(C) 2011 Gerd Gäde. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The presented work is a hybrid of an overview and an original research paper on peptides belonging to the adipokinetic hormone (AKH) family that are present in the corpora cardiaca of Chrysomeloidea. First, we introduce the AKH/red pigment-concentrating hormone (RPCH) peptide family. Second, we collate the available primary sequence data on AKH peptides in Cerambycidae and Chrysomelidae, and we present new sequencing data (from previously unstudied species) obtained by liquid-chromatography coupled with ion trap electrospray ionisation mass spectrometry. Our expanded data set encompasses the primary structure of AKHs from seven species of Cerambycidae and three species of Chrysomelidae. All of these species synthesise the octapeptide code-named Peram-CAH-I (pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide). Whereas this is the sole AKH peptide in Cerambycidae, Chrysomelidae demonstrate a probable event of AKH gene duplication, thereby giving rise to an additional AKH. This second AKH peptide may be either Emppe-AKH (pGlu-Val-Asn-Phe-Thr-Pro-Asn-Trp amide) or Peram-CAH-II (pGlu-Leu-Thr-Phe-Thr-Pro-Asn-Trp amide). The peptide distribution and structural data suggest that both families are closely related and that Peram-CAH-I is the ancestral peptide. We hypothesise on the molecular evolution of Emppe-AKH and Peram-CAH-II from the ancestral peptide due to nonsynonymous missense single nucleotide polymorphism in the nucleotide coding sequence of prepro-AKH. Finally, we review the biological significance of the AKH peptides as hyperprolinaemic hormones in Chrysomeloidea, i.e. they cause an increase in the circulating concentration of proline. The mobilisation of proline has been demonstrated during flight in both cerambycid and chrysomelid beetles.
Cerambycidae, Chrysomelidae, adipokinetic hormone, structure elucidation, mass spectrometry, phylogenetic relatedness
Peptides belonging to the AKH/ RPCH family are produced in neurosecretory cells of either the corpora cardiaca (CC) of insects or of the X-organ cells in the eyestalks of decapod crustaceans (see reviews by
The primary structure of peptides from the AKH/RPCH family have been used as an additional data set to aid in the construction of phylogenies in insect orders (
In the present study we focus on the AKHs from certain beetle families, namely the superfamily Chrysomeloidea. Here, we review the previously published data on AKHs in this superfamily and add hitherto unpublished structural data on AKHs of more species from the two subfamilies, Chrysomelidae and Cerambycidae, with the aim of drawing preliminary conclusions on the putative value of this data set for scientific speculation on relatedness of these families. We also review the current knowledge on the function of AKHs in both subfamilies with respect to the control of substrate availability, especially the amino acid proline.
2. The AKH/RPCH peptide family in ChrysomeloideaThe order Coleoptera constitutes the most species-rich taxon on Earth with approximately 350 000 described species and comprises, therefore, about 40 % of all insect species and about 25 % of all animal species (
The cerambycids Ceroplesis capensis Linnaeus, 1764 and Promeces longipes (Olivier, 1795) were collected in September 2009 and 2010 in the Cedarberg close to Clanwilliam, South Africa, while the eucalyptus borer Phoracantha recurva Newman, 1840 was found in the cellar of a house in Cape Town, S. Africa after the purchase and storage of logs from eucalyptus trees which were apparently infested with borer larvae and pupae (2009). The chrysomelid Chrysolina kuesteri (Helliesen, 1912) was collected at the beginning of November 2007 in a vineyard in Frickenhausen, Germany. The cerambycid Leptura maculata Poda, 1761 and the chrysomelid Chrysolina fastuosa Scopoli, 1763 were collected in June 2011 in the forest adjacent to the campus of the University of Konstanz in Germany. For collection details of the cerambycids Ceroplesis thunbergii Fåhraeus, 1872, Phryneta spinator (Fabricius, 1792) and Morimus funereus Mulsant, 1863, see
We followed standard laboratory procedures to identify and obtain the primary structure of AKHs from the CC of Chrysomeloidea species. In all cases, the CC were dissected into 80% methanol and extracts were prepared by sonification according to
For the remaining species: Ceroplesis capensis, Promeces longipes, Phoracantha recurva, Leptura maculata, Chrysolina kuesteri and Chrysolina fastuosa, peptide material in the CC extracts were analysed directly by liquid chromatography-mass spectrometry (LC-MS) using a quadropole ion trap mass spectrometer equipped with an electrospray ionisation (ESI) source (instruments and methods as described in detail elsewhere (see
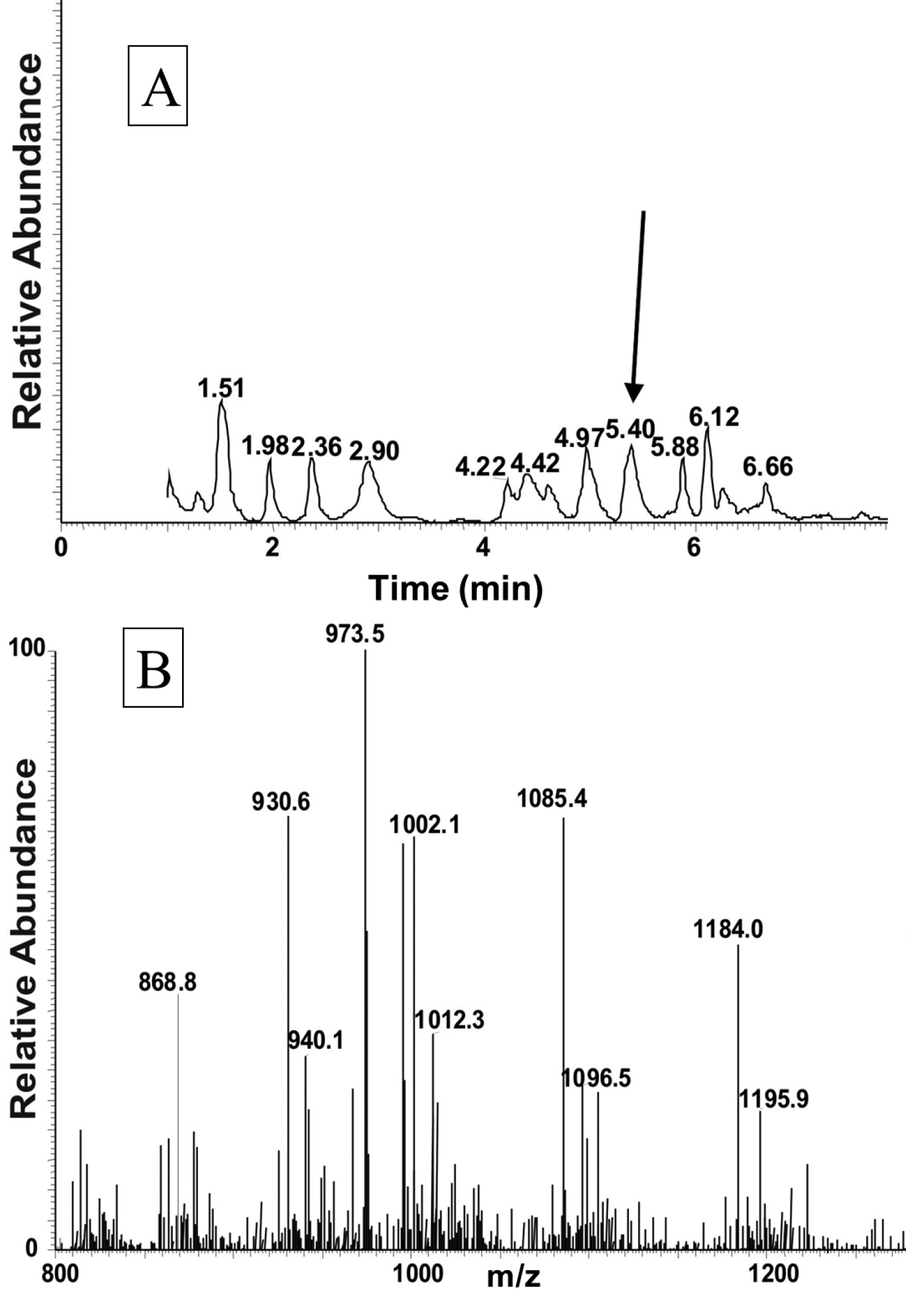
LC-MS analysis of an extract from the corpora cardiaca of Ceroplesis capensis. Material from the CC of the cerambycid beetle, Ceroplesis capensis was extracted with 80% methanol and analysed by LC-MS. A The total ion chromatogram B A full scan positive electrospray ionisation (ESI) mass spectrum of the peak shown in (A) with a retention time of 5.40 min.
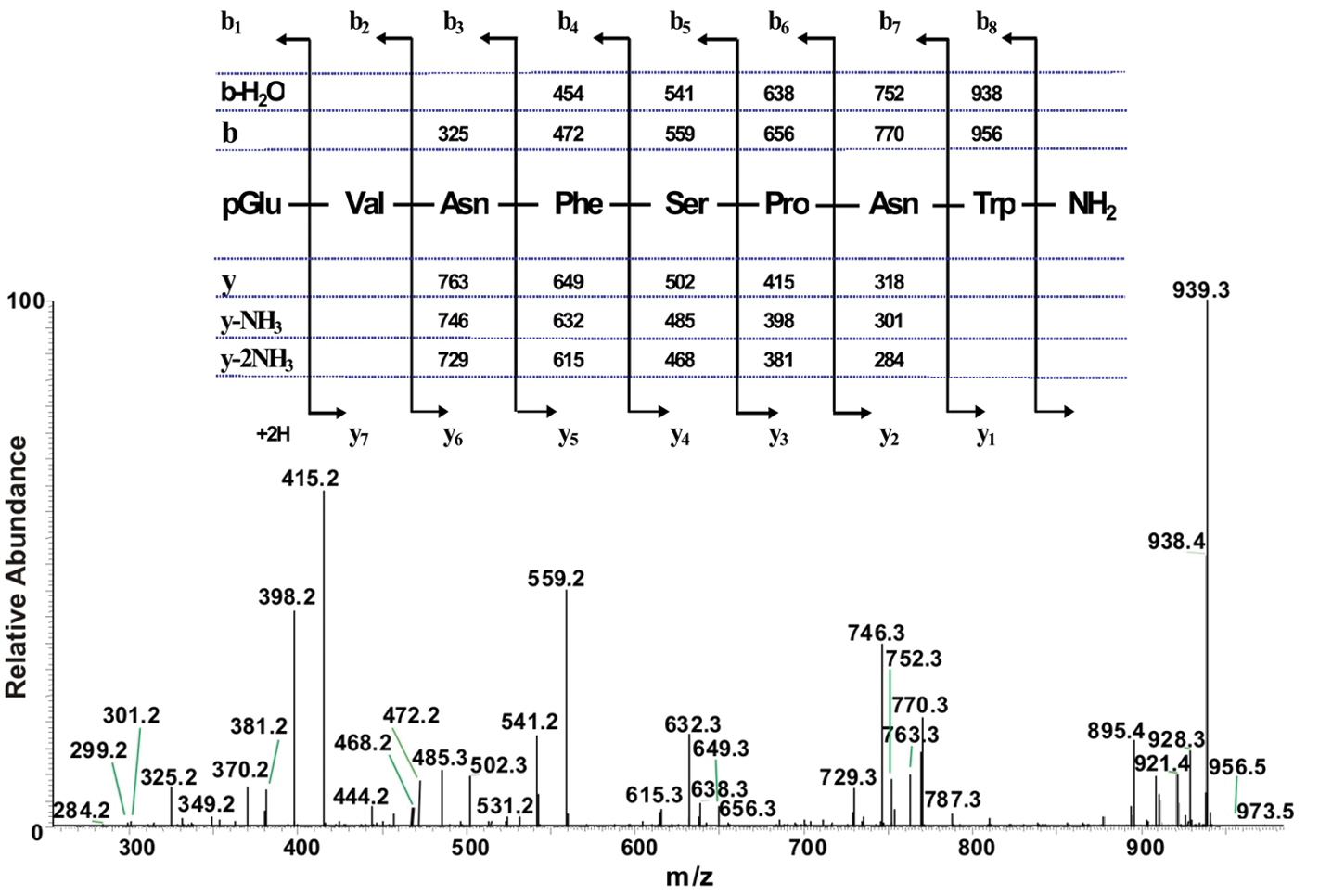
The total ion chromatogram of about 0.5 pair equivalent of CC from Ceroplesis capensis showed one peak of interest at a retention time of 5.40 min (Fig. 1A); this peak displays inter alia an ion with highest abundance at m/z 973.5 for the [M + H]+ ion (Fig. 1B). The primary sequence of the peptide was deduced from the MS2 spectrum obtained by collision-induced dissociation (CID) of the precursor shown in Fig. 2. The characteristic y-type and b-type product ions, in conjunction with diagnostic ions (i.e. y-NH3, y-2NH3 and b-H2O) enabled the assignment of an [M + H]+ ion as a member of the AKH family (see inset in Fig. 2). Although the alignment of the first two amino acids cannot be inferred from the recorded MS2 spectrum, we know from the general signature structure of AKHs that pyroglutamate is typically at the N-terminus, which leaves only a valine residue at position two to fill the gap of the measured 99 atomic mass units. Thus, the sequence of the AKH synthesised in the CC of Ceroplesis capensis is assigned as pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide which is identical to that of Peram-CAH-I (Periplaneta americana-cardioacceleratory hormone-I), which was found for the first time in the cockroach Periplaneta americana (Linnaeus, 1758) (
The collision-induced dissociation ESI mass spectrum of an AKH peptide in Ceroplesis capensis, and its deduced amino acid sequence. The CID MS2-ESI spectrum of the ion MH+ (m/z 973.5) in Fig. 1B. The inset shows the assigned peptide sequence (corresponding to that of Peram-CAH-I), together with the theoretical calculated masses for b-type and y-type diagnostic fragment ions which are observed in the MS2 mass spectrum.
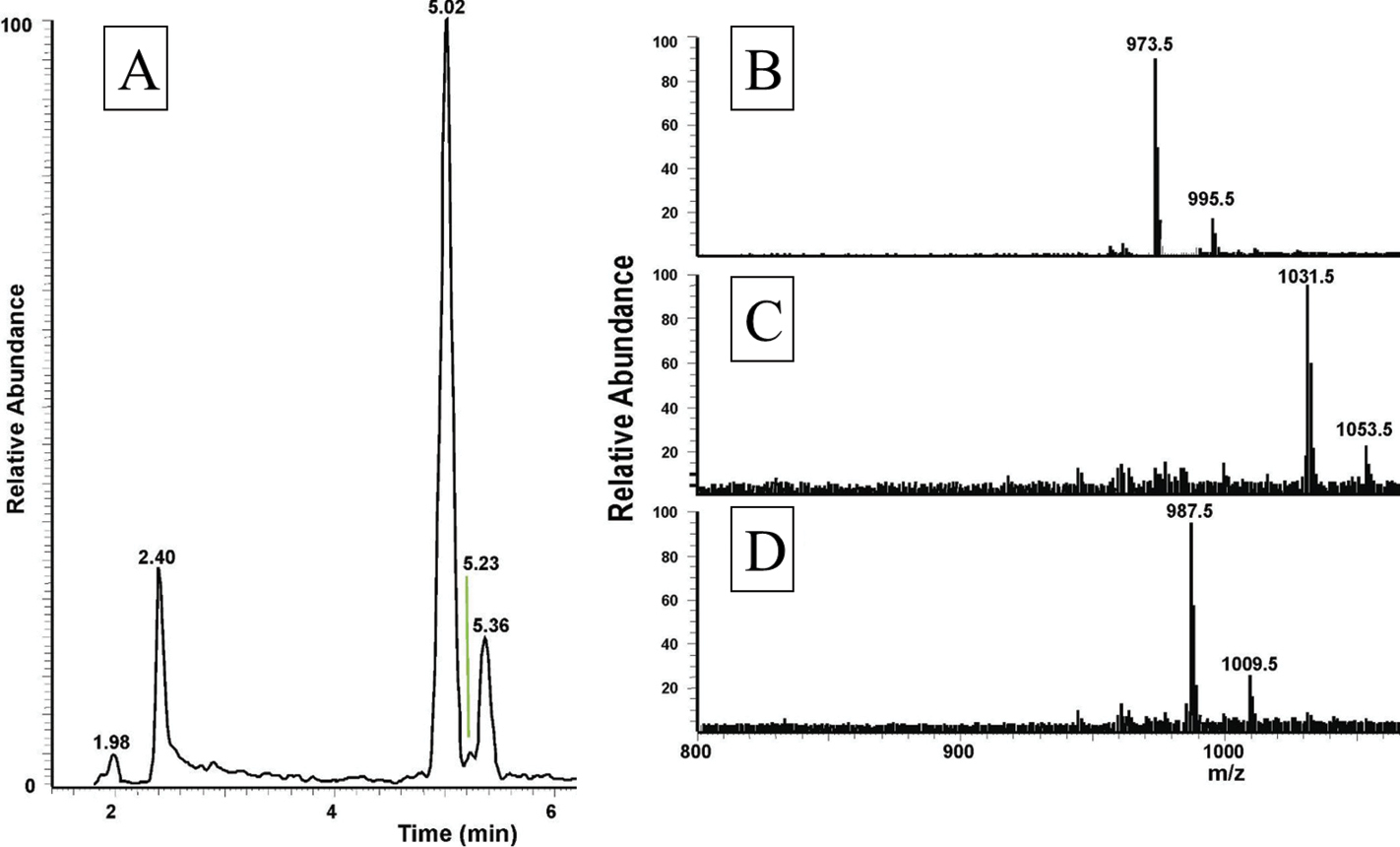
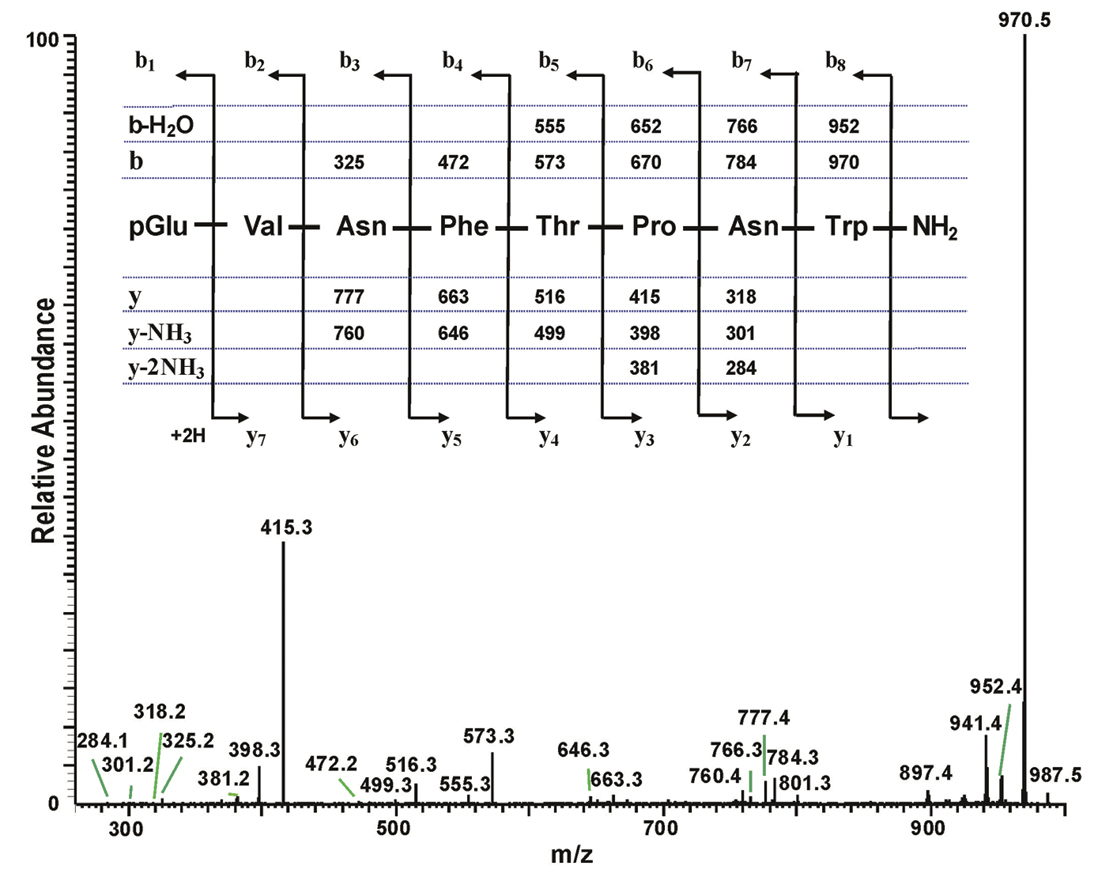
The total ion chromatogram of about 0.8 pair equivalent of CC from Chrysolina kuesteri show three peaks of interest at retention times of 5.02 min (highest abundance), 5.23 min (lowest abundance) and 5.36 min (intermediate abundance) (Fig. 3A); these peaks display ions at m/z 973.5, 1031.5 and 987.5, respectively for the [M + H]+ ions (Figs 3B-D). The MS2 spectra obtained by CID of the respective precursors were recorded. Because the spectrum for [M + H]+ at m/z 973.4 was almost identical to the one for Ceroplesis capensis (Fig. 2), the data are not illustrated here, and the underlying AKH was easily assigned as Peram-CAH-I. The MS2 spectrum for [M + H]+ at m/z 987.5 is given in Fig. 4; the interpretation was straightforward, and a peptide with the primary structure of pGlu-Val-Asn-Phe-Thr-Pro-Asn-Trp amide was assigned, which had previously been found in the CC of the mantid Empusa pennata (Thunberg, 1815) called Emppe-AKH (
LC-MS analysis of an extract from the corpora cardiaca of Chrysolina kuesteri. Material from the CC of the chrysomelid beetle, Chrysolina kuesteri was extracted with 80% methanol and analysed by LC-MS. (A) The total ion chromatogram. Full scan positive ESI mass spectra of the peaks shown in (A) with a retention time of 5.02 min (B), 5.23 min (C) and 5.36 min (D).
The collision-induced dissociation ESI mass spectrum of an AKH peptide in Chrysolina kuesteri, and its deduced amino acid sequence. The CID MS2 ESI spectrum of the ion MH+ at m/z 987.5 (see Fig. 3D). The inset shows the sequence of the assigned peptide (corresponding to that of Emppe-AKH), together with the theoretical calculated masses for b-type and y-type diagnostic fragment ions which are observed in the MS2 mass spectrum.
The collision-induced dissociation ESI mass spectrum of an additional putative AKH peptide in Chrysolina kuesteri, and its deduced amino acid sequence. The CID MS2 ESI spectrum of the ion MH+ at m/z 1031.5 (see Fig. 3C). The inset shows the sequence of the assigned peptide (corresponding to an intermediate processing form of Peram-CAH-I, i.e. a non-amidated nonapeptide with a Gly residue at position 9). Also shown are the theoretical calculated masses for b-type and y-type diagnostic fragment ions which are observed in the MS2 mass spectrum.
In the cases detailed above, the assigned amino acid sequence of the native AKHs was confirmed by comparing the retention times, the [M + H]+ ions and the respective CID-MS2 spectra with those of the appropriate synthetic peptides; chromatographic and mass spectral properties were always identical (data not shown). For use in such confirmatory studies, synthetic peptides Peram-CAH-I and -II (Periplaneta americana cardioacceleratory hormone-I and -II) had been purchased from Peninsula Laboratories Inc. (now Bachem Americas Inc., California, USA), while the peptide Emppe-AKH (Empusa pennata adipokinetic hormone) had been custom-synthesised by Dr. R. de Milton (Medical School, UCT, South Africa).
2.3 Primary sequence diversity of AKHsTable 1 summarises the structural information of AKHs in chrysomeloid beetles known previously from Edman sequencing (
Adipokinetic hormone sequences in Cerambycidae and Chrysomelidae. Primary sequences of peptides of the adipokinetic hormone family in the corpora cardiaca of various species belonging to the families Cerambycidae and Chrysomelidae.
| Family | Species | Amino acid sequence | Code name of peptide |
|---|---|---|---|
| Cerambycidae | Phryneta spinator | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I |
| Ceroplesis thunbergii | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Ceroplesis capensis | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Promeces longipes | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Phoracantha recurva | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Morimus funereus | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Leptura maculata | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| Chrysomelidae | Leptinotarsa decemlineata | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I |
| pGlu-Leu-Thr-Phe-Thr-Pro-Asn-Trp amide | Peram-CAH-II | ||
| Chrysolina kuesteri | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| pGlu-Val-Asn-Phe-Thr-Pro-Asn-Trp amide | Emppe-AKH | ||
| Chrysolina fastuosa | pGlu-Val-Asn-Phe-Ser-Pro-Asn-Trp amide | Peram-CAH-I | |
| pGlu-Val-Asn-Phe-Thr-Pro-Asn-Trp amide | Emppe-AKH |
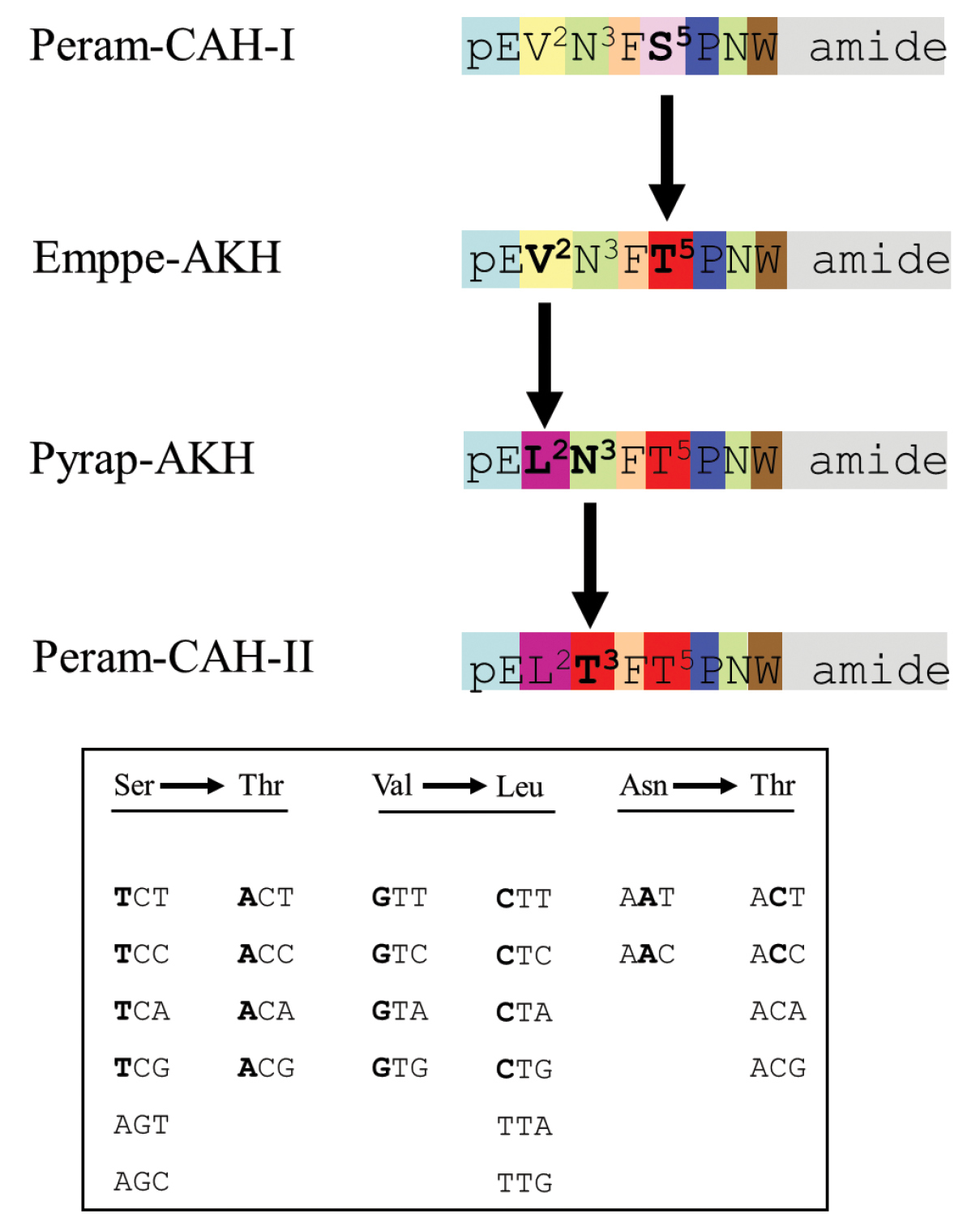
From the data in Table 1, it seems that Peram-CAH-I is the ancestral peptide. We propose that the following molecular evolution (Fig. 6) may have occurred by conservative nucleotide substitutions involving nonsynonymous missense single nucleotide polyphemisms (SNPs) in the DNA:
Putative molecular evolution of adipokinetic peptides in chrysomeloid beetles. A proposed scheme of the molecular evolution of adipokinetic peptides to give rise to the different structures observed in investigated chrysomeloid beetles. The inset shows the genetic code for the respective amino acids involved. Differences in amino acids or code nucleotides are given in bold lettering.
(1) T in position one of the genetic code for serine (S5) in Peram-CAH-I was replaced by A to code for threonine (T5) in Emppe-AKH;
(2) G in position one of the genetic code for valine (V2) in Emppe-AKH was replaced by C to now code for leucine (L2) in Pyrap-AKH (Pyrrhocoris apterus-adipokinetic hormone);
(3) A in position two of the genetic code for asparagine (N3) in Pyrap-AKH was replaced by C and now codes for threonine (T3) in Peram-CAH-II (Fig. 6).
The proposed scenario outlined above, appears to be the most likely one. An alternative route may have involved the molecular change from Emppe-AKH to Peram-CAH-II via another intermediate peptide where N3 in Emppe-AKH is first changed to T3; we find this to be highly unlikely since none of the 50 completely known AKH structures, to date, has the combination of pGlu-Val-Thr at the N-terminus that is required from such an intermediate AKH.
The evolutionary steps proposed in Fig. 6 lead to the peptide Pyrap-AKH, which exists in certain heteropteran and homopteran bugs, in a pamphagid grasshopper and in the red flour beetle, Tribolium castaneum (Herbst, 1797) (
Previously, we conducted two sets of experiments to determine which substrate is mobilised by the secretion of an AKH in chrysomeloid beetles.
In the first set of experiments, we injected the cerambycid beetles, Ceroplesis thunbergii and Phryneta spinator, with their own CC extract or a low dose of the identified peptide, Peram-CAH-I, in a synthetic form and measured the concentration of total lipids, total carbohydrates and proline in the haemolymph before injection and 90 min thereafter (
In the second set of experiments we examined the effect of active flight on substrate levels in the haemolymph of chrysomeloid beetles. In this set-up, the beetles were tethered to a flight mill that was modified to allow lift-generating flight; after a 1 min period of such active flying, the beetle was detached from the mill and kept at rest. In both cerambycid species, Ceroplesis thunbergii and Phryneta spinator, a small but significant decrease in the concentration of carbohydrates was measured in the haemolymph upon 1 min of flight; there was, however no changes measured in the levels of total lipids. In contrast, the proline concentration in these cerambycid species diminished by 20 to 30 % upon 1 min of flight; following an hour of rest subsequent to a short period of active flight, however, the proline concentration returned to pre-flight levels in the haemolymph (
Based on these earlier investigations with chrysomeloid species, we have, thus, shown that proline is used in conjunction with carbohydrates as metabolic fuel to power flight activity (
This study was funded in part by grants from the National Research Foundation (Pretoria, South Africa; grants FA2007021300002 and IFR2008071500048 to GG) and the University of Cape Town Research Council (grants to GG and HGM). We are indebted to P. Simek (Ceske Budejovice, Czech Republic) for contributing the mass spectrometric measurements and A. Bellmann (Bremen, Germany) for identifying the Chrysolina species, as well as H. Martz and D. Gustav (Konstanz, Germany) for help with collecting and identifying beetle species in Konstanz.