(C) 2011 Ulrich Kotthoff. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Miocene Randeck Maar (southwestern Germany) is one of the only sites with abundant material of fossil honey bees. The fauna has been the focus of much scrutiny by early authors who recognized multiple species or subspecies within the fauna. The history of work on the Randeck Maar is briefly reviewed and these fossils placed into context with other Tertiary and living species of the genus Apis Linnaeus (Apinae: Apini). Previously unrecorded specimens from Randeck Maar were compared with earlier series in an attempt to evaluate the observed variation. A morphometric analysis of forewing venation angles across representative Recent and Tertiary species of Apis as well as various non-Apini controls was undertaken to evaluate the distribution of variation in fossil honey bees. The resulting dendrogram shows considerable variation concerning the wing venation of Miocene Apini, but intergradation of other morphological characters reveals no clear pattern of separate species. This suggests that a single, highly variable species was present in Europe during the Miocene. The pattern also supports the notion that the multiple species and subspecies proposed by earlier authors for the Randeck Maar honey bee fauna are not valid, and all are accordingly recognized as Apis armbrusteri Zeuner.
Apoidea, Anthophila, Apinae, Apis, honey bees, taxonomy, Tertiary, morphometrics
Honey bees (Apini Latreille: Apis
Linnaeus) are among the most familiar of animals, with a tight
association with humans since their domestication and use worldwide in
agricultural ecosystems as crop pollinators (e.g.,
The earliest definitive members of the tribe Apini are known from the Oligocene of France and Germany. These comprise Apis henshawi Cockerell, and under some classificatory schemes Apis vetusta Engel, from Rott and Enspel, Germany (e.g.,
William Scheuthle was the first to discover honey bees at
the Randeck Maar (Early Miocene, southwest Germany) in 1926, and in
1928 he and Ludwig Armbruster, a prominent apiculturist of the day,
excavated more material. Finally, in 1938 the accumulated material was
first formally described based on an examination of 72 specimens (
The abundance of material from Randeck Maar represents a
wonderful opportunity to evaluate more critically these fossil honey
bees, since from most localities only one or a very few specimens are
typically available. Unfortunately, several of the diagnostics used for
the determination of extant Apis species or subspecies cannot be used for the differentiation of fossil Apini, even when excluding the obvious biochemical attributes. For example, Apis cerana, Apis mellifera, and their subspecies, along with Apis koschevnikovi Enderlein and Apis nigrocincta
Smith, are generally recognized from differences in size, coloration
of setae and integument, distribution and proportions of setal bands on
the metasoma, length of the proboscis, sternal and leg podite
proportions, the presence or absence of a distal abscissa to M in the
hind wing (absent in Apis mellifera),
structure of the drone legs and endophallus, and behavioral aspects
such as the time of drone mating flights, structure of brood cell caps,
or the position of a worker while wing-flapping in front of the hive
(e.g.,
In order to circumvent these extreme limitations in studying fossil Apini, herein we follow the approach of
While we are well cognizant of the fact that dendrograms
resulting from cluster (phenetic) analyses cannot be equated with
phylogenies owing to the inability of such methods to distinguish
plesiomorphic and apomorphic features or homologies from analogies, and
that these are more useful at the level of tokogenetic relationships
(e.g.,
The number of Recent species of Apis
and their respective diagnoses has been a matter of debate over the
last couple of decades. Interpretations vary between six or seven
species on the conservative end (
The Randeck Maar is located in southwest Germany, southeast of Stuttgart at the escarpment of the Swabian Alb (48°71'N, 9°31.8'E,
750m elevation) and originated during the Miocene. During this epoch,
the Mesozoic rocks of the Swabian Alb were penetrated by numerous
volcanic dykes leading to phreatomagmatic eruptions when the rising
nepheline-melilithitic magma contacted groundwater (
Modern honey bee diversity (all bees are workers and to the same scale). A Apis mellifera Linnaeus B Apis koschevnikovi Enderlein C Apis nigrocincta Smith D Apis cerana Fabricius E Apis dorsata Fabricius F Apis florea Fabricius G Apis andreniformis Smith. After
The fossil material studied originates from the
Staatliches Museum für Naturkunde, Stuttgart (SMNS), the Heimatmuseum
Göppingen Jebenhausen (HMJ), and the Paläontologisches Museum Nierstein
(PMN) (Figs 2–5). Additional Apis armbrusteri specimens are present in the Urwelt-Museum Hauff but were already considered in detail by
Fossils were examined with a stereomicroscope, while
drawings were prepared using a camera lucida. Photographs were taken
with a digital camera and the software “Analysis Pro Version 3.1” (SIS)
used for distance and angle measurements. The software “PAST” version
1.75b (
FWVA measurements of all specimens documented herein and
with well-preserved forewings were subjected to a cluster analysis
together with measurements of representative Recent Apini from Europe (Apis mellifera; eleven specimens) and Asia (Apis florea Fabricius; four specimens; Apis dorsata; twelve specimens; Apis cerana;
14 specimens). So as to expand our comparative treatment we included
other Miocene and Oligocene honey bees that had sufficiently
well-preserved forewings to permit meaningful measurement and
comparison. These included the European material of Apis henshawi (ten specimens;
While it would have been ideal to make the analysis more
robust with the inclusion of more of Armbruster’s original material,
this was not possible. Most of the specimens described by
Although the venation of drones does not differ
significantly from that of workers, in order to completely eliminate
potential caste differences two drones of Apis cerana
from Pakistan were added to the analysis as a control. While the drones
were separated from workers, they were still more similar to
conspecific workers than to specimens of any other taxon. Further tests
which included drones of Apis mellifera found similar results (
List of not-yet described specimens of Apis armbrusteri presented in this work.
| Museum/number | body length (mm) | forewing length (mm) | hindwing length (mm) | Sediment/Annotations | |
|---|---|---|---|---|---|
| Wing venation not well preserved: | |||||
| HMJ | A 817 | 12.6 | 7.5 | - | light varve layer |
| SMNS | 64674/17a | 21.6 | - | - | dark grey limestone |
| SMNS | 64674/21a/b | 16.9 | 8.9* | 6.0* | dark grey limestone |
| SMNS | 64674/28 | 15.0 | - | - | dark varve layer |
| SMNS | 64674/31 | 13.9 | - | - | dark varve layer |
| SMNS | 64674/38 | 16.0 | - | - | light limestone |
| SMNS | 64674/50a | 24.3 | >15 | - | dark varve layer |
| PMN | SSN10RM12 | 16.9 | 9.1 | - | light varve layer |
| Wing venation well preserved: | |||||
| SMNS | 64674/11a | 13.2 | >7.9 | - | light varve layer |
| SMNS | 64674/11b & 11c | 14.1 | >8.7* | - | dark varve layer |
| SMNS | 64674/12a & 12b | 15.7 | 9.9 | 7.3 | dark grey calcareous marl |
| SMNS | 64674/18 | 17.4 | 9.7* | 7.8* | dark varve layer |
| SMNS | 64674/19 | 17.0 | >10.3 | - | dark varve layer |
| SMNS | 64674/30 | - | 8.4 | - | dark varve layer |
| SMNS | 64674/35 | >15.0** | >11.2 | dark varve layer | |
| SMNS | 64674/36 | >14.7** | 10.0 | dark varve layer | |
| SMNS | 64674/49 | 9.9** | >9.0 | light varve layer | |
| SMNS | 64675 | 14.3 | 8.1 | dark varve layer | |
* distal part reconstructed based on similar complete wings of other specimens
** head missing
Apis armbrusteri Zeuner
Refer to
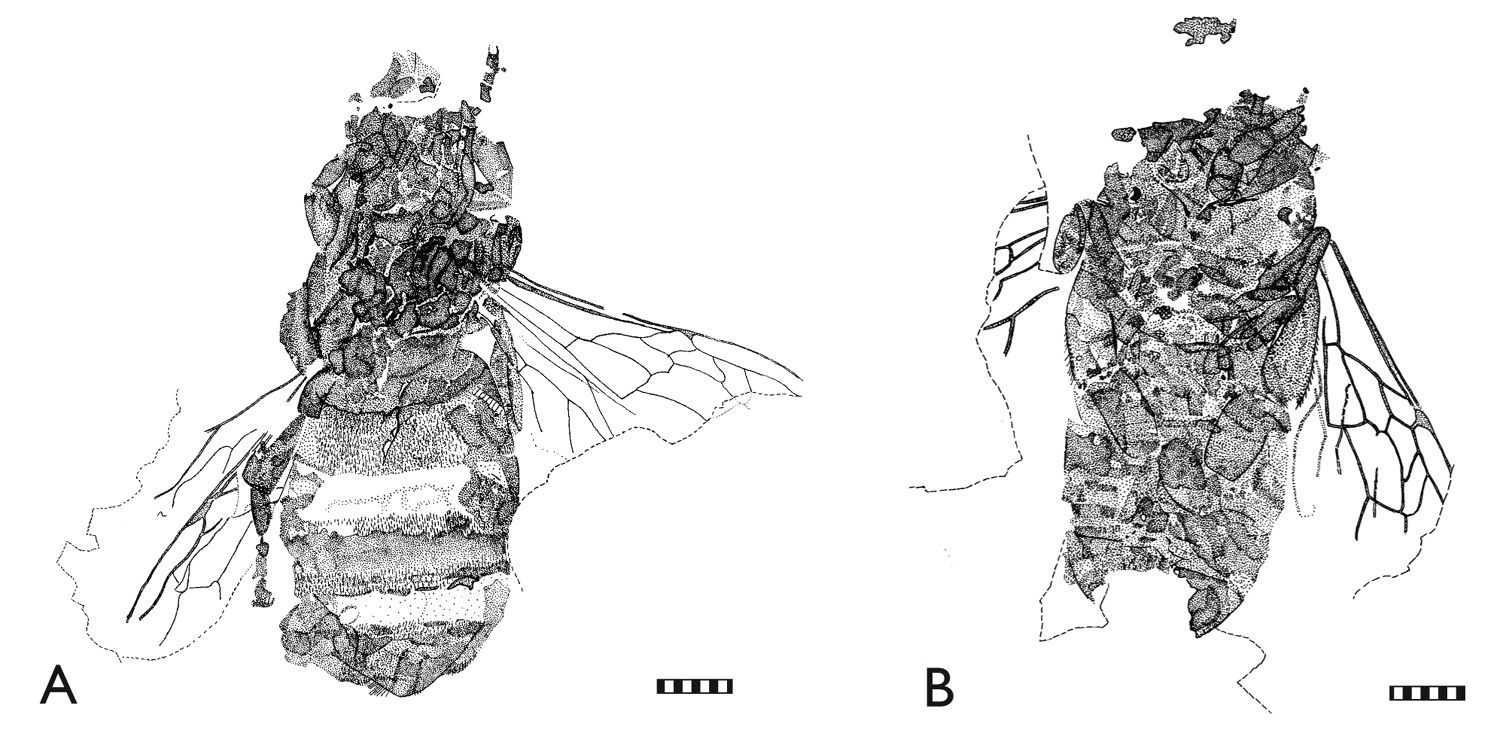
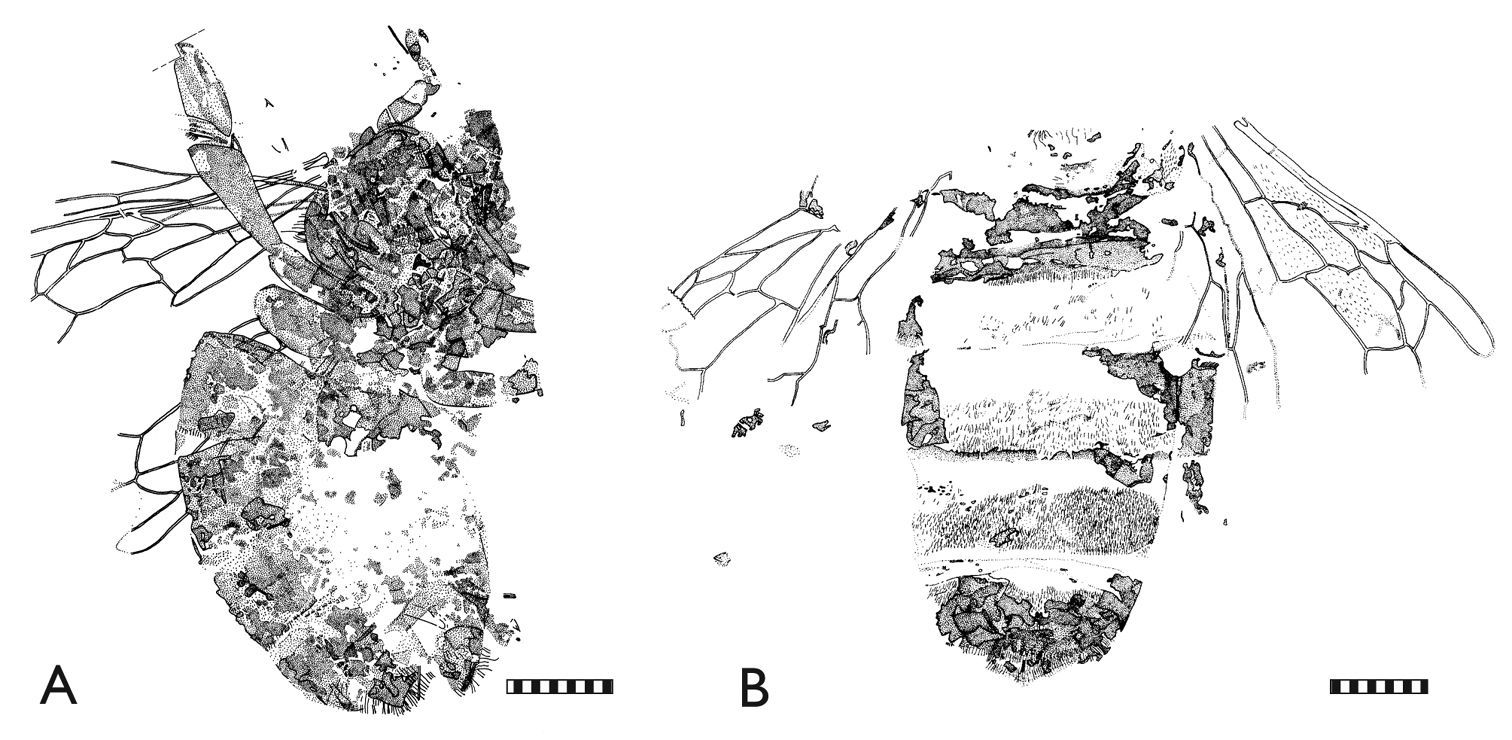
Photomicrographs of representative Randeck Maar honey bees (Apis armbrusteri Zeuner). A SMNS 64675 (neotype) [Morphotype D] B SMNS 64674/12 [Morphotype D] C SMNS 64674/11b [Morphotype D?] D SMNS 64674/11a [Morphotype CM] E SMNS 64674/21 F SMNS 64674/28. Scale bar = 2 mm.
Photomicrographs of representative Randeck Maar honey bees (Apis armbrusteri Zeuner). A SMNS 64674/18 [Morphotype D] B SMNS 64674/49 [Morphotype D] C SMNS 64674/30 [Morphotype D] D SMNS 64674/35 [Morphotype D] E SMNS 64674/19 [Morphotype D] F SMNS 64674/36 [Morphotype CM]. Scale bar = 2 mm.
Representative Randeck Maar honey bees (Apis armbrusteri Zeuner). A SMNS 64675 (neotype) [Morphotype D] B SMNS 64674/11a [Morphotype CM]. Scale bar = 2 mm.
Representative Randeck Maar honey bees (Apis armbrusteri Zeuner). A SMNS 64674/19 [Morphotype D] B SMNS 64674/30 [Morphotype D]. Scale bar = 2 mm.
HMJ A 817. Metrics: body length 12.6; metasoma 7.3; mesosoma 3.2; head 1.8; forewing 7.5. Descriptive notes: Ventral view on light varve layer; preservation exceptionally poor; legs missing except for a few fragments, apparently preserving only metatibiae, which are relatively long and slender; wings only fragmentarily preserved, fragments match forewings of other Apis armbrusteri; no counterpart.
SMNS 64674/17a. Metrics: body length 21.6. Descriptive notes: Ventral view on dark grey limestone; fossil re-crystallized and fragmented; metasoma highly deformed and obviously swollen, resulting in extra-ordinary high body length; forewing venation poorly preserved; left hind leg positioned parallel to metasoma, revealing a slender metabasitarsus; head ventrally directed; compound eyes small in proportion to head and far apart from each other; counterpart is SMNS/17b, which is even more poorly preserved.
SMNS 64674/21a. Metrics: body length 16.9; metasoma 9.9; mesosoma 4.7; head 2.8; mesofemur 1.2; mesotibia 1.6; mesobasitarsus 1.6; metafemur 2.1; metatibia 3.2; metabasitarsus 2.7; forewing (reconstructed) 8.9; hind wing (reconstructed) 6.0.Descriptive notes: Ventral view on dark grey limestone; parts of dorsal cuticle apparent; right forewing well preserved, but folded; metasoma very long (probably resulting from swelling in water after death) and well preserved, but not completely exposed from matrix; sting apparatus apparent; left metatibia long and slender; metabasitarsus very long and broadened; setae preserved in some areas of metabasitarsus; glossa and galeae evident between mandibles; counterpart is SMNS 64674/21b.
SMNS 64674/28. Metrics: body length 15.0; metasoma 9.0; mesosoma 4.7; head 2.1; glossa 2.9; profemur 2.2; protibia 1.6; probasitarsus 1.3; mesofemur 2.3; mesotibia 1.9; metabasitarsus 2.5. Descriptive notes: Lateral view of inner surface on dark varve layer; fossil slightly turned ventral; right forewing venation only partly visible; fragment of left forewing preserved; metatibia and metabasitarsus appear flattened and short; mesoscutum broken; mandibles well preserved; glossa appears protruded; counterpart (SMNS, not registered) exhibits a few dorsal elements, fragments of left forewing, and parts of the other lateral side.
SMNS 64674/31. Metrics: body length 13.9; metasoma 7.6; mesosoma 3.8; head 3.1; metafemur 2.8; metatibia 2.9; metabasitarsus 1.8. Descriptive notes: Laterally embedded on dark varve layer; head turned upwards; mandibles well preserved; right hind leg exposed above metasoma; metatibia and metabasitarsus appear short and broadened; metabasitarsus partly covered by metasoma; wings not preserved; no counterpart.
SMNS 64674/38. Metrics: body length 16.0; metasoma 9.0; mesosoma 5.5; head 2.8; metatibia 2.7; metabasitarsus 1.8. Descriptive notes: Laterally embedded on light limestone; fragmentary preservation of head and mesosoma; metabasitarsus of presumably left hind leg flat and short; metasoma obviously swollen and compressed; wings not preserved.
SMNS 64674/50a. Metrics: body length 24.3; forewing >15; mesosoma+head 8.8. Descriptive notes:
Largest specimen known among Miocene honey bees from Randeck Maar;
preserved on dark varve layer; metasoma, especially first metasomal
segment, very well preserved; mesosoma poorly preserved except for
slightly arched mesoscutum; legs missing; wings oriented parallel to
metasoma, wing venation not apparent; compound eyes especially well
preserved; counterpart is SMNS 64674/50b. Remarks: Even though
the metasoma may have swollen due to postmortem processes, the specimen
is extraordinarily large. Additionally, the forewing length indicates
that this specimen approximated an Apis dorsata
worker in size. The average forewing length of the specimens presented
here is 9.6 mm, thus the wing of SMNS 64674/50 is >50% longer. The
second largest forewing length of specimen SMNS 64674/35 is more than 3
mm shorter. Due to this difference in size, we believe SMNS 64674/50
may have been an Apis armbrusteri queen. According to
PMN SSN10RM12. Metrics: body length 16.9; metasoma 10.0; mesosoma 4.8; head 2.5; mesofemur 1.5; mesotibia 1.8; metafemur 2.2; metatibia 2.7; metabasitarsus 1.9; scape (reconstructed) 0.6; flagellum 2.6; forewing 9.1. Descriptive notes: Dorsoventrally embedded and very well preserved on light varve layer; antennae very well preserved; third submarginal cell of forewing broader than that of Apis cerana or Apis mellifera (unfortunately the wings could not be analyzed in detail); metatibiae and metabasitarsi rather slender; specimen somewhat resembles Apis henshawi.
Specimens with well preserved forewingsSMNS 64674/11a [Morphotype CM]. Metrics:
body length (reconstructed) 13.2; metasoma 7.0; mesosoma 3.7; head 2.4;
mesofemur 2.1; metafemur 1.7; metabasitarsus 2.3; forewing >7.9
(most distal part not apparent). Descriptive notes: Dorsoventrally compressed, ventral view on light varve layer (Figs 2D, 4B);
posterior metasoma not preserved, head only partially preserved; a
fragment, possibly part of head, lies next to specimen; mesosoma,
left forewing, and legs completely preserved; extremities ventrally
exposed; one setal row of left metabasitarsus evident, number of setae
estimated at 28–30; metatibia and metabasitarsus laterally flattened and
short in proportion to width; some setae of rastellum preserved at
metatibial apex; forewing with third submarginal cell extraordinarily
long; 1m-cu with a small distal process in second medial cell; hind wing
with a distal process of vein M preserved; no counterpart. Remarks:
This specimen is small in comparison to other bees from the Randeck
Maar. The forewing length of SMNS 64674/11a (>7.9, but probably not
>8.5 mm) is similar to a small Apis cerana
worker (7.5–9 mm) and shorter than the average forewings from the
Miocene honey bees from Randeck (9.6 mm). The long third submarginal
cell is reminiscent of Apis cerana. A. mellifera,
and their relatives, but the process of 1m-cu is not present in these
modern species, while this aberrant veinal stub appears in several
individuals of Apis armbrusteri. The distal process of M in the hind wing does not occur in Apis mellifera but is present in all other Recent Apis species (
SMNS 64674/11b [Morphotype D?]. Metrics: body length 14.1; metasoma 8.5; mesosoma 3.5; head 2.5; metatibia 2.2; metabasitarsus 1.9; forewing (reconstructed) > 8.7; mandible 1.5. Descriptive notes: Dorsoventrally compressed on dark varve layer (Fig. 2C); anterior part (head in particular) slightly rotated around axis of body length; mandibles, compound eyes, and antennae well preserved; antennae with at least nine, perhaps 10, flagellomeres preserved; parts of the legs compressed close to body; left hind leg positioned lateral of metasoma; metatibia and metabasitarsus do not appear flattened or shortened in respect to homologous appendages of SMNS 64674/11a; part of metasoma re-crystallized, probably pyritized; parts of sting apparatus apparent; forewing 1m-cu with a very short process; counterpart is SMNS 64674/11c. Remarks: The wing venation of this specimen is generally similar to that of Apis dorsata; however, the third submarginal cell is not completely preserved. The shape and relative length of the metatibia and metabasitarsus are also similar to those of Apis dorsata but the specimen is smaller than typical workers of this species.
SMNS 64674/12b [Morphotype D]. Metrics: body length 15.7; metasoma 9.7; mesosoma 4.3; head 1.8; profemur 1.5; protibia 1.5; probasitarsus 1.1; mesofemur 1.6; mesotibia 1.6; metatibia 2.4; metabasitarsus 1.8; forewing 9.9; hind wing 7.3. Descriptive notes: Laterally compressed on dark grey calcareous marl (Fig. 2B); body parts well preserved except for head; wing venation outstandingly well preserved; third submarginal cell rather short and meeting 2m-cu strongly distad; 1m-cu broken, with a short medioapical process projecting into second medial cell; mesosomal cuticle partly fragmented; distal five segments of metasoma stressed horizontally owing to postmortem processes; metabasitarsi fragmented but revealing a relatively slender shape; setae clearly preserved distally on metasoma; counterpart 64674/12a not preserving further details. Remarks: The shape of the short submarginal cell and relatively slender shape of the metabasitarsi are reminiscent of those of Apis dorsata. While the length of the forewing exceeds the typical length of Apis mellifera, it does not reach the length of Apis dorsata.
SMNS 64674/18 [Morphotype D]. Metrics:
body length 17.4; metasoma 10.2; mesosoma 3.8; head 3.3; metafemur 2.4;
metatibia 3.2; metabasitarsus 2.3; metabasitarsal width 0.9; forewing
(reconstructed) 9.7 (minimal); hind wing (reconstructed) 7.8 (minimal). Descriptive notes:
Ventral aspect on dark varve layer, very well preserved but metasoma
probably swollen during rest in water column and subsequently compressed
during compaction, with metasoma appearing artificially lengthened and
broadened; specimen length was probably ~15 mm in life (reaching
general size of Apis dorsata); forewing venation well preserved (Fig. 3А);
third submarginal cell short and broad; process of 1m-cu present;
rastellum of left metatibia apparent; setal comb rows of right
metabasitarsus consist of 20–25 setae; left metabasitarsus shorter and
broader than that of SMNS 64674/11b but more slender than that of
64674/11a; sting very well preserved; no counterpart; next to head is
wing of Bombylius sp. (
SMNS 64674/19 [Morphotype D]. Metrics: body (without head) 17.0; metasoma 8.3; mesosoma 5.6; metatibia 2.9; metabasitarsus 2.3; forewing (reconstructed) >10.3. Descriptive notes: Partly laterally, partly dorsoventrally compressed on dark varve layer (Figs 3Е, 5А); head missing; right hind leg extended laterally; right metatarsus not completely excavated from matrix; rastellum well preserved; wing venation of right forewing well preserved except for apicalmost area; third submarginal cell short; 1m-cu with long medioapical process projecting into second medial cell; one hind wing compressed under right forewing; distal abscissa of M apparently present; left forewing obscured by metasoma; sting preserved but sting device not apparent owing to re-crystallization in center of metasoma; no counterpart. Remarks: The body size of this individual is quite large and cannot be explained solely by broadening and lengthening of the metasoma from postmortem swelling given that the terga are positioned close to and largely overlapping each other. The right forewing has a short, broad third submarginal cell somewhat similar to Apis henshawi, with a general length presumably reaching a similar proportion to that of Apis dorsata. The forewing is generally similar to the forewing of SMNS 64674/18. The metabasitarsus is relatively slender.
SMNS 64674/30 [Morphotype D]. Metrics: metasoma 9.1; metabasitarsus 1.8; forewing 8.4. Descriptive notes: Parts of metasoma and fragments of mesosoma, legs and wings preserved on dark varve layer (Figs 3Ц, 5B); metasoma mainly represented by setae and tergal fragments, presumably ventral view of dorsal elements; no counterpart. Remarks: The wings are similar to those of Apis dorsata and Apis henshawi, and the fragments of the hind legs indicate that the metatibia and metabasitarsus were slender, similar to those of Apis dorsata.
SMNS 64674/35 [Morphotype D]. Metrics: body length (metasoma + mesosoma) 15.0; metasoma 9.5; mesosoma 4.8; metatibia 3.3; metabasitarsus 1.9; forewing >11.2. Descriptive notes: Laterally compressed, with head missing, on dark varve layer (Fig. 3D); mesosoma fragmentarily preserved; mesoscutum turned upwards; one forewing well preserved in basal part; one hind leg obscured by dorsal part of metasoma; other hind leg positioned on top of ventral part of metasoma; metatibia slender; metabasitarsus apparently broad and short, but more slender than that of SMNS 64674/11a. Remarks: The complete forewing was perhaps 12 mm long in life and therefore as long as an Apis dorsata forewing.
SMNS 64674/36 [Morphotype CM?]. Metrics: body (metasoma + mesosoma) 14.7; metasoma 9.7; mesosoma 4.9; forewing (reconstructed) 10.0. Descriptive notes: Laterally compressed, with head missing, on dark varve layer; mesosoma and hind legs only fragmentarily preserved; right (presumably, could be left) metabasitarsus positioned along ventral part of metasoma, very broad and short; metasoma not well preserved; terga not in contact with each other; one forewing very well preserved (Fig. 3F), revealing long third submarginal cell; distal absicssa of vein M apparently present in hind wing; no counterpart. Remarks: The forewing of this specimen is very similar to those of Apis cerana and Apis mellifera. The shape of the metabasitarsus is also reminiscent of these species but possibly the metabasitarsus was deformed during fossilization. The size of the specimen exceeds the typical size of both Apis cerana and Apis mellifera.
SMNS 64674/49 [Morphotype D]. Metrics: body length (without head) 9.9; metasoma 4.7; mesosoma 4.6; mesofemur 1.9; mesotibia 1.6; mesobasitarsus 1.6; metafemur 1.8; metatibia 2.9; metabasitarsus 1.6; forewing (reconstructed) >9.0. Descriptive notes: Interior apparent in ventral view on light varve layer (Fig. 3B); head not preserved; terga still connected (indicating that metasoma was barely swollen); terga slightly laterally inflected; sting apparatus well preserved; setae of a single setal comb row evident in basal part of left mesobasitarsus, consisting of ~25 setae; metabasitarsus slender and somewhat triangular in shape; forewing venation preserved except for distalmost part; no counterpart. Remarks: While the lengths of the mesosoma and forewings are comparable to those of other bees from the Randeck Maar, the metasoma is noticeably shorter. The forewing length and venation appear similar to that of Apis dorsata. In addition, the slender metabasitarsi are reminiscent of Apis dorsata, even though the specimen does not approximate this species in size.
SMNS 64675 (neotype) [Morphotype D]. Metrics: body length 14.3; metasoma 8.1; mesosoma 3.4; head 2.6; metatibia 2.4; metabasitarsus 1.1; forewing 8.1. Descriptive notes: Dorsoventrally compressed on dark varve layer (Figs 2A, 4A);
sterna fragmentarily preserved, setae of sterna nearly completely
preserved; wax mirrors apparent as orange-brown areas (cf.
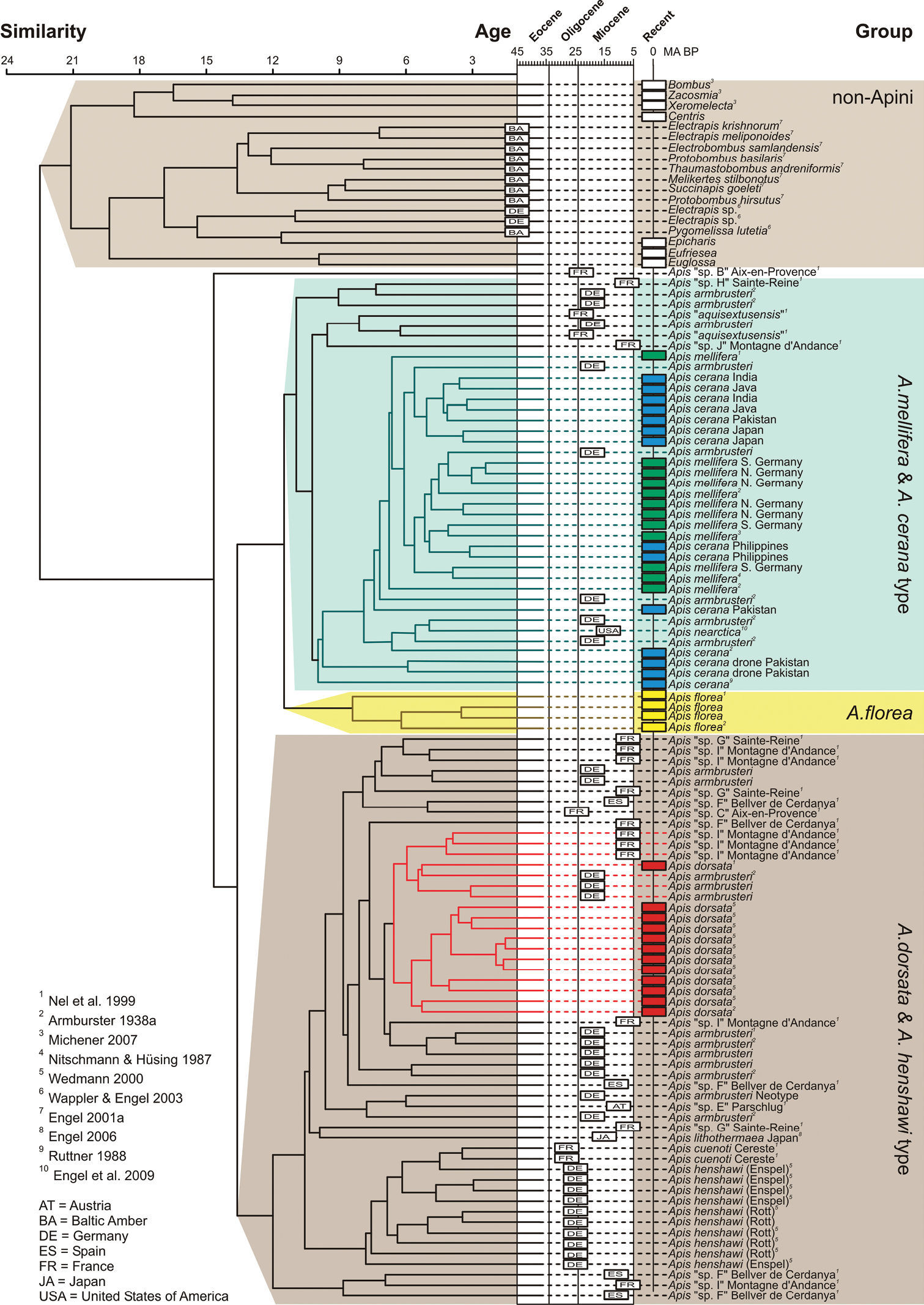
As to be expected given the unique venation of honey bees, all non-Apini were grouped together relative to Apis in the FWVA cluster analysis (Fig. 6). Among the Apini,
all Recent forms were grouped in general accordance with their
systematic position, and independent of whether the measurements were
based on the literature or newly measured forewings. This underlines
both the utility of the method and the quality of metrics and drawings
made by different authors (e.g.,
The dendrogram (Fig. 6) reveals two main clusters, the first of which comprises the FWVAs of mellifera/cerana and, on a subbranch of their own, the specimens of Apis florea. The second major cluster consists of FWVAs of Apis dorsata and Apis henshawi,
with both species well segregated from each other. These groups do not
necessarily represent clades given that undoubtedly some grouping is
based on symplesiomorphies. In regard to the specimens of Apis armbrusteri, most specimens group with Apis dorsata, while some specimens (e.g., SMNS 64674/11a and SMNS 64674/36) are positioned within the cerana/mellifera group (Fig. 6).
The dendrogram supports the observations described above, that at
least some of the specimens newly documented herein are superficially
more similar in forewing venation to cerana/mellifera-like beesthan to Apis dorsata, the latter phylogenetically outside of the Apis s.str. clade (
Apis henshawi from the Oligocene grouped nearest to those Apis dorsata and dorsata-like fossils (Fig. 6). Not surprisingly, Apis cuenoti from the Oligocene of Céreste groups within Apis henshawi, generally supporting the synonymy of these taxa (
The probably Late Oligocene-aged specimens from
Aix-en-Provence, however, showed a different clustering pattern. Some
of the specimens of the debated species “Apis aquisextana” (
The Apini
from the Miocene are, independent of their geographical origin,
scattered across the principle clusters (i.e., the branches on which
either mellifera/cerana or Apis dorsata and Apis henshawi
are positioned). In addition to the specimens SMNS 64674/11a and
64674/39, six other individuals from the Miocene of Randeck showed a
forewing venation more similar to that of cerana/mellifera-like bees than to that of Apis dorsata.
The distribution of Armbruster’s specimens (based on his 1938a
photographs and figures) in the dendrogram was independent of the “Hauffapis”-species designated by
Dendrogram resulting from FWVA cluster analysis described in the text. Recent specimens of Apis and associated clusters are marked in the following colors: red, Apis dorsata Fabricius; yellow, Apis florea Fabricius; green, Apis mellifera Linnaeus; blue, Apis cerana Fabricius; cyan, cerana/mellifera, morphotype.
Naturally, as noted previously, dendrograms cannot be
interpreted as phylogenies owing to an inability to distinguish homology
from analogy and plesiomorphy from apomorphy. This is immediately
evident in that, while all Apis group together, the corbiculate Apinae do not form a cluster, nor do the Centridini, or other well-defined taxa based on larger suites of characters (Fig. 6). Moreover, Apis florea phylogenetically lies outside of an Apis s.str.+Megapis clade (
The newly documented honey bees from the Randeck Maar exhibit, similar to the specimens described by
We conservatively suggest that European Apini of the Miocene exhibited a considerable morphological diversity, even somewhat more so than in modern congeners. This is supported by the fact that among the specimens from Randeck, even those within morphotype D, showed a remarkable variation in body size, which cannot be explained solely by postmortem effects, by caste differences, or biological phenomena. The heterogeneity is further supported by the considerable variability in leg shape and the varying presence or absence of the small process of 1m-cu, all of which are apparently independent of the two morphotypes recognized on the basis of FWVA.
Noteworthy, our results show a much lower variability for the Oligocene Apini from Germany and France. As shown by the
We thank the personnel of the Landesanstalt für Bienenkunde, Universität Hohenheim; Fachgruppe Biologie, Universität Bonn; and Institut für Bienenkunde, Universität Frankfurt; as well as several apiculturists for providing extant honey bee specimens. We are also grateful to Jes Rust, Sonja Wedmann, James Nebelsick, and Oliver Friedrich for discussions; to the Staatliches Museum für Naturkunde Stuttgart, the Paläontologisches Museum Nierstein, the Urweltmuseum Hauff, Holzmaden, and the Städtisches Naturkundliches Museum Göppingen for access to fossils from the Randeck Maar; and to two anonymous reviewers for their thoughtful comments on an earlier version of the manuscript. Partial support was provided by US National Science Foundation grant DEB-0542909 (to MSE). This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.
Forewing venation angle measurements. (doi: 10.3897/zookeys.96.752.app) File format: Microsoft Word.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.