(C) 2011 Jesús Gómez-Zurita. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The study of external morphology of the New Caledonian leaf beetle Dematochroma foaensis Jolivet, Verma & Mille (Chrysomelidae, Eumolpinae, Colaspoidini) substantiates its new combination into the genus Rhyparida Baly (Chrysomelidae, Eumolpinae, Nodinini). The species is redescribed here to highlight characters important for suprageneric diagnosis. This is the second species of Nodinini found in New Caledonia, otherwise rich in species of Colaspoidini, raising questions about the paucity of Rhyparida and this tribe in New Caledonian fauna, when they are dominant in surrounding archipelagoes, and very rich in potential source areas such as Australia and New Guinea. Some alternative explanations for this pattern are advanced, serving as alternative hypotheses until our knowledge on the ecology of these species improves or supported phylogenetic scenarios become available for this group.
Rhyparida, Dematochroma, New Caledonia, island disharmony, new combination
Generic attributions of New Caledonian Eumolpinae are currently in need of revision.
Habitus of three species of Dematochroma a Male holotype of Dematochroma piceum Baly from Lord Howe Island (N.H.M., London) b male of Dematochroma laboulbenei (Montrouzier) from Thio, New Caledonia (voucher no. IBE-JGZ-NC-0112; I.B.E., Barcelona), and c male of Dematochroma antipodum (Fauvel) from L’Aoupinié, New Caledonia (voucher no. IBE-JGZ-NC-0144; I.B.E., Barcelona). Scale bar = 5 mm.
Size as a systematic criterion is liable to taxonomic confusion. In my initial steps to understand the systematic structure of New Caledonian Eumolpinae above the species level, both using morphological and DNA-based criteria, stood out one example in need of additional study. The 6 mm long species described as Dematochroma foaensis Jolivet, Verma & Mille, 2007a: 43 belongs into a distantly related suprageneric rank compared to Dematochroma or most other New Caledonian Eumolpinae. Indeed, after Stethotes bertiae Jolivet, Verma & Mille, 2007b: 81 it is the second representative reported from this archipelago as belonging into the tribe Nodinini, as opposed to Colaspoidini, where Dematochroma and most other New Caledonian species appear to belong. The species shows highly divergent characters as compared to Dematochroma sensu auctorum or any other Eumolpinae in New Caledonia. These include the lack of dorsal longitudinal groove on pygidium, meso- and metatibiae with preapical emargination, and bifid claws. A closer analysis of morphology of several specimens showed it to present the characters considered by previous authors to diagnose the genus Rhyparida Baly, 1861 (e.g.,
Type material: (1) Holotype, one male, La Foa, 21°44S, 165°54E, 10 February 2004, M’bouéri R. M. leg. (Museum National d’Histoire Naturelle, Paris); (2) Paratype, one female, Ouégoa, Mandjélia, 20.39683°S, 164.53218°E, 787m, 7–8 February 2005, S. Cazères & C. Mille leg. (Museum National d’Histoire Naturelle, Paris). Other material: (3) two females, Caavatch (=Kaavac), 5 February 1977, Dr. J. Balogh leg. (Hungarian Natural History Museum, Budapest); (5) three females, Province Sud, Camp Brun, 14 March 1994, on Melaleuca quinquenervia, M. Schöller leg. (M. Schöller coll., Berlin); (4) one female, Province Nord, Hienghene 20.69545°S, 164.94274°E, 24m, 8 April 2008, J. Gómez-Zurita leg. (J. Gómez-Zurita coll., voucher no. NC-0110, Institute of Evolutionary Biology, Barcelona).
Habitus (Fig. 2). Body stout, elongated oval (6.1 mm long, 3.4 mm wide), moderately convex. Ground color orange testaceous, with infuscate head sutures, inverted triangle on frons, apical antennal segments, margins and discal markings on pronotum, scutellum, elytral suture, humeri, medially for short distance on third and seventh elytral intervals, apex of femora, basal half of tibiae, episterna and ventral thoracic segments; mandibles black.
Habitus of Rhyparida foaensis (Jolivet, Verma & Mille). Scale bar = 5 mm.
Head large, deeply inserted into pronotum, nearly to upper eye margin; surface very delicately microreticulated; vertex weakly convex, very finely, rather densely and homogeneously punctured, with very fine median longitudinal impression, becoming progressively larger, on depressed longitudinal area on frons, joined apically to transversally widely obtuse fronto-clypeal suture. Clypeus wider than long, subtrapezoidal, depressed apically, with deep median semicircular apical emargination, flanked laterally by shortly produced denticles; surface microreticulated, with larger, deeper punctures than those on vertex, bearing minute, very fine setae anteriorly. Labrum as long as wide; surface finely microreticulated; sides feebly convergent towards round anterior angles; apex depressed and weakly emarginated; anterior angles with one pair of nearly adjacent fine golden setae; two setae anteriorly on disc. Genae very short, with some fine setae below eye margin. Eyes very big, dorsoventrally elongated; deeply emarginated at inner border for antennal insertion; supraocular margin furrowed, furrow not surpassing eye margin above, with long, yellowish dorsal seta. Space for antennal insertion concave, slightly raised dorsally above clypeus level; microreticulated, unpunctured, with one anterior, oblique fine golden seta. Antennae long and slender, reaching basal third of elytra; scape long, weakly flattened and arched antero-posteriorly; second antennomere elongated, slightly clavate, weakly curved, 0.66x as long as first; third segment straight, as long as second; antennomeres 4–5 subcylindrical, slightly shorter than scape, narrow and slender; 6–10 as long as scape, slightly widened towards apex, densely setose; apical antennomere longest, sharply pointed and paler at apex. Maxillary palpi short, slender; apical palpomere elongated, subconical.
Pronotum transverse, 0.58× as long as wide between posterior angles, shorter than head, transversally convex, especially at anterior angles; posterior border weakly bisinuated with weakly projecting median lobe, finely margined with premarginal line of dense dot-like impressions; posterior angles laterally projecting as small teeth continuing basal margin, with large apical setigerous pore; anterior border nearly straight, finely margined at sides, with margin broader and more imprecisely defined at middle; anterior angles laterally and slightly obliquely projecting as small teeth with large setigerous pore at apex; sides broadly curved, wider behind middle; lateral margins relatively wide, flat, glossy, with internal row of dense round impressions; pronotal surface delicately microreticulated, rather uniformly and densely covered by shallow, moderate punctures, smaller, almost disappearing near borders. Anterior border of hypomeron more or less straight, regularly continuing profile of anterior border of pronotum with that of prosternum, both remaining largely separated by anterior margin of hypomeron (see Fig. 4f in
Scutellum as long as broad at base, sides straight, weakly divergent at basal 2/3, curved at obtuse angle to obtusely pointed apex; surface finely alutaceous, unpunctured. Elytra slightly broader than base of pronotum; humeri round, slightly callose; sides very feebly curved, with maximum width behind middle, and regularly curved to broadly round apex; margins feebly explanate, entirely visible from above; surface shiny, with dense unordered minute punctures and regular series of strong punctures separated at most by distance equal to their diameter; short scutellar striae of some 14 punctures starting before middle of scutellum and obliquely directed to suture; sutural striae reaching from base of elytra to sutural angles, joining marginal striae at inner edge of explanate margin of elytra; four longitudinal discal striae from base of elytra joining successively to apical ends of ninth, eighth, seventh and sixth striae on preapical declivity of elytra; basal ends of striae 6–8 behind humeri and of premarginal stria 9 behind middle of elytra; short premarginal posthumeral striae, curved and convergent with elytral margin before middle of elytra; space between striae 7 and 8, medially and at lateral declivity of elytra occupied by two additional shorter longitudinal striae convergent at both ends; darkened sutural interval, humeri, elongated spots medially on disc on third interval and more advanced at lateral declivity of elytra on seventh interval between stria 7 and internal row of additional posthumeral striae. Epipleura flat, unpunctured, shiny, broad basally and gradually narrowing toward apex; only visible laterally below humeri. Species fully winged.
Profemora spindle-shaped at basal 3/4, nearly cylindrical at apical quarter; extremely finely alutaceous with scattered minute punctures and very short appressed setae on basal 3/4 and coarser punctures and longer setae at apical 1/4. Protibiae very slightly curved inward, gradually widened toward apex; with several fine longitudinal ridges and longitudinal series of semierect golden setae at intervals; apex concave, obliquely cut for tarsal insertion, densely setose internally. Protarsi 0.6× as long as protibiae; first tarsomere slightly expanded laterally, longer than wide at concave apex; second shorter than first, triangular with broadly concave apex; third deeply and narrowly bilobed; fifth longer than tarsomeres 2–3, slender, subparallel, ventrally curved; claws bifid, weakly divergent, long, sharp, with short, sharp inner teeth. Median and hind legs very similar to anterior legs, but tibiae straight, with conspicuous preapical emargination externally, margined by fringe of erect golden setae and apex not densely setose internally. Abdominal ventrites finely microsculptured, shiny, narrow, strongly transverse, with posterior border increasing concavity from ventrites one to four, finely but more or less uniformly punctured and with very fine, short whitish setae; sides corrugated; anterior process between metacoxae of first abdominal ventrite broader than long, regularly curved; last abdominal ventrite very feebly emarginated.
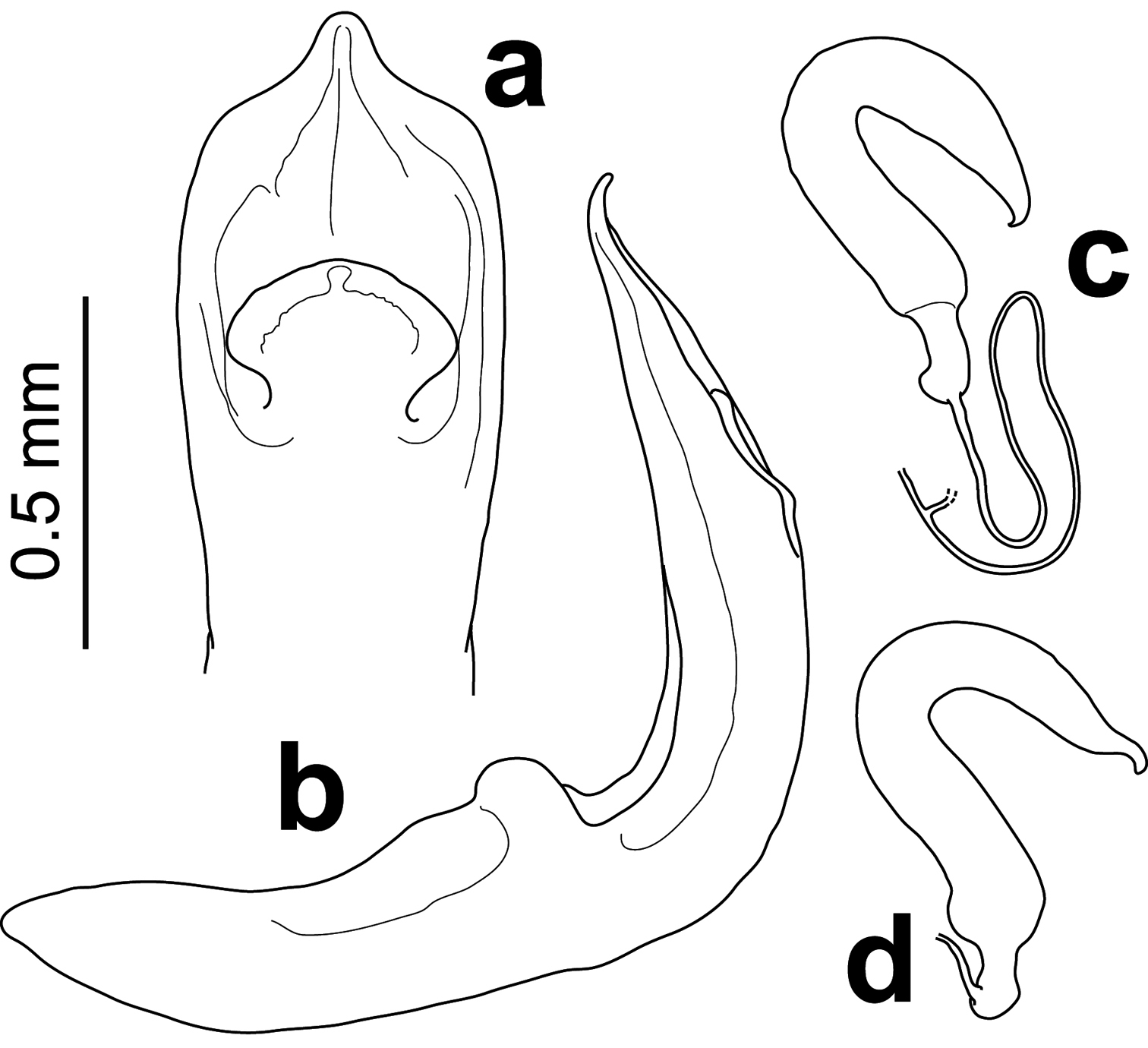
Median lobe of the aedeagus (Fig. 3a, b) strongly bent at right angle near base, dorso-ventrally flattened and nearly straight at apical 2/3; sides slightly divergent, reaching maximum width at mid-level of ostium, feebly converging before abruptly tapering at obtuse angle before apex; apex anteriorly prolonged as blunt median triangular denticle curved dorsally; median dorsal flap broad, spatula-like, with short narrow base 0.5× as wide as broadest point medially, before regularly curved nearly semicircular apex. Spermatheca (Fig. 3c, d) U-shaped with pump slightly shorter than receptacle, gradually narrowing towards curved pointed apex; proximal end slightly broadened before narrow elongated basal appendix attached prebasally to very fine, transparent spermathecal duct; spermathecal gland apparently attached to spermathecal duct distally from spermatheca at 1.5× its length.
Male (a dorsal b lateral) and female c, d genitalia of Rhyparida foaensis (Jolivet, Verma & Mille).
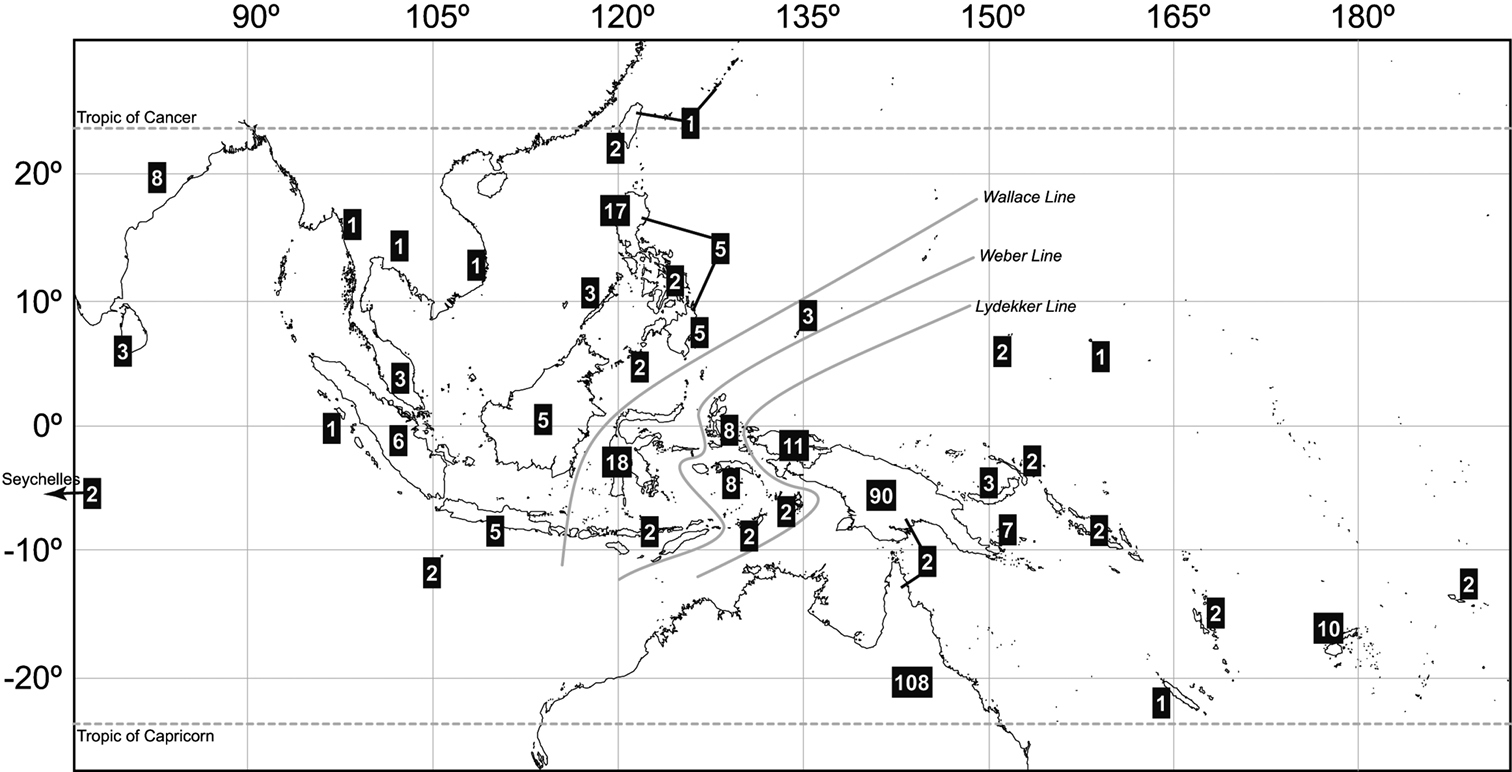
As it occurs with most Eumolpinae genera, the objective limits of Rhyparida need to be revised and it is possible that profound changes will affect the systematics of the group (C.A.M. Reid, Australian Museum, pers. comm.). However, before this revision is attempted, following the latest treatments of the genus by several specialists, it is possible to draw some preliminary conclusions about the diversity and biogeography of the genus. Clavareau’s (1914) catalogue lists 166 species of Rhyparida, an increase of 34.3% over the account by
Distribution and diversity of Rhyparida Baly species worldwide.
It is largely elusive understanding why such a diverse genus like Rhyparida is so rare in New Caledonia, considering the old age of the island, its relatively large size, its ecological diversity and its relative proximity to species-rich source areas such as New Guinea and Australia, as compared to Fiji, for instance, comparatively rich in species of Rhyparida. Island disharmony is a well-known biogeographic pattern, and very common in the case of insects in Pacific islands (see
If differences in ability for dispersal compared to other eumolpines are not obvious, another possibility is that successfully colonizing Rhyparida (or other Nodinini for that matter) were outcompeted by local stable populations of Colaspoidini, in this case. Again, and considering the generally eclectic ecologies of these animals, their notable success in similar geographic scenarios also rich in other eumolpines, and the diversity of suitable habitats offered by New Caledonian ecosystems, it is difficult to admit that such a fierce antagonism and exclusion can affect settlement chances for representatives of an entire beetle tribe.
Yet another possibility is that ecological requirements for Nodinini, or Rhyparida in particular, are actually stricter than considered a priori, and not available in New Caledonia, compared to the mainland or surrounding oceanic islands. This hypothesis could be evaluated examining for instance the association of Rhyparida species to specific soils, types of vegetation or specific plants throughout its range and confirming the absence (or rarity) of these conditions in New Caledonia, remarkable and quite unique for its geologic and mineral characteristics (
A last possibility about the paucity of Nodinini in New Caledonia and worth consideration here is that there may be several species in the archipelago still awaiting discovery. Considering the intense sampling in the recent past and the conspicuous characters diagnosing this tribe, although it is likely that new species will be discovered, it appears improbable that the catalogue of New Caledonian Nodinini will grow to a number of species comparable to that found in Fiji or even Samoa, the later with at least eight species among Rhyparida, Stethotes and Stygnobia (
The number of questions that this intriguing pattern suggest and the few, speculative answers available, highlight the importance of further research on New Caledonian fauna, from biodiversity and ecological surveys to phylogenetic analyses which will help understanding the history of colonization and diversification on this remote biodiversity hotspot.
Replacement names for the genus RhyparidaDuring the course of this study, several homonyms were detected affecting the genus Rhyparida Baly, which need name replacements to avoid ambiguity. Rhyparida leana nom. n. (after Arthur Mills Lea) is proposed as replacement name for the Australian species Rhyparida apicipennis Lea, 1915, name preoccupied by a species from Fergusson Island (Papua New Guinea) described by
I express my gratitude to Pierre Jolivet (Museum Nationale d’Histoire Naturelle, Paris), Ottó Merkl (Magyar Természettudományi Múzeum, Budapest), Max Barclay and Beulah Garner (Natural History Museum, London), and Matthias Schöller (Berlin) for kindly allowing me to study the Eumolpinae material discussed here. Anabela Cardoso (Institut de Biologia Evolutiva CSIC-UPF, Barcelona) and José A. Jurado-Rivera (IMEDEA, CSIC-UIB, Palma de Mallorca) collected the specimen of Dematochroma laboulbenei (Montrouzier) figured in this work. Pierre Jolivet and an anonymous referee reviewed the original version of the manuscript and helped me improve it. I also want to thank Lyubomir Penev (Bulgarian Academy of Sciences / Pensoft Publishers, Sofia) and Michael Schmitt (Ernst-Moritz-Arndt-Universität, Greifswald) for considering this work a suitable contribution for this special ZooKeys issue on leaf beetle research. This study received the support of a travel grant from the Committee of Research and Exploration (Contract No. 8380-07, National Geographic Society) and from the Percy Sladen Memorial Fund (Linnean Society of London).
References