(C) 2010 Deborah S. Kent. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Previous descriptions of adult Austroplatypus incompertus (Schedl) are completed by the addition of descriptions and illustrations of the adults and, in particular, their maxillary palps. I describe and illustrate the non-adult phases of the life cycle and provide a key to the larval instars. The sexual dimorphism of Austroplatypus incompertus is atypical and includes a latitudinal cline which obeys Bergmann’s rule. The taxonomic position of the genus within the Platypodinae is clarified. Platypus incostatus Schedl is recognised as the male of the species, and hence a new synonym of Austroplatypus incompertus.
Curculionidae, Platypodinae, Austroplatypus, Australia, external morphology, larvae, sexual dimorphism, latitudinal cline
Studying and accurately describing the external morphology of insects is important because it is the external details that characterise and give indications of the animals’ biology, ecology and social behaviour. In addition, insects are often identified solely by external adult morphology and this is reflected in the formal taxonomic descriptions of most species.
Studies of the external morphology of platypodines have
generally concentrated on taxonomic research and consequently have
mainly dealt with descriptions of specific parts of the external
morphology of adults. In contrast, relatively few papers (
The lack of comprehensive morphological descriptions within the platypodines is typified by Austroplatypus incompertus
(Schedl). This species was discovered in the early 1950s and named in
1968. Describing and identifying it has been problematic as specimens
are unusually difficult to collect. Adult beetles are not attracted to
light and the only way to obtain all developmental stages is to cut them
from living trees. Consequently there has been a dearth of material for
description or comparison. In fact, until the current study, no
well-documented collection of both adult and larval Austroplatypus incompertus material had been made. This has no doubt contributed to the small number of papers dealing with the taxonomy and biology of Austroplatypus incompertus (
The research reported in this paper had two broad aims. The first was to verify and complement existing descriptions of the external morphology of Austroplatypus incompertus. This necessitated descriptions of the egg, larval instars and the pupa as well as a description of adult sexual dimorphism and the adult maxillary palps. In addition immature stages and adults were illustrated with line drawings and scanning electron micrographs.
An integral part of these descriptions were measurements
such as head capsule widths and adult body lengths. However, such
measurements are problematic if variation exists among populations. The
increase in mature larval head capsule width of Platypus subgranosus Schedl from north to south (
The second aim was to integrate the new material presented here with existing taxonomic descriptions and discuss the current taxonomic position of Austroplatypus incompertus.
Material and methodsThe source materials for this paper were voucher
specimens collected during the study as well as material already held in
the Forestry Commission of NSW Insect Collection (FCNI). In their
entirety these specimens encompassed the currently known geographical
and host tree species range of Austroplatypus incompertus (
Collections examined
AM Australian Museum
ANIC Australian National Insect Collection
BM The Natural History Museum, London
FCNI Forestry Commission of NSW Insect Collection
NMV National Museum of Victoria
SAM South Australian Museum.
Material examinedUnless otherwise noted all specimens mentioned below are part of FCNI.
Austroplatypus incompertus (Schedl)
Holotype female: New South Wales: Eden, 23.x.1953 (NMV), LH Bryant. Ex. Eucalyptus sieberana F. Muell. (= Eucalyptus sieberi L. Johnson).
Paratypes: Victoria: Woodhouse Creek, N of Omeo,
xi.1964 (1♀ NMV); near Omeo, xi.1965 (2♀ NMV). (Note: the distribution
of the paratypes examined at NMV does not agree with
Other material examined: New South Wales: Dorrigo, 23.iii.1954 (1♀) [Note: same information as Holotype of Platypus incostatus]; Styx River State Forest, 24.ix.1992 (11♀), 16.x.1992 (7♀); 20.i.1993 (1♀, 1♂); Mt Boss State Forest, 27.iii.1958 (2♀), Bellangry State Forest, 12.xi.1965 (5♀); 7.xii.1988 (13♀); Bellangry Timber Mill, Wauchope, 9.xii.1988 (1♀); Manning River National Forest, Taree, 10.viii.1965 (5♀), 8.ix.1965 (2♀), 9.ix.1965 (1♀), 19.vi.1967 (3♂); Coopernook State Forest, 11.viii.1965 (1♀); Ourimbah State Forest, Wyong, 20.xi.1984 (1♀), 18.iii.1988 (6♀, 5♂), 13.x.1988 (1♀), 22.xi.1988 (22♀), 17.i.1989 (1♀), 24.ii.1989 (3♀, 1♂), 16.vi.1989 (11♀, 1♂); Mt Wilson, Blue Mtns, iv.1986 (3♂ AM); Banshea State Forest, near Oberon, 7.x.1965 (5♀), 18.iii.1970 (3♀, 1♂); Cumberland National Forest, West Pennant Hills, 19.viii.1965 (1♀), 30.viii.1965 (1♀), 30.ix.1965 (1♀), 28.x.1965 (3♀), 12.iv.1967 (1♀), 19.iv.1967 (1♀, 1♂), 26.ix.1967 (1♀), 6.xii.1967 (1♀); iii-v.1968 (1♀, 6♂), 8.iv.1969 (1♀, 4♂), 30.iii.1988 (1♂), 5.iv.1988 (1♀), 8.iv.1988 (2♀), 14.iv.1988 (15♀, 1♂), 19.iv.1988 (1♀), 20.iv.1988 (1♀), 21.iv.1988 (1♂), 13.xii.1988 (2♀), 23.ii.1989 (1♀), 1.iii.1989 (1♀), 30.iii.1989 (23♂), 3.iv.1989 (11♀, 2♂), 4.iv.1989 (1♀, 4♂), 5.iv.1989 (1♀, 1♂), 10–12.iv.1989 (9♀, 8♂), 11.iv.1989 (1♀), 2.iv-6.v.1990 (75♀, 55♂), 14.vi.1990 (4♀), 25.x.1990 (3♀), 18–19.iv.1991 (1♂), 20–21.iv.1991 (1♀, 1♂); 4–14.iv.1991 (3♂), 4–24.iv.1991 (24♀, 13♂), 30.iv-1.v.1991 (1♀), 4–5.iv.1992 (1♂), 6.iv.1992 (1♂), 7.iv.1992 (2♀, 6♂), 6–7.iv.1992 (3♀, 11♂), 7–8.iv.1992 (2♂), 11–12.iv.1992 (4♀, 11♂), 13.iv.1992 (17♀, 38♂), 14.iv.1992 (19♀, 1♂), 15.iv.1992 (6♀, 7♂), 15–18.iv.1992 (9♀), 18.iv.1992 (3♀, 2♂), 18–21.iv.1992 (2♀, 1♂), 24.iv.1992 (1♂), 25–28.iv.1992 (9♀, 4♂), 29.iv.1992 (1♀), 30.iv.1992 (4♀, 1♂), 1–11.v.1992 (12♀, 1♂), 6–8.v.1992 (1♀); Broughton’s Lookout, 15 kms S of Wombeyan Caves, 27.viii.1979, (1♂ AM); Nullica State Forest, Eden, 19.xi.1991 (1♀); Nalbaugh State Forest, Bombala, 21.xi.1991 (7♀); Bondi State Forest, Bombala, 21.x.1965 (4♀); Bombala, 13.iii.1991 (3♀, 6♂); Eden, 23.x.1953 (1♀)[Note: same information as Holotype], 25.vii.1989 (4♀); Naghi State Forest, Eden, 20.x.1965 (4♀).
Victoria: Lightning Creek, N of Omeo, viii.1965 (1♂ NMV); Woodhouse Creek, near Omeo, viii.1965 (2♀, NMV); Swifts Creek, 20 miles (32 km) S of Omeo, 1966 (2♀ NMV; 2♀ SAM; 1♀ ANIC), 14.iv.1967 (3♀, 1♂).
Platypus incostatus Schedl
Holotype male: New South Wales: Dorrigo, 23.iii.1954 (BM), J Cartwright. Ex. Eucalyptus laevopinea R.T. Baker.
Specimen preparation – General
All specimens of immature life stages were fixed to preserve their shape and size and thus ensure that descriptions and measurements were accurate and comparable. Eggs, larvae and pupae were fixed in KAA [kerosene (7%), glacial acetic acid (16%) and 95% ethanol (77%)] for 5–30 minutes depending on size and developmental stage; eggs and small larvae required the shortest fixing period and the fifth instar and pupae the longest. The fixed specimens were then passed through immersion stages of several hours each in 90% and 85% ethanol before eventual permanent storage in 80% ethanol. Adults were killed either by freezing or by immersion in 80% ethanol and then air-dried and mounted on card points.
Specimen preparation - Scanning electron microscopy
For scanning electron microscopy, specimens were fixed in 2% glutaraldehyde in phosphate buffer (pH 6.9) for 24 hours, transferred through a series of increasing ethanol concentrations and then stored in absolute ethanol until coating. Just before coating, the specimens were placed in either ethyl acetate or acetone and then critical-point dried, after which they were sputter-coated with platinum or gold.
Observations
Observations of all life cycle stages of the beetle were
made using stereo-dissecting and compound microscopes and a Cambridge
S120 scanning electron microscope with a Robinson detector. These
observations formed the basis for the descriptions of the external
structures. The morphological terminology for larval descriptions
follows that used by Browne (1961, 1972) and
Sex determination of adults
Adult beetles were sexed based on external morphological differences (
Measurements
Both adult and larval measurements were made using a stereo-dissecting microscope fitted with a scaled graticule. One measurement was recorded for larvae: the width of the head capsule at its widest point. Three measurements were recorded for adults: dorsal prothorax length (measured along middle of the prothorax) and width (measured at its widest point, the posterior edge of the femoral emargination), and elytral length. Although total length is commonly reported in taxonomic descriptions, this measurement proved to be unreliable due to post-mortem head deflection and was therefore not recorded. Some adult beetles could not be measured because of their position on the card mounts.
Statistical analysis
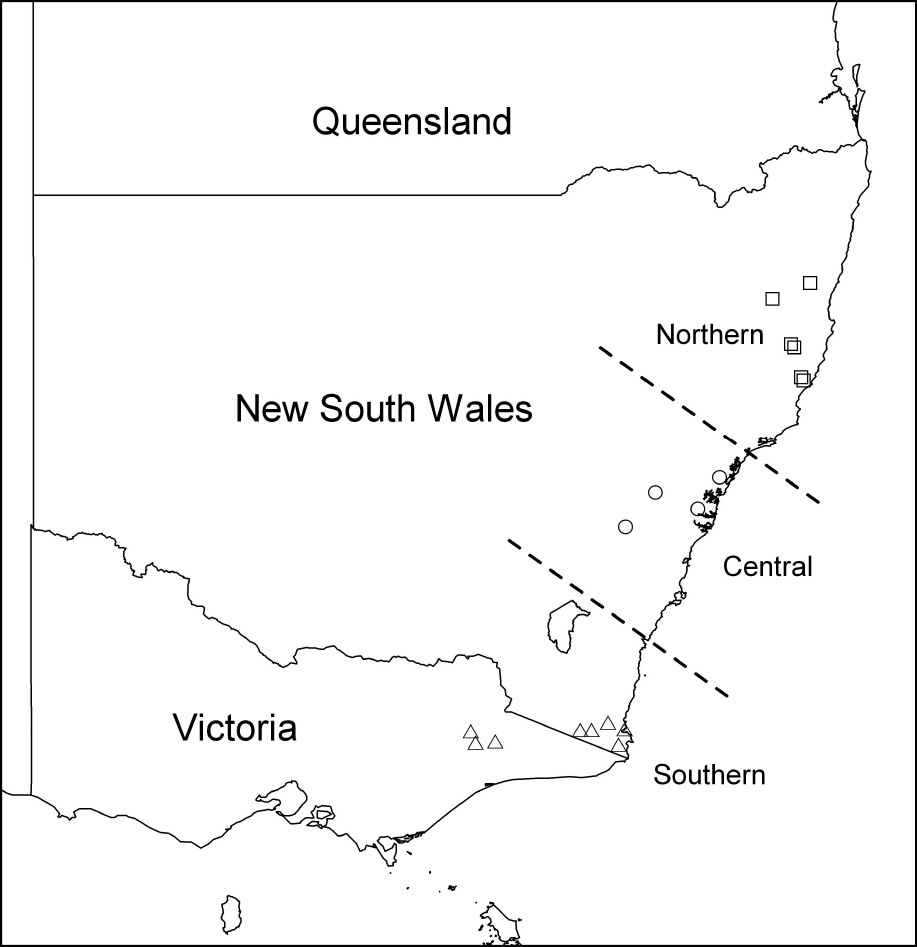
Analysis of variance (ANOVA) was carried out on the measurements of the fifth instar larval head widths to determine whether there was a difference in specimens from different localities (the localities from which study material was derived fell naturally into three well separated latitudinal groups - Fig. 1). Similarly, measurements of adult beetles were analysed using ANOVA to determine whether there were differences between sexes, or between adult females from different localities or different host species (there being too few males from the range of localities and hosts to permit analysis). The analyses of females were restricted to prothorax length, as this was the most repeatable measurement. Where appropriate, Post hoc Tukey Honest Significant Difference (HSD) multiple comparison tests were performed to determine which means differed significantly.
Latitudinal distribution of adults and larvae from which measurements were taken.
Descriptions
Egg (n = 40) length 0.70 mm ± 0.06, width 0.45 mm ± 0.04 (SD). Elongate, translucent white, shiny, without obvious sculpturing.
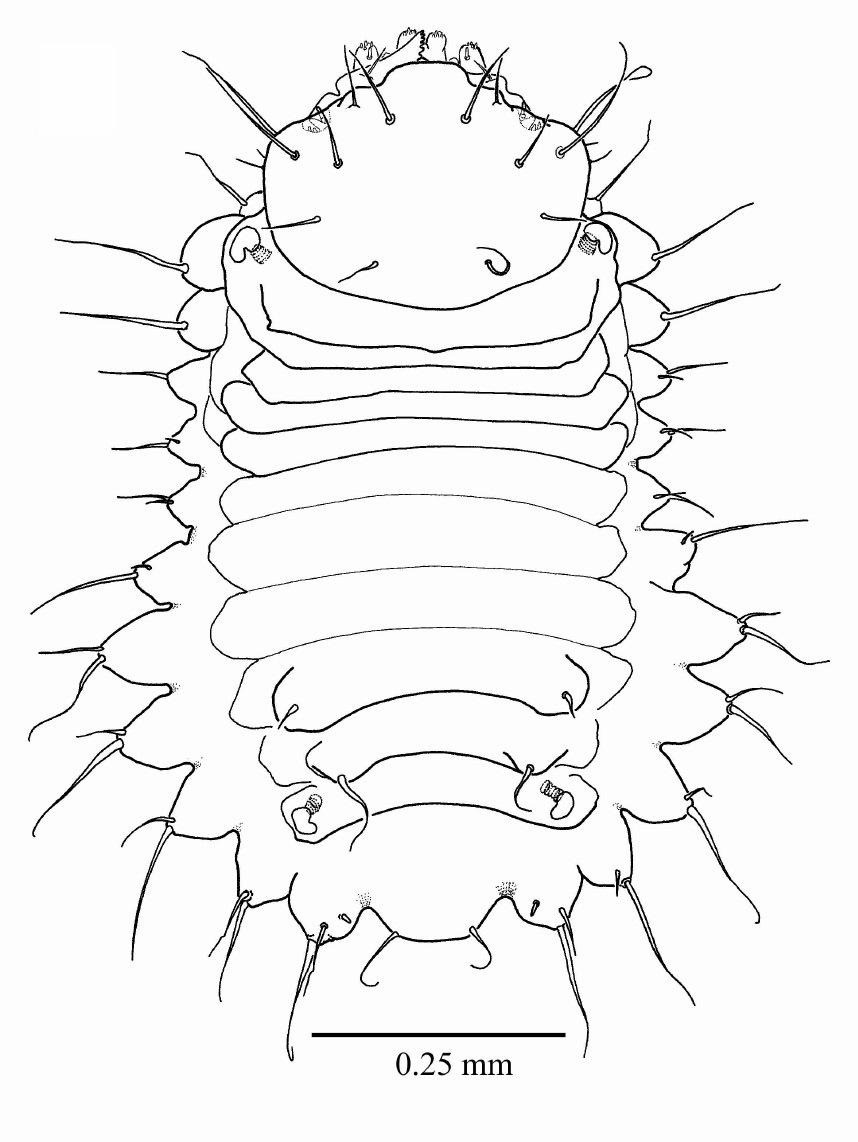
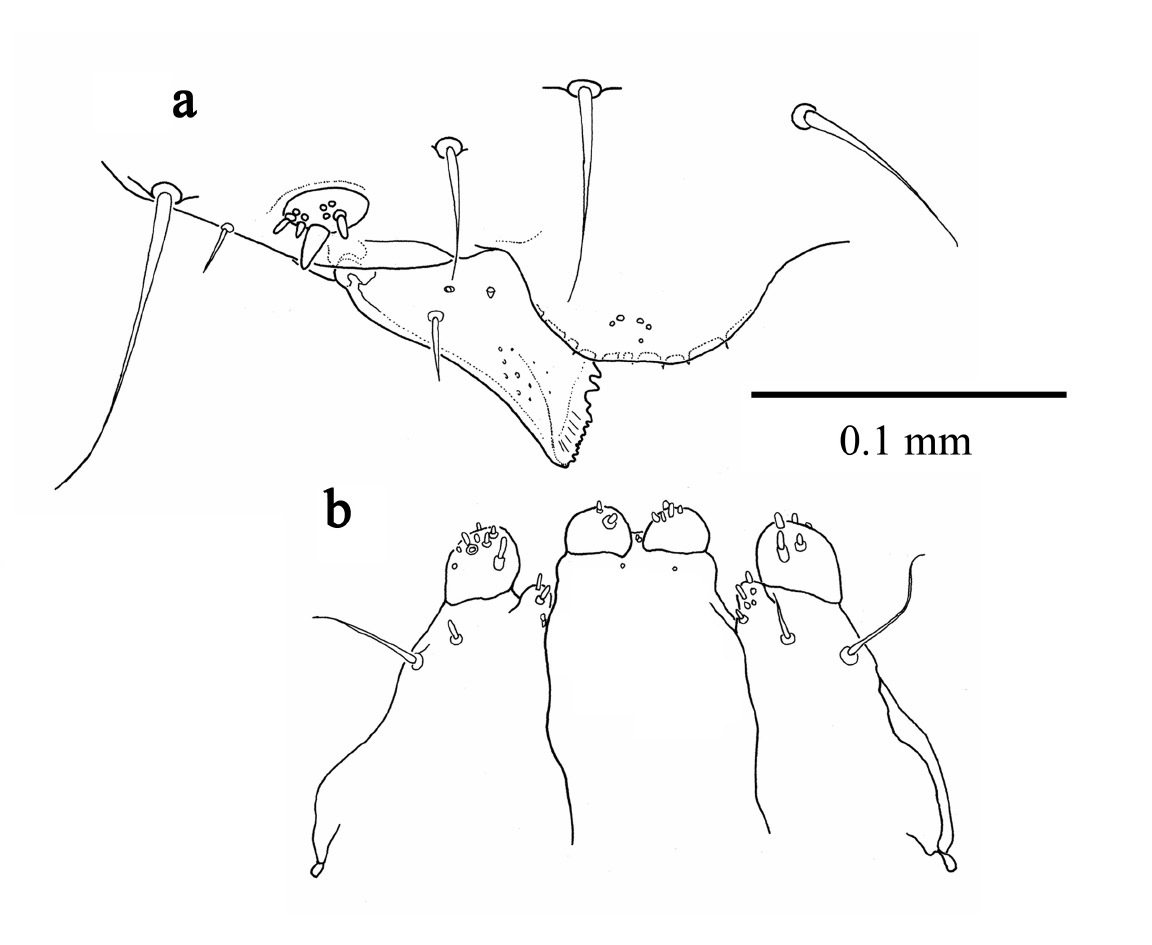
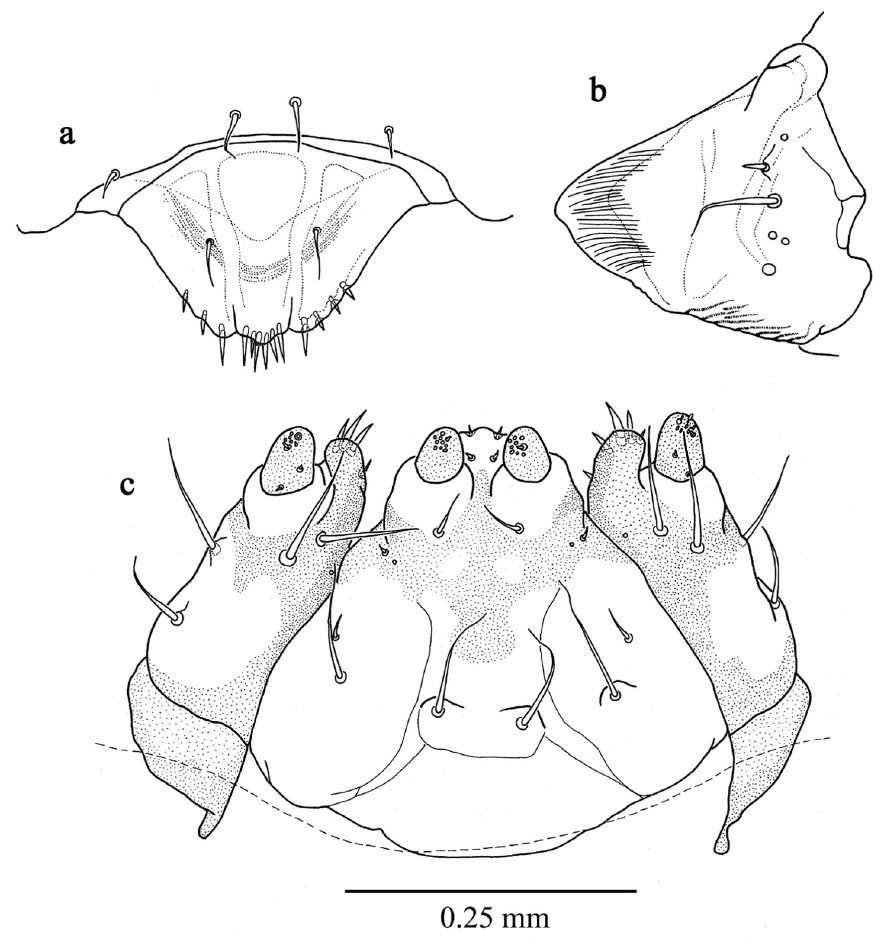
First instar larva (Figs 2, 3a, b) length 1.183 mm ± 0.2 (SD) (n = 5), maximum body diameter much smaller than that of gallery. Maximum width on 5th abdominal segment. Body hyaline, white, shape ovoid and hump-backed dorsally. Head exserted, width of head capsule 0.303 mm ± 0.012 (n = 10), wider than long, greatest width over bulbous antero-lateral margins. Head setae prominent, 1 posteriorly to each antero-lateral margin, nearly twice as long as any other. Antennae small, one each side of epicranium in unpigmented portion between mandibular condyles (Fig. 3a). Mandibles lightly sclerotised and with comb-like teeth (Fig. 3a). Maxillary palpi 1-segmented; labial palpi 1-segmented (Fig. 3b). Meso- and metathoracic segments each enlarged into pseudopods. All thoracic segments bearing a single prominent lateral seta on each side. Abdominal tergites 6 and 7 each bear a single prominent seta on a dorsolateral protuberance on each side. All sternites with lateral protuberances, each bearing two setae, those of segments 5–9 enlarged into pseudopods (Fig. 2). Only two pairs of spiracles, one on prothorax and the second on abdominal segment eight.
First instar larva, habitus, dorsal.
First instar larva a antenna, labrum and mandible, dorsal b maxillae and labium, dorsal.
Second instar larva (Fig. 4) slightly larger than first instar, but same general form. Head more elliptical, less angular than first instar. Head capsule width 0.353 mm ± 0.028 (SD) (n = 11). Mandibles similar. The main difference between the first two instars is the presence of nine pairs of spiracles, one each on the prothorax and one each on eight abdominal segments.
Second instar larva, habitus, dorsal.
Third instar larva (Fig. 5a, b) slightly larger than the second instar, but still smaller than gallery diameter. Head capsule width 0.581 mm ± 0.077 (SD) (n = 32). Body still hump-backed but more flattened ventrally and not as translucent as first two instars. Head distinctly narrower than prothorax and more rounded in shape in comparison to the first two instars. Pseudopods no longer as prominent. Spiracles as in second instar. Mouthparts as in Fig. 5a, b.
Third instar larva a mandible, labrum and epistoma, dorsal b maxillae and labium, ventral.
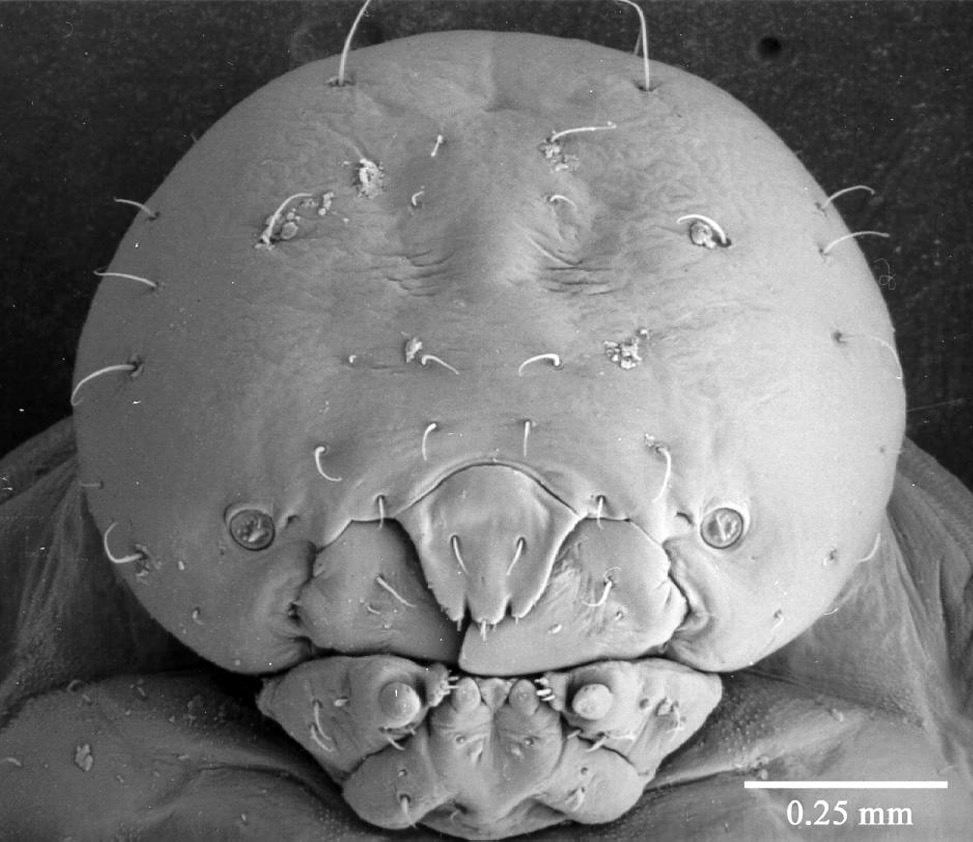
Fourth instar larva (Fig. 6) body stout, more or less closely fitting the galleries. Head clearly narrower than width of pronotum. Pseudopods not evident. Head capsule slightly wider than long, width 0.868 mm ± 0.071 (SD) (n = 49). Labrum and mandibles as in Fig. 6. Mandibles similar to third instar, only slightly chitinized and still bearing small teeth along apical and subapical edges. Pronotum lacking any chitinized pattern of ridges (see below).
Fourth instar larva, antennae, mandibles, labrum and epistoma, dorsal.
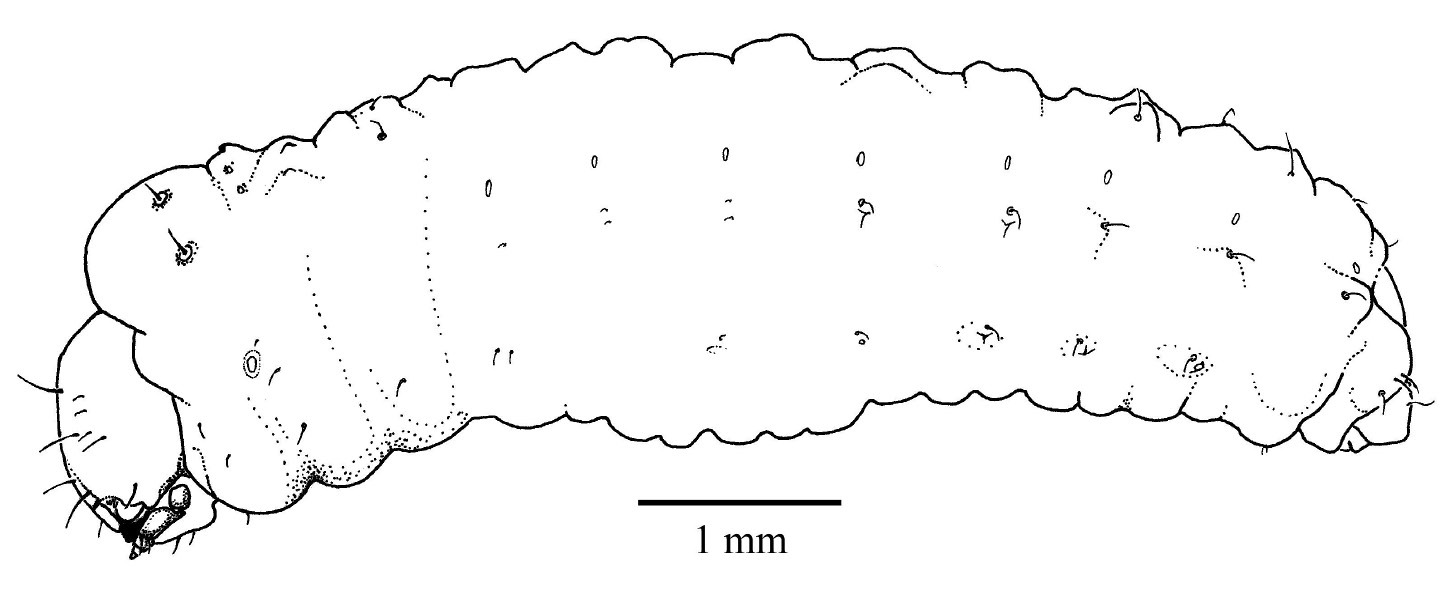
Fifth instar larva (Figs 7, 8, 9a, b, c, 10, 11) see
Fifth instar larva, habitus, lateral.
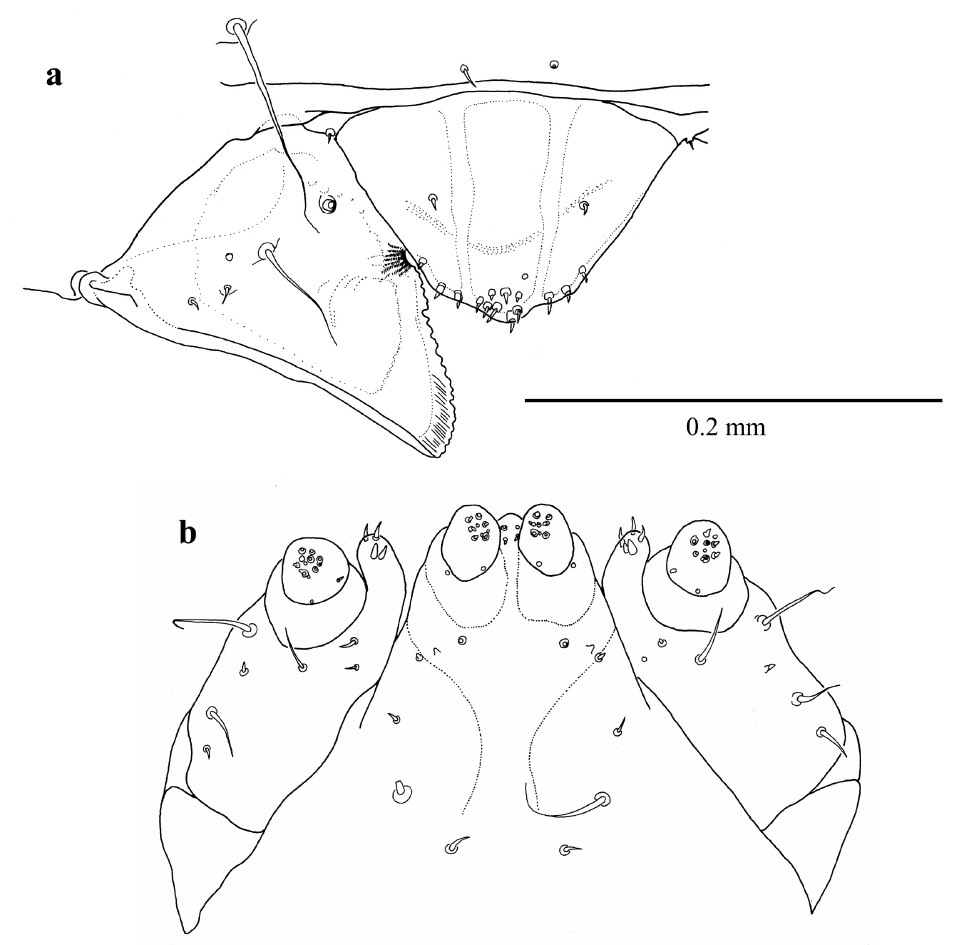
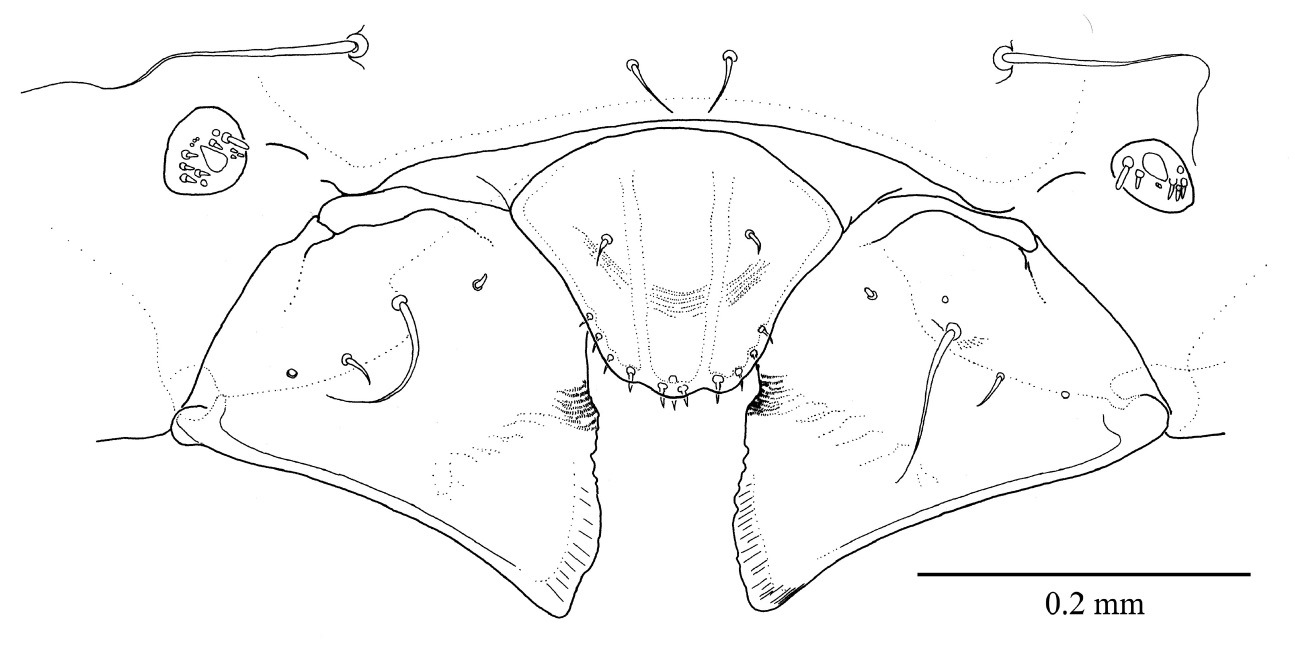
Fifth instar larva, head, ventral.
Fifth instar larva a labrum and epistoma, dorsal b mandible, dorsal c maxillae and labium, ventral.
10 Fifth instar larva, pronotum, dorsal 11 Fifth instar larva, thoracic spiracle.
Five distinct larval instars are morphologically discernible using the following key
| 1. | Body ovoid, trapezoidal, rhomboidal, hump-backed dorsally; body width distinctly less than diameter of gallery (Figs 2, 4) | 2 |
| – | Body elongate, not markedly hump-backed; body width almost the same as that of the gallery (Fig. 7) | 4 |
| 2. | Prominent pseudopods on meso- and meta-thoracic segments and on abdominal segments 5–8, head broader than long (Figs 2, 4) | 3 |
| – | Pseudopods not very prominent on any body segment, head more round | third instar |
| 3. | Only two pairs of spiracles, both the same size, one pair on prothorax and one on abdominal segment 8, head very broad, transversely oblong (Fig. 2) | first instar |
| – | Nine pairs of spiracles, one pair on prothorax and one pair on each side of first eight abdominal segments, head more elliptical (Fig. 4) | second instar |
| 4. | Pronotum lacking any brown chitinized pattern; mandibles slightly chitinized with teeth on cutting margin (Fig. 6) | fourth instar |
| – | Pronotum with brownish chitinized patterned consisting of two pairs setae, surrounded by irregular ridges (Fig. 10); mandibles heavily chitinized, apex bluntly pointed and free of small teeth (Fig. 9b) | fifth instar |
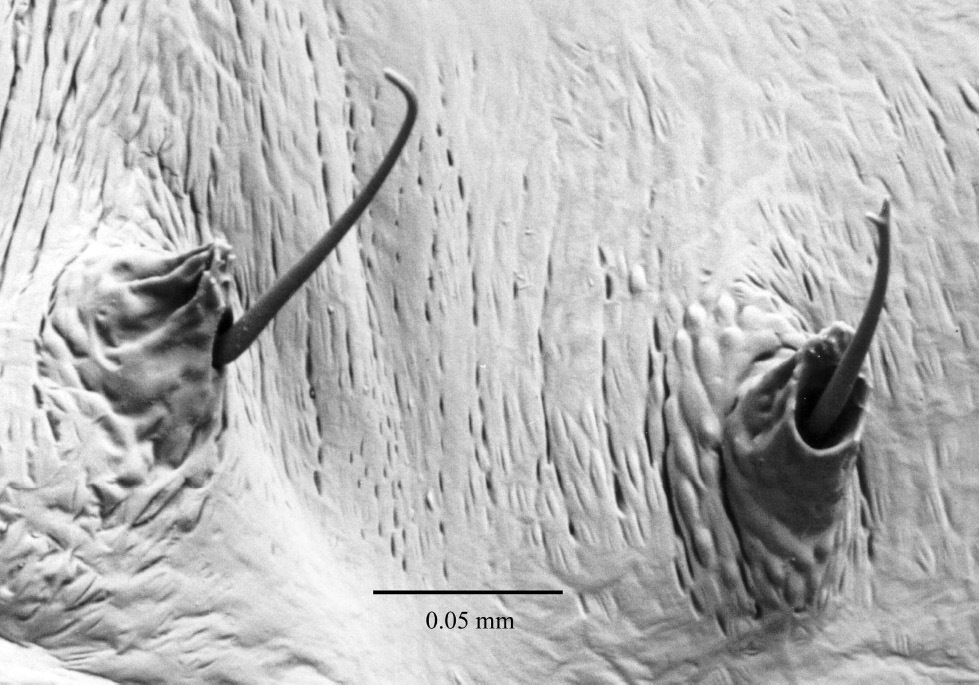
Pupa (Fig. 12) cuticle white and glabrous, setae coarse, arising laterally from an armed tubercle (Fig. 13), larger and more numerous on head and prothorax than on abdomen. Rostrum not reaching fore coxae. Antennal club smooth. Sex indeterminable until the darkening of the cuticle of the young adult appears through the pupal skin. At this point females can be identified by the appearance of mycangia in the centre of the prothorax.
Pupa, habitus, lateral.
Pupa, thoracic setae, dorsal.
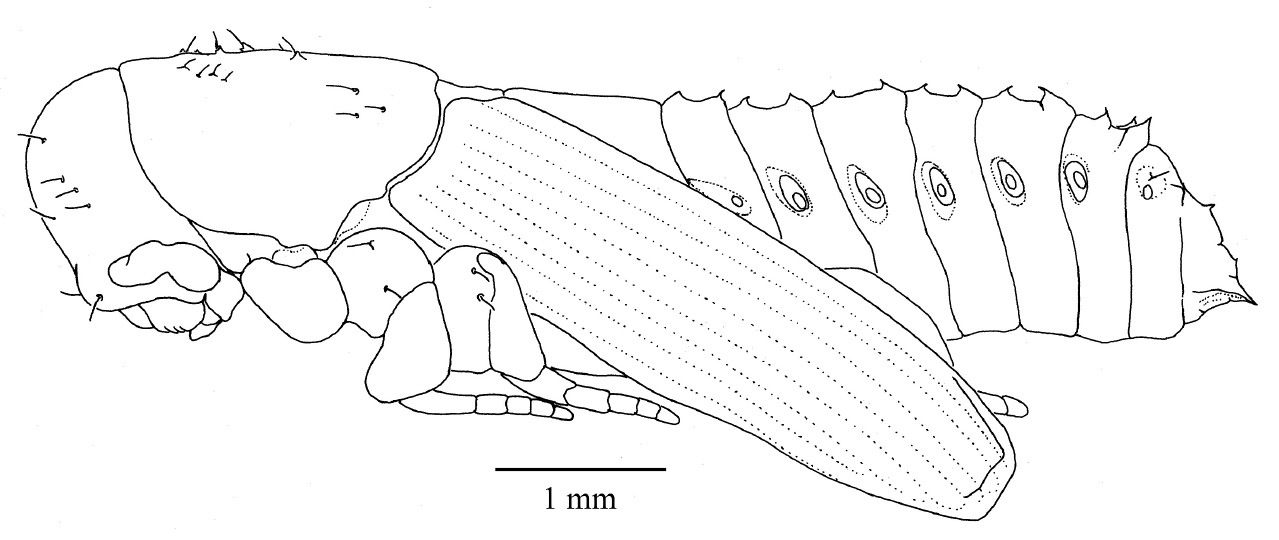
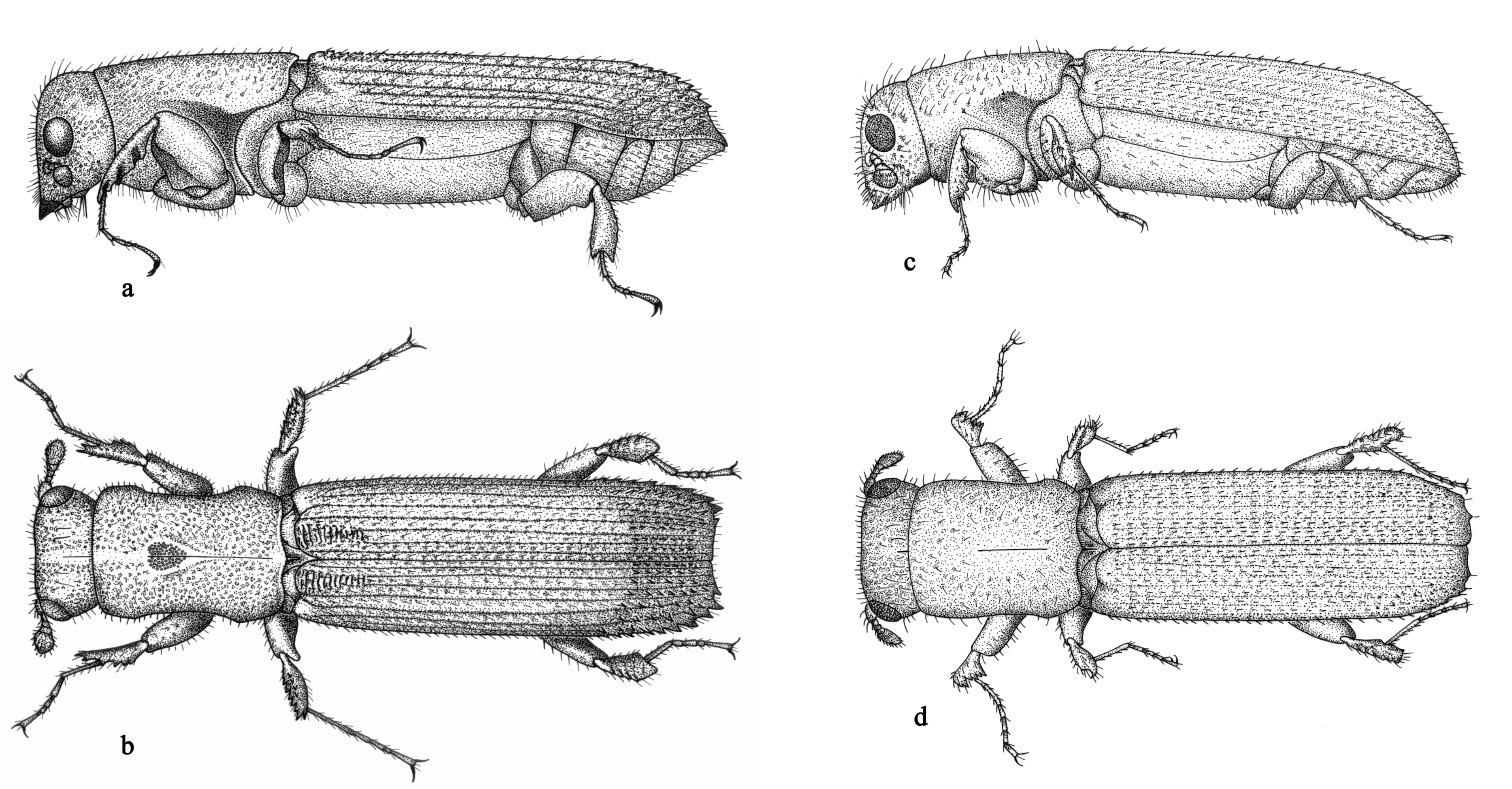
Adults (Fig. 14a, b, c, d)
have the typical elongate cylindrical form of platypodines, with a
length of approximately 6 mm and a diameter of approximately 2 mm. All
types examined at NMV were females. This is in contradiction to Schedl’s
tentative assignment of them as all male (
Adult female (a&b) and male (c&d) (lateral and dorsal respectively).
Provided one is aware of these problems Schedl’s 1968 and
1972b descriptions of adult beetles are fairly complete. Since they
have already been published do not require repeating in this paper.
Inadequacies in the original description of the adult head (
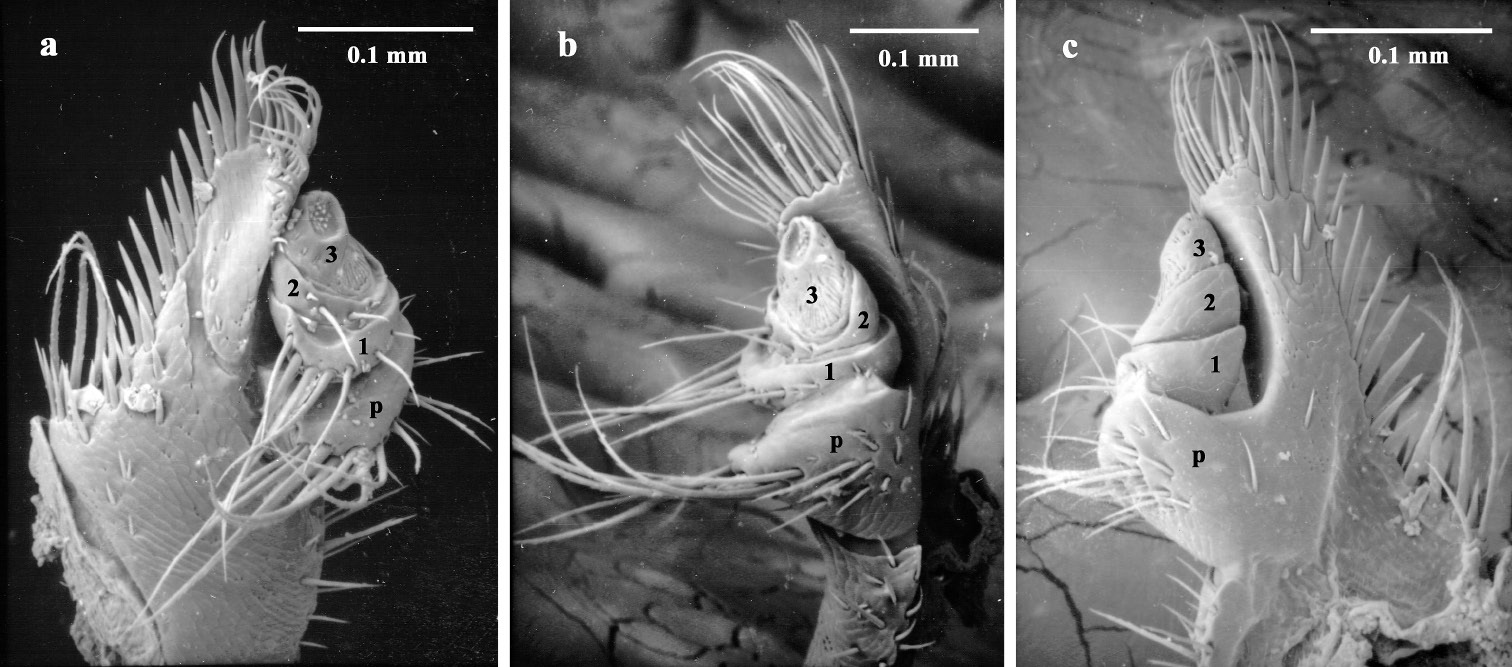
Maxillary palps (Fig. 15a, b, c) of adult Austroplatypus incompertus are three segmented. This is in contrast to a previous report that they have four segments (
Adult maxillary palp a ventral b lateral c dorsal (segments numbered 1–3 and palpiger – p).
Sexual dimorphism
The sexes are dimorphic in Austroplatypus incompertus with the most obvious difference being the shape and sculpturing of the elytra. In the female the elytral declivity is abrupt and armed with prominent spines, while in the male the elytral apices are more rounded with only very small spines. This difference between the sexes is easily discernible with the naked eye and can be used to sex individuals in the field. Additional differences between the sexes can be seen using a stereo-dissecting microscope:
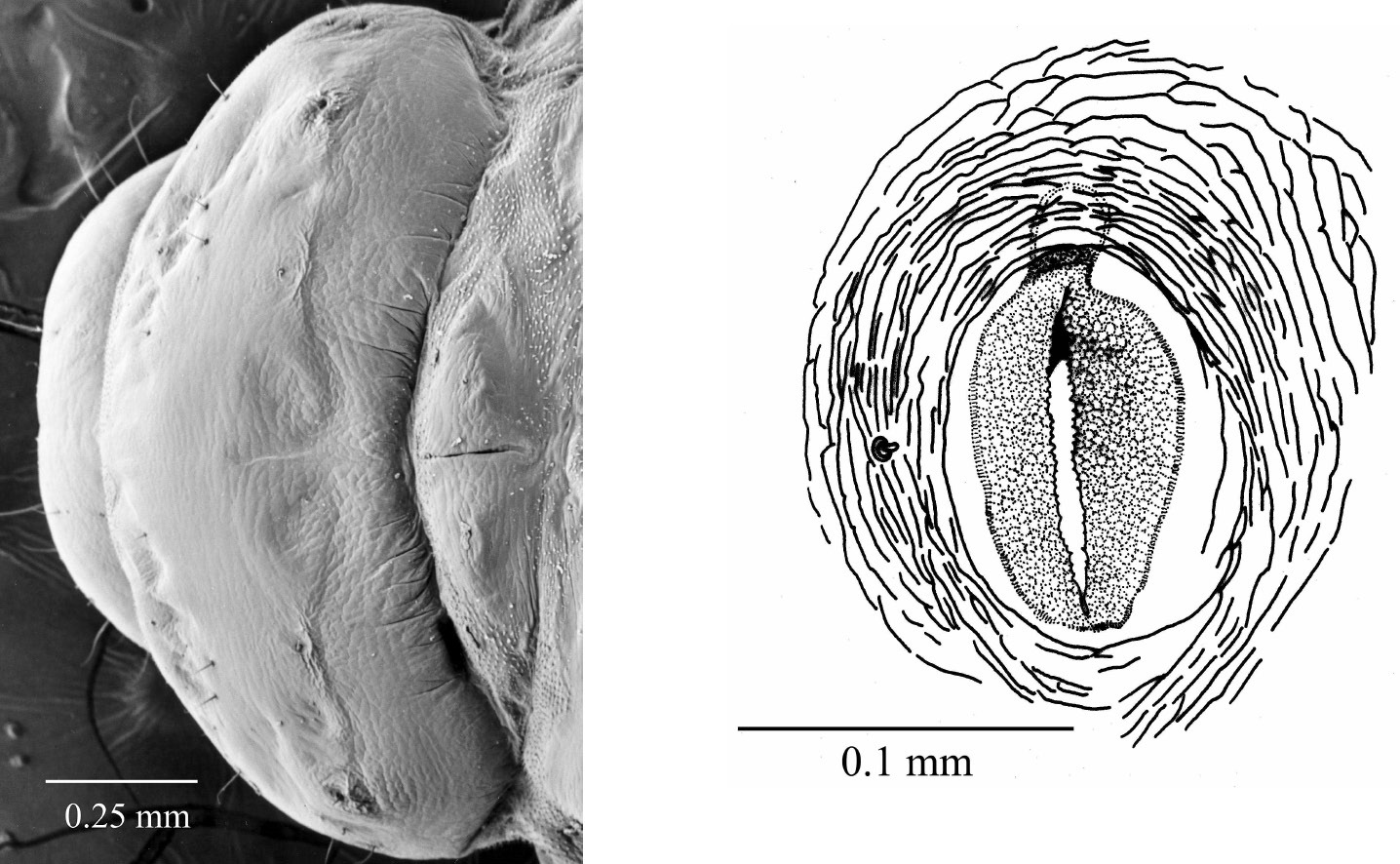
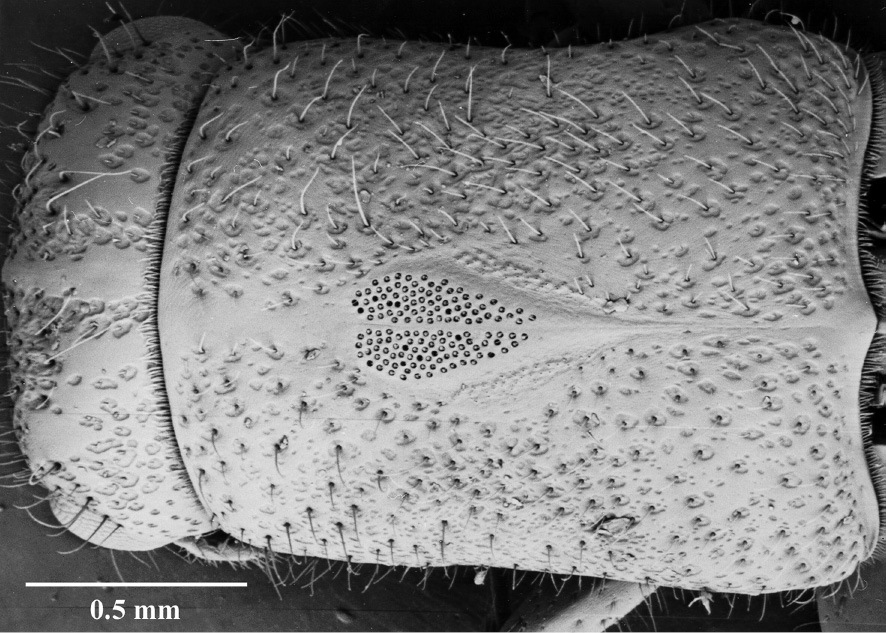
– The presence in the female of mycangia in the centre of the prothorax (Fig. 16) and their absence in the male;
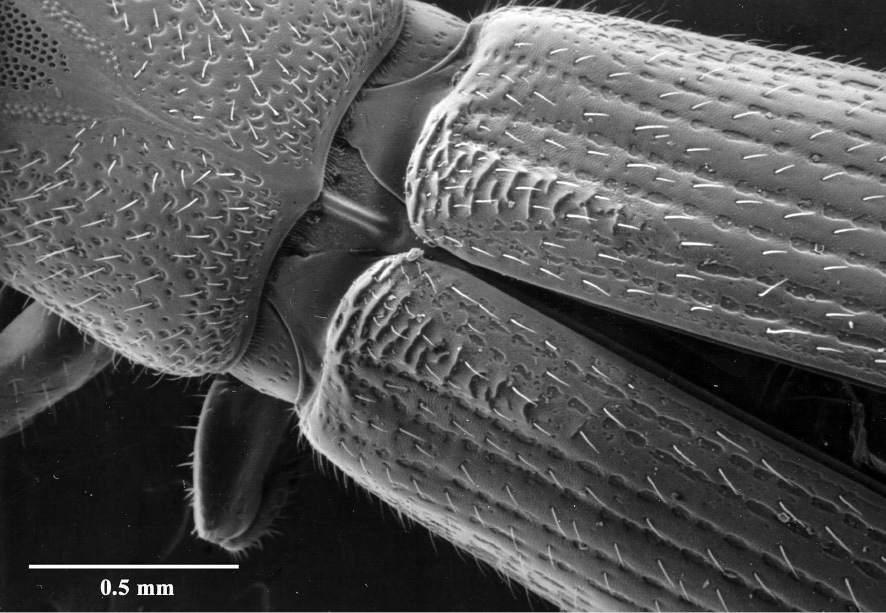
– The presence in the female of a series of ridges at the base of the elytra (Fig. 17) and their absence in the male. The ridges are located between the 3rd and 4th interstices and form a series of backwardly directed ridges, twice as wide at the anterior edge (straddling both the 4th and 3rd interstices) and tapering to half that (just the 3rd) for approximately a sixth of the total length of the elytra.
Mycangia of female.
Elytral ridges of female.
Size differences
Larvae
Only fifth instar larvae were present in sufficient numbers to allow analysis of variance. There were highly significant differences in head capsule width among the different latitudinal groups [P < 0.001, DF = (2, 543)] with the width increasing from north to south (Table 1).
Head capsule width of fifth instar for different latitudinal groups.
| Latitudinal group | n | Mean head capsule width (mm) |
|---|---|---|
| Northern | 84 | 1.015 a |
| Central | 292 | 1.087 b |
| Southern | 171 | 1.230 c |
Adults
Between sexes. Although both the prothorax and the elytra were measured, the prothorax proved to be better suited to regular measurement because of its flatness, especially along its length. Measurements based on the prothorax were also less likely to be subject to error, compared to elytral length, as the latter can be difficult to measure if the elytra are opened after the death of the specimen. Even so, the results of the analysis of the three different measurements pooled across all hosts and localities showed that all were highly significantly different between the sexes [prothorax length P < 0.001, DF = (1, 604); prothorax width P < 0.001, DF = (1, 603) and elytral length P < 0.001, DF = (1, 604)], with males being smaller than females.
Because all three measurements were significantly different between the sexes but prothorax length was the most suitable and reliable measurement, subsequent analyses were restricted to this variable. Males were present in too few numbers across all localities and hosts to permit analysis.
Between localities. There was a highly significant size difference between female beetles from the different latitudinal groups [P < 0.001, DF = (2, 428)] with prothorax length increasing from north to south (Table 2).
Mean prothorax length of Austroplatypus incompertus females for each of the three latitudinal groups.
| Latitudinal group | n | Mean head capsule width (mm) |
|---|---|---|
| Northern | 70 | 1.621 a |
| Central | 314 | 1.745 b |
| Southern | 47 | 1.872 c |
Between hosts. There was also a highly significant difference between beetles from the different host tree species [P < 0.001, DF = (8, 418)], and Post hoc Tukey HSD multiple comparisons placed the hosts into three groups (Table 3).
Mean prothorax length of Austroplatypus incompertus females for each of the host tree Eucalyptus species.
| Host species | n | Prothorax length (mm) | Host distribution |
|---|---|---|---|
| Eucalyptus andrewsii | 16 | 1.533 a | Northern tablelands of NSW & adjacent areas of Queensland |
| Eucalyptus cameroni | 2 | 1.630 a b | Northern tablelands & ranges of NSW |
| Eucalyptus laevopinea | 15 | 1.681 b | Central & northern tablelands of NSW and immediately adjacent areas of Queensland |
| Eucalyptus pilularis | 300 | 1.723 b | Coastal NSW and southeast Queensland |
| Eucalyptus obliqua | 33 | 1.811 c | Northern tablelands & south coast of NSW, coast & ranges Victoria, Mt Lofty Ranges South Australia and Tasmania. |
| Eucalyptus agglomerata | 36 | 1.817 c | Central & southern coast of NSW and adjacent areas of Victoria |
| Eucalyptus fastigata | 6 | 1.856 c | Tablelands, ranges & coastal escarpments of NSW and adjacent parts of Victoria |
| Eucalyptus sieberi | 16 | 1.879 c | Tablelands & coast of NSW and eastern Victoria |
| Eucalyptus delegatensis | 3 | 1.914 c | Southern ranges of NSW and eastern Victoria |
Immature stages
Five distinct larval instars could be distinguished on
the basis of their morphology and their head capsule widths, as is the
case with other platypodines (
Sexual dimorphism
Sexual dimorphism in Austroplatypus incompertus
is reversed, compared with the situation in other platypodines. Males
are significantly smaller than females, females have elytral
modifications, in the form of an elytral declivity modified for both
cleaning and defence, which males do not, and only females possess
mycangia. In most platypodines, the sexes are similar in size or the
male is only slightly smaller, males alone possess elytral
modifications or modifications are much more strongly developed in
males, and males either have no mycangia or a reduction in the number
of mycangia compared with females (
Size differences
Fifth instar larval head capsule widths and all three adult body measurements of Austroplatypus incompertus display a size variation consistent with Bergmann’s rule which states that body size increases at higher latitudes (
Austroplatypus incompertus (Schedl)
Platypus incompertus,
Austroplatypus incompertus,
Platypus incostatus, Schedl, 1972b, Papua New Guinea Agricultural Journal. 23, 68, syn. n.
Platypus incompertus was described from six specimens, which Schedl thought to be male. An examination of the type material of Austroplatypus incompertus (held in the National Museum of Victoria) revealed that it consisted of females only. Platypus incompertus was subsequently placed in a new genus, Austroplatypus, by
Armed with the knowledge that Austroplatypus incompertus displays atypical sexual dimorphism, the discrepancy between the original descriptions of both Austroplatypus incompertus (
Because the sexes in most platypodines are either similar in size or the female is slightly larger the distinctly smaller size of the single Austroplatypus incostatus specimen described by Schedl probably convinced him that he was dealing with two different species. However, the current study has shown that not only is there a significant size difference between the sexes, but also a significant size difference between beetles from different parts of their distributional range. The original type material that was used to describe Austroplatypus incompertus came from the southern part of the distribution(Eden, NSW and Woodhouse Creek north of Omeo and near Omeo Victoria), whereas the single type specimen of Platypus incostatus is from the most northern part of the distribution (Dorrigo, NSW). Thus, Schedl would have been looking at a male specimen from the smallest end of the size range, compared with female specimens from the larger end of the range. This situation would have suggested to him that he was dealing with two species.
Position of Austroplatypus within Platypodinae
The position of Austroplatypus incompertus in the family Platypodinae is still unclear.
I wish to thank Roger Beaver and Gregory Gowing for critically reviewing an earlier draft of this paper and Lawrence Kirkendall for valuable comments which improved the final version.