(C) 2010 Peter H.W. Biedermann. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Strongly female-biased sex ratios are typical for the fungalfeeding haplodiploid Xyleborini (Scolytinae, Coleoptera), and are a result of inbreeding and local mate competition (LMC). These ambrosia beetles are hardly ever found outside of trees, and thus male frequency and behavior have not been addressed in any empirical studies to date. In fact, for most species the males remain undescribed. Data on sex ratios and male behavior could, however, provide important insights into the Xyleborini’s mating system and the evolution of inbreeding and LMC in general.
In this study, I used in vitro rearing methods to obtain the first observational data on sex ratio, male production, male and female dispersal, and mating behavior in a xyleborine ambrosia beetle. Females of Xyleborinus saxesenii Ratzeburg produced between 0 and 3 sons per brood, and the absence of males was relatively independent of the number of daughters to be fertilized and the maternal brood sex ratio. Both conformed to a strict LMC strategy with a relatively precise and constant number of males. If males were present they eclosed just before the first females dispersed, and stayed in the gallery until all female offspring had matured. They constantly wandered through the gallery system, presumably in search of unfertilized females, and attempted to mate with larvae, other males, and females of all ages. Copulations, however, only occurred with immature females. From galleries with males, nearly all females dispersed fertilized. Only a few left the natal gallery without being fertilized, and subsequently went on to produce large and solely male broods. If broods were male-less, dispersing females always failed to found new galleries.
inbreeding, haplodiploidy, ambrosia beetles, all male broods, LMC, subsociality
In panmictic (randomly mating) species, natural selection usually favors balanced sex ratios (
A prominent group of beetles living under the bark is the weevil sub-family Scolytinae, that includes species with varying mating systems (
The few observational studies on Xyleborini that exist suggest that these beetles behave subsocially (
If males do not play an important role for gallery
maintenance and protection, male numbers should be minimized in order
to lower the cost of LMC. The optimal number should be affected by the
maximum number of females one male is able to fertilize, the timing of
male production, male survivorship and longevity, as well as male
dispersal (see
The objective of this study was to observe males of Xyleborus saxesenii
inside their galleries for the first time to report their number and
hatching time relative to females, as well as to determine whether or
not males disperse and if they share in gallery maintenance and
protection. Furthermore, I aimed at clarifying the existence of all
male broods, which has been remarked upon in several studies on Xyleborini (e.g.
Study species
In the study species Xyleborinus saxesenii Ratzeburg, the ambrosial complex is typically dominated by the anamorphic fungus Ambrosiella sulfurea Batra (
Laboratory breeding and data collection
Xyleborinus saxesenii beetles were bred in artificial agar-sawdust based “standard medium” in glass tubes (
Observations
I scanned the beetles in 70 observable galleries
every second to third day and recorded male and female numbers,
allocating the individuals to different developmental stages (eggs,
larvae, male and female pupae, immature females, mature females, and
males). Male and female pupae and adults were easy to differentiate
because of their strong sexual dimorphism in size (Fig. 1, see figures in
Parts of a brood chamber of Xyleborinus saxesenii in artificial medium. 1st and 2nd/3rd instar larvae, several pupae, two immature females and a male are visible. The male is mounting an immature female.
I measured the breeding success (defined as the successful start of egg-laying within 40 days) and brood size of 311 daughters out of 33 families (median = 5 daughters/family, range = 1–41) to determine whether they differ between daughters of galleries with males and male-less ones. Subsequently, I correlated the secondary sex ratio of 62 daughter-families that had successfully produced a brood with the secondary sex ratio of their mothers’ families to test whether secondary sex ratios might be heritable.
Additionally, I observed the behavior of eight males
from different galleries for 10 min and calculated the proportion of
time spent on different behaviors. I differentiated between shuffling
(moving frass and feces with the legs under the body and with the
abdomen), digging (excavating new tunnels), cannibalism (feeding on a
larva, pupa, or adult beetle), courtship behavior (grooming an egg,
larva, pupa, or adult beetle with maxillae and labium), walking,
cropping (feeding on the fungal layer covering gallery walls), and
mating attempt (mounting or copulating with a female) (
Statistical analyses
The association between numbers of male and female
offspring was explored using linear regression. The number of males
within a brood of a given size should have a binomial distribution under
random sex determination. To test if this was the case, I compared the
variance of the male numbers observed with that expected if the numbers
of males were binomially distributed using a Chi²-test (
Sex ratio and breeding success
Males were extremely scarce in Xyleborinus saxesenii galleries (mean abundance per gallery = 0.96, SE = 0.09, N = 70; Fig. 2). Male production by mothers was not random, as their overall number did not resemble a Poisson distribution. They produced more one- and two-male broods, fewer male-less and three-male broods than expected, and no four-male brood, which suggests the optimal number is one or two males per brood. Nearly one third of the mothers produced no males (N = 21).
Occurrence of males in laboratory galleries of Xyleborinus saxesenii. The maximum number of males per gallery across the observation period is reported (N = 70 galleries). The data significantly differs from the expected Poisson distribution.
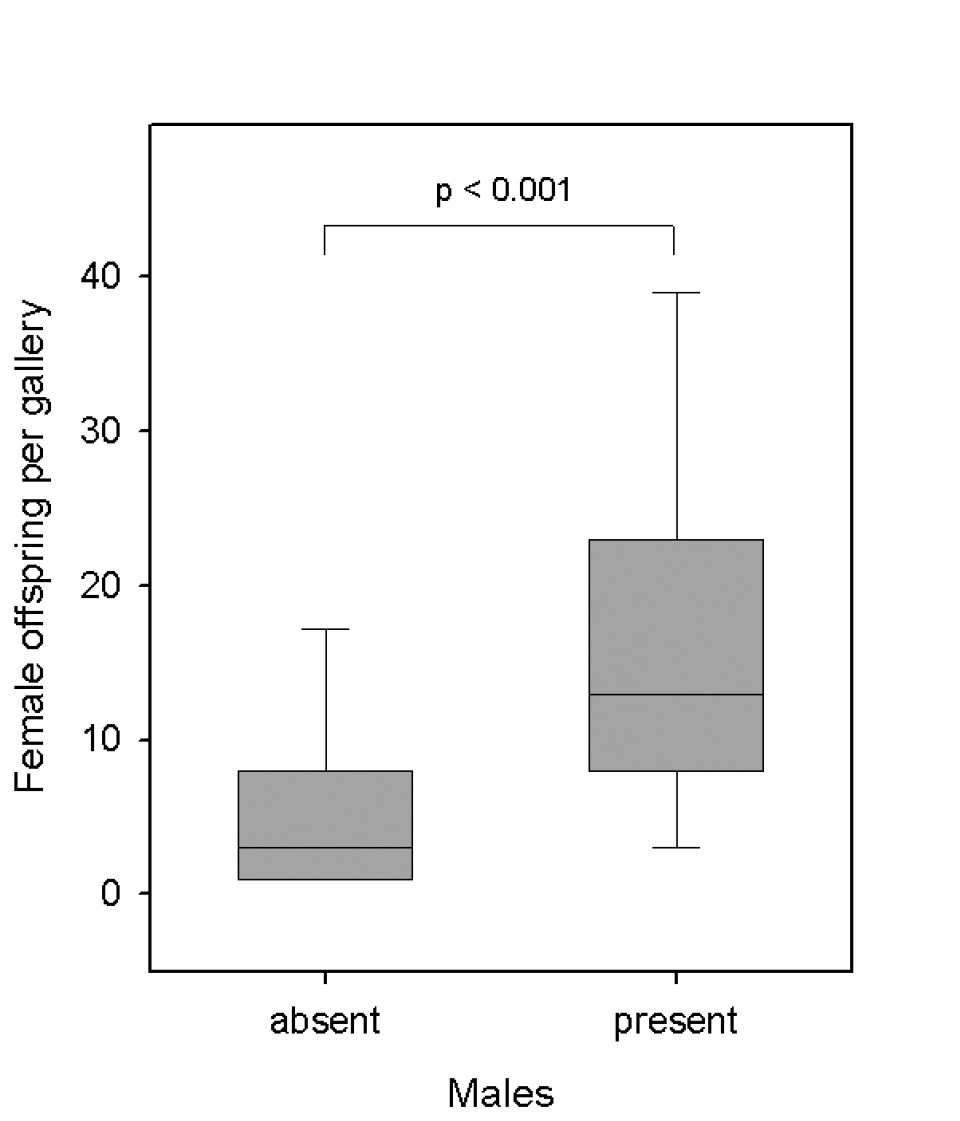
Families with males were significantly larger than families without males (Fig. 3). In accordance with this finding, males were absent from 50% of the galleries with only ten daughters or fewer, whereas only 8.3% of the galleries with more than ten daughters were male-less. However, overall the number of males per gallery was only weakly affected by the number of adult females (Linear regression: F = 3.824, p = 0.055, DF= 1, N = 70; Fig. 4). The residual mean squared error of the number of males (MSE = 0.597) was much smaller than the mean variance expected under binomial distribution (0.9398; Chi²-test: χ² = 43.2, DF = 68, p > 0.05), which indicates that the production of males was not random, but relatively constant and precise (around one or two males per brood).
Presence of males in relation to the productivity of laboratory galleries of Xyleborinus saxesenii. Box-whisker plots with median, 90%, 75%, 25%, and 10% quartiles are shown. Mann-Whitney U-test: T = 423, p < 0.001, N = 21 + 49 galleries.
The number of males in relation to the number of females in laboratory galleries of Xyleborinus saxesenii. Each gallery is represented by one data point (N = 70). Linear regression (males = 0.785 + (0.0119 * females)): F = 3.824, p = 0.055, DF = 1.
Breeding success (i.e., the likelihood to lay at least one egg within 40 days) of daughters per family ranged between 0% and 78% (mean = 15.2%, median = 0, N = 311 daughters from 33 families), and was 0% for all male-less broods. A statistical difference was however not detectable because of the high variation of breeding success across all families tested (U-test: T = 77, p = 0.067, N = 26 vs. 7).
The secondary sex ratios of the 62 successfully founded daughter-galleries did not correlate with the secondary sex ratios of their mothers galleries (Spearman’s rank correlation: r = 0.038, p = 0.771, N = 62).
Male presence and behavior
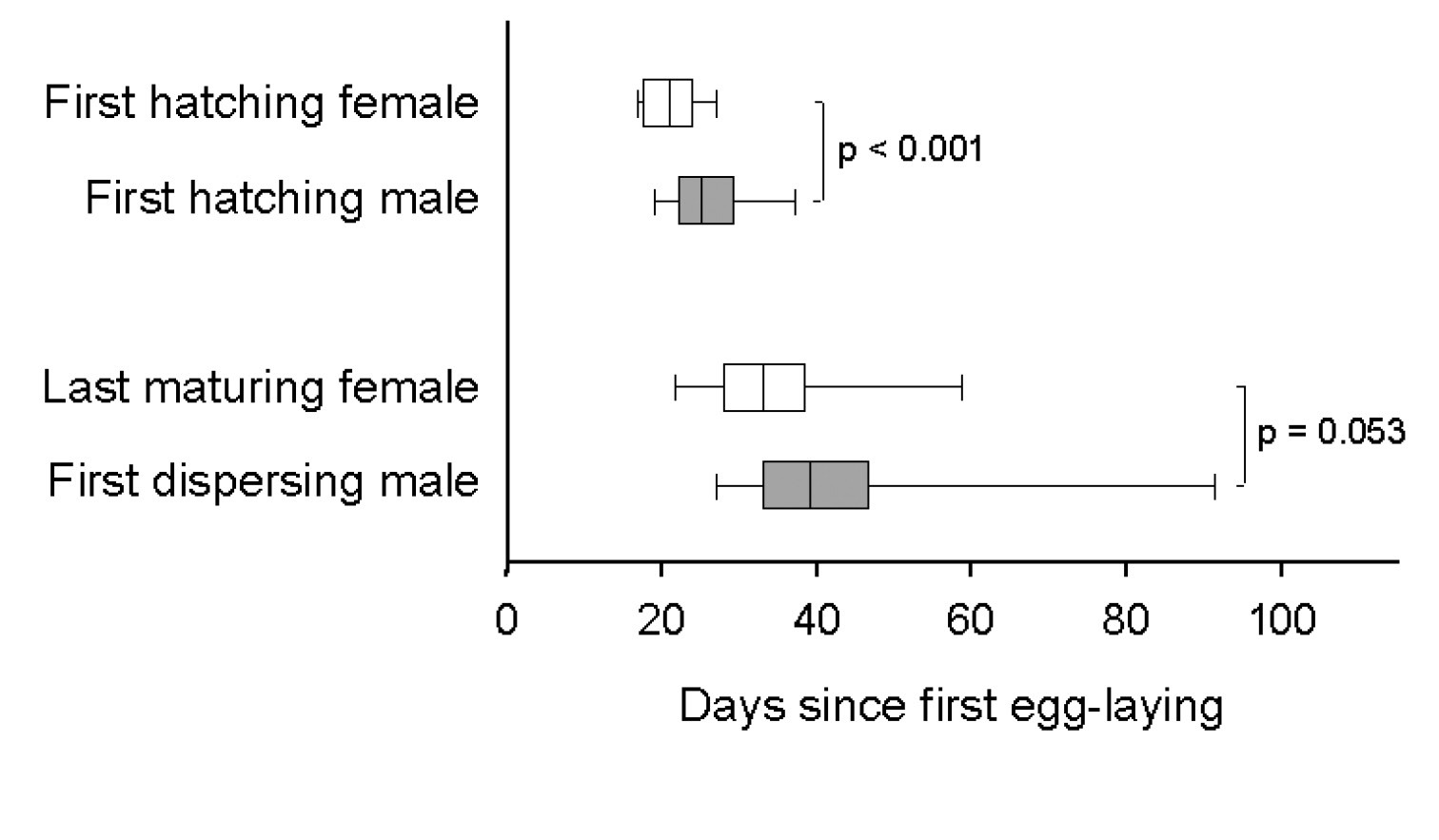
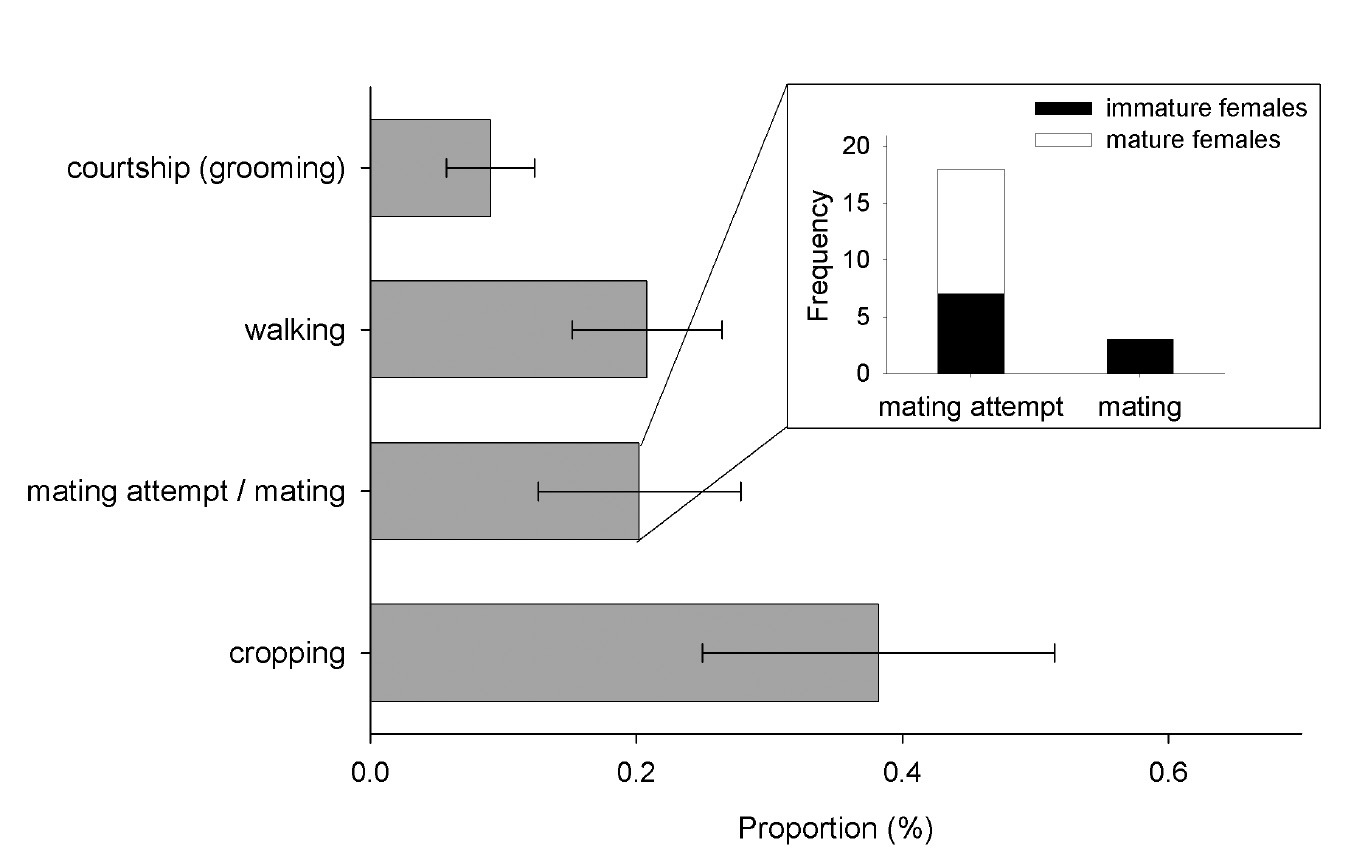
The first male per gallery usually eclosed a few days after his first sister did so (Fig. 5), but before her maturation (N = 29 galleries). Following eclosion, males spent most of their time with cropping fungus and walking through the gallery system. When encountering a possible partner, the male started to courtship her, which resembles grooming the body of the female, and afterwards attempted to mate with her. Females seemed relatively reluctant, and thus mating attempts usually lasted several minutes and were rarely successful. Thirty mating attempts were observed, during which males mounted immature (N = 7 of 30 times) and mature females (N = 11 of 30 times) as well as pieces of wood, larvae, and other males (N = 12 of 30 times). Successful copulations, however, were only observed with three immature females, which hints towards a preference to mate with them (Fisher’s exact test: p = 0.09, N = 21; Fig. 6). Only two females were found to emerge unfertilized from their galleries (indicated by the production of an exclusively male brood later on); they emerged from galleries with brood sex ratios of 2:16 and 1:86, respectively, which indicates that one male is capable of fertilizing up to 60 sisters on its own (no unfertilized females were detected in a gallery with a sex ratio of 1:60). Other male behaviors which were rarely exhibited included digging, cannibalism, and the shuffling of saw-dust and feces with the legs (all with median = 0%).
Timing of male and female hatching, maturation, and dispersal in laboratory galleries of Xyleborinus saxesenii. The correlation of the first male and female hatching from the pupal stage and the correlation between the last date a not-fully sclerotized female was seen, and the first male dispersal event (N = 29 galleries) were determined where possible and contributed one data point per gallery to this graph. Box-whisker plots with median, 90%, 75%, 25%, and 10% quartiles are shown. Mann-Whitney U-tests: T = 1066, p < 0.001, N = 29 + 29 galleries (female vs. male hatching); T = 325.5, p = 0.053, N = 26 + 13 galleries (female maturation vs. male dispersal).
Behaviors of males in laboratory galleries of Xyleborinus saxesenii. Males (N = 8 galleries) were observed for 10 min and proportion of time spent with the different behaviors was calculated. The grey bars show the mean +/- SE for each behavior across all males. Male mating behaviors were further split into mating attempt and mating, and the age class of the female partner is reported.
Although males are wingless, some of them (at least one male in 13 out of 29 galleries) dispersed, i.e., were found on the surface of the medium, when all offspring had matured and no new eggs were laid. Males did not disperse randomly together with their sisters throughout gallery life, but in most cases the first male dispersal occurred after the last female (in a gallery) had matured (Fig. 5).
DiscussionUnder inbreeding conditions, natural selection should
favor the production of that number of males that maximizes the mean
number of inseminated females dispersing from a brood. Thus, in case
males are able to inseminate only a limited number of females, the
numbers of males and females should be correlated (e.g.
At least one male per family should, however, be
expected under the assumption of strict inbreeding. Contrary to that,
30% of all families were male-less; a proportion which is not untypical
for Xyleborini (19% of all families in Xylosandrus germanus Reiter in the field;
Relatively independent from the fact if secondary
manipulations of the sex ratio occur, male numbers should be higher
under conditions that allow outbreeding, as has been shown in Xyleborinus germanus (
Males hatched significantly later than their first sisters did, which contrasts with findings in Xyleborinus affinis, whose males hatch before females (
Unfertilized females produced solely male broods, but
did not mate with their sons to subsequently produce “normal” mixed
broods. This expectation was based on observations of Coccotrypes dactyliperda Fabricius (Scolytinae) females that do so (
My data shed light on the cryptic life and mating system of xyleborine ambrosia beetles within their galleries. In the future, molecular studies will be crucial for determining whether the observed inter- and intraspecific variance in sex ratios is an adaption to outbreeding in some Xyleborini or a hint for the existence of sex ratio distorters, for example, their symbionts. My data provide a starting point for future studies dealing with factors that influence xyleborine sex ratios and the frequency of outbreeding events. Ambrosia beetles are one of the best model systems for studying the evolution of inbreeding, haplodiploidy, sociality and symbioses, but at the same time one of the least known. Before comparative studies on different species can be done to address the issues mentioned, further basic data on the behaviors of these beetles inside their galleries have to be collected.
ConclusionMy data suggest that males in Xyleborinus saxesenii Ratzeburg are extremely successful in locating and fertilizing all their sisters in the natal gallery. On average there is only about one male per family relatively independent of the number of sisters and the maternal brood sex ratio. Apparently, this is sufficient to fertilize all females, probably because the morphology of the gallery system with a central “brood chamber” makes it easy for males to locate them. Only about 3% of the females from broods with males disperse unfertilized from the natal nest and subsequently produce all male broods, but do not mate with one of their sons to produce a mixed brood afterwards.
Despite the fact that males are not the first to hatch within a brood, they do so before the first females mature, and only disperse from the natal gallery once the last female has finished her development. They do not engage in gallery maintenance, gallery protection and brood care, but constantly wander the gallery, presumably in search of unfertilized females. Although males attempt to mate with all individuals independent of age and sex, they were observed to copulate only with immature females.
I want to thank J. Hulcr and A. Cognato for giving me the chance to be part of this festschrift. I hold S. L. Wood’s accomplishments in great esteem, and I regret that I have never had the chance to meet him in person. This manuscript emerged at the Institute of Ecology and Evolution, University of Bern, Switzerland, under the supervision of M. Taborsky. Additionally to discussions with him, it profited a lot from suggestions made by T. Turrini, R. Roeper, K. Peer, E. Ott, and two anonymous reviewers. During parts of the project, I was financially supported by a grant of the Roche Foundation and a DOC grant of the Austrian Academy of Science.