(C) 2010 Jans Morffe. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Hystrignathus dearmasi sp. n. (Oxyurida: Hystrignathidae) is described from an unidentified passalid beetle (Coleoptera: Passalidae) from Panama. It resembles Hystrignathus cobbi Travassos & Kloss, 1957 from Brazil, by having a similar form of the cephalic end, extension of cervical spines and absence of lateral alae. It differs from the latter species by having the body shorter, the oesophagus and tail comparatively larger, the vulva situated more posterior and the eggs ridged. This species constitutes the first record of a nematode parasitizing a Panamanian passalid.
Nematoda, Hystrignathidae, Hystrignathus, Passalidae, Passalus, Panama
The family Hystrignathidae includes a large number of monoxenous nematodes from passalid beetles. At present, more than 100 species have been described from North America, Mexico, Cuba, Lesser Antilles, Brazil, Africa, Madagascar and Australasia.
The type genus of the family, Hystrignathus
Leidy, 1850, is characterized by having a single cephalic annule,
the cervical cuticle armed with opposite rows of spines, procorpus
clavate and genital tract didelphic-amphidelphic (
The family Passalidae
in Panama comprises about 60 species belonging to 16 genera (de Armas,
pers. comm.). Despite such diversity there are no records of parasitic
nematodes from Panamanian passalid beetles. In general, parasitological
surveys of passalids are scarce in Central America. The few studies
that have been carried out are restricted to the area of the Yucatan
peninsula, Mexico (
In this paper a new species of Hystrignathus from Panama is described. It constitutes the first record of a parasitic nematode from passalid beetles for this country.
Material and methodsTwo specimens of an unidentified small, blackish passalid beetle were collected by hand on rotting logs from the Summit National Park, Panama Province, Panama.
Hosts were killed by decapitation and the last abdominal segments were removed in order to extract the guts that were fixed and conserved in 70% ethanol. Intestines were dissected as soon as possible in Petri dishes with 70% ethanol under a stereomicroscope. The nematodes found were removed and fixed in 70% ethanol.
Nematodes were transferred and cleared in glycerine via
slow evaporation method and mounted in the same medium. The edges of the
coverslips were sealed using nail polish. Measurements were taken as in
The type-material is deposited in the Colección Helmintológica de las Colecciones Zoológicas (CZACC) from the Instituto de Ecología y Sistemática, Havana, Cuba and the Coleçao Helmintologica do Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, Brazil.
Systematics Genus Hystrignathus Leidy, 1850urn:lsid:zoobank.org:act:62307E25-9A1E-4CE0-9571-B5AE5B7E733C
Fig. 1 A–H, Fig. 2 A–E♀ holotype, Panama, Panama Province, Summit National Park; in unidentified short, blackish Passalidae; 25.IX.2009; L. F. de Armas coll.; CZACC 11.4604. Paratypes: 6 ♀♀, same data as holotype, CZACC 11.4605–11.4610; 2 ♀♀, CHIOC, same data as holotype.
Holotype (female) a = 8.62, b = 4.89, c = 7.24, V% = 57.46, total length = 1.810, maximum body width = 0.210, first cephalic annule (length×width) = 0.013×0.055, stoma length = 0.045, procorpus length = 0.295, isthmus length = 0.025, diameter of basal bulb = 0.090, total length of oesophagus = 0.370, nerve ring to anterior end = 0.213, excretory pore to anterior end = 0.480, vulva to posterior end = 0.770, anus to posterior end = 0.250, eggs = 0.095–0.110×0.043–0.048 (0.099 ± 0.007×0.046 ± 0.002 n = 4).
Paratypes (females) (n = 8): a = 7.81–9.37 (8.61 ± 0.58 n = 8), b = 4.17–5.35 (4.84 ± 0.51 n = 6), c = 6.19–8.16 (7.14 ± 0.59 n = 8), V% = 54.27–60.00 (56.69 ± 2.05 n = 7), total length = 1.300–1.780 (1.549 ± 0.179 n = 8), maximum body width = 0.158–0.210 (0.180 ± 0.019 n = 8), first cephalic annule (length×width) = 0.010–0.015×0.048–0.055 (0.013 ± 0.001×0.052 ± 0.003 n = 7), stoma length = 0.038–0.045 (0.041 ± 0.004 n = 8), procorpus length = 0.230–0.273 (0.248 ± 0.016 n = 8), isthmus length = 0.020 (n = 1), diameter of basal bulb = 0.070–0.085 (0.077 ± 0.005 n = 8), total length of oesophagus = 0.300–0.350 (0.325 ± 0.019 n = 6), nerve ring to anterior end = 0.175–0.190 (0.184 ± 0.007 n = 4), excretory pore to anterior end = 0.420–0.450 (0.433 ± 0.015 n = 4), vulva to posterior end = 0.590–0.770 (0.686 ± 0.073 n = 7), anus to posterior end = 0.190–0.250 (0.218 ± 0.023 n = 8), eggs = 0.088–0.103×0.038–0.055 (0.097 ± 0.005×0.046 ± 0.004 n = 16).
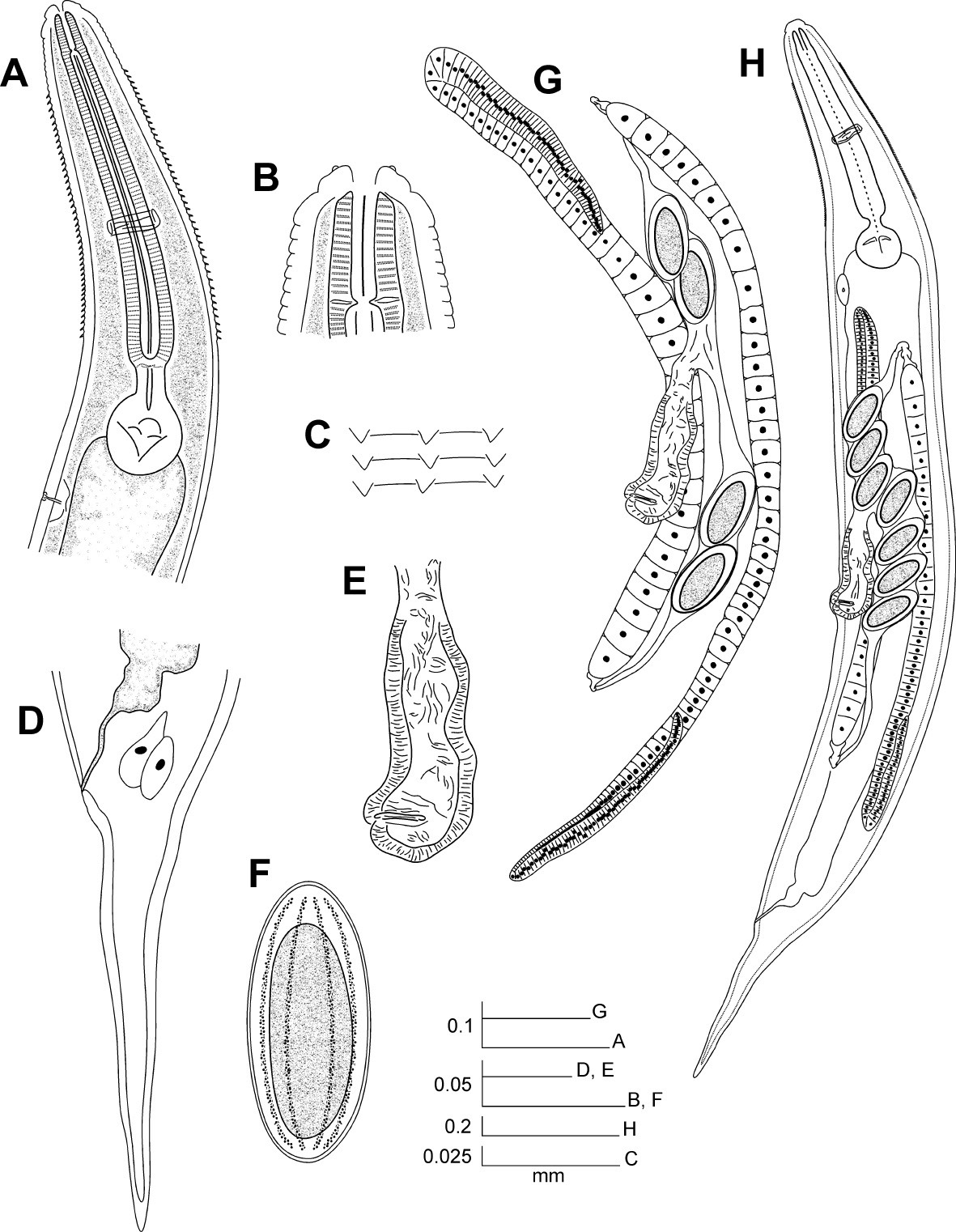
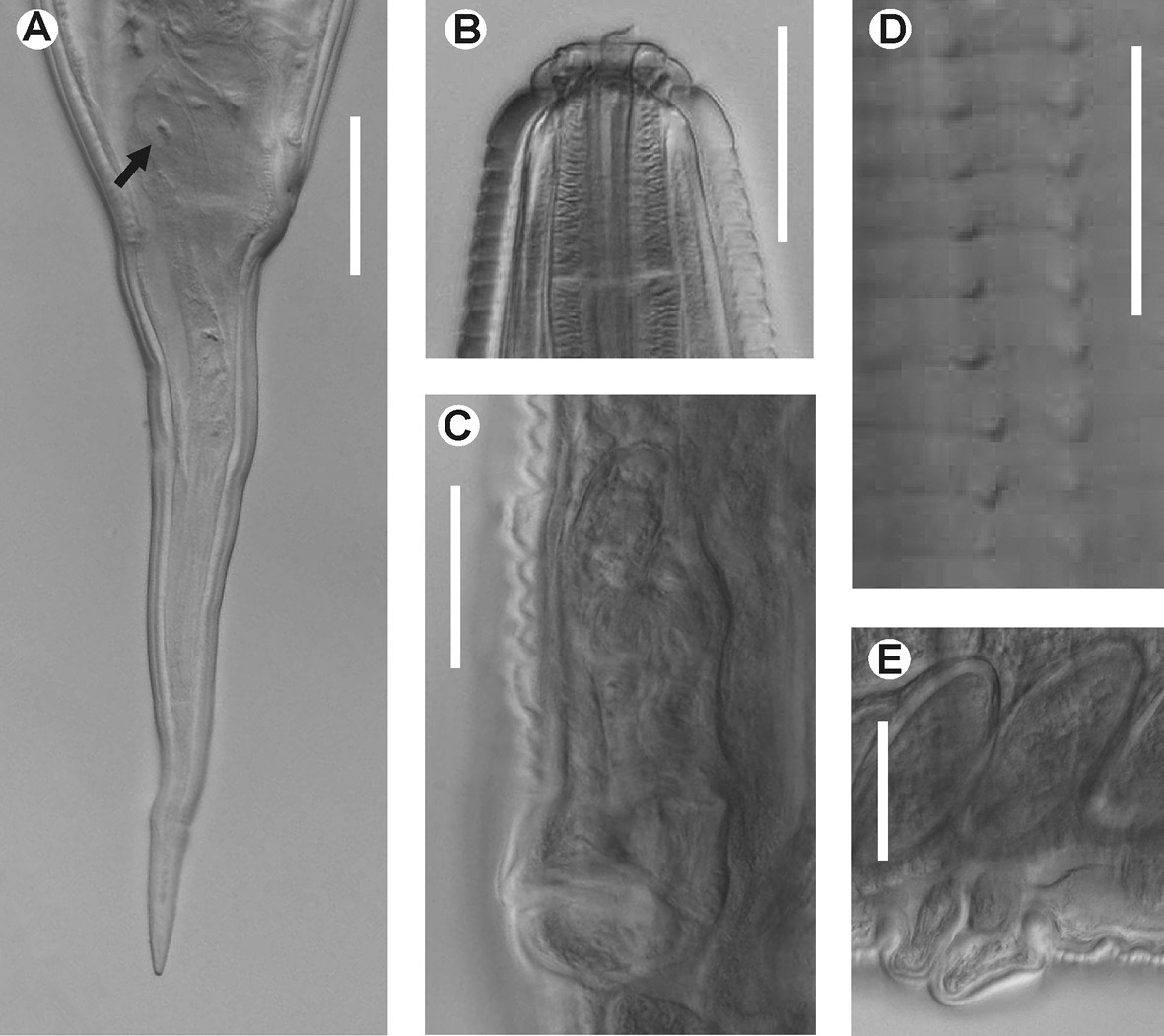
Female body robust, slightly fusiform. Cuticle strongly annulated in spiny region (annule c.5 µm width) and less in rest of body. Cervical cuticle armed with spines from some distance beyond stoma (distance about length of stoma) almost to end of procorpus. Spines arranged initially in c. 16 apposite rows that do not seem to increase consistently where they terminate. Anterior spines short and wide, scale-like, becoming sharply pointed but still short toward end of rows. Sub-cuticular longitudinal striae present. Lateral alae absent. Head bearing 8 paired papillae, set-off from body by single groove. First cephalic annule cone-like and truncated, not inflated, c.1.5 head lengths long. Stoma short, wide, about 4 first annule lengths long, surrounded by oesophageal collar. Oesophagus consists of muscular procorpus whose diameter increases slightly and gradually, well set-off from short isthmus. Intestine simple, sub-rectilinear, its fore region inflated. Rectum short, anus not prominent. At least with 2, large, ovoid, rectal glands with central nuclei at level of rectum. Nerve ring encircles procorpus at about its midpoint. Excretory pore located at about half of body width posterior to basal bulb. Vulva a median transverse slit slightly displaced towards posterior half of body, lips very prominent. Vagina muscular, forwardly directed. Genital tract didelphic-amphidelphic. Ovaries reflexed. Anterior ovary shorter, reflexed just posterior excretory pore, posterior ovary reflexed at slightly more than body width before anus. Both flexures about 2 body-widths long. Eggs ovoid, numerous, bearing 8 longitudinal, slightly prominent ridges on shell. Tail comparatively short, conical, attenuated, sharply pointed. Male unknown.
Hystrignathus dearmasi sp. n. female. A Esophageal region, lateral view B Cephalic region and stoma C Cervical spines D Tail, lateral view E Vulva, ventro-lateral view F Egg G Genital tracts H Entire nematode.
Hystrignathus dearmasi sp. n. female. A Tail, lateral view (arrow shows therectal glands) B Cephalic region and stoma C Vulva, ventro-lateral view D Cervical spines E Prominent lips of the vulva, lateral view. Scale bars: A, B, C, E. 0.05 mm; D. 0.025 mm.
Hystrignathus dearmasi sp. n. is similar to Hystrignathus cobbi
Travassos & Kloss, 1957 from Brazil, since both have a similar
form of the cephalic end, spines commencing posterior to the stoma
(feature unique in the genus) and the apparent absence of lateral alae (
Hystrignathus heliae Travassos & Kloss, 1957, from Brazil, has a similar cephalic end, but can be differentiated by the length of the stoma, which hardly surpasses the base of the first cephalic annule, and spines starting at the end of the cephalic annule. In Hystrignathus dearmasi sp. n. the stoma is notably longer and the spines commence at some distance posterior to it.
Unidentified, short, blackish passalid beetle (Coleoptera: Passalidae).
Gut caeca.
Summit National Park, Panama Province, Panama.
The specific epithet honours Dr. Luis F. de Armas Chaviano, an eminent Cuban aracnologist and the collector of the type-host.
Note: In the following key we omit two species of Cuban hystrignathids formerly placed in the genus Hystrignathus, because they will be published in the future as new combinations.
| 1. | Rows of spines commencing to some distance posterior to the stoma | 2 |
| – | Rows of spines commencing just after the end of the first cephalic annule | 3 |
| 2. | Tail very short (c = 14.48–16.61); eggs with smooth shell | Hystrignathus cobbi Travassos & Kloss, 1957 |
| – | Tail longer (c = 6.19–8.16); eggs with less prominent ridges on the shell | Hystrignathus dearmasi Morffe & García sp. n. |
| 3. | One ovary atrophied | Hystrignathus inegalis Van Waerebeke & Remillet, 1982 |
| – | Both ovaries well developed | 4 |
| 4. | First cephalic annule long and notably inflated | 5 |
| – | First cephalic annule shorter and less inflated | 7 |
| 5. | Eggs with smooth shell | 6 |
| – | Eggs with ridged shell | Hystrignathus splendidus Morffe & García, 2010 |
| 6. | Oesophagus longer than the tail | Hystrignathus tarda (Artigas, 1928) |
| – | Oesophagus as longer as the tail | Hystrignathus inflatus Travassos & Kloss, 1957 |
| 7. | Stoma not extending further than end of the first cephalic annule | 8 |
| – | Stoma extending further than end of the first cephalic annule | 9 |
| 8. | Spines ending at the level of the excretory pore; tail longer (c =8.63) | Hystrignathus paulistanus Cordeira, 1981 |
| – | Spines ending at the end of the basal bulb; tail shorter (c = 9.61) | Hystrignathus papillophorus Cordeira, 1981 |
| 9. | Eggs with a ridged shell | 10 |
| – | Eggs with a smooth shell | 15 |
| 10. | First cephalic annule very short, much less than half the stoma length | 11 |
| – | First cephalic annule longer, about half the stoma length | Hystrignathus metropolitanus Cordeira, 1981 |
| 11. | Lateral alae surpass the level of the vulva | 12 |
| – | Lateral alae do not surpass the level of the vulva | 13 |
| 12. | Tail markedly attenuate and comparatively short (c = 6.0–7.6) | Hystrignathus egalis Van Waerebeke & Remillet, 1982 |
| – | Tail markedly subulate and comparatively large (c = 3.64–4.81) | Hystrignathus rescens Travassos & Kloss, 1958 |
| 13. | Spines terminate at a short distance (less than a body-width) posterior to basal bulb | 14 |
| – | SSpines terminate at a longer distance (about a body-width) posterior to basal bulb | Hystrignathus ferox Hunt, 1982 |
| 14. | Lateral alae end at the level of the vulva; tail comparatively larger (c = 3.38–3.98) | Hystrignathus rosario García, Ventosa & Morffe, 2009 |
| – | Lateral alae end before the level of the vulva; tail comparatively shorter (c = 5.71–6.86) | Hystrignathus rugosus Travassos & Kloss, 1958 |
| 15. | Spines terminate before the basal bulb | 16 |
| – | Spines terminate after the basal bulb | 19 |
| 16. | Lateral alae present | 17 |
| – | Lateral alae not present | Hystrignathus popiliophagus Guerrero, 1980 |
| 17. | Spines cease at the end of the bulb; tail very short (c = 7.88–10.66) | Hystrignathus heliae Travassos & Kloss, 1957 |
| – | Spines cease before the end of the bulb; tail longer (c ≤ 7) | 18 |
| 18. | Lateral alae end just before the anus | Hystrignathus insularis Van Waerebeke, 1973 |
| – | Lateral alae end at certain distance before the anus | Hystrignathus meridensis Guerrero, 1980 |
| 19. | Spines terminate at the level of the excretory pore | 20 |
| – | Spines terminate slightly anterior to the excretory pore | Hystrignathus rigidus Leidy, 1850 |
| 20. | Stoma very short, hardly surpassing the end of the first cephalic annule | Hystrignathus pearsoni Travassos & Kloss, 1958 |
| – | Stoma longer, clearly surpassing the end of the first cephalic annule | Hystrignathus spinosus Travassos & Kloss, 1957 |
We are indebted to Dr. Luis F. de Armas (Instituto de Ecología y Sistemática, Havana, Cuba) for collecting the hosts and revision of manuscript. To MSc. Yamir Torres (Instituto de Ecología y Sistemática) for his technical assistance with the micrographs. To Dr. Pedro Herrera (Instituto de Ecología y Sistemática) for the revision of the English manuscript. This work was supported by IDEAWILD and the project DB-06 “Colecciones Zoológicas, su Conservación y Manejo”, Ministerio de Ciencia, Tecnología y Medio Ambiente, Cuba.