm, width (T1): 0.9 mm. Later instars (4 and 5) tissue feeders, with cylindrical bodies; maximum length: 4.7 mm, width: 0.7 mm; body colour pale green to white with notal plates and pinnacula smooth, reduced and unpigmented (indistinct).
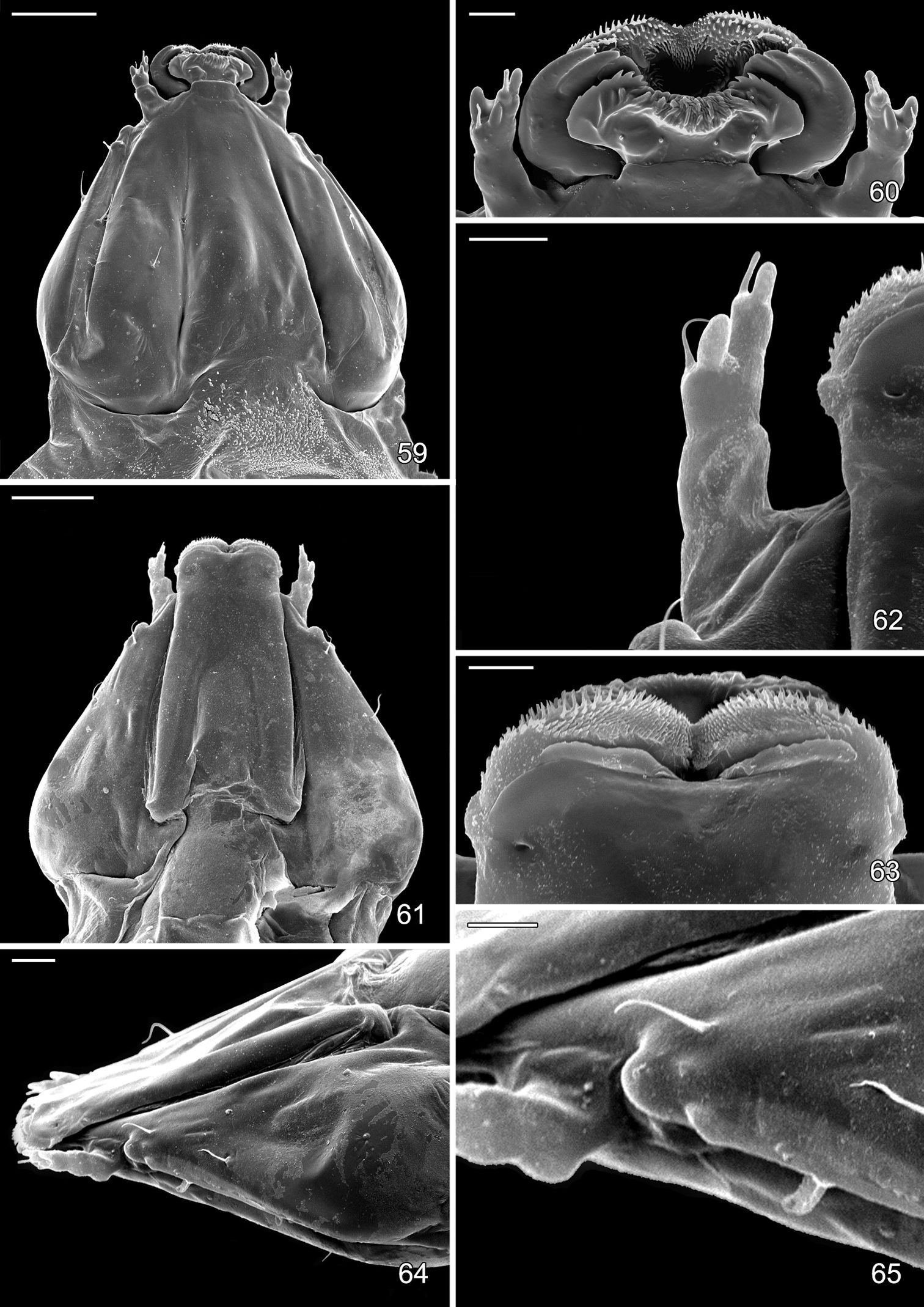
Head: Maximum width (third instar) 0.4 mm; greatly depressed, triangular. Most setae lost or reduced; 3 pairs of stemmata arranged in a lateral, anterior cluster on head. Labrum (Fig. 60) short and broad, bilobed, with 2 pairs of extremely reduced, peglike dorsal setae; anterior margin broadly concave, roughened, with 4–5 minute dentations along inner margin of lateral lobes. Mandibles broadly rounded, flattened, with 2 short cusps lateral to relatively large inner plate. Labium smooth, lateral margins subparallel; anterior margin shallowly notched at middle; spinneret absent. Maxillary and labial palpi absent. Hypopharynx broad, densely covered with minute spines along anterior margin; with margin slightly excavated at middle. Antenna 3-segmented, with short basiconic sensilla as shown (Fig. 62). Body: Setae generally reduced. Legs, prolegs, and crochets absent.
Head: Approximately round with full complement of mouthparts; brown; maximum width (fifth instar) 0.35 mm. Frons elongate, ~ 0.85× the distance to epicranial notch. Ecdysial line terminating near epicranial notch. Chaetotaxy (Figs 91–92) relatively complete; all three MD setae present, arising caudad to P1. P1 arising adjacent to ecdysial line. P2 reduced, arising slightly caudad to reduced L1. Setae AF1–2 absent. A2 arising near A3 in a line between P1 and A3. C1 and 2 reduced, closely adjacent. Four stemmata present. Antenna 3-segmented; first segment moderately long; sensilla as shown in Fig. 70. Labrum (Figs 68, 95) strongly bilobed with raised median portion on each lobe; M1 absent; numerous secondary spines visible from inner, ventral perimeter of labrum. Mandible (Figs 71, 96) with three large median cusps and one smaller median and two lateral cusps; mandibular setae variable (1–2) and located on anterior surface. Hypopharynx with dense, well developed dorsal spines. Maxilla as shown in Fig. 69. Spinneret a relatively short tube with a simple, rounded apex. Labial palpus with a relatively long basal segment bearing one short sensillum and a much shorter (~ 0.25× length of basal segment) apical bearing a single long apical sensillum ~ 2× length of apical segment. Thorax: Setae XD1 and 2 short, of equal lengths on prothorax (T1). SD1 elongate, immediately ventral to XD2; SD2 absent on T1, present on T2–3L group bisenose on T1–3. SV unisetose on T1–3. Legs (Fig. 76) relatively short but fully developed; coxae widely separated, with 4 coxal setae; pretarsal claw moderately curved. Abdomen: D and SD groups bisetose on A1–8, 10; unisetose on A9; L group bisetose on A1–5, unisetose on A6–10. Prolegs present on A3–5, 10; crochets of A3–5 consisting of 17–24 small hooks arranged in a uniordinal circle; anal proleg with crochets consisting of 15–18 small hooks arranged in a uniordinal semicircle opened caudally (Fig. 79). Anal plate with 4 pairs of setae.
(Figs 36–40). The mine begins as an elongate serpentine track (Fig. 37) which enlarges to an elongate-oval, whitish blotch Fig. 36, 38) located on one side of the midrib and usually on the under (abaxial) side of the leaflet. Eventually the mine becomes slightly tentiform due to the silk laid down by the later instar larvae.
(Table 1). Fabaceae: Robinia pseudoacacia L. (
(Figs 36–40). The egg of Macrosaccus robiniella
is deposited externally usually some distance from the leaf edge or
midrib. Five larval instars have been observed by counting head capsules
within mines in North America and Belgium.
In addition to Hymenoptera parasitoids, other Lepidoptera larvae have been noted within the mines of Macrosaccus robiniella (
(Table 4). Fifty seven species (including two unidentified) ofHymenoptera, the great majority of which are members of Eulophidae (
(Figs 82–89, 97, 98). Maximum length 3.6 mm; width 0.9 mm. Vertex with frontal process (cocoon cutter) relatively short, broadly triangular, acute (Figs 81, 82). Forewing extending to anterior margin of A6; antenna slightly longer to middle of A6; hindleg extending to A7. Abdomen mostly covered dorsally and ventrally with dense, minute spines; dorsum of A2–7 with a single anterior row of short, stout spines (Figs 83, 98); caudal half of sternum 7 with a transverse ridge (accessory cremaster) bearing ~ 18–21 mostly longitudinal rows of short, blunt spines (Figs 84, 85). Cremaster of A10 greatly reduced, nearly absent, consisting of 1–2 pairs of minute tergal spines.
Parasitoids of Macrosaccus robiniella.
| Parasitoid name | Family | Country | Reference |
|---|---|---|---|
| Achrysocharoides cilla (Walker, 1839) | Eulophidae | Hungary |
|
| Achrysocharoides gahani (Miller, 1962) | Eulophidae | Italy |
|
| Achrysocharoides gahani (Miller, 1962) | Eulophidae | Switzerland |
|
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Austria |
|
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Germany |
|
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Hungary |
|
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | Italy |
|
| Achrysocharoides robiniae Hansson & Shevtsova, 2010 | Eulophidae | U.S.A. |
|
| Achrysocharoides robinicolus Hansson & Shevtsova, 2010 | Eulophidae | U.S.A. |
|
| Ageniaspis testaceipes (Ratzeburg, 1848) | Encyrtidae | Hungary |
|
| Apanteles nanus Reinhard, 1880 | Braconidae | Italy |
|
| Astichus trifasciatipennis (Girault, 1913) | Eulophidae | Italy |
|
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Czech Republic |
|
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Hungary |
|
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Italy |
|
| Baryscapus nigroviolaceus (Nees, 1834) | Eulophidae | Switzerland |
|
| Chrysocharis laomedon (Walker, 1839) | Eulophidae | Italy |
|
| Chrysocharis nephereus (Walker, 1839) | Eulophidae | Switzerland |
|
| Chrysocharis pentheus (Walker, 1839) | Eulophidae | Hungary |
|
| Chrysocharis pentheus (Walker, 1839) | Eulophidae | Switzerland |
|
| Cirrospilus elegantissimus Westwood, 1832 | Eulophidae | Italy |
|
| Cirrospilus lyncus Walker, 1838 | Eulophidae | Hungary |
|
| Cirrospilus talitzkii Bouček, 1961 | Eulophidae | Hungary |
|
| Cirrospilus variegatus (Masi, 1907) | Eulophidae | Italy |
|
| Cirrospilus viticola (Rondani, 1877) | Eulophidae | Hungary |
|
| Cirrospilus viticola (Rondani, 1877) | Eulophidae | Italy |
|
| Closterocerus cinctipennis Ashmead, 1888 | Eulophidae | U.S.A. |
|
| Closterocerus sp. | Eulophidae | Czech Republic |
|
| Closterocerus trifasciatus Westwood, 1833 | Eulophidae | Hungary |
|
| Closterocerus trifasciatus Westwood, 1833 | Eulophidae | Italy |
|
| Colastes braconius Haliday, 1833 | Braconidae | Italy |
|
| Colastes braconius Haliday, 1833 | Braconidae | Switzerland |
|
| Elachertus inunctus Nees, 1834 | Eulophidae | Italy |
|
| Eupelmus urozonus Dalman, 1820 | Eupelmidae | Hungary |
|
| Hockeria unicolor Walker, 1834 | Chalcididae | Italy |
|
| Horismenus fraternus (Fitch, 1856) | Eulophidae | U.S.A. |
|
| Mesochorus sp. | Ichneumonidae | USA |
|
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Czech Republic |
|
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Hungary |
|
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Italy |
|
| Minotetrastichus frontalis (Nees, 1834) | Eulophidae | Switzerland |
|
| Necremnus hungaricus (Erdös, 1951) | Eulophidae | Hungary |
|
| Neochrysocharis formosus (Westwood, 1833) | Eulophidae | Hungary |
|
| Pediobius bucculatricis (Gahan, 1927) | Eulophidae | Canada |
|
| Pediobius liocephalatus Peck, 1985 | Eulophidae | Canada |
|
| Pediobius saulius (Walker, 1839) | Eulophidae | Hungary |
|
| Pediobius saulius (Walker, 1839) | Eulophidae | Italy |
|
| Pholetesor circumscriptus Nees, 1834 | Braconidae | Italy |
|
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Czech Republic |
|
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Hungary |
|
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Italy |
|
| Pholetesor nanus (Reinhard, 1880) | Braconidae | Switzerland |
|
| Pholetesor ornigis Weed, 1887 | Braconidae | U.S.A. |
|
| Pnigalio agraules (Walker, 1839) | Eulophidae | Switzerland |
|
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Hungary |
|
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Italy |
|
| Pnigalio pectinicornis (Linnaeus, 1758) | Eulophidae | Switzerland |
|
| Pnigalio soemius (Walker, 1839) | Eulophidae | Hungary |
|
| Pnigalio soemius (Walker, 1839) | Eulophidae | Italy |
|
| Pteromalus chrysos Walker, 1836 | Pteromalidae | Italy |
|
| Pteromalus sp. | Pteromalidae | Czech Republic |
|
| Sympiesis acalle (Walker, 1848) | Eulophidae | Hungary |
|
| Sympiesis acalle (Walker, 1848) | Eulophidae | Italy |
|
| Sympiesis dolichogaster Ashmead, 1888 | Eulophidae | Switzerland |
|
| Sympiesis gordius (Walker, 1839) | Eulophidae | Hungary |
|
| Sympiesis gordius (Walker, 1839) | Eulophidae | U.S.A |
|
| Sympiesis marylandensis Girault, 1917 | Eulophidae | U.S.A. |
|
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Czech Republic |
|
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Hungary |
|
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Italy |
|
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | Switzerland |
|
| Sympiesis sericeicornis (Nees, 1834) | Eulophidae | U.S.A. |
|
Genitalia, Macrosaccus morrisella. 18–19 Male. 18 Genital capsule, ventral view 19 Aedeagus 20–22 Female. 20 Lateral view 21 Detail of signa within corpus bursae 22 Segments 7–10, ventral view.
Lithocolletis robiniella Clemens: Lectotype ♀ (present designation): “14”; “Lithocolletis robiniella Clemens, Type ! A.B. 1902; Type 7505 Lithocolletis robiniella B. Clemens”; “Lectotype ♀ by D. R. Davis”, (ANSP). [The abdomen, right forewing, and distal part of right hindwing are missing].
Paralectotypes 3 ♂ and 1 specimen without abdomen “Syntype”, “Lithocolletis robiniella Clem. 1/4”, “Clemens det. ex Clemens coll.”, “Stainton coll. Brit. Mus. 1893–134”; same labels except nrs. 2/4, 3/4 and 4/4. The specimen with nr. 4/4 carries an extra label in Stainton’s handwriting: “Lithocolletis robiniella Clemens, Proc. N. S. Phil. 1859 p. 319, n.s. unlike any European species”, (BMNH).
Argyromiges pseudacaciella Fitch: Lectotype ♀ (present designation): “Argyromiges Pseudacaciella; Type No. 514 U.S.N.M”; “Lectotype ♀ by D. R. Davis.” (USNM).
BELGIUM: Province of Antwerp: Postel: 15 ♂, 23 ♀, 7 Sep 2009, em. 15–22 Sep 2009, J. and W. De Prins, leafmine on Robinia pseudoacacia, USNM slides 34257, 34258, 34263, DNA/BOLD ID RDOPO090-09, GenBank GU669590, DNA/BOLD ID RDOPO091-09, GenBank GU669591, (USNM). CANADA: ONTARIO: Ancaster: 1 ♂, 24 Jul 1964, T. N. Freeman, Host: Robinia pseudoacacia, 64–20, (CNC). Bobcaygeon: 1 ♂, 23 Jul 1932, J. McDunnough, reared on Robinia, (CNC). Ottawa: 1 ♂, 26 Aug 1955, G. G. Lewis, Host: B. locust, 55–8, (CNC). Walsh: 1 ♂, 23 Sep 1966, T. N. Freeman, Host: Bl. Locust, (CNC). UNITED STATES: DISTRICT OF COLUMBIA: 1 ♀, 7 Jul 1879, Host: Robinia, V.T. Chambers, (USNM); 3 ♂, 2 ♀, 9 Aug 1898, (USNM); 1 ♀, 24 Aug 1899 (USNM); 4 UNK, 18 Sep 1899, Host: Robinia, (USNM); Rock Creek Park: 1 ♂, 22 May 1984, W. E. Steiner, (USNM). ILLINOIS: Adams Co: Quincy: 3 ♂, 3 ♀, 15–21 Feb 1948; 2 ♂, 2 ♀, 6 Apr 1947; J. P. Nielson (INHS). Coles Co: Fox Ridge State Park: 1 ♂, 1 Jun 1991, em. 9 Jun, 1991; 1 ♀, 29 Jun 1991, em. 5 Jul 1991; 3 ♂, 1 ♀, 14 Jul 1991, em. 16–20 Jul 1991, T. Harrison, leafmine on Robinia pseudoacacia, (INHS); 1 ♂, 7 Jun 1991, at UV light T. Harrison, (INHS). Putnam Co: 1 ♀, 15 Apr 1996; 1 ♂, 24 Apr 1963; 1 ♂, 29 Apr 1969; 1 ♂, 2 Jun 1966; 1 ♀, 11 Jul 1959; 1 ♀, 3 Sep 1951, M. O. Glenn, (INHS); 1 ♀, 3 May 1953; 1 ♂, 1 ♀, 10 May 1953; 1 ♂, 26 Aug 1961; 2 ♂, 10 Sep 1948; 2 ♂, 10 Oct 1948, M. O. Glenn, reared from Robinia pseudoacacia, (INHS). Vermilion Co: Kickapoo State Recreation Area: 1 ♂, 13 Jun 1991, em. 14 Jun 1991, T. Harrison, leafmine on Robinia pseudoacacia, (INHS). KENTUCKY: Fayette Co: Lexington: 1 ♂, 6–13 Oct 1975, malaise trap, (USNM). MARYLAND: Garret Co: Deep Creek. Lake State Park: 15 ♂, 12 ♀, 16 Sep 1990, em. 23 Sep – 7 Oct 1990, D. and S. Davis, DRD 821, Host: Robinia pseudoacacia L. USNM slides 33282, 30903, 30895, 30894, DNA/BOLD ID RDOPO088-09, GenBank GU669592, DNA/BOLD ID RDOPO089-09, GenBank GU669593, (USNM). Montgomery Co: Fort Washington, vicinity Henson Creek: 1 ♂, 19 Sep 1990, em. 13 Oct 1993, D. Davis, DRD 1376, Host: Robinia pseudoacacia L., (USNM). MASSACHUSETTS: Essex Co: Beverly: 1 ♂, 2/69, Burgess, (BMNH). MICHIGAN: Clinton Co: T6N-R1W S10: 3 ♂, 2 Oct 1997, em. 9–13 Oct 1997, R. J. Priest, Host: Robinia pseudoacacia L., (USNM). Wayne Co: Detroit: 1 ♂, 2 ♀, 20 Nov 1995, T. Wallenmeier, (USNM). MISSOURI: Boone Co: Columbia: 1 ♂, 25 Nov 1995, slide USNM 17047, 1 ♀, 14 Dec 1969, W.S. Craig, under bark of sycamore, (USNM). NEW HAMPSHIRE: Cumberland Co: Hampton: 1 ♀, 16 Feb 1906, S.A. Shaw, (USNM). NEW JERSEY: Burlingnton Co: Moorestown: 2 ♂, 22 Aug 1902, W.D. Kearfott, Host: Locust, (USNM). NEW YORK: Specific locality unknown: 1 ♀, lectotype, Argyromiges pseudacaciella Fitch, (USNM). Clinton Co: Peru: 2 ♂, 2–18 May 1977, R. Weires, caught in pheromone trap, slide USNM 20912, (USNM). Essex Co: Crown Point: 2 ♂, 4–20 May 1977, 1 ♂, 20 May-17 Jun 1977, R. Weires, caught in pheromone trap, slide USNM 20910, (USNM). Livingston Co: Letchworth State Park: 12 ♂, 8 ♀, 21–22 Jun 1986, E. R. Hoebeke, reared from mines of Robinia pseudoacacia, (CU). Thompkins Co: Ithaca: 1 ♂, 15 Feb; 1 ♂, 1 ♀, 8 Apr 1945, Renwick, (CU). NORTH CAROLINA: Macon Co: Highlands, 3865’: 6 ♂, 5 ♀, 1–24 Aug 1958, R. W. Hodges, (CU); 11 ♂, 5 ♀, 27 Jul-25 Aug 1958, R.W. Hodges, (USNM). OHIO: Hamilton Co: Cincinnati: 1 ♂, 29 Apr 1905, 6806, (CNC); 1 ♀, 29 Apr 1903, 7 ♂, 4 May 1904, 1 ♂, 23 July 1903, 1 ♂, 1 ♀, 25–27 Sep 1902, 1 ♂, 1 ♀, 30 Sep 1911, slide USNM 97837, 2 ♀, 10–22 Oct 1903, 3 ♂, 15–20 Nov 1903, Annette F. Braun, (USNM). PENNSYLVANIA: Specific locality unknown: 1 ♀, lectotype, Lithocolletis robiniella Clemens, (ANSP). Allegheny Co: Oak Station: 1 ♂, 1 ♀, 1 Oct 1910, Fred Marloff, (CU); 1 ♀, 10 Apr 1910, 3 ♂, 5 ♀, 3–22 May 1910, 1 ♀, 12 Jun 1908, Fred Marloff, (USNM). Erie Co: Girard: 3 ♂, 1 ♀, 9 Oct 1920, reared from black locust, (CU). Franklin Co: Mont Alto: 1 ♀, 5 Oct 1971, reared Black locust seedling, slide USNM 17166, (USNM). Indiana Co: Strongstown: 1 ♀, 23 Sep 1971, reared Black locust seedling, (USNM). Monroe Co: Sciota: 1 ♂, 21 Jul 1965, T. N. Freeman, Host: Robinia pseudoacacia, 65–27, (CNC). SOUTH CAROLINA: Oconee Co: Cherry Hill Rec. Area, Rt.107, 2000’ [610m]: 2 ♂, 1 ♀, 11 Aug 1958, R.W. Hodges, (CU); 3 ♂, 11 Aug 1958, R.W. Hodges, slide USNM 17017, (USNM). TEXAS: 1 ♀, Boll, (USNM). VIRGINIA: Arlington Co: Rosslyn: 1 ♂, A. Busck, underside mine on Hog peanut, (USNM). Madison Co: Shenandoah Nat. Park, Skyline: 3 ♀, 12 Aug 1972, E. Jäckh, Host: Robinia pseudoacacia L., (USNM). WEST VIRGINIA: Marion Co: Morgantown: 5 ♂, 3 ♀, [no date], Host: Robinia, slide USNM 97835, (USNM). WISCONSIN: Dane Co: Madison: 1 ♂, 1 ♀, 24 Aug 1958, L. J. Bayer, Host: Robinia, (USNM).
Macrosaccus robiniella occurs naturally over much of eastern North America from Ontario, Canada south to South Carolina and west to Missouri and Texas. Macrosaccus robiniella was first reported in Europe in 1983, near Basel, Switzerland (
Discovery and general distribution of Macrosaccus robiniella in Europe.
| Country | First year of occurrence | Reference to the first record |
|---|---|---|
| Albania | not recorded |
|
| Austria | 1991 |
|
| Belgium | 2001 |
|
| Bosnia and Herzegovina | 1999 |
|
| Bulgaria | 2001 |
|
| Croatia | 2000, unpublished observations, Aleš Laštůvka & Hana Šefrová, pers. comm. |
|
| Czech Republic | 1992 |
|
| Denmark | 2003 |
|
| France | 1984 |
|
| Germany | 1988–1989 |
|
| Hungary | 1992 |
|
| Italy | 1988 |
|
| Lithuania | 2007 |
|
| Moldova(Pridnestrovje) | 2006 |
|
| Netherlands | 1999 |
|
| Poland | 1999 from |
|
| Romania | 2002 |
|
| Serbia | 1998 |
|
| Slovakia | 1992 |
|
| Slovenia | 1994 |
|
| Spain | 2001 |
|
| Switzerland | 1983 |
|
| Ukraine | 2002 |
|
Thesynonymousnames Lithocolletis robiniella Clemens and Argyromiges pseudacaciella Fitch were both published in 1859. The month of publication for robiniella
is clearly indicated as November in the Proceedings of the Academy of
Natural Sciences of Philadelphia for that year. The month of publication
for pseudacaciella Fitch cannot be determined as precisely. With
the assistance of Tim McCabe of the New York State Museum, we were
able to resolve an approximate date of printing for the Fifth report of
Fitch’s Report on the noxious, beneficial and other insects of the
state of New York (
Thus, available evidence now suggests that pseudacaciella Fitch preceded the publication of robiniella Clemens by a few months. Because it is known that (1)
Neither the type locality nor the number of specimens examined were provided by Fitch for Argyromiges pseudacaciella. The same is true for the other two species of Gracillariidae Fitch proposed in 1859, Argyromiges morrisella, and Argyromiges uhlerella. Because it is believed that most of Fitch’s collecting occurred within the vicinity of his “bug house” (still standing and now a historical site) in Salem, New York, it is likely that the type locality for all three species may be from this general area (McCabe, in litt.).
http://species-id.net/wiki/Macrosaccus_morrisella
Figs 1, 5, 41–44, 18–22, Tables 1, 2The forewing pattern of this species differs from that of Macrosaccus robiniella and Macrosaccus neomexicanus in possessing a more distinct basal white streak, in having the dorsal strigulae oriented less obliquely, and with the basal white dorsal stigula more pronounced, and from Macrosaccus gliricidius by the darker ground colour. The forewing pattern most resembles that of Macrosaccus uhlerella but differs in the more pronounced basal white streak which is absent or barely evident in Macrosaccus uhlerella. The male genitalia are most similar to that of Macrosaccus robiniella, particularly with regard to the more abruptly constricted apical third of the valva. The female genitalia differ from the latter in lacking the minute longitudinally oriented striae and spicules in the walls of the corpus bursae.
Adult(Fig. 5). Forewing length 2.3–2.8 mm.
Head: Vestiture of head and antenna similar to Macrosaccus robiniella.
Thorax: Dark brown to fuscous dorsally, sometimes with a coppery to purplish luster; shiny white ventrally; tegula dark brown, with white suffusion anteriorly. Forewing pattern similar to Macrosaccus robiniella except basal two costal strigulae less oblique; a slender white, slightly oblique streak usually well developed extending distad from tegula at base of wing to sometimes as far as first dorsal strigula; 3 white dorsal strigulae usually present, but these oriented less obliquely than in Macrosaccus robiniella; basal strigula white, but sometimes obscure; median strigula connected to second costal strigula to form a narrow white fascia; black apical spot present as in Macrosaccus robiniella; cilia light grey to white. Hindwing, including fringe, uniformly grey. Legs similar to Macrosaccus robiniella in colour pattern.
Abdomen: Similar to Macrosaccus robiniella, dark fuscous dorsally and white ventrally with greyish suffusion on anterior portion of segments 2–7 laterally and sometimes ventrally on A8.
Male genitalia (Figs 18, 19): Similar to Macrosaccus robiniella, with valva gradually constricting before apex. Saccus a long, slender rod ~ 1.75× length of valva. Aedeagus long and slender, ~ 3.0× length of valva, with phallobase slightly more enlarged than in Macrosaccus robiniella.
Female genitalia (Figs 20–22): Ductus bursae long and slender, nearly equal to length of of elongate corpus bursae. Accessory bursae ~ 2/3 the length of corpus bursae, arising from near anterior 1/3 of ductus bursae. Corpus bursae elliptical, with series of small, scattered dentate spicules concentrated over caudal 2/3; longitudinal folds or striae not evident along walls; walls of anterior end (distal 1/3) of corpus bursae entirely membranous.
Similar to that of Macrosaccus robiniella.
(Figs 41–44) The mine begins as an elongate serpentine track on the under (abaxial) side of the leaflet. This enlarges to an elongate-oval, whitish blotch which eventually becomes strongly tentiform (Fig. 43).
(Table 1). Fabaceae: Amphicarpa bracteata (L.) Fernald, (=Amphicarpa monoica (L.) Nutt., = Falcata comosa (L.) Kuntze, = Amphicarpa comosa (L.) Loudon), (
Argyromiges morrisella Fitch: Lectotype ♂, (present designation): “Type; Argyromiges morrisella Ft.; Figured by Miss A. Braun, Feb. 1908; Lectotype ♂, Macrosaccuss morrisella (Fitch) by D. R. Davis.” (USNM). [Abdomen is missing].
Lithocolletis texanella Zeller: Holotype ♂, “Type 1336”; “Dallas, Texas”, (MCZ). Paratype ♂, “Type”, “Lithoc. Texanella Z. B1/77”, “Dallas, Texas”, “Zeller Coll., Walsingham Collection 1910–427”, “Lithocolletis morrisella Fitch”, (BMNH).
Lithocolletis amphicarpeaeella Chambers: Lectotype ♂, (present designation): “Type, 1326”; “Kentucky [crossed out], Chambers”; “Lithocolletis amphicarpeaeella Cham.”; “Lectotype♂, Lithocolletis amphicarpeaeella Chambers, by D. R. Davis”, (MCZ); Paralectotype ♂, “N J, Chambers 1/77” “Lith. Amphicarpeaeella Nr. 3, Syntype, select KRT”, (BMNH).
Note: In the lower right corner of drawer Mi 4423, a label with the following handwritten text is present: “Nb. Chambers’ syntypes. The specimens bearing the serial numbers 1–68, and the specimens of G. hermannella var. lingulacella immediately following, were received by Stainton from Chambers. The numbers refer to a list made by Chambers; many of the specimens are likely to be syntypes. See Stainton foreign correspondence, letters nos. 94, 97 and 111 (by Chambers) and Stainton’s “translations” of Chambers’ awful handwriting, nos. 95, 99 and 96 respectively (the letters are out of chronological sequence). – KRT, 1980”.
CANADA: MANITOBA: 1 ♀, Aweme: 31 Aug 1931, Host: Strophostyles pauciflora (=Strophostyles leiosperma), R. M. White, (CNC). ONTARIO: Pt. Pelee: 1 ♀, 10 Oct 1967, T. N. Freeman, Host: Hog Ranot 63–46, (CNC). Simcoe: 1 ♂, 15 Sep 1955, T. N. Freeman, Host: Amphicarpa monoica (=Amphicarpa bracteata) 65–85, (CNC). Toronto: 2 ♂, 2 ♀, 5.22, Parish; 1 ♂, 6.22, Parish, (BMNH). QUEBEC: Fairy Lake: 1 ♂, 28 Aug 1955, Host: Amphicarpa 55–178, G. G. Lewis, (CNC). UNITED STATES: COLORADO [no specific locality provided]: 1 ♂, Type 1326, [Kentucky crossed out] Chambers, Lectotype Lithocolletis amphicarpeaeella Cham. ♂, (MCZ); 1 ♂, 1 ♀, Type 1326, [Kentucky crossed out] Chambers, Paralectotype Lithocolletis amphicarpeaeella Cham., (MCZ). CONNECTICUT: Hartford Co: Southington: 1 ♂, 3 Sep 1981, C. T. Meier, Host: Amphicarpa bracteata, Leaflet, (USNM). KENTUCKY: 1 ♀, Chamb., 132, (MCZ). ILLINOIS: Adams Co: Quincy: 1 ♀, 15 Feb 1948; 2 ♂, 3 ♀, 6–20 Apr 1947; J. P. Nielson, (INHS). Coles Co: Fox Ridge State Park: 1 ♀, 1 Jun 1991, em. 9 Jun 1991; 1 ♂, 1 ♀, 7 Jun 1991, em. 10 Jun 1991; 2 ♂, 3 ♀, 17 Jun 1991, em. 18–25 Jun 1991; 14 Jul 1991, em. 15 July 1991; 1 ♂, 2 ♀, 2 Aug 1992, em. 6–9 Aug 1992, T. Harrison, Host: Amphicarpa bracteata, (INHS). Putnam Co: 2 ♂, 1 ♀, 15 Jan 1941; 1 ♀, 8 Oct 1964; 2 ♂, 3 ♀, 13–30 Sep 1967; 4 ♂, 1 ♀, 2–8 Oct 1969, underside tentiform mine Amphicarpa monoica, M. O. Glenn, (INHS). MARYLAND: Montgomery Co: 2 mi. S. Laytonsville: 1 ♂, DOA 8 Oct 2009, D.R. Davis, Host: Amphicarpa bracteata, (USNM). Little Bennett Regional. Park: 3 ♂, 3 Aug 2002, em. 14 Aug 2002, D. R. and S. R. Davis, 3 ♂, 5 ♀, 6 Aug 2006, em. 10–16 Aug 2006 DNA/BOLD ID RDOPO081-09, GenBank GU669599, DNA/BOLD ID RDOPO083-09, GenBank GU669598 ; 2 ♂, 7 Aug 2010, em. 16–18 Aug 2010, D. R. and M. M. Davis, DRD slide 2664, Host: Amphicarpa bracteata, slides USNM 34180, 34181, 34182, DNA/BOLD ID 00715473, DNA/R50BOLD ID 00715475, (USNM). Plummers Island: 1 ♂, 5 Nov 1914, Shannon, (USNM). Prince Georges Co: Seton Belt Woods: 1 ♂, 11 Jul 1977, E. Jäckh, (USNM). MICHIGAN: Calhoun Co: 4 ♂, T1S-R6W Sec 15: 31 Aug 1996, em. 7–11 Sep 1996, lot RJP654.17-18, Host: Amphicarpa bracteata. Clinton Co: T05N-R01 R01W S24: 28 Aug 1991, em. 7–11 Sep 1996, R. J. Priest, lot RJP 932.6-7, Host: Amphicarpa bracteata, (USNM). NORTH CAROLINA: Macon Co: Highlands, 3865’: 1 ♂, 31 Jul 1958, R. W. Hodges, (USNM). NEW YORK: Thompkins Co: Ithaca, Six Mile Creek: 3 ♂, 1 ♀, 8 Apr 1945, (CU); 1 ♂, 10 Jul 1960, 1 ♂, 10 Jul 1960, R. W. Hodges, Host: Amphicarpa monoica (L.), (USNM). OHIO: Hamilton Co: Cincinnati: 1 ♂, em. 23 Aug 1908, Annette F. Braun, B361, (MCZ); 1 ♀, em. 16 Jul 1908, 1 ♂, em. 2 Aug 1908, 1 ♂, em. 23 Aug 1908, 1 ♂, em. 24 Aug 1908, 1 ♂, em. 25 Aug 1908, 1 ♂, 1 ♀, em. 2 Sep 1908, 1 ♂, 5 Sep 1918, slides USNM 30891, 30892, 97446, 97447, (USNM). TEXAS: 3 ♂, 1 ♀, Boll, (BMNH). Dallas Co: Dallas: 2 ♂, (holotype and paratype of Lithocolletis texanella Z.), type 1336, Boll, (MCZ); “Lithoc Texanella Z, B 1/77, type”, (BMNH); 2 ♀, (Lithocolletis texanella Z.), (BMNH); 3♂, 3♀ (Lithocolletis texanella Z.) in coll. Stainton (BMNH).
Macrosaccus morrisella occurs widespread across eastern North America from Manitoba and Ontario, Canada, south and west to Texas and Colorado (
Chambers in his 1877 description of Lithocolletis amphicarpeaeella expressed doubt if this was a new species or new variety (as he did on the same page and line for “Lithocolletis amorphaeella
n.sp.? or var.?”). Their descriptions included long, detailed
comparisons of the forewing patterns of these two new moths as well of robiniella and “texana” (misspelled). No locality for either name was mentioned except for Colorado in the title of the publication (
The holotype specimen of Lithocolletis texanella Zeller upon examination was found to be a male and not a female as stated originally by
urn:lsid:zoobank.org:act:3FD42A3B-6E1B-4788-82F7-D9B2AF3D3354
http://species-id.net/wiki/Macrosaccus_neomexicanus
Figs 1–6, 23–27, 45–50, Tables 1, 2As discussed in the diagnosis of Macrosaccus robiniella, this species most resembles the former in general appearance. They differ in distribution, host preference, in genital morphology (see diagnoses of Macrosaccus robiniella), and possibly overall size, with the wingspan of neomexicanus being slightly larger.
(Fig. 6). Forewing length 2.7–3.5 mm.
Head: Vestiture of head and antenna similar to Macrosaccus robiniella; apical segment of antenna white to grey.
Thorax: Dark brown dorsally, with whitish suffusion anteriorly and laterally; white ventrally; tegula dark brown, with pale grey to white suffusion anteriorly. Forewing and hindwing patterns very similar to Macrosaccus robiniella. Vestiture of legs similar to Macrosaccus robiniella.
Abdomen: Similar to Macrosaccus robiniella, dark fuscous dorsally and white ventrally with greyish suffusion on anterior portion of segments 2–7 laterally and sometimes ventrally on A8.
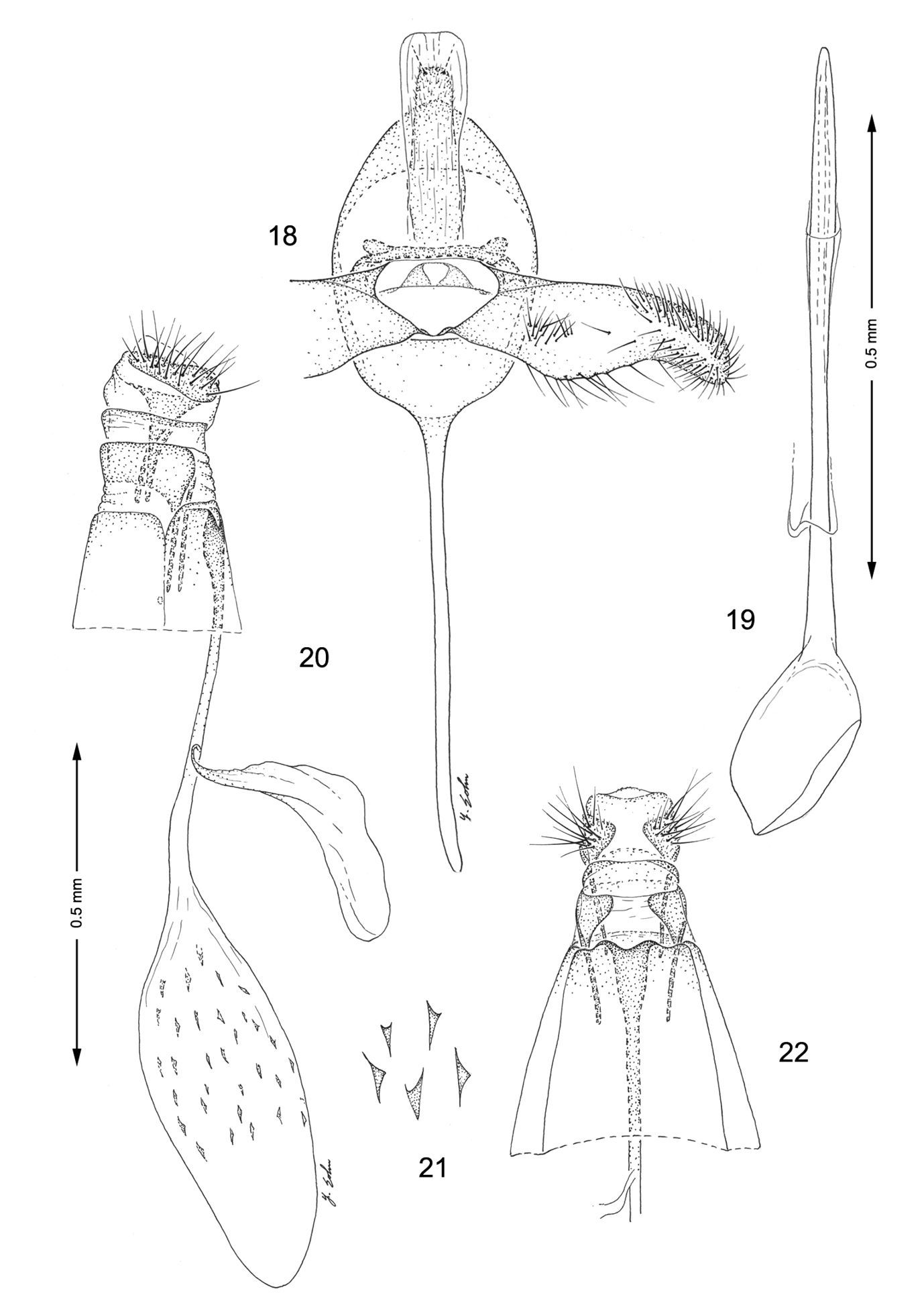
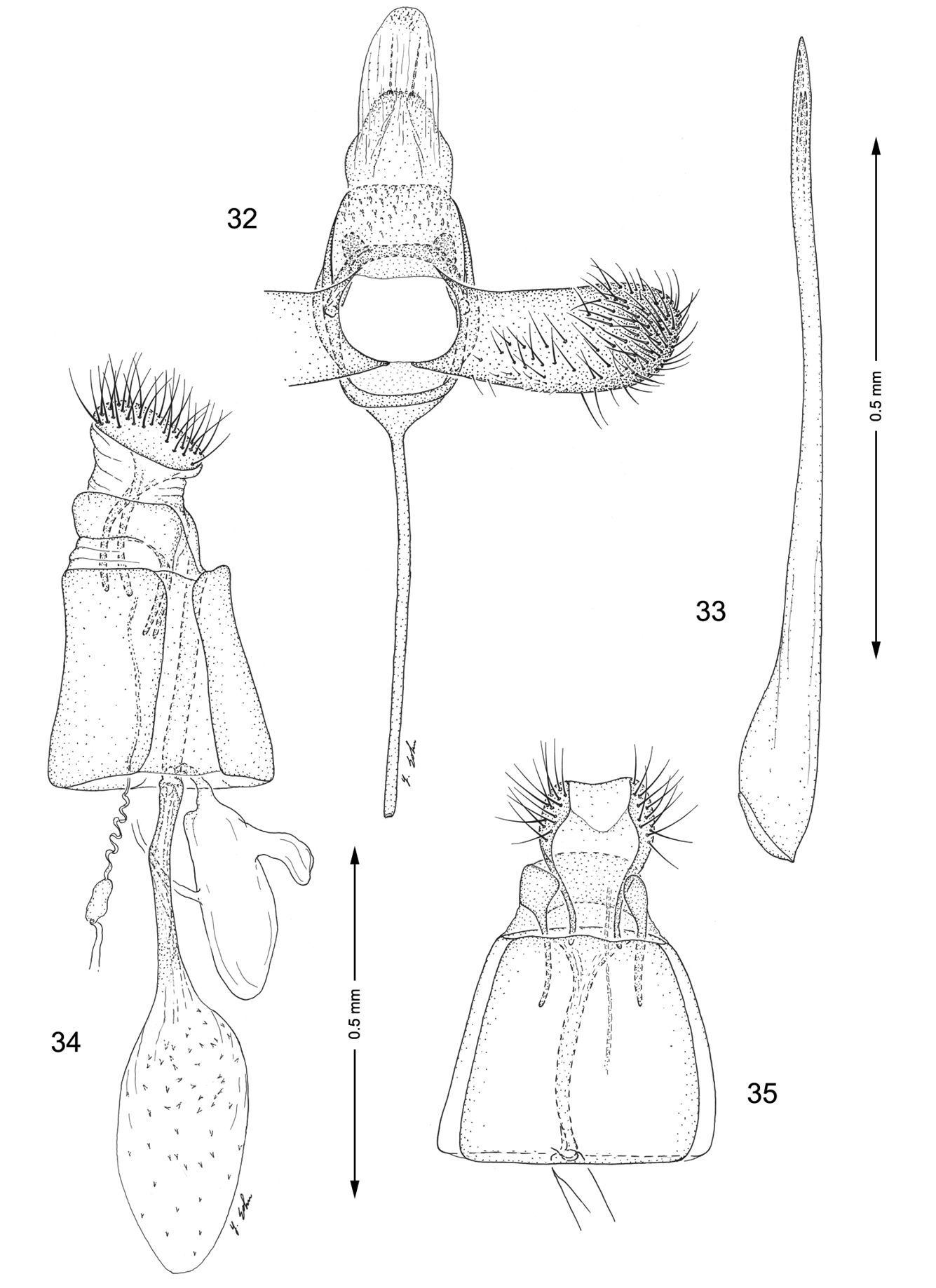
Male genitalia (Figs 23, 24): Valva relatively simple, gradually narrowing before apex without abrupt constriction; apex narrowly rounded, densely setose, particularly along costal margin. Saccus a slender, elongate rod ~ 1.3× length of valva. Aedeagus very long and uniformly slender, ~ 2.5× length of valva.
Female genitalia (Figs 25–27): Ductus bursae moderately long and slender, ~ 1/3 the length of elongate corpus bursae. Accessory bursae nearly as long as corpus bursae, arising from junction of ductus bursae and corpus bursae; with a smaller lateral pouch arising ~ midway along side of accessory bursae. Corpus bursae relatively slender, anterior end only slightly broader; a dense scattering of minute spicules encircling middle; remaining walls of corpus bursae entirely membranous.
Genitalia, Macrosaccus neomexicanus. 23–24 Male. 23 Genital capsule, ventral view 24 Aedeagus 25–27 Female. 25 Lateral view 26 Detail of signa within corpus bursae 27 Segments 7–10, ventral view.
Similar to that of Macrosaccus robiniella.
(Figs 46–50). The mine begins as a relatively short, serpentine track which enlarges to an elongate-oval, whitish blotch located on the under (abaxial) side of the leaflet. As the larva develops and begins laying down silk, the mine becomes strongly tentiform, causing the upper (adaxial) surface to roll over (Figs 48, 49).
(Table 1). Fabaceae: Robinia neomexicana Gray. The host is a moderately small, spiny shrub growing to as high as 5 meters and usually forming dense thickets (Fig. 45). It occurs from California to Texas and north to Wyoming.
(Figs 46–50). Some collections of this species from southern Arizona have been from dense infestations. In such populations, oviposition tends to be concentrated on fewer available leaflets with as many as 45 short, initial serpentine mines observed on a single leaflet. These soon coalesce resulting in a single large blotch covering nearly the entire lower side of the leaflet (Fig. 47). Larval mortality is probably high under these conditions. One large composite mine opened contained 14 live and 11 dead, late instar larvae and no pupae. A maximum of 11 cocoons with pupae (5 on one side of the midrib and 6 on the other side) were found in one leaflet (DRD rearing lots 541, 541.1, Fig. 50).
♂: UNITED STATES: ARIZONA: Cochise Co: Carr Canyon: Huachuca Mts: 20 Sep 1985, em. 27 Sep 1985, HOST: Robinia neomexicana, R. S. Wielgus, digital image captured, (USNM).
UNITED STATES: ARIZONA: Coconino Co: North Rim Grand Canyon: 1 ♀, 15–16 Aug 1978, em. 17 Aug – 6 Sep 1978, G. Deschka, HOST: Robinia neomexicana, (BMNH). Cochise Co: Carr Canyon: Huachuca Mts: 70 ♂, 57 ♀, 20 Sep 1985, em. 21 Sep - 2 Oct 1985, R.S.Wielgus, DRD541, HOST: Robinia neomexicana, slides USNM 28416, 30890, 34267, (USNM). NEW MEXICO: Catron Co: Rocky Canyon., Gila Nat. Forest: 1 ♂, 1969, em. 6 Aug 1969, D. & M. Davis, DRD642.2, Host: Robinia neomexicana, (USNM). Otero Co: Deerhead Campground, ca. 2 mi. S. Cloudcroft: 1 ♂, 1 ♀, 18–19 Jul 1969, em. 2 Aug 1969, D. & M. Davis, DRD 642, Host: Robinia neomexicana, DNA/BOLD ID RDOPO084-09, GenBank GU669596, DNA/BOLD ID RDOPO085-09, GenBank GU669597, (CCDB, USNM). Sandoval Co: Pakitza Campground, 4 mi. E. Ponderosa, Santa. Fe Nat. Forest: 7 ♂, 9 ♀, em. 4–11 Aug 1969, D. & M. Davis, DRD 642.1, Host: Robinia neomexicana, slides USNM 34183, 34184, (USNM).
Known only from the southwestern United States from Arizona and New Mexico.
The specific name is derived from the specific name of its plant host. The specific epithet is an adjective in the nominative singular.
http://species-id.net/wiki/Macrosaccus_uhlerella
Figs 7, 8, 28, 29–31, 51–53, Tables 1, 2The forewing pattern of this species is most similar to that of Macrosaccus morrisella in having the basal strigulae less oblique than those present in Macrosaccus robiniella and Macrosaccus neomexicanus, but it differs from Macrosaccus morrisella in lacking the distinct basal white streak typical of the latter. The male genitalia of Macrosaccus uhlerella are distinct in possessing the most modified, slender valvae (Fig. 28) of any member of Macrosaccus.
(Figs 7, 8). Forewing length 2.2–2.8 mm.
Head: Vestiture of head and antenna similar to Macrosaccus robiniella and Macrosaccus morrisella.
Thorax: Light to dark brown to fuscous dorsally, sometimes with a slight orange luster and a suffusion of fuscous posteriorly; shiny white ventrally; tegula usually orange brown, occasionally with fuscous suffusion posteriorly. Forewing mostly light brownish orange with 4 white costal strigulae, each usually with pale to dark fuscous borders; pattern similar to Macrosaccus morrisella except without a distinct slender, oblique, white streak from tegula at base of wing; 3 white dorsal strigulae usually present, but these oriented less obliquely than in Macrosaccus robiniella; basal strigula white, but sometimes obscure; median strigula connected to second costal strigula to form a narrow white fascia as in 4; dorsal half of wing with black scaling variably present between strigulae; a large black apical spot present similar to that of Macrosaccus robiniella and Macrosaccus morrisella; cilia light grey to white. Hindwing, including fringe, uniformly grey. Legs similar to Macrosaccus robiniella in colour pattern.
Abdomen: Similar to Macrosaccus robiniella, dark fuscous dorsally and white ventrally with greyish suffusion laterally on anterior portion of segments 2–7 and sometimes ventrally on A8.
Male genitalia (Figs 28, 29): Distal half of valva abruptly constricted to ~ 1/3 the width of basal half; saccular lobe broadly produced, truncate. Saccus a long, slender rod ~ 1.3× length of valva. Aedeagus long and slender, ~ 3.0× length of valva, with phallobase only slightly more enlarged than aedeagus.
Female genitalia (Figs 30, 31): Ductus bursae long and slender, slightly longer (~ 1.2×) than length of elongate corpus bursae. Accessory bursae spherical, ~ half the length of corpus bursae, arising from approximately midway along ductus bursae. Corpus bursae elliptical, with series of small, dentate spicules arranged in faint longitudinal folds or striae; walls of anterior end (distal 1/6) of corpus bursae membranous.
Genitalia, Macrosaccus uhlerella. 28–29 Male. 28 Genital capsule, ventral view 29 Aedeagus 30–31 Female. 30 Lateral view 31 Segments 7–10, ventral view.
Not examined.
(Figs 51–53). The mature mine is an elongate-oval, whitish blotch located on the under (abaxial) side of the leaf usually near the edge of the leaflet. Eventually, as the mine becomes tentiform, the leaf edge is slightly curled (Fig. 51).
(Table 1). Amorpha fruticosa L., (
Argyromiges uhlerella Fitch [type material and deposition not stated, believed lost]: [New York]. Lithocolletis amorphaeella Chambers: Lectotype (present designation), ♀: “Type 1327; Chambers, Colorado; Lithocolletis amorphaeella Cham.; Lectotype ♀, Lithocolletis amorphaeella Chambers, by D. Davis; ♀ genitalia on slide 4530, D.R. Davis”, (MCZ), [head, right wings missing]. Lithocolletis amorphae Frey and Boll: Type material not stated, deposition unknown; [Texas].
UNITED STATES: COLORADO: Specific locality unknown: 1 ♀, lectotype, Lithocolletis amorphaeella Cham., DRD slide 4530, (MCZ). ILLINOIS: McDunnough Co: 3 mi. East of Good Hope, Short Fork Seep, Ti7N-R2W, Section 27–28: 2 ♂, 2 ♀, 7 Aug 2010, emerged 11–17 Aug 2010, leaf mine on Amorpha fruticosa, J. Wiker and T. Harrison, slide USNM 33918, (USNM). Putnam Co: 1 ♂, 1 ♀, 29 Mar 1938, DRD slide 4508; 1 ♂, 18 Aug 1939; 1 ♂, 8 Sep 1940, DRD slide 4507; 2 ♂, 20 Sep 1967, reared from underside leafmine Amorpha fruticosa; 2 ♂, 25 Sep 1940; 1 ♂, 5 Oct 1946, DRD slide 4506, reared from leafmine, Amorpha fruticosa, M. O. Glenn, (INHS). Vermilion Co: Kickapoo State Recreation Area: 1 ♀, 13 Jun 1991, moth iss. 24 Jun 1991, T. Harrison, (INHS). MISSOURI: Boone Co: Colombia: 1 ♀, 14 Dec 1969, W. S. Craig, under bark of sycamore, (USNM). TEXAS: Specific locality unknown: 4 ♀, from Amorpha fruticosa, (BMNH). Dallas Co: Dallas: 2 ♂, 3 ♀, Boll, slide BM 4003, (BMNH); Dallas, Boll. 1876, 1 ♂, 4 ♀, bred from Amorpha fruticosa, Stainton collection 1893-134, (BMNH).
Macrosaccus uhlerella is known to occur from Colorado, Illinois, Missouri, New York, and Texas.
For over 120 years Argyromiges uhlerella Fitch has been regarded as the senior synonym of amorphaeella Chambers. The inadequacy of the original description of Argyromiges uhlerella
(quoted below), together with the disappearance of any type material,
has caused some uncertainty regarding this insect’s identity. In his
review of the insects feeding on Robinia pseudoacacia,
No type material of “Argyromiges” uhlerella is believed to exist. In 1977, during a search for Fitch’s Lepidoptera types deposited in the USNM, Tim McCabe found a pin bearing Fitch’s label 8158 (the type number for uhlerella) in the main collection. The moth was missing and was presumed destroyed. The pin with that number has since disappeared. Because the name uhlerella has been used consistently as the valid name for this taxon since before 1899, we believe that this usage should continue even though some doubt now exists regarding the correct application of the name.
Argyromiges uhlerella
“337. Uhler’s leaf-miner, Argyromiges Uhlerella, new species.”
“This resembles Pseudacaciella, but is throughout of paler color, the fore wings being golden gray, with five white spots along their outer sides, of which the hindmost ones are small, the others quite large and bordered with blackish upon their anterior sides; and the black dot on the tip of the wings is here replaced by a short black stripe thrice as long as it is wide; whilst the hind wings and their fringes are pale silvery gray. These marks will suffice to distinguish this from the two preceding species.”
urn:lsid:zoobank.org:act:19A1403C-D313-42BE-AE90-588AF6876F0D
http://species-id.net/wiki/Macrosaccus_gliricidius
Figs 1, 9, 32–35, 54–58, Tables 1, 2The forewing pattern of this species is similar to Macrosaccus robiniella and Macrosaccus neomexicanus in possessing 4 white costal and 3 dorsal, mostly sharply oblique strigulae, with a median fascia often formed by the junction of the 2nd costal and median dorsal strigulae. The pale golden brown ground colour of Macrosaccus gliricidius is distinctly paler than that of the other species. The forewing of Macrosaccus gliricidius also differs from other Macrosaccus in possessing a small, elongate white subapical spot and a more reduced dark fuscous apical spot. The male valva of Macrosaccus gliricidius is distinct in having the distal half more broadly rounded than that of other Macrosaccus.
(Fig. 9). Forewing length 2.2–2.6 mm.
Head: Vestiture of head and antenna similar to Macrosaccus robiniella except vertex generally paler and with more white scales concentrated toward occiput.
Thorax: Dorsum with a narrow, median, longitudinal band of light golden brown bounded laterally with white; tegula light golden brown; venter white. Forewing pale golden brown with 4 equally spaced, oblique, white costal strigulae and 3 white, dorsal strigulae, each bordered by dark brown scales; 2nd costal strigula connected to median dorsal strigula; subapical dorsal strigula directed inward toward small, white subapical spot; dark fuscous apical spot poorly developed, with a more elongate subapical spot immediately basad to rudimentary apical spot; fringe mostly pale greyish white, with narrow, dark brown median band and broad, grey inner band. Hindwing, including fringe, uniformly grey. Foreleg mostly dark fuscous dorsally, white ventrally, with 2 white annuli around basal tarsomeres; midleg mostly white with oblique bands of white extending dorsally over tibia; tarsomeres more broadly banded with white dorsally; hindleg mostly white with much of tibia fuscous dorsally, and with 3 broad fuscous annuli dorsally over tarsomeres.
Abdomen: dark brown dorsally and laterally along anterior margins of A3–7; white ventrally.
Male genitalia (Figs 32, 33): Valva simple, becoming slightly broader near apex; apex broadly rounded, setose; saccus a slender, elongate rod ~ 1.6× length of valva. Aedeagus very long and slender, ~ 3.5× length of valva; phallobase moderately enlarged.
Genitalia, Macrosaccus gliricidius. 32–33 Male. 32 Genital capsule, ventral view 33 Aedeagus 34–35 Female. 34 Lateral view 35 Segments 7–10, ventral view.
Leafmines of Macrosaccus robiniella on Robinia pseudoacacia. 36 Abaxial blotch mines, with kind permission of György Csóka 37 Early instar, abaxial serpentine mines, with kind permission of György Csóka 38 Abaxial blotch mine 39 Adaxial view of Fig. 38 40 Opened mine with 2 cocoons.
Leafmines of Macrosaccus morrisella on Amphicarpa bracteata. 41 Abaxial tentiform blotch mine 42 Detail of abaxial tentiform blotch mine 43 Two abaxial blotch mines at leaf base 44 Adaxial view of Fig. 43.
Habitat andleafmines of Macrosaccus neomexicanus on Robinia neomexicana. 45 Mixed pine-juniper habitat of Robinia neomexicana, Kaibab National Forest, Coconino Co., Arizona, ~2130 m 46 Multiple early instar serpentine and blotch mines on abaxial leaf surface 47 Later stage abaxial mines after multiple blotch mines begin to coalesce 48 Late stage tentiform blotch mine, adaxial view 49 Completely folded leaf resulting from double tentiform mines, adaxial view 50 Opened (with ventral leaf epidermis removed) aggregate blotch mines with 8 pupal cocoons, abaxial view.
Leafmines of Macrosaccus uhlerella on Amorpha fruticosa. 51 Abaxial tentiform blotch mine 52 Flat blotch mine 53 Detail of abaxial tentiform blotch mine. Photographs by T. Harrison.
Female genitalia (Figs 34, 35): Ductus bursae long and slender, ~ 1.25× the length of corpus bursae. Accessory bursae ~ 0.8× the length of corpus bursae, arising from near middle of ductus bursae at a point where the ductus is slightly constricted; a smaller lateral pouch arising near caudal end of accessory bursae. Corpus bursae elliptical, with numerous acute spicules somewhat evenly scattered over much of inner surface but less dense near anterior end.
(Figs 56, 58).Similar to that of Macrosaccus robiniella.
(Figs 54–58).The mine begins as an elongate serpentine track which abruptly enlarges to an elongate-oval, whitish blotch located on either the upper (adaxial) or lower (abaxial) side of the leaflet. When present on the under side, the blotch mines usually develop along the midrib. Only the upper side blotch mines occurred directly on top of the midrib (Fig. 55, Cave, in litt.).
(Table 1). Fabaceae: Gliricidia sepium (Jacq.). Gliricidia sepium isa small to medium-sized, thornless tree growing to a height of 10–12 meters. It is believed to have originated in Central America and has been introduced into many tropical countries around the world. It can be grown as dense hedges and is frequently used as “living fences”.
Eulophidae: Zagrammosoma multilineatum (Hansson & Cave, 1993).
♂: HONDURAS: Dept. Francisco Morazán: Guaimaca, Rio Morazán, 14°32'N, 86°51'W: 26 Jul 1992, em. 5 Aug 1992, R. D. Cave, DRD1165, Host: Gliricidia sepium, digital image captured, (USNM).
HONDURAS: Dept. Francisco Morazán: Guaimaca, Rio Morazán, 14°32'N, 86°51'W: 2 ♂, 4 ♀, 26 Jul 1992, em. 5 Aug 1992, R. D. Cave, DRD1165, Host: Gliricidia sepium, slides USNM 34118–34121, BOLD ID RDOPO086-09, GenBank GU669594, BOLD ID RDOPO087-09, GenBank GU669595, (USNM). San Antonio de Oriente, El Zamorano: 1 ♀, 21 Jul 1988, R. Cave, Host: Gliricidia sepium, (USNM). Tegucigalpa: Steven Passoa, (USNM). Dept. Olancho: Juticalpa, Sta. Cruz: 2 ♂, 1 ♀, 5 Aug 1988, R. Cave, Host: Gliricidia sepium, slides USNM 30888, 30889, 30893, (USNM). FRENCH WEST INDIES: GUADELOUPE: Lamentin, Chaude Ravine: 1 ♂, 10 ♀, 10 May 2004, Jean Etienne, Host: Gliricidia sepium, slides USNM 34186, 34187, 34265, DNA/BOLD ID RDOPO366-10, GenBank HM382079, DNA/BOLD ID HM382080, GenBank HM382080, (USNM).
Known from Central America (Honduras) and the West Indies (Guadeloupe).
The species name is derived from the generic name of its host, Gliricidia. The specific epithet is a noun in the nominative singular.
Leafmines of Macrosaccus gliricidius on Gliricidia sepium. 54 General damage to host 55 Adaxial blotch mine 56 Late instar tissue feeding larva 57 Open blotch mine with single cocoon 58 Pupa with cocoon removed. Photographs by R. Cave.
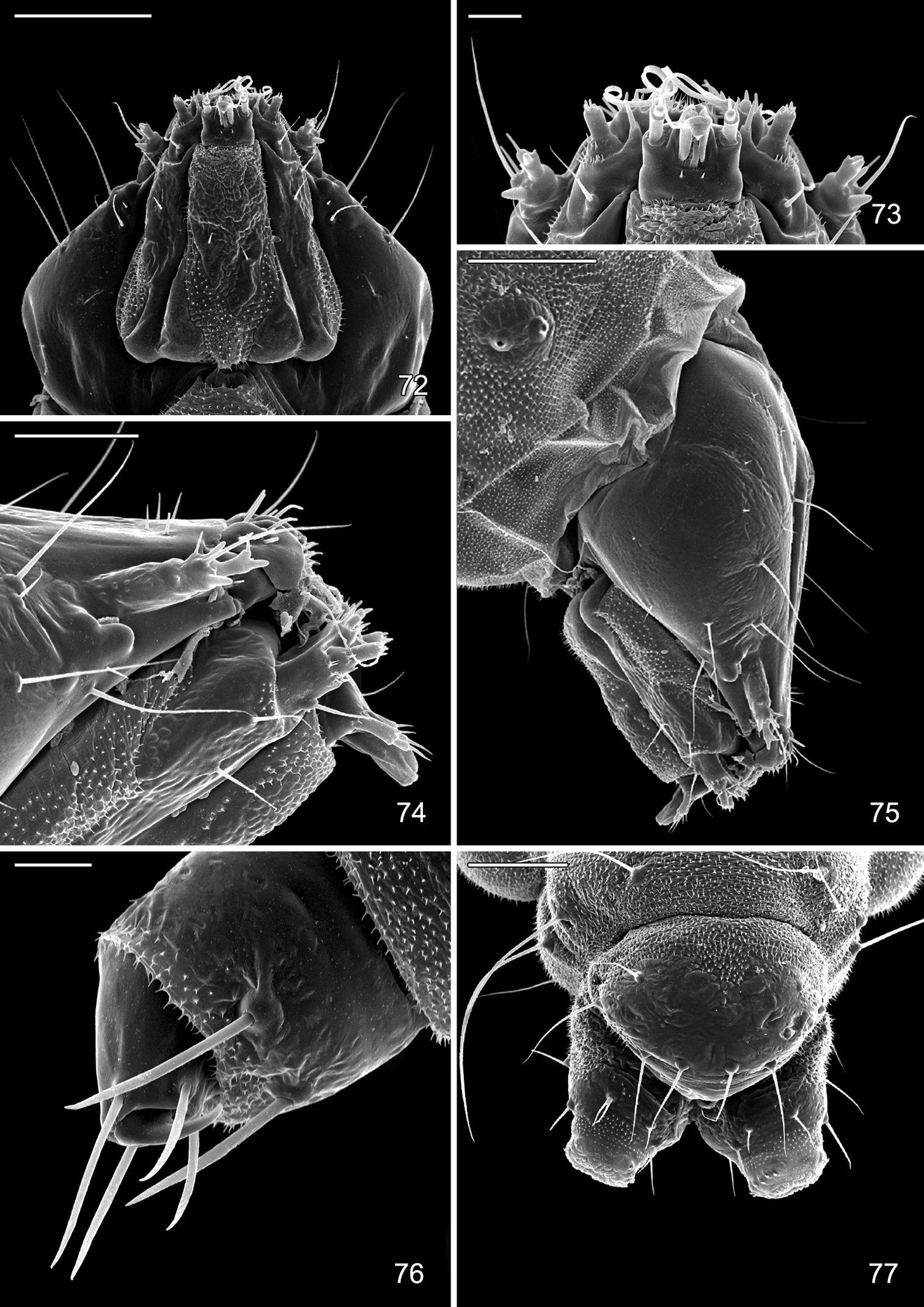
Sap feeding larval instar of Macrosaccus robiniella. 59 Head, dorsal view (50 µm) 60 Detail of mouthparts, antenna, dorsal view (10 µm) 61 Head, ventral view (50 µm) 62 Antenna dorsal view (10 µm) 63 Detail of mouthparts, ventral view (10 µm) 64 Head, lateral view (20 µm) 65 Detail of stemmatal area, lateral view (20 µm). (Scale lengths in parentheses).
Late tissue feeding instar larva of Macrosaccus robiniella. 66 Ventral view (1 mm) 67 Head, dorsal view (100 µm) 68 Mouthparts, dorsal view (50 µm) 69 Maxilla, anterior view (5 µm) 70 Antenna, lateral view (10 µm) 71 Mandible, dorsal view (10 µm). (Scale lengths in parentheses).
Late tissue feeding instar larva of Macrosaccus robiniella. 72 Head, ventral view (100 µm) 73 Detail of mouthparts, ventral view (20 µm) 74 Lateral view of mouthparts (50 µm) 75 Lateral view of head (100 µm) 76 Thoracic leg (20 µm) 77 Abdominal segments 9, 10, dorsal view (100 µm). (Scale lengths in parentheses).
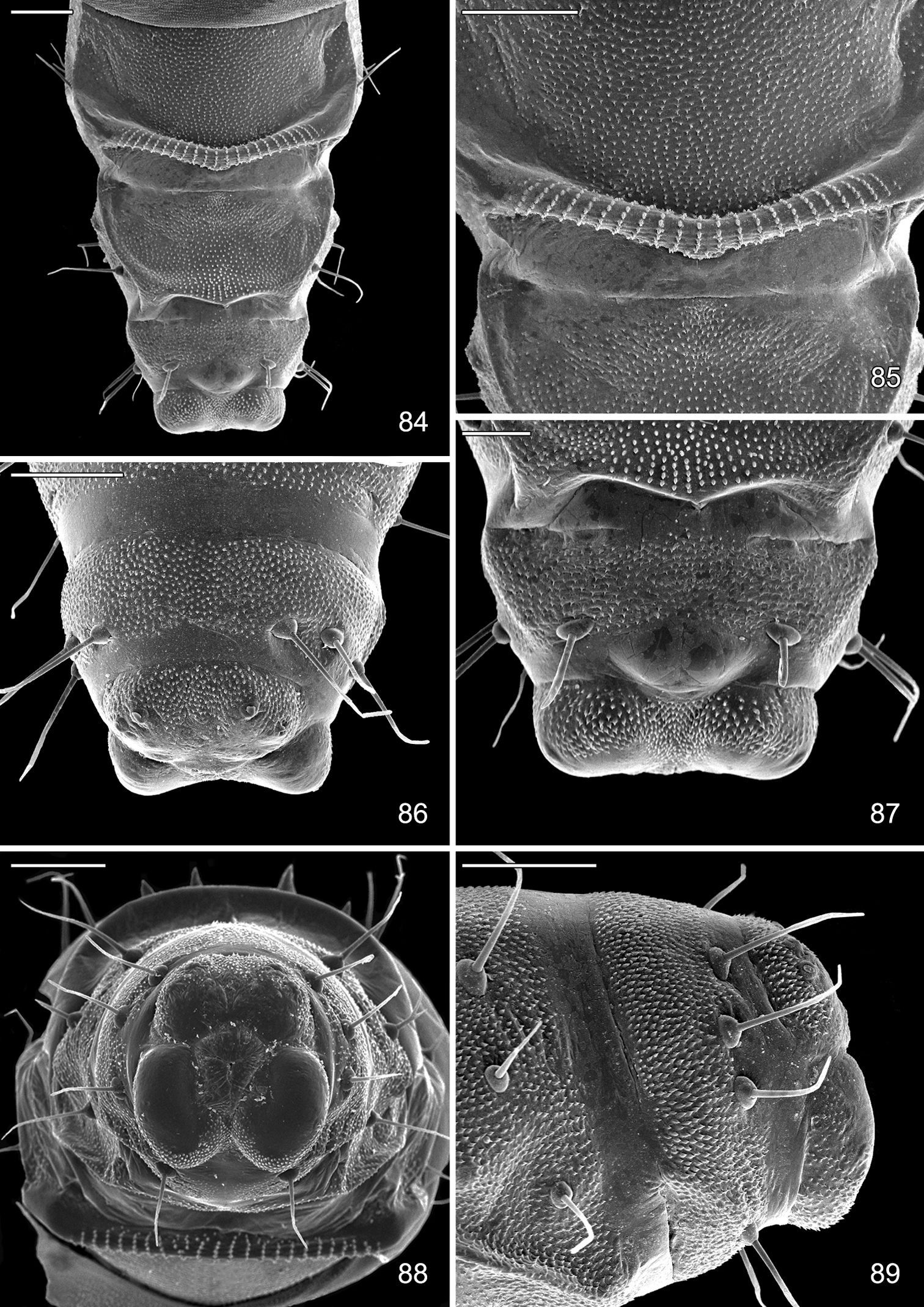
Late tissue feeding instar larva and pupa of Macrosaccus robiniella. 78–80 Larva 78 Abdominal segments 9, 10, ventral view 79 Anal proleg 80 Abdominal segments 9, 10, lateral view. 81–83 Pupa 81 Head, ventral view 82 Lateral view 83 Anterior row of dorsal abdominal spines. (Scale lengths in parentheses).
Pupa of Macrosaccus robiniella. 83 Abdominal segments 7, 8, 9+10, ventral view (100 µm) 85 Detail of accessory cremaster, abdominal sternum 7 (100 µm) 86 Abdominal segments 8, 9+10, dorsal view (100 µm) 87 Abdominal segments 8, 9+10, ventral view (50 µm) 88 Caudal view of abdomen (100 µm) 89 Abdominal segments 8, 9+10, lateral view (100 µm). (Scale lengths in parentheses).
Late tissue feeding instar larva and pupa of Macrosaccus robiniella. 90–96 Larval chaetotaxy. 90 Lateral schematic of prothorax, mesothorax, and abdominal segments 1, 2, 5–10 91 dorsal view of head 92 Ventral view 93 Lateral view 94 Dorsal view of abdominal segments 8–10 95 Labrum, dorsal view 96 Mandible. 97–98 Pupa. 97 Ventral view 98 lateral view. (Bar scale for figures as indicated).
We wish to thank Young Sohn of the Department of Entomology, Smithsonian Institution for the line illustrations and Patricia Gentili-Poole of the Department of Entomology, Smithsonian Institution, who assisted with graphics and final preparation of plates. Eliane De Coninck, Royal Museum for Central Africa, Belgium kindly assisted in photographing the preimaginal stages of Macrosaccus robiniella. Mignon Davis of the Department of Entomology, Smithsonian Institution recorded specimen data and assisted with fieldwork and specimen curation. Special thanks are due to Tim McCabe, New York State Museum, and Jean Sheviak, Capital District Library Council, Albany, NY, USA for their assistance in establishing a more precise publication date for the 1859 New York State Agricultural Society publication by Asa Fitch. Tim McCabe was also helpful in providing information regarding the gracillariid names proposed by Fitch. We are indebted to Linda Butler and Vicki Kondo, Division of Plant and Soil Sciences, West Virginia University, Morgantown, WV, USA; Ronald Cave, Department of Entomology and Nematology, University of Florida, Fort Pierce, FL, USA; Jean Etienne, Institut national de la Recherche agronomique, Centre Antilles Guyane, Domaine Duclos, Guadeloupe; Terry Harrison, Department of Entomology, University of Illinois at Urbana-Champaign, Illinois, USA; E. Richard Hoebeke, Cornell University, Ithaca, New York, USA; Jean-François Landry, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada; Steven Passoa, Plant Protection and Quarantine, APHIS, USDA, Columbus, Ohio, USA; Phillip Perkins, Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; Ron Priest, Department of Entomology, Michigan State University, USA; Paul Tinerella, Insect Collection Manager, Illinois Natural History Survey, Champaign, Illinois, USA; Kevin Tuck, Natural History Museum, London, United Kingdom; Jason Weintraub, Academy of Natural Sciences, Philadelphia, USA, and Ronald Wielgus, Kneeland, California, USA for the gift or loan of essential material and pertinent information. We kindly thank Zdenek Laštůvka and Hana Šefrová, Mendel University of Agriculture and Forestry, Brno, Czech Republic; David Lees, Centre de Recherche d’Orléans, INRA, France; Bernard Landry, Muséum d’histoire naturelle, Genève, Switzerland; Alberto Zilli, Museo civico di Zoologia, Rome, Italy; Rumen Tomov, Faculty of Agriculture, Sofia, Bulgaria; Sergey Sinev, Zoological Institute of the RAS, St. Petersburg, Russia; Constantin Neţoiu, Institutul de Cercetări şi Amenajări Silvice, Romania; Gabrijel Seljak, Slovenia; György Csóka, Department of Forest Protection, Forest Research Institute, Mátrafüred, Hungary; Alexey Bidzylia, Zoological Museum of Kiev Taras Shevchenko National University, Ukraine, and the members of the Flemish Entomological Society, Belgium, Willy De Prins in particular, for very valuable information on the distribution of Macrosaccus robiniella in Europe. Terry Harrison was especially helpful in rearing additional specimens of Macrosaccus uhlerella and photographing their larval mines. We also are grateful to György Csóka, Department of Forest Protection, Forest Research Institute, Mátrafüred, Hungary for his permission to use two images (Figures 36, 37) of Macrosaccus robiniella. We cordially thank John Noyes, Natural History Museum, London, UK for providing some of the references on the parasitoids of Macrosaccus robiniella. The DNA barcode sequences were generated at the Biodiversity Institute of Ontario with funding to Paul D.N. Hebert from Genome Canada through the Ontario Genomics Institute, NSERC, and the Ontario Ministry of Research and Innovation. Stephanie Kirk, Jeremy deWaard, and Megan Milton of the Biodiversity Institute of Ontario, and Jean-François Landry also were helpful in assisting with specimens for barcoding.Apostolis Pekas(Westerlo, Belgium) is kindly acknowledged for explaining the finesses of old Greek spelling, which were implemented into the formation of the genus-group name Macrosaccus. Belgian Science Policy Office is gratefully thanked for supporting financially the taxonomic study on Gracillariidae. DRD wishes to acknowledge the former Smithsonian Research Foundation, Smithsonian Research Initiatives, and the Research Opportunity Fund for supporting his research on the biology of leaf-mining Lepidoptera.