(C) 2011 Vivian Flinte. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Species richness and abundance of seven Plagiometriona species on their host plants were studied along a single trail in the mountainous Serra dos Órgãos National Park in the State of Rio de Janeiro, Brazil. Six sites were chosen along an altitudinal gradient ranging from 1300 m to 2050 m, where all Solanaceae host plants were inspected in search of adults every two months from June 2006 to June 2007. Species richness did not vary clearly with altitude, but abundance increased up to 1800 m, where the highest mean host plant density was found, and abruptly decreased at the last elevational site. Most species showed a restricted distribution and just one occurred across the entire gradient. For at least four species, altitudinal distribution seems to be strongly related to host plant availability, while for the others it is difficult to access which factors are decisive, due to their low numbers. Only in October all species were found in the field, although February was the month with the highest total abundance. Over the course of the study, the greatest abundances were recorded from October to February, comprehending the hottest and rainiest months, and the lowest abundances were found from June to August, which include the coldest and driest months. Thus, species seasonal distribution, supported by other studies in the same area, seems to be related to the local climate.
Species richness, abundance of individuals, population fluctuation, host plant, altitude, phenology, Chrysomelidae, Cassidinae, Solanaceae
Chrysomelidae is one of the richest families of Coleoptera, comprised of almost 37, 000 described species (
The majority of Cassidinae are specialized feeders (narrowly oligophagous), with few species which could be considered either truly monophagous or polyphagous in habits. In the neotropical region, cassidoid Cassidinae are mainly associated with host plants in the dicotyledenous families Convolvulaceae, Asteraceae, Bignoniaceae, Boraginaceae, Lamiaceae and Solanaceae. It is interesting to note how few potential plant families present in any particular area are actually exploited by these beetles. Of those plant families that are, most are members of a single clade of Eudicotyledonaes (
It is believed that multiple factors, operating across a hierarchy of spatial and temporal scales, shape species distributions (
On tropical mountains, abiotic factors are likely to have even greater effects on community structure, so patterns of population fluctuation similar to subtropical and even temperate regions may emerge, with occurrence periods of insects well defined throughout the year. Increasing altitude brings lower temperatures, increased precipitation (rain or snow), lower partial pressure of gases, higher wind speed and turbulence, and greater extremes in radiation input (
There are only few studies with Brazilian Chrysomelidae on elevational gradients, and data obtained so far show that abundance and richness patterns vary with altitude among study areas.
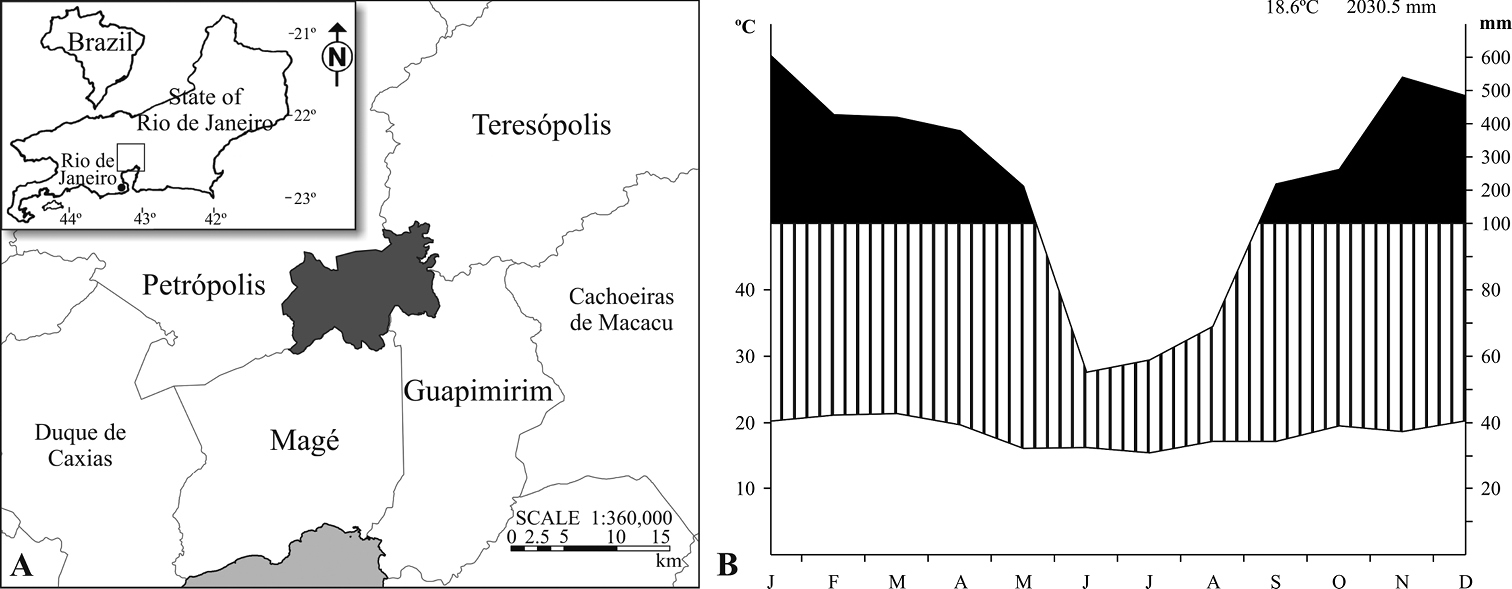
Our study was conducted in Serra dos Órgãos National Park (22°32'S, 43°07'W), which encompasses an area of 20, 024 ha, extending over four counties in southeast Brazil, State of Rio de Janeiro (Fig. 1A), within the tropical Atlantic domain. The climate is marked by mild summers, winters of high precipitation and temperature reduction with altitude (
A Location of Serra dos Órgãos National Park in the State of Rio de Janeiro, Brazil and B climatic diagram (meteorological station at 980 m altitude, from the National Institute of Meteorology), compiled for the period of January 2007 to December 2008. Striped area= humid period; Black area= superhumid period.
Surveys were conducted at six sites of different altitudes (approximately 1300 m, 1500 m, 1600 m, 1700 m, 1800 m and 2050 m) in Teresópolis County, specifically on the Pedra do Sino trail, which has an altitudinal variation of more than 1000 m, ranging from 1100 m to 2263 m. Accordingly, the two lowest sites are found in montane forest, the highest occupies the high altitude grasslands, with intermediatesites in high-montane forest. Trail conditions (incident light, tree cover, humidity) were undoubtedly different from the interior of the forest, but these varied nevertheless along the chosen sites with changing phytophysiognomies. Besides, although host plant characteristics will probably not be the same, open areas such as clearings and trails may be preferable over intact canopy forest, since many Cassidinae, including various Plagiometriona species, are associated with secondary growth plants (Windsor, 1992).
Within a wider project on Chrysomelidae diversity and distribution started in 2005 in the Park, seven Cassidinae species were chosen for the present study: Plagiometriona ambigena (Boheman, 1855) (Fig. 2A), Plagiometriona dodonea (Boheman, 1855) (Fig. 2B), Plagiometriona dorsosignata (Boheman, 1855) (Fig. 2C), Plagiometriona sahlbergi (Boheman, 1855) (Fig. 2D), Plagiometriona stillata (Boheman, 1855) (Fig. 2E), Plagiometriona tredecimguttata (Boheman, 1862) (Fig. 2F) and Plagiometriona sp. 7 (Fig. 2G), all of which present very similar patterns of elytral coloration, form and body size. Within these species, the biggest individuals belong to Plagiometriona dorsosignata and the smallest to Plagiometriona stillata (mean of 69 and 52 mm, respectively; n= 10 each species). Some of these species (Plagiometriona dodonea, Plagiometriona dorsosignata, Plagiometriona stillata and Plagiometriona tredecimguttata) were also studied by
Species studied at the Serra dos Órgãos National Park: Plagiometriona ambigena A Plagiometriona dodonea B Plagiometriona dorsosignata C Plagiometriona sahlbergi D Plagiometriona stillata E Plagiometriona tredecimguttata F and Plagiometriona sp. 7G
To describe species richness on each plant species and altitude, only adults were considered because eggs, larvae and pupae of the studied Plagiometriona are very similar to each other and to other species of the same genus not mentioned here, but which can be found on the same plant species (this problem was already described by
Adult individuals of the focal Plagiometriona species fed one or more of seven different Solanaceae species: Aureliana fasciculata Sendtn., Capsicum mirabile Mart., Solanum campaniforme Roem. & Schult., Solanum enantiophyllanthum Bitter, Solanum megalochiton Mart., Solanum swartzianum Roem. & Schult. (Solanoideae: Solaneae) and Cestrum bracteatum Link & Otto (Cestroideae: Cestreae) (Table 1).
Host plant records based on larval “no choice” feeding tests for the seven Plagiometriona species in the study (from
| Host plants / Cassidines | Aureliana fasciculata | Capsicum mirabile | Cestrum bracteatum | Solanum campaniforme | Solanum enantiophyllanthum | Solanum megalochiton | Solanum swartzianum |
|---|---|---|---|---|---|---|---|
| Plagiometriona ambigena | x | x | |||||
| Plagiometriona dodonea | x | x | |||||
| Plagiometriona dorsosignata | x | ||||||
| Plagiometriona sahlbergi | x | ||||||
| Plagiometriona sp. 7 | x | ||||||
| Plagiometriona stillata | x | x | |||||
| Plagiometriona tredecimguttata | x | x | x | x |
Because the host plants Aureliana fasciculata and Solanum campaniforme had very similar vegetative forms, it was not possible to reliably distinguish them in the field outside their reproductive season. Therefore, data from beetles associated with these plants were grouped in the present study.
Beetles were deposited in the collection of the Laboratório de Ecologia de Insetos at the Federal University of Rio de Janeiro, but some specimens were also deposited in the collection of the Department of Biodiversity and Evolutionary Taxonomy, Institute of Zoology, University of Wroclaw, Poland. After curation, plants were deposited in the Herbarium of the Federal University of Rio de Janeiro and in the Rio de Janeiro Botanical Garden.
Temporal and altitudinal distributionSurveys were conducted every two months from June 2006 to June 2007 by Sama de Freitas and one additional of four undergraduation students. At each site, two transects of 200 m × 0.5 m (length × width) were made, one at each side of the border of the trail. Within each transect, host plants were carefully surveyed for adults of the focal Plagiometriona species, and the number of individuals per plant and the number of each plant species was recorded in every site.
To obtain mean plant density for each altitude, we summed the number of plant individuals of each species in the transect per month, and divided that number by the number of surveys (seven months). In order to describe the temporal distribution of the species we considered the total abundance of each species per survey. Finally, beetle altitudinal distribution was calculated from the total number of adults sampled over the course of the study for each species and elevational site. Host plant quality was not considered in this study.
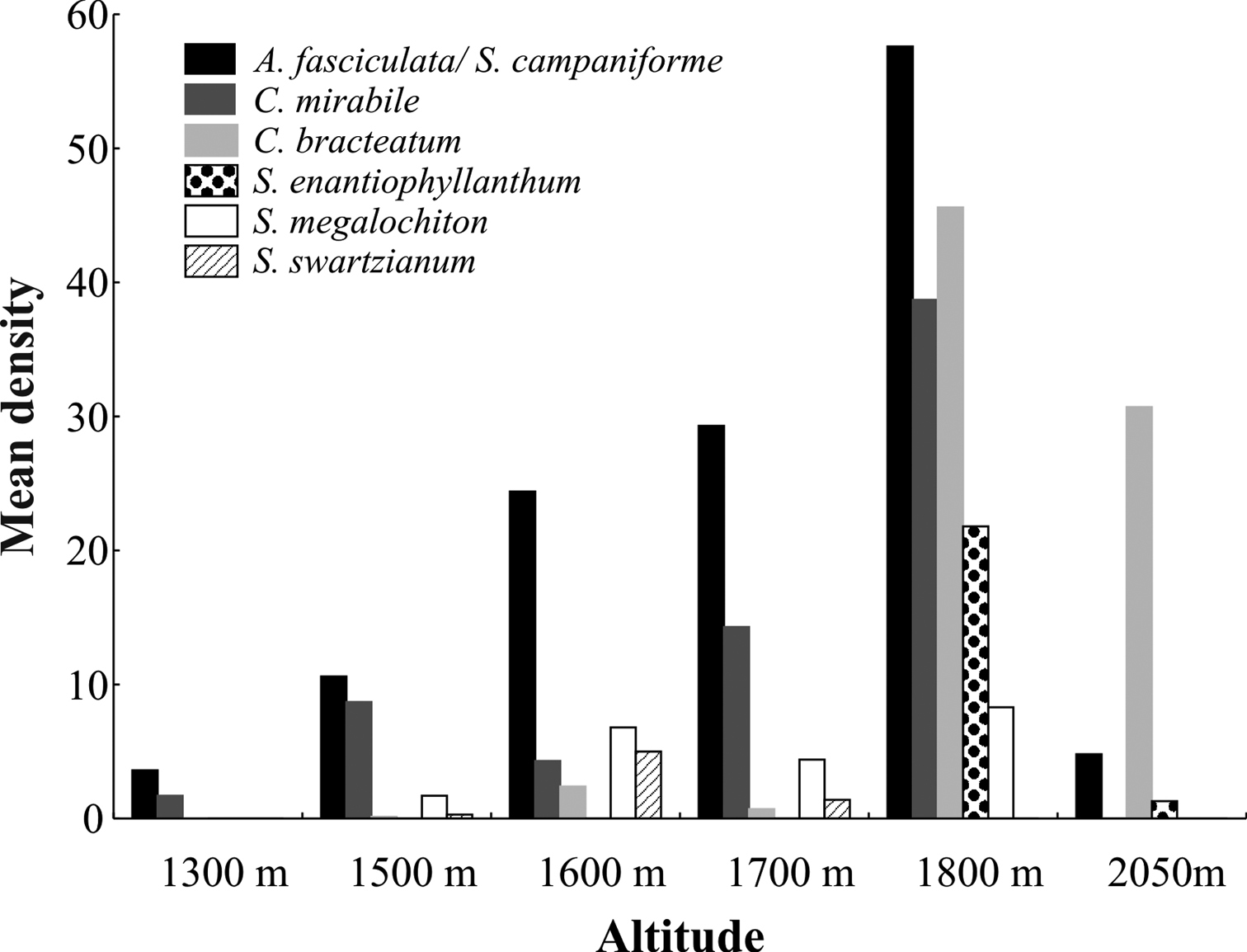
Results and discussion Spatial distribution of host plantsAt no altitudinal site did all seven host plant species co-occur. Plant richness was highest with five species co-occurring at 1600 m, 1700 m and 1800 m, and lowest at 1300 m and 2050 m where only two and three species co-occurred, respectively (Fig. 3). Aureliana fasciculata / Solanum campaniforme occurred along the whole elevational gradient, while the other species were more restricted in their altitudinal distribution. In general, host plant density was highest at 1800 m for all species, except Solanum swartzianum, which showed higher density at 1600 m.
Mean host plant density of the studied Plagiometriona species at different altitudes.
Our field observations were consistent with the data presented by
Relative adult occurrence (in percentage) of the seven Plagiometriona species associated with Solanaceae at the study site.
| Host plants / Cassidines | Aureliana fasciculata / Solanum campaniforme | Capsicum mirabile | Capsicum bracteatum | Solanum enantiophyllanthum | Solanum megalochiton | Solanum swartzianum |
|---|---|---|---|---|---|---|
| Plagiometriona ambigena | 50.0 | 50.0 | ||||
| Plagiometriona dodonea | 33.3 | 66.7 | ||||
| Plagiometriona dorsosignata | 100.0 | |||||
| Plagiometriona sahlbergi | 1.5* | 98.5 | ||||
| Plagiometriona sp. 7 | 3.7 * | 3.7 * | 92.6 | |||
| Plagiometriona stillata | 100.0 | |||||
| Plagiometriona tredecimguttata | 70.9 | 25.5 | 2.9 | 0.7* |
Except for Plagiometriona ambigena, the other oligophagous species showed a clear preference for one of their host plants, with more than 50% of individuals being recorded on a single host species (Table 2). Because plants on the border of the trail are sometimes very close to each other, some individuals were eventually found on non-host plants, probably during dispersal or because of disturbances that may take place during the inspection of the feeding plants. Thus, very low percentage values of occurrence on “new” host plants were not considered true associations and must be confirmed by laboratory rearing. The occurrence of Plagiometriona stillata on only one of its described hosts in field may be an artifact due to the small number of individuals this species ever observed during the study period.
Temporal and altitudinal distribution of Plagiometriona spp.Abundance of the seven focal species varied considerably along the year. The lowest values were recorded in June, gradually increasing until peaking in February and then decreasing again (Table 3). Thus, the peak happens in the middle of the summer, when precipitation and temperatures are high, while the low numbers occur during months of lower rainfall and milder temperatures (Fig. 1B).
Abundance (per month and total) of the seven Plagiometriona species studied from June 2006 to June 2007 at the study site, and species richness per month. Darker shade in gray indicates the month in which the most abundant species had the highest numbers of individuals.
| Months / Cassidines | 2006 | 2007 | Total abundance | |||||
|---|---|---|---|---|---|---|---|---|
| J | A | O | D | F | A | J | ||
| Plagiometriona ambigena | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 4 |
| Plagiometriona dodonea | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 |
| Plagiometriona dorsosignata | 4 | 16 | 6 | 15 | 24 | 3 | 2 | 70 |
| Plagiometriona sahlbergi | 3 | 15 | 30 | 22 | 58 | 9 | 0 | 137 |
| Plagiometriona sp. 7 | 0 | 2 | 1 | 3 | 19 | 0 | 2 | 27 |
| Plagiometriona stillata | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Plagiometriona tredecimguttata | 4 | 10 | 26 | 37 | 39 | 18 | 7 | 141 |
| Total abundance | 11 | 44 | 68 | 77 | 142 | 30 | 11 | 363 |
| Species richness | 3 | 5 | 7 | 4 | 5 | 3 | 3 | |
In spite of the low number of studied species, changes in species richness could also be observed during the period; in June 2006 and 2007 and April 2007 just three of the four most abundant species were found in the field and only in October all seven species were recorded together (Table 3). Once again, the lowest numbers occur in the coldest and driest months, and the highest during the rainy season with warmer temperatures.
Three species, Plagiometriona dorsosignata, Plagiometriona sahlbergi and Plagiometriona tredecimguttata were the most abundant species during the study. Also, Plagiometriona dorsosignata and Plagiometriona tredecimguttata were the only two species present in all surveyed months. Although Plagiometriona sp. 7 occurred practically throughout the year, the total number of individuals recorded was very small, with the exception of the February survey when its abundance was the highest observed. Plagiometriona ambigena, Plagiometriona dodonea and Plagiometriona stillata were rarely observed over the entire study (Table 3). The two latter were only recorded once and in October.
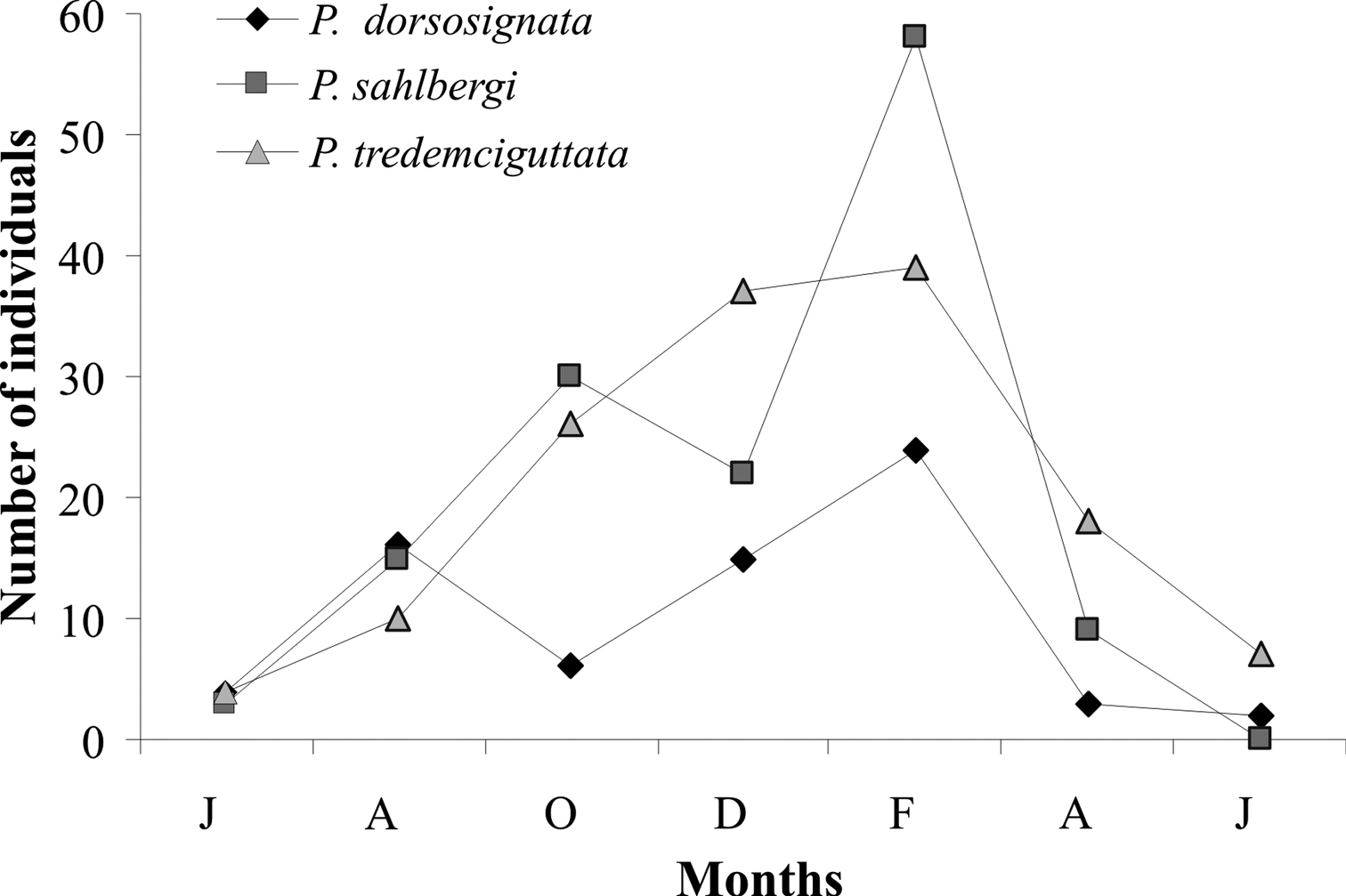
Plagiometriona dorsosignata, Plagiometriona sahlbergi, and Plagiometriona tredecimguttata numbers varied similarly during the study period. Their abundance was very low in June 2006, peaked in February 2007 and decreased again in April (Fig. 4). Increasing numbers during the study may reflect temperature and precipitation increases, since the highest numbers of individuals were found from October to February, comprehending the warmest and most rainy months, while the lowest were recorded from June to August, the coldest and driest period (Fig. 1B). This increase in cassidine activity during the warm and rainy season was also described by
Population fluctuation of Plagiometriona dorsosignata, Plagiometriona sahlbergi and Plagiometriona tredecimguttata from June 2006 to June 2007 at the study site.
Most insect species living in temperate zones become active during spring and summer, overwintering in diapause (
Changes in community composition per elevational site were observed with increasing altitude. Considering the small number of study species, richness showed no clear pattern with altitude, starting with three species at the three lowest sites, to four species at intermediate elevations (1700 m and 1800 m), and decreasing to two species at the highest site (Table 4).
Abundance of the seven Plagiometriona species at the different altitudinal sites, number of species per altitude and total of sites in which each species was recorded at the study area. Darker shade in gray indicates the altitudinal sites in which the most abundant species had the highest numbers of individuals.
| Altitudes / Cassidines | 1300 m | 1500 m | 1600 m | 1700 m | 1800 m | 2050 m | Total abundance | Total sites |
|---|---|---|---|---|---|---|---|---|
| Plagiometriona ambigena | 0 | 0 | 3 | 1 | 0 | 0 | 4 | 2 |
| Plagiometriona dodonea | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 2 |
| Plagiometriona dorsosignata | 0 | 2 | 0 | 41 | 27 | 0 | 70 | 3 |
| Plagiometriona sahlbergi | 0 | 0 | 5 | 4 | 96 | 32 | 137 | 4 |
| Plagiometriona sp. 7 | 0 | 0 | 0 | 0 | 27 | 0 | 27 | 1 |
| Plagiometriona stillata | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Plagiometriona tredecimguttata | 1 | 14 | 7 | 13 | 103 | 3 | 141 | 6 |
| Total abundance | 4 | 17 | 15 | 61 | 252 | 35 | 383 | |
| Species richness | 3 | 3 | 3 | 4 | 4 | 2 |
Significant differences occurred in the spatial distribution of Plagiometriona species along the altitudinal gradient; with some species, such as Plagiometriona sp. 7 and Plagiometriona stillata restricted to a single altitude, while others showed a wide elevational distribution, Plagiometriona tredecimguttata being the most remarkable example, occurring on all six elevational sites. Most species were restricted in their altitudinal range, occurring at two, three or four sites, normally adjacent to each other (Table 4).
A general increase in the total abundance of beetles throughout the altitudinal gradient was recorded, the highest numbers of individuals being found at 1700 m and 1800 m, 61 and 252, respectively, followed by a sharp decrease in abundance at the highest site (Table 4). This pattern was evident in the distributions of each of the three most numerous species.
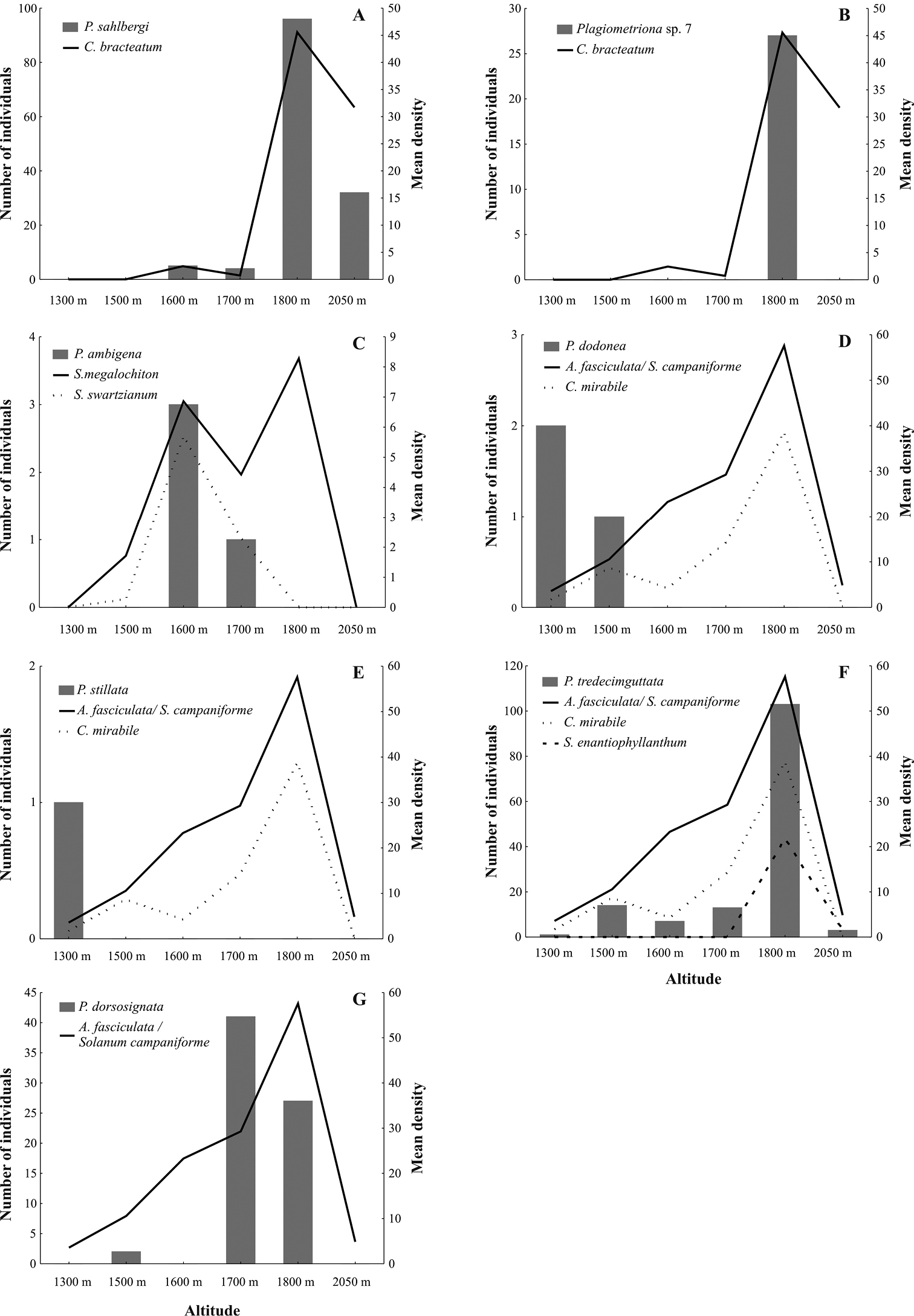
Plagiometriona sp. 7 and Plagiometriona sahlbergi feed on the same host plant, Cestrum bracteatum, which was observed from 1600 to 2050 m. The highest density of Plagiometriona bracteatum occurred at 1800 m (Fig. 5A), where 57.3% (n= 557) of all its individuals were recorded. At this site we also observed the highest abundance of Plagiometriona sahlbergi, which was found at the same altitudinal range as its host plant (Fig. 5A). Thus, spatial distribution of Plagiometriona sahlbergi along the elevational gradient seems to be greatly influenced by the availability of its host plant. Individuals of Plagiometriona sp. 7 were only found at 1800 m (Fig. 5B), suggesting that their occurrence may be responding to the high density of their host at this altitude. Meanwhile, at the highest elevational site, where plant density is still considerable, the species absence may be explained by the lack of physiological adaptations necessary to survive at such altitudes. With increasing altitude, abiotic factors such as temperature and precipitation can influence physiological and morphological changes in insect populations in the short term (e.g. variations in the life cycle, fecundity and size of individuals) and over evolutionary time (e.g. such as high numbers of apterous or brachypterous individuals, polymorphisms) (
Figure 5. Altitudinal distribution of the seven Plagiometriona species (bars) and their host plants (lines) from June 2006 to June 2007 at the study site: Plagiometriona sahlbergi A Plagiometriona sp. 7 B Plagiometriona ambigena C Plagiometriona dodonea D Plagiometriona stillata E Plagiometriona tredecimguttata F and Plagiometriona dorsosignata .
Plagiometriona ambigena, Plagiometriona dodonea and Plagiometriona stillata were restricted to a small altitudinal range, occurring at only one or two sites, while their host plants showed broader distribution along the elevational gradient (Fig. 5C, D and E, respectively). Thus, it is possible that these three species also have low tolerance to the harsher climatic conditions at higher sites, which prevent them from having a wider altitudinal range. Another possibility may be sampling error, in that their small numbers prevented us from recording them at different altitudes.
Plagiometriona tredecimguttata occurred throughout the entire elevational gradient of the study (Fig. 5F), but not in even numbers. Low abundances were recorded at the lowest and highest sites of the gradient, with numbers peaking at 1800 m. In this way, species density increased with altitude to 1800 m, after which it decreased, following variation in host plant density (Fig. 5F). Therefore, the distribution of Plagiometriona tredecimguttata appears strongly related to host availability.
Plagiometriona dorsosignata was found from 1500 m to 1800 m, with its abundance peaking at 1700 m (Fig. 5G). Its host plant Aureliana fasciculata is potentially distributed along the entire length of the transect, but more densely between 1700 m and 1800 m, where the beetle is also more abundant.
A general pattern observed within the seven study species seems to be the complete or near absence of most of the species at the highest altitudinal site. Temperatures below 0°C are commonly recorded at the highest elevations in the Park (
Since many Cassidinae are associated with host plants on open habitats (
We are grateful to Lech Borowiec (University of Wroclaw, Poland) for identifying the beetle species, and Lucia d’Ávila Freire de Carvalho (Rio de Janeiro Botanical Garden) and Luciano Bianchetti (Embrapa/Brasília) for identifying the Solanaceae species. We thank Cecília Cronemberger and ICMBio / Parque Nacional da Serra dos Órgãos for field research support and the undergraduation team of the Laboratório de Ecologia de Insetos for help during the surveys. Our research permits were 214/2005 and 246/2006. VF was funded by a graduation scholarship from CAPES and FAPERJ (Nota 10), and from the Graduation Program in Ecology (PPGE), Federal University of Rio de Janeiro. SF received an undergraduate scholarship from Apoio UFRJ. Our sincere thanks go to Donald Windsor (Smithsonian Tropical Research Institute, Panama) for valuable suggestions on the manuscript. We are also thankful to two anonymous referees for all improvements proposed.