(C) 2011 Eduard Petitpierre. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
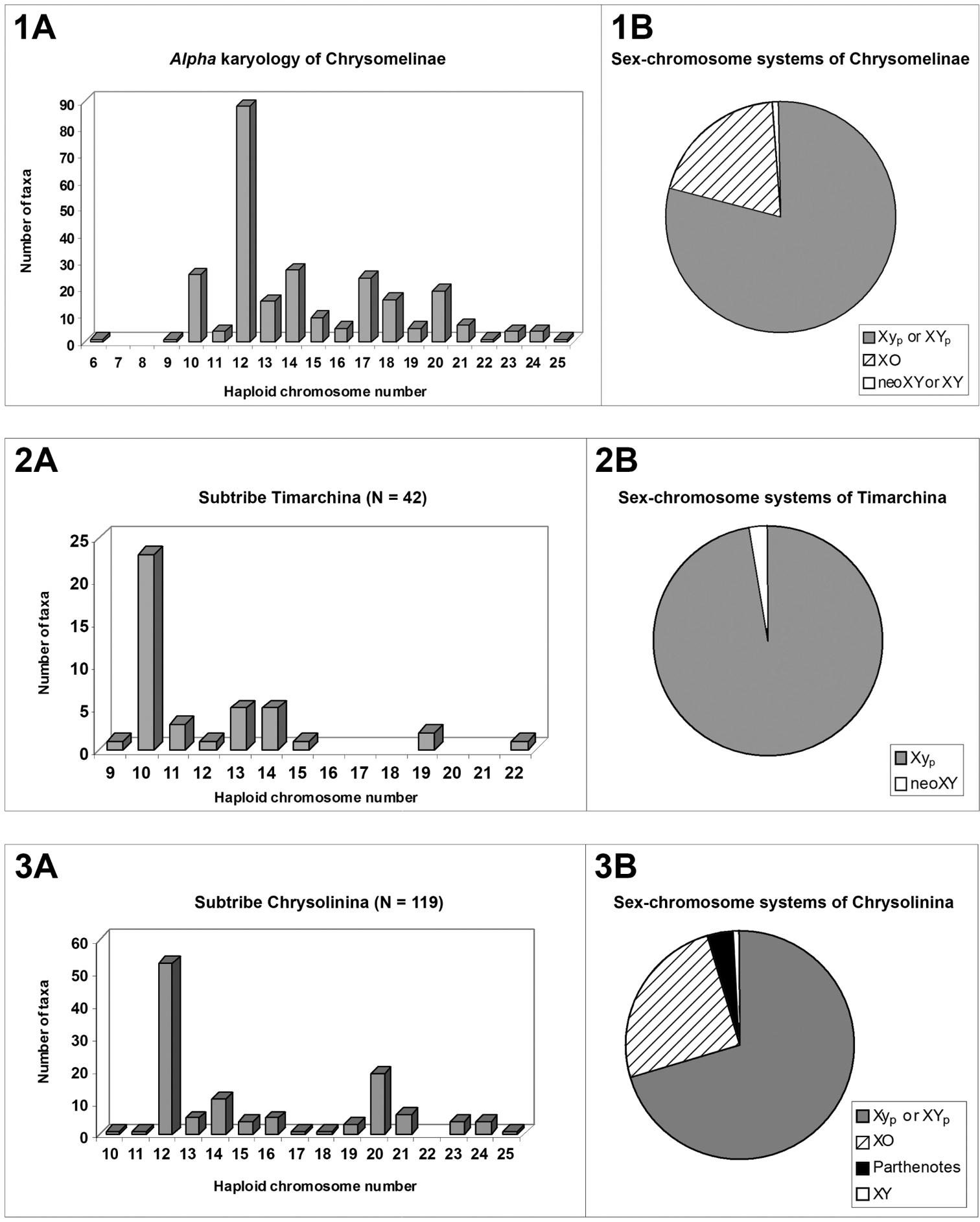
Nearly 260 taxa and chromosomal races of subfamily Chrysomelinae have been chromosomally analyzed showing a wide range of diploid numbers from 2n = 12 to 2n = 50, and four types of male sex-chromosome systems. with the parachute-like ones Xyp and XYp clearly prevailing (79.0%), but with the XO well represented too (19.75%). The modal haploid number for chrysomelines is n = 12 (34.2%) although it is not probably the presumed most plesiomorph for the whole subfamily, because in tribe Timarchini the modal number is n = 10 (53.6%) and in subtribe Chrysomelina n = 17 (65.7%). Some well sampled genera, such as Timarcha, Chrysolina and Cyrtonus, are variable in diploid numbers, whereas others, like Chrysomela, Paropsisterna, Oreina and Leptinotarsa, are conservative and these differences are discussed. The main shifts in the chromosomal evolution of Chrysomelinae seems to be centric fissions and pericentric inversions but other changes as centric fusions are also clearly demonstrated. The biarmed chromosome shape is the prevalent condition, as found in most Coleoptera, although a fair number of species hold a few uniarmed chromosomes at least. A significant negative correlation between the haploid numbers and the asymmetry in size of karyotypes (r = -0.74) has been found from a large sample of 63 checked species of ten different genera. Therefore, the increases in haploid number are generally associated with a higher karyotype symmetry.
chromosomes, leaf-beetles, diploid numbers, modal numbers, sex-chromosome systems, intrageneric evolution, karyotype symmetry
The subfamily Chrysomelinae is a large cosmopolitan taxon of nearly 2000 species (
The current cytogenetic findings in this subfamily cover a total of 259 taxa and chromosomal races, that is between 8, 6% and 13.0% of those described, which have been surveyed at least with the first level of chromosomal knowledge, usually called α-karyology (
The cytogenetic data were mostly obtained by testis dissection of adult or pupa male specimens, which were fixed, teased, squashed, and finally treated by using conventional staining procedures. A great majority of the cells used for these analyses were in meiotic metaphase I stages, which provide the male meioformula, so including the number of autosomal bivalents, male sex-chromosome system, and the possible presence of accessory chromosomes. In addition, less than 50% of the analysed species were also studied in their karyotype architecture from spermatogonial cells in mitotic metaphases or, more seldom, from meiotic metaphase II cells. These more in-depth analyses, gave up worth information on the size and shape of all chromosomes of specific karyotypes, at this second level of cytogenetic resolution known as ß-karyology (
Other cytogenetic findings of an even much finer resolution, such as those on genome size, C and/or Ag-banding, and fluorescent in-situ hybridization (FISH), have been reported in so few species that they should not be discussed in the frame of this contribution.
Although all the recent and also ancient authors accept the reality of the subfamily Chrysomelinae, the number and names of its tribes and subtribes differ strikingly among them. Thus, very recently,
The Chrysomelinae show a wide variation of diploid chromosome numbers and meioformulas, from 2n = 12 and 5 + neo XY, respectively, in the South American Doryphora quadrisignata (Vidal, 1984), to 2n = 50 and 24 + Xyp in the European Chrysolina rufoaenea (Petitpierre and Mikhailov, 2009). These shifts in number are almost always due to structural chromosome rearrangements, because only a few polyploidy parthenotes have been recognized to date, all of them restricted to the genus Calligrapha, (
Basic chromosomal data on higher taxa of Chrysomelinae
Conversely, the parachute-like sex-chromosome system (Xyp), of a non-chiasmate nature, is clearly prevailing in the subfamily (79.0%) as shown in fig. 1B. This system consists mostly of a large X and a small y-chromosome, looking such as this configuration at metaphase I, or more rarely, two large X and Y chromosomes (XYp), held together by a non-nucleolar argyrophilic substance (
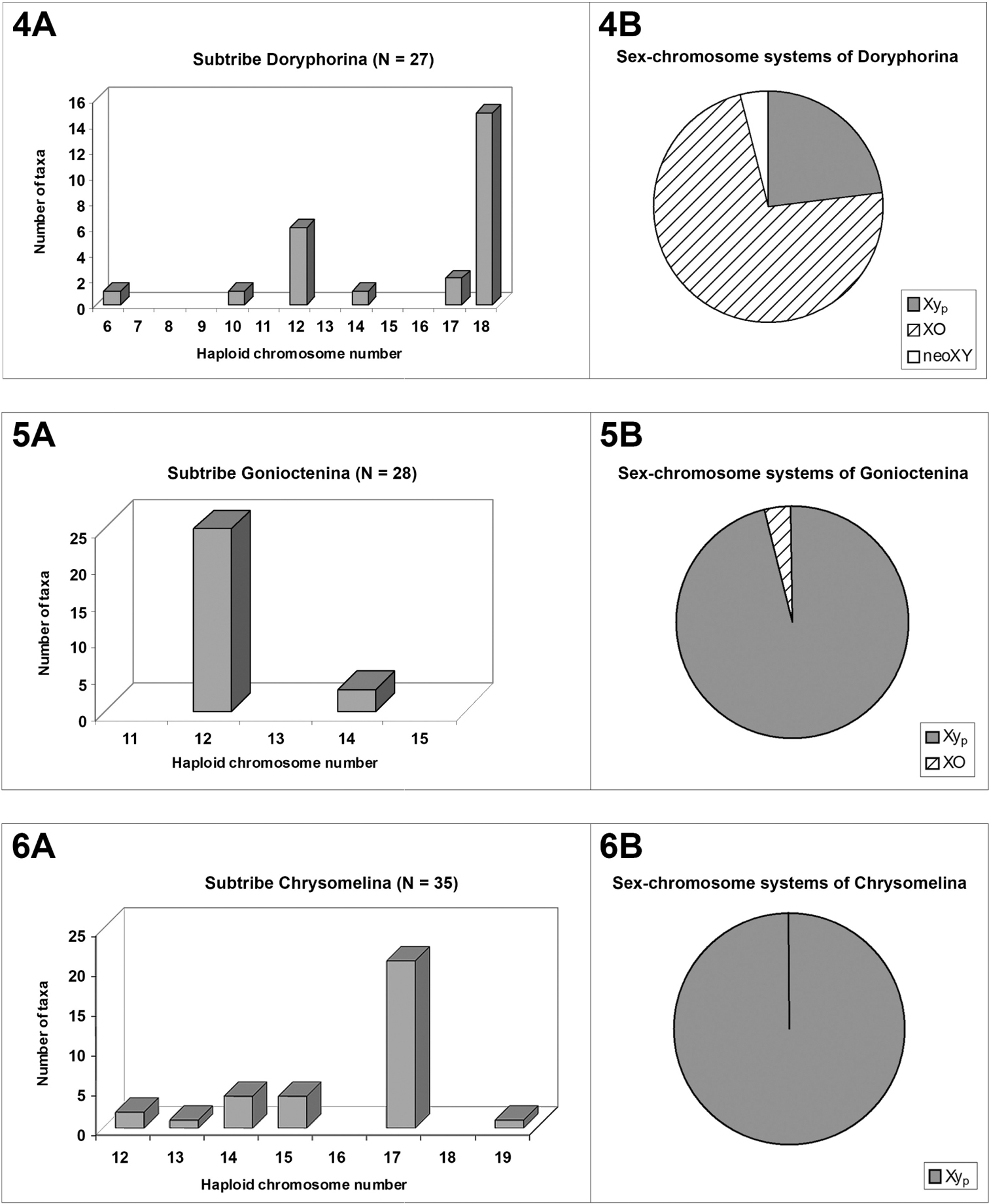
Although the modal number of n = 12 chromosomes has been found in five out of the six reported subtribes, it is very seldom in Timarchina (fig. 2A) and Chrysomelina (fig. 6A), and it does not occur to date in the poorly surveyed Entomoscelina, with only seven analyzed species (
Basic chromosomal data on higher taxa of Chrysomelinae
The Timarchina subtribe shows a striking modal value of n = 10, and 9 + Xyp meioformula (figs. 2A-2B), which are the modal and presumably the possible plesiomorphous state for this group, as well as for the whole beetles of the suborder Polyphaga (
The subtribe Doryphorina displays a 2n(♂) = 35 modal chromosome number and 17 + XO meioformula (figs. 4A–4B), but this can be attributed to a biased sampling on the species of Leptinotarsa, all but one sharing these values (
On the contrary, in subtribe Chrysomelina the modal number and meioformula are 2n = 34 and 16 + Xyp, respectively (Figs. 6A and 6B), shared by 65.7% of the 35 surveyed species in twelve genera, and we assumed that this should possibly be the ancestral condition (Petitpierre and Segarra, 1985) for this taxon, but with our present enlarged screening of species and genera, it is more uncertain due to the absence of this 2n = 34(Xyp) karyotype and meioformula in half of the twelve sampled genera.
If we study the α-karyology of chrysomelines at the genus level, we find genera with high chromosomal diversity, as measured by standard deviation (SD) of their male diploid chromosome numbers, for example Chrysolina with SD = 8.67 in 72 sampled taxa and chromosomal races, Timarcha with SD = 4.33 in 42 taxa, and Cyrtonus with SD = 6.33 in 15 taxa, whereas other genera have zero or a low diversity such as Paropsisterna with SD = 0 in 10 taxa, Chrysomela with SD = 0 in 9 taxa, Oreina with SD = 1.15 in 12 taxa, and Leptinotarsa with SD = 2.77 in 16 taxa. The differences between “variable” and “conservative” genera in their chromosome numbers, were tentatively explained according with the ability for dispersal of flying vs. flightless species genera, and the number of host-plant families they are able to feed, being both characters in a presumed relationship with the size of local populations and thereby with the chances of fixation for new chromosomal shifts (
The chromosomes may show a huge variable morphology in size and shape, some species have karyotypes made of very few chromosomes of a large size while others have karyotypes of many small chromosomes and there are not evidences of any advantages of ones over others, although minute chromosomes are more easily lost at meiosis if a chiasma fails to be formed, and very large acro- or telocentric chromosomes can be cut across before they have been properly separated at anaphase (
Some 80 among the 259 presently know taxa or chromosomal races of chrysomelines have been examined at the level of ß-karyology i.e. by identifying size and shape of individual chromosomes in each karyotype. Such kind of studies have been mainly carried out in certain genera, the North American Calligrapha (
Karyotypes can also be classified as symmetrical in size when all chromosomes have similar magnitudes, and asymmetrical when there are two clearly distinct size classes, and these two alternatives can also be applied to chromosome shape, uniarmed chromosomes for asymmetrical and biarmed ones for symmetrical karyotypes (
For the sake of simplicity we should only consider here the asymmetry vs. symmetry in chromosome size but not in shape. The karyotypes of Chrysomelinae offer examples of both types but more often of intermediate states, that is, with chromosomes of gradually decreasing sizes. In order to measure the degree of asymmetry of a karyotype we have used the standard deviation (SD) of each chromosome relative length with respect to the averaged % length taken from the total complement length (TCL) (
These cytogenetic results are reported in Table 1 and they were used to obtain the coefficient of correlation (r) between these two cytological parameters, haploid chromosome number and SD of karyotype asymmetry, which was clearly negative with a highly significant likelihood, r = - 0.74 (P > 0.99). In brief, the increase in haploid chromosome number is generally associated with a decrease in asymmetry, or in other words, the karyotypes are more symmetrical when they have more chromosomes, a clear trend which has also been reported in other beetles like the weevils (Curculionidae) by
Haploid chromosome number (n) and SD of karyotype asymmetry
| n | DS | n | DS | ||
|---|---|---|---|---|---|
| Timarcha balearica | 11 | 3.57 | Cyrtonus cobosi | 14 | 3.36 |
| Timarcha calceata | 15 | 2.30 | Cyrtonus contractus | 14 | 2.19 |
| Timarcha cyanescens | 10 | 4.71 | Cyrtonus elegans | 14 | 2.11 |
| Timarcha erosa vermiculata | 10 | 6.28 | Cyrtonus plumbeus | 14 | 2.29 |
| Timarcha fallax | 10 | 4.66 | Oreina ludovicae | 12 | 3.32 |
| Timarcha intermedia | 10 | 3.41 | Calligrapha alni | 12 | 3.73 |
| Timarcha lugens | 10 | 4.02 | Calligrapha amator | 12 | 3.42 |
| Timarcha marginicollis | 10 | 4.09 | Calligrapha bidenticola | 12 | 3.32 |
| Timarcha pimelioides | 14 | 4.01 | Calligrapha californica corepsivora | 12 | 4.76 |
| Timarcha recticollis | 10 | 4.72 | Calligrapha confluens | 12 | 3.32 |
| Timarcha rugosa | 13 | 3.45 | Calligrapha multipunctata bigsbyana | 12 | 3.39 |
| Timarcha sicelidis | 10 | 5.27 | Calligrapha philadelphica | 12 | 3.14 |
| Timarcha strangulata | 14 | 1.68 | Calligrapha pnirsa | 12 | 3.41 |
| Chrysolina affinis baetica | 12 | 1.65 | Calligrapha pruni | 12 | 3.95 |
| Chrysolina americana | 12 | 1.98 | Calligrapha rowena | 12 | 3.42 |
| Chrysolina bankii | 12 | 2.12 | Calligrapha verrucosa | 12 | 3.16 |
| Chrysolina bicolor | 12 | 1.60 | Labidomera clivicollis | 17 | 2.36 |
| Chrysolina carnifex | 20 | 1.29 | Labidomera suturella | 16 | 2.11 |
| Chrysolina coerulans | 12 | 3.05 | Leptinotarsa behrensi | 18 | 1.84 |
| Chrysolina costalis | 12 | 2.54 | Leptinotarsa decemlineata | 18 | 1.51 |
| Chrysolina femoralis | 12 | 1.65 | Leptinotarsa defecta | 18 | 1.83 |
| Chrysolina gypsophilae | 16 | 2.76 | Leptinotarsa haldemani | 18 | 1.38 |
| Chrysolina haemoptera | 20 | 1.54 | Leptinotarsa heydeni | 18 | 1.52 |
| Chrysolina helopioides | 24 | 1.51 | Leptinotarsa juncta | 18 | 1.20 |
| Chrysolina herbacea | 12 | 2.56 | Leptinotarsa lineolata | 18 | 1.62 |
| Chrysolina hyperici | 19 | 1.37 | Leptinotarsa peninsularis | 18 | 1.83 |
| Chrysolina kuesteri | 11 | 5.41 | Leptinotarsa rubiginosa | 18 | 1.36 |
| Chrysolina latecincta | 12 | 4.74 | Leptinotarsa texana | 18 | 1.45 |
| Chrysolina umbratilis | 15 | 3.08 | Leptinotarsa tumamoca | 18 | 1.55 |
| Phratora tibialis | 17 | 1.63 | Leptinotarsa typographica | 18 | 1.48 |
| Henicotherus porteri | 14 | 2.14 | Leptinotarsa undecimlineata | 17 | 2.67 |
| Araucanomela wellingtonensis | 14 | 4.49 |
I would like to acknowledge to Dr. M.A. Conesa (UIB, Palma de Mallorca) for his valuable help in the process of making the histogram figures and the table.