(C) 2011 Kaniyarikkal Divakaran Prathapan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The biology, host plants, and pest status of Podontia Dalman, 1824 species are reviewed. Natural history of Podontia congregata Baly, 1865 a flea beetle endemic to southern India, is reported for the first time. It is distributed from the Western Ghats Mountains westward to the plains. Clusiaceae is reported as a new host plant family for Blepharida-group species, with Garcinia gummi-gutta (L.) N. Robson (Clusiaceae) as the host plant for Podontia congregata. Pentatomid bugs attack the larvae but not eggs, pupae, or adults. A new egg parasitoid species, Ooencyrtus keralensis Hayat and Prathapan, 2010 (Hymenoptera: Encyrtidae), was discovered. Aspects of Podontia congregata host selection, life cycle, and larval fecal defenses are consistent with its inclusion in the Blepharida-genus group.
Leaf beetles, Podontia congregata, Pest, Garcinia, Clusiaceae, India
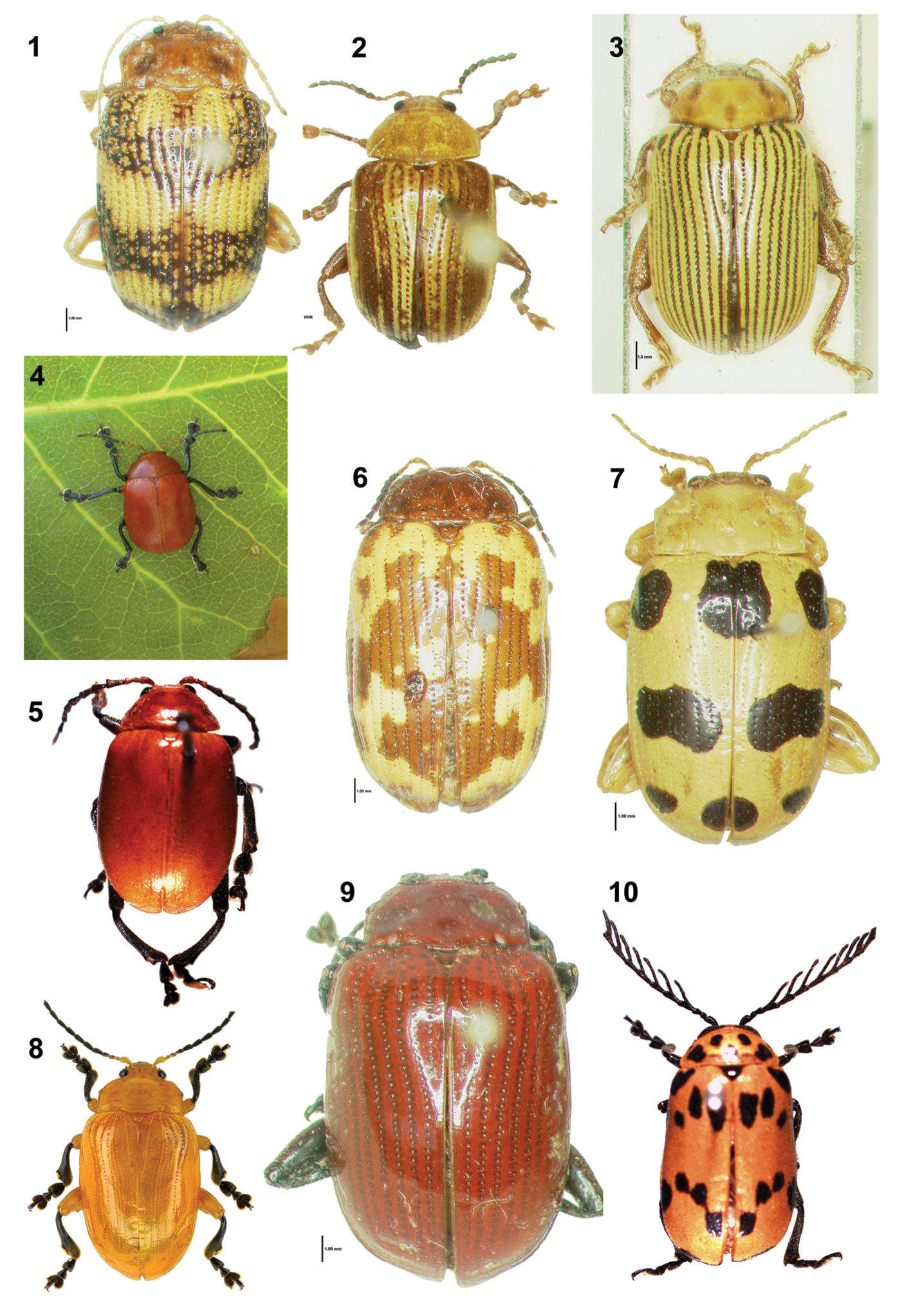
The Blepharida-group of genera consists of robust and brightly colored flea beetles (Figs 1–10).
Habitus of adults of Blepharida-groupgenera, size <2 cm long. 1. Asiophrida marmorea (Wiedemann) (photo by C.-w. Shin). 2. Blepharida rhois (Forster)(photo by C.-w. Shin). 3. Blepharida vittata Baly (photo by C.-w. Shin). 4. Crimissa cruralis Stål (Photo by M. Tavares). 5. Diamphidia femoralis Gerstaecker (photo by C.S. Chaboo). 6. Ophrida spectabilis (Baly) (photo by C.-w. Shin). 7. Podontia affinis (Gröndal) (photo by C.-w. Shin). 8. Podontia lutea (Olivier) (photo by C.-F. Lee). 9. Podontia rufocastanea Baly (photo by C.-w. Shin). 10. Polyclada flexuosa Baly (photo by C.S. Chaboo).
Species in the Blepharida-group are documented most commonly on host plants in the Anacardiaceae, Bignoniaceae, Burseraceae, and Sapindaceae (Table 1). However, there are several single species records from Apocynaceae, Caesalpiniaceae, Elaeocarpaceae, Fabaceae, Lythraceae, Meliaceae, Moraceae, and Verbenaceae, which raise interesting questions about diet evolution as well as the distinct possibility of questionable host reports. Additionally,
In Blepharida, Diamphidia, Podontia, and Polyclada, larvae retain their feces directly on the dorsum. This coating acts as a deterrent to attacking enemies such as ants (
The genus Podontia Dalman 1824 (Figs 7–9) comprises 14 Asian species ranging from Indonesia to Indo-China, with one species occurring in northern Australia (
Here, we review the biology of Podontia and other Blepharida-group genera and provide the first natural history account of Podontia congregata Baly, 1865. An endemic to the southern Western Ghats and adjoining areas, Podontia congregata is the largest flea beetle in southern India, ranging from 11.5 to 14.7 mm in length. Our study is based on both field and laboratory observations.
Host plants of species of the Blepharida-group. Known questionable records are indicated by “(?)”. Plant names follow the
| Species | Host plant | Reference |
|---|---|---|
| Asiophrida Medvedev | ||
| Asiophrida marmorea (Wiedemann) | Anacardiaceae: Spondias L. sp. |
|
| Apocynaceae: Holarrhena pubescens Wall. (=antidysenterica (L.) Wall) |
|
|
| Burseraceae: Garuga pinnata Roxb. |
|
|
| Garuga Roxb. sp. |
|
|
| Asiophrida (Trichophrida) hirsuta (Wiedemann) | Burseraceae: Boswellia serrata Roxb. ex Colebr. |
|
| Asiophrida scaphoides (Baly) | Anacardiaceae: Rhus L. |
|
| Burseraceae: Canarium L. |
|
|
| Blepharida Chevrolat | Anacardiaceae |
|
| Anacardiaceae: Cotinus Mill. |
|
|
| Rhus L. sp. |
|
|
| Schinus L. sp. |
|
|
| Burseraceae |
|
|
| Bursera Jacq. ex L. |
|
|
| Bursera schlechtendalii Engl. |
|
|
| Burseraceae: Commiphora Jacq. sp. |
|
|
| Sapindaceae: Allophylus L. sp. |
|
|
| Matayba Aubl. |
|
|
| Blepharida alternata Jacoby | Bursera arborea L. Riley |
|
| Bursera attenuata L. Riley |
|
|
| Bursera bicolor Engl. |
|
|
| Bursera chemapodicta Rzed. & E. Ortiz |
|
|
| Bursera citronella McVaugh & Rzed. |
|
|
| Bursera cuneata Engl. |
|
|
| Bursera excelsa Engl. |
|
|
| Bursera fragilis S. Watson |
|
|
| Bursera heteresthes Bullock |
|
|
| Bursera instabilis McVaugh & Rzed. |
|
|
| Bursera palmeri S. Watson |
|
|
| Bursera submoniliformis Engl. |
|
|
| Blepharida atripennis Horn | Bursera epinnata (Rose) Engl. |
|
| Bursera odorata T.S. Brandeg |
|
|
| Bursera ruticola Pérez-Navarro |
|
|
| Blepharida balyi Bryant | Bursera copallifera (Sessé & Moc. ex DC.) Bullock |
|
| Bursera bipinnata (DC.) Engl. |
|
|
| Bursera discolor Rzed. |
|
|
| Bursera diversifolia Rose |
|
|
| Bursera Jacq. ex L. sp. |
|
|
| Blepharida bryanti Furth | Bursera excelsa (Kunth) Engl. |
|
| Blepharida condrasi (Weise) | Rhus tripartita (Ucria) Grande |
|
| Blepharida conspersa (Horn) | Bursera epinnata (Rose) Engl. |
|
| Bursera filicifolia T. S. Brandeg. |
|
|
| Bursera hindsiana Engl. in DC. |
|
|
| Blepharida flavocostata Jacoby | Bursera aspleniifolia T. S. Brandeg. |
|
| Bursera bicolor Engl. |
|
|
| Bursera biflora (Rose) Standl. |
|
|
| Bursera bipinnata (DC.) Engl. |
|
|
| Bursera bonetii Rzed. |
|
|
| Bursera copallifera (DC.) Bullock |
|
|
| Bursera hintonii Bullock |
|
|
| Bursera sarukhanii Guevera & Rzed. |
|
|
| Bursera schlechtendalii Engl. |
|
|
| Bursera submoniliformis Engl. |
|
|
| Bursera velutina Bullock |
|
|
| Bursera xochipalensis Rzed. |
|
|
| Blepharida florhi Jacoby | Bursera bipinnata (DC.) Engl. |
|
| Blepharida gabrielae Furth | Bursera aptera Ramirez |
|
| Bursera discolor Rzed. |
|
|
| Bursera fagaroides Engl. |
|
|
| Bursera paradoxa Guevera & Rzed. |
|
|
| Bursera trifoliolata Bullock |
|
|
| Bursera Jacq. ex L. sp. |
|
|
| Blepharida hinchahuevosi Furth | Anacardiaceae: Pseudosmodingium perniciosum (Kunth) Engl. |
|
| Blepharida humeralis Furth | Bursera submoniliformis Engl. |
|
| Blepharida irrorata Chevrolat | Sapindaceae: Allophylus cominia Sw. |
|
| Allophylus occidentalis Radlk. |
|
|
| Matayba Aubl. |
|
|
| Bursera simaruba (L.) Sarg. |
|
|
| Blepharida johngi Furth | Bursera glabrifolia (Kunth) Engl. |
|
| Bursera Jacq. ex L. sp. |
|
|
| Blepharida judithae Furth | Bursera ariensis (Kunth) McVaugh & Rzed. |
|
| Blepharida lineata Furth | Bursera crenata P. G. Wilson |
|
| Bursera denticulata McVaugh & Rzed. |
|
|
| Bursera kerberi Engl. |
|
|
| Bursera trimera Bullock |
|
|
| Blepharida maculicollis Furth | Bursera submoniliformis Engl. |
|
| Bursera xochipalensis Rzed. |
|
|
| Blepharida marginalis Weise | Rhus natalensis Bernh. ex Krauss, Rhus tripartita DC., Rhus vulgaris Meikle |
|
| Blepharida melanoptera (Fall) | Bursera infernidialis Guevera & Rzed. |
|
| Bursera laxiflora S. Watson |
|
|
| Blepharida multimaculata Jacoby | Bursera aptera Ramirez |
|
| Bursera discolor Rzed. |
|
|
| Bursera fagaroides (Kunth) Engl. |
|
|
| Bursera fagaroides var. purpusii (Brandegee) McVaugh & Rzed. |
|
|
| Bursera paradoxa Guevera & Rzed. |
|
|
| Bursera trifoliolata Bullock |
|
|
| Bursera Jacq. ex L. sp. |
|
|
| Blepharida natalensis Baly | Rhus lancea L.f. |
|
| Rhus zeyheri Sond. |
|
|
| Blepharida nigromaculata Jacoby | Rhus L.sp. |
|
| Blepharida nigrotesselata Baly | Rhus L. sp. |
|
| Blepharida pallida Blake | Bursera arborea (Rose) Riley |
|
| Bursera aloexylon (Scheide ex Schlecht.) Engl. |
|
|
| Bursera bipinnata (DC.) Engl. |
|
|
| Bursera coyucensis Bullock |
|
|
| Bursera cuneata (Schlecht.) Engl. |
|
|
| Bursera excelsa (Kunth) Engl. |
|
|
| Bursera glabrifolia Engl. |
|
|
| Bursera grandifolia (Schlecht.) Engl. |
|
|
| Bursera heteresthes Bullock |
|
|
| Bursera instabilis McVaugh & Rzed. |
|
|
| Bursera kerberi Engl. |
|
|
| Bursera penicillata (DC.) Engl. |
|
|
| Bursera sarcopoda P. G. Wilson |
|
|
| Rhus L. spp. |
|
|
| Blepharida parallela Furth | Bursera discolor Rzedowski |
|
| Bursera schlechtendalii Engl. |
|
|
| Blepharida rhois (Forster) | Anacardiaceae: Cotinus obovatus Raf. Sullivan |
|
| Rhus L. |
|
|
| Rhus aromatica Aiton |
|
|
| Rhus copallina Linnaeus |
|
|
| Rhus cotinus Nutt. |
|
|
| Rhus microphylla Engl. |
|
|
| Rhus trilobata Nutt. |
|
|
| Rhus typhina Linnaeus |
|
|
| Rhus vernix Linnaeus |
|
|
| Rhus L. spp. |
|
|
| Schinus terebinthifolius Raddi |
|
|
| Schinus L. sp. |
|
|
| Apocynaceae: Catharanthus (=Vinca) roseus (L.) G. Don |
|
|
| Pinaceae: Pinus palustris Mill. |
|
|
| Rosaceae: strawberry |
|
|
| Blepharida sacra (Weise) | Rhus natalensis Bernh. ex Krauss |
|
| Rhus tenuinervis Engl. & Gilg. (non-host) |
|
|
| Rhus tripartita DC. |
|
|
| Rhus vulgaris Meikle |
|
|
| Blepharida schlechtendalii Furth | Bursera aptera Ramirez |
|
| Bursera heteresthes Bullock |
|
|
| Bursera schlechtendalii Engl. |
|
|
| Blepharida singularis Jacoby | Bursera Jacq. ex L.sp. |
|
| Blepharida sonorstriata Furth | Bursera laxiflora S. Watson |
|
| Blepharida sparsa (Clark) | Bursera kerberi Engl. |
|
| Bursera submoniliformis Engl. |
|
|
| Bursera Jacq. ex L.sp. |
|
|
| Blepharida unami Furth | Bursera fagaroides (H. B. K.) Engl. |
|
| Bursera Jacq. ex L. sp. |
|
|
| Blepharida variegatus Furth | Bursera submoniliformis Engl. |
|
| Blepharida verdea Furth | Bursera lancifolia (Schlecht.) Engl. |
|
| Bursera morelensis Ramirez |
|
|
| Bursera rzedowskii C. A.Toledo |
|
|
| Blepharida vittata Baly | Rhus L. sp. |
|
| Blepharida xochipala Furth | Bursera mirandae C.A. Toledo |
|
| Bursera Jacq. ex L.sp. |
|
|
| Blepharida sp. | Bursera cuneata (Schlecht.) Engl. |
|
| Blepharida sp. | Bursera schlechtendalii Engl. |
|
| Blepharida sp. | Pseudoosmodingium perniciosum (Kunth) Engl. |
|
| Blepharida sp.1 | Bursera glabrifolia Engl. |
|
| Blepharia sp. 2 | Bursera chemapodicta Rzed. & Ortiz |
|
| Blepharida sp. 3 | Bursera vejar-vazquezii Miranda |
|
| Blepharida sp. 4 | Bursera biflora (Rose) Standl. |
|
| Bursera longipes (Rose) Standl. |
|
|
| Blepharida sp. 5 | Bursera xochipalensis Rzed. |
|
| Blepharida sp. 1a | Rhus L.sp., Commiphora Jacq.sp. |
|
| Blepharida sp. 2a | Bignoniaceae: Rhizogum ebovatum? |
|
| Blepharida sp. 3a | Commiphora mollis (Oliv.) Engl. |
|
| Blepharida sp. 6 | Bursera ribana Rzed. & Calderón |
|
| Blepharida sp. 7 | Bursea suntui C.A. Toledo |
|
| Crimissa Stål | Anacardiaceae (?) |
|
| Anacardiaceae: Anacardium L.; Mangifera L. |
|
|
| Crimissa cruralis Stål | Anacardium occidentale L. |
|
| Crimissa sp. | Anacardium occidentale L. |
|
| Bignoniaceae |
|
|
| Diamphidia Gerstaecker | Burseraceae |
|
| Commiphora Jacq. sp. |
|
|
| Diamphidia femoralis Gerstaecker | Commiphora Jacq. sp. |
|
| Diamphidia nigroornata Stål | Commiphora Jacq. sp. |
|
| Commiphora africana (A. Rich.) Engl. |
|
|
| Commiphora angolensis Engl. |
|
|
| Commiphora glandulosa Schinz |
|
|
| Diamphidia simplex Péringuey | Commiphora africana (A. Rich.) Engl. |
|
| Diamphidia vittatipennis Baly | Commiphora africana (A. Rich.) Engl. |
|
| Commiphora tenuipetiolata Engl. |
|
|
| Diamphidia sp. | Sclerocarya caffra Sond. |
|
| Elithia Chapuis | Anacardiaceae |
|
| Euplectroscelis Crotch | Burseraceae |
|
| Bursera Jacq. ex L. sp. |
|
|
| Bursera microphylla A. Gray |
|
|
| Euplectroscelis xanti Crotch | Bursera microphylla A. Gray |
|
| Bursera odorata Brandegee |
|
|
| Notozona Chevrolat | Anacardiaceae (?) |
|
| Rhus L. sp. (?) |
|
|
| Burseraceae |
|
|
| Bursera Jacq. ex. L.sp. |
|
|
| Notozona histrionica Chevrolat | Bursera simaruba (L.) Sarg. |
|
| Notozona nicaraguensis Jaq. | Bursera simaruba (L.) Sarg. |
|
| Ophrida Chapuis | Anacardiaceae |
|
| Apocynaceae |
|
|
| Burseraceae |
|
|
| Boswellia Roxb. ex. Colebr., Canarium L., Garuga Roxb. |
|
|
| Ophrida hirsuta Stebbing | Boswellia serrata Roxb. |
|
| Ophrida nigrovaria (MacLeay) | Canarium australianum F. Muell. |
|
| Ophrida scaphoides (Baly) | Anacardiaceae: Rhus succedanea L. |
|
| Burseraceae: Canarium L. |
|
|
| Ophrida spectabilis (Baly) | Anacardiaceae: Rhus chinensis Mill.; Gall nut, Sumac |
|
| Rhus punjabensis J.L. Stewart |
|
|
| Rhus trichocarpa Miq. |
|
|
| Rhus verniciflua Stokes |
|
|
| Oprhida xanthospilota (Baly) | Continus coggygria Scop. |
|
| Podontia Dalman | Anacardiaceae |
|
| Anacardiaceae: Mangifera L., Rhus L., Spondias L., Toxicodendron Mill. |
|
|
| Rhus L. |
|
|
| Burseraceae |
|
|
| Burseraceae: Canarium L. |
|
|
| Caesalpiniaceae (?) |
|
|
| Elaeocarpaceae: Elaeocarpus L. sp. |
|
|
| Moraceae: Ficus L.sp. (?) |
|
|
| Theaceae: Thea L. sp. (?) |
|
|
| Podontia affinis (Gröndal) | Anacardiaceae: Spondias L. sp. |
|
| Spondias dulcis Forster |
|
|
| Podontia congregata Baly | Clusiaceae: Garcinia gummi-gutta (L.) N. Robson | New Family Record, this paper |
| Podontia dalmani Baly | Meliaceae: Melia L.sp. |
|
| Caesalpiniaceae |
|
|
| Podontia lutea (Olivier) | Canarium L. sp. |
|
| Anacardiaceae: Rhus L. sp. |
|
|
| Rhus succedanea L. |
|
|
| Toxicodendron Mill. sp. |
|
|
| Podontia quatuor-decimpunctata (L.) | Anacardiaceae: Mangifera L. sp. |
|
| Spondias L. sp. |
|
|
| Spondias cyatherea Sonn. |
|
|
| Spondias dulcis Forster |
|
|
| Spondias pinnata (L.f.) Kurz (= Spondias mangifera Willd.) |
|
|
| Burseraceae: Canarium L. |
|
|
| Moraceae: Ficus elastica Roxb. ex Hornem. |
|
|
| Ficus L. |
|
|
| “fruit trees"(native & imported) |
|
|
| Lythraceae: Duabanga grandiflora Walp |
|
|
| Lythraceae: Duabanga sonneratioides Buch. |
|
|
| Lythraceae: Sonneratia apetala Buch.-Ham. | http://banglapedia.search.com.bd/HT/B_0385.html | |
| Podontia soriculata (Swartz) | Thea boheae (?) |
|
| Polyclada Chevrolat | Anacardiaceae |
|
| Pseudospondias Engl. |
|
|
| Rhus L. |
|
|
| Sclerocarya caffra Sond. |
|
|
| Sclerocarya birrea (A.Richt.) Hochst. |
|
|
| Burseraceae: Commiphora Jacq. |
|
|
| Fabaceae: Dalbergia L. sp. (?) |
|
|
| Verbenaceae: Clerodendrum L. sp. (?) |
|
|
| Polyclada flexuosa Baly | Sclerocarya birrea sub. sp. caffra Sonder |
|
| Procalus Clark | Anacardiaceae |
|
| Lithraea Miers ex Hook. & Arn., Schinus L. |
|
|
| Lithraea caustica (Molina) Hook. & Arn. |
|
|
| Schinus latifolius Engl. |
|
|
| Schinus montanus Engl. |
|
|
| Schinus patagonicus (Phil.) I.M. Johnst. |
|
|
| Schinus polygamus (Cav.) Cabrera |
|
|
| Schinus velutinus (Turcz.) I.M. Johnst. |
|
|
| Procalus lenzi (Harold) | Lithraea caustica (Molina) Hook. & Arn. |
|
| Schinus polygamus (Cav.) Cabrera |
|
|
| Procalus malaisei Bechyné | Lithraea caustica (Molina) Hook. & Arn. |
|
| Procalus mutans (Blanchard) | Lithraea caustica (Molina) Hook. & Arn. |
|
| Schinus montanus Engl. |
|
|
| Procalus reduplicatus Bechyné | Lithraea caustica (Molina) Hook. & Arn. |
|
| Procalus silvai Jerez | Schinus patagonicus (Phil.) I.M. Johnst. |
|
| Procalus viridis (Philippi & Philippi) | Lithraea caustica (Molina) Hook. & Arn. |
|
| Schinus latifolius Engl. |
|
|
| Schinus montanus Engl. |
|
|
| Schinus polygamus (Cav.) Cabrera |
|
|
The biology for most Podontia species is unknown; however, host data on Podontia affinis, Podontia lutea, and Podontia quatuordecimpunctata (Linnaeus) indicate that these species severely defoliate anacardiaceous trees. For example, Podontia affinis (kedongdong spring-beetle) ranges from Indonesia to China and is a pest in Indonesia, where its larvae attack the foliage of Spondias dulcis Forster (Anacardiaceae; =Spondias cytherea Sonn., ambarella or kedongdong tree;
The golden leaf beetle, Podontia lutea is large sized (~2 cm, Fig. 8) and its attractive coloration promotes its use in cheap Lucite jewelry. The limited available data indicates biology like other Blepharida-group members (
Podontia quatuordecimpunctata is the best-known Podontia species because both adults and larvae defoliate the tree Spondias dulcis. This tree, commonly known as the mak-ok, hog plum, or golden apple tree, is cultivated for its edible fruits in Indonesia, Malaysia, India, Thailand, and the Caribbean (Figs 11–15; Table 1 and references therein).
Podontia quatuordecimpunctata on the host tree, Spondias dulcis Forster (Anacardiaceae; mak-ok, ambarella, kedongdong) in Thailand 11 Host plant 12 The colorful adult, ~ 2 cm long 13 A larva completely covered by feces 14 Larva, partially covered by feces 15 A juvenile pentatomid bug (Heteroptera: Pentatomidae) attacking a fecal-covered larva, with the beak inserted through the fecal cover. (Photos by S. Damrongsiri).
Asiophrida Medvedev comprises 20 species in three subgenera (
The biology of Blepharida, with 55 species, is currently the best known among Blepharida-group genera. Life cycle data have been published for Blepharida rhois (Forster) (as Blepharida dorothea Mignot) (
Crimissa cruralis Stål, the red cashew beetle, is a major pest of cultivated cashew in Brazil, Anacardium occidentale L. (Fig. 4;
The nine known species of Diamphidia are distributed along the eastern coast from Ethiopia to South Africa and into Namibia (Fig. 5;
The austral-oriental genus Ophrida Chapuis consists of four or five species (
The 12 species of Polyclada Chevrolat are distributed along east Africa, from South Africa to the Arabian Peninsula (
The South American genus Procalus Clark comprises nine species that are associated with Anacardiaceae (Table 1;
Immature stages of Euplectroscelis Crotch, Furthia Medvedev, Neoblepharella (Medvedev) [=Blepharella Medvedev, which was previously occupied as a genus of tachinid flies (
Documented enemies of Podontia species.
| Species | Life stage | Enemy | Source |
|---|---|---|---|
| Podontia | Egg, larva | Coleoptera: Coccinellidae: Aiolocaria Crotch sp. |
|
| Podontia affinis (Gröndal) | Not indicated | Hymenoptera: Encyrtidae: Ooencyrtus podontiae (Gahan) |
|
| Egg | Hymenoptera: Encyrtidae: Ooencyrtus podontiae (Gahan) |
|
|
| Not indicated | Nematoda: Mermithidae: Mermis Dujardin sp. |
|
|
| Not indicated | Sphaeriales: Hypocreaceae: Cephalosporium Corda sp. |
|
|
| Podontia congregata Baly | Egg | Hymenoptera: Encyrtidae: Ooencyrtus keralensis Hayat & Prathapan |
|
| Larva | Heteroptera: Pentatomidae: Eucanthecona parva (Distant) | This paper (Figs 22, 23) | |
| Podontia lutea (Olivier) | Egg, larva | Coleoptera: Coccinellidae: Aiolocaria mirabilis (Motschulsky) |
|
| Fungi: Laboulbeniales: Laboulbenia podontiae Thaxter |
|
||
| Podontia quatuordecimpunctata (Linnaeus) | Adult | Arachnida: Lynx spider |
|
| Adult | Aves: Corvus splendens Vieillot; Acridotheres tristis (L.) |
|
|
| Egg, larva | Mantodea |
|
|
| Egg | Hymenoptera: Braconidae: Apanteles Foerster, Meteorus Haliday; Trichogrammatidae: Trichogramma Westwood |
|
|
| Egg | Hymenoptera: Chalcididae |
|
|
| Egg | Hymenoptera: Eulophidae: Pediobius Walker sp. |
|
|
| Egg | Hymenoptera: Encyrtidae: Ooencyrtus corbetti Ferr. |
|
|
| Larva | Heteroptera: Pentatomidae | This paper (Fig. 15) | |
| Larva | Nematoda: Mermithidae: Mermis Dujardin sp. |
|
|
| Larva | Fungi: Laboulbeniales: Laboulbenia podontiae Thaxter |
|
|
| Larva | Fungi: Sphaeriales: Hypocreaceae: Cephalosporium Corda sp. |
|
One of us (KDP) studied natural populations of Podontia congregata on its host tree, Garcinia gummi-gutta, under field conditions during several visits in 2008–2010 in Vallamkulam, Pathanamthitta, Kerala, India. We also reared beetles in cages for laboratory observations. We examined beetle specimens obtained from the Department of Entomology, College of Horticulture, Mudigere, India (see Fig. 16).
Cage-reared beetle populations were maintained under ambient conditions at Vellayani, Trivandrum, Kerala, India. Individuals from these cage-reared populations were introduced onto field plants of the host for observations. Although Podontia congregata is absent in Vellayani, the host tree grows naturally on the banks of Vellayani Lake.
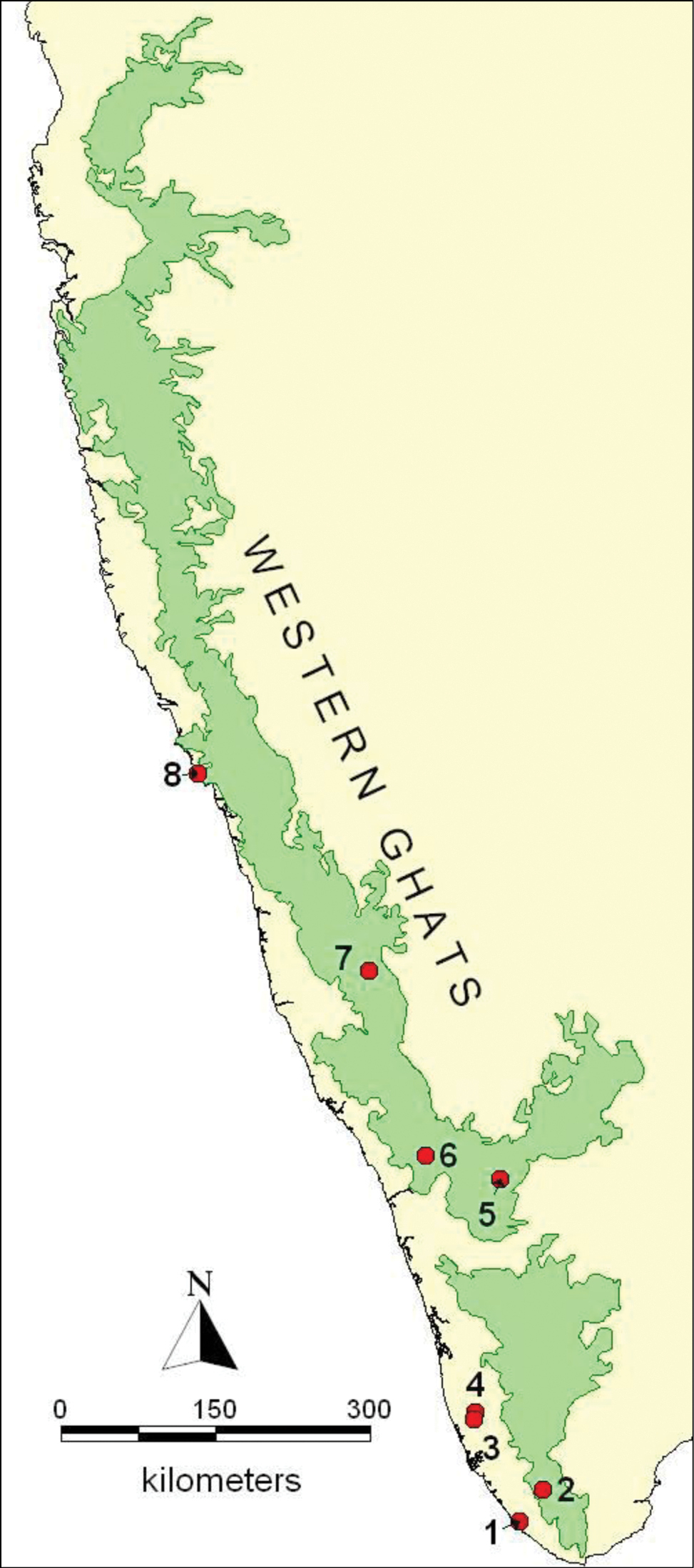
The Western Ghats Mountains in south India with the localities Vellayani (1), Pomudi (2), Pandanad (3), Vallamkulam (4), Conoor (5), Meppadi (6), Mudigere (7) and Karwar (8) where Podontia congregata has been recorded in the present study and in
Habitat 1. India: Kerala State: Pathanamthitta District, Vallamkulum (76°36'18.4"E, 9°22'29.5"N; 12 - 20 m above msl). This is a typical urbanized village in Kerala, where the majority of the agricultural holdings are below 0.5 ha. Homestead farming, a hallmark of the settlement pattern in Kerala, comprises a diverse assortment of crop trees (e.g., Garcinia gummi-gutta), shrubs and herbs, which enhances biodiversity conservation in this densely populated village. This rather hot and humid locality is endowed with a few rivulets to the extent that rice fields can remain submerged during the rainy season. Mature Garcinia gummi-gutta trees are common on the banks of paddy fields and rivulets.
Habitat 2. India: Kerala State: Trivandrum District, Vellayani (76°59'8.3"E, 8°25'47.5"N; 18 m above msl). This is a watershed bordered by small hillocks that drain into Vellayani Lake, which is the second largest freshwater lake in Kerala. Banana and vegetable cultivation dominate the low-lying paddy fields, while a coconut-based cropping system is practiced on the hillocks. Perhaps because it is not preferred for culinary purposes in southern Kerala, Garcinia gummi-gutta is generally uncommon in southern Kerala homesteads and particularly so in Trivandrum. A local preference for dried tamarind fruit (Fabaceae: Tamarindus indica Linnaeus) may explain the low abundance of the host plant here.
Habitat 3. India: Kerala State: Alappuzha District: Pandanad (76°35'0.7"E, 9°19'15.1"N; 12 m above msl), located ~8 km south of Vallamkulam. This is an urbanized village similar to Habitat 1.
Habitat 4. India: Kerala State: Trivandrum District: Ponmudi (77°06'43.7"E, 8°45' 19.9"N; 872 m above msl), a hill station, near the southern end of the Western Ghats mountains. A century ago Ponmudi was covered with pristine wet ever green forests and is a hot spot of biodiversity in peninsular India. However, agricultural plantations, tourism, and commercial tree felling has altered the landscape significantly.
Laboratory conditions. Laboratory culture of Podontia congregata was started at Vellayani from nearly half a dozen adults and several larvae collected at Vallamkulam. Adults were confined in a cage of 30 cm3. We offered food and oviposition sites by supplying branches of the host plant, with the cut end placed in water in a glass bottle. Leaves with eggs were transferred to Petri dishes. Larvae were reared on branches in cages or plastic containers, as well as in Petri dishes. Wet soil was provided for pupation. Rearing was carried out at an ambient temperature of about 22–32°C. About two dozen laboratory reared adults and larvae were introduced onto a naturally growing Garcinia gummi-gutta tree at Vellayani during October–December, 2008, and the different life stages were observed.
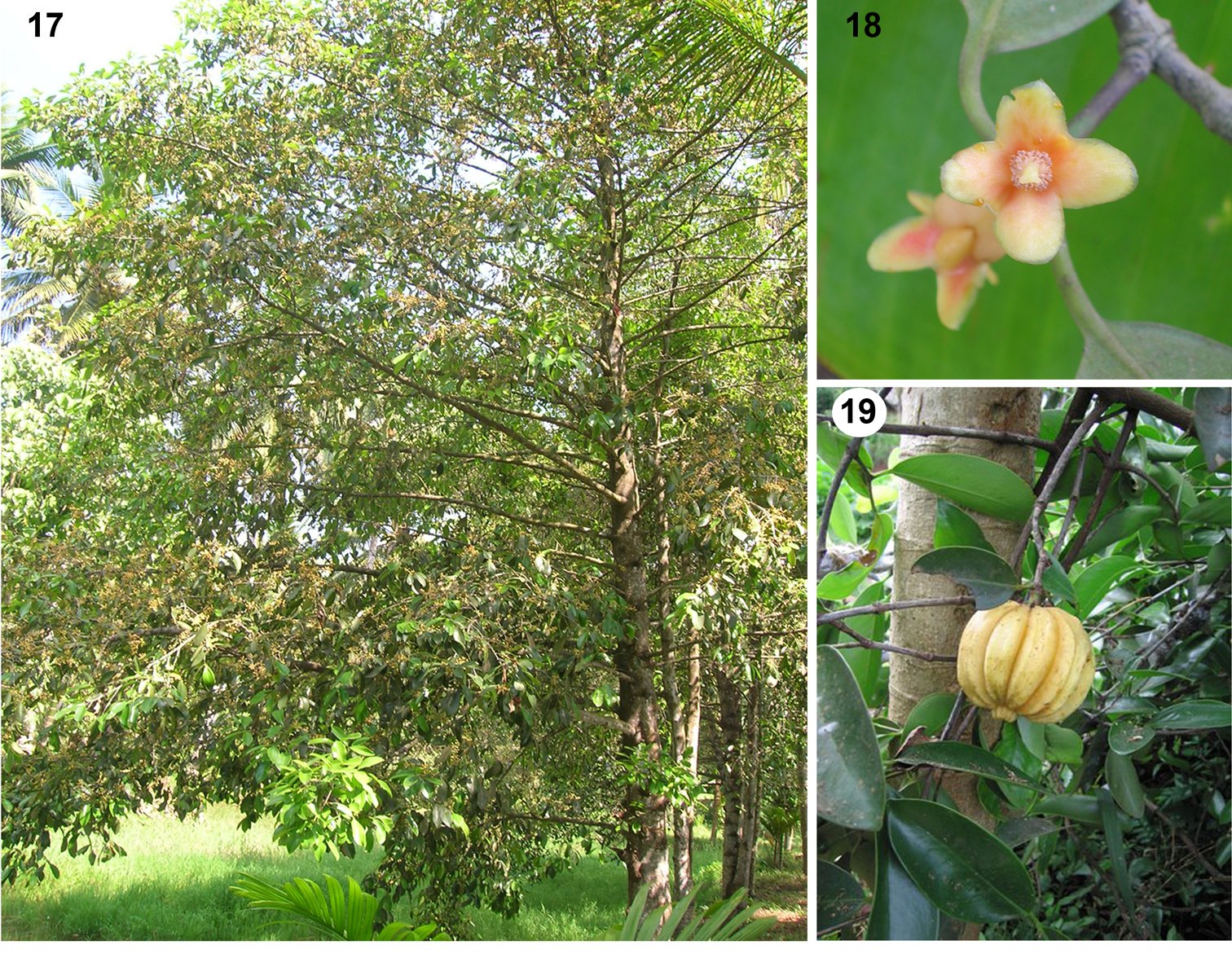
Natural history of the host plant. Garcinia gummi-gutta (Figs 17–19) grows well in the high rainfall areas of the southern Western Ghats Mountains, India. This medium-sized tree (Fig. 17), locally known as kodampuli, is found naturally along banks of rivers, lakes and inundated paddy fields, and is common in Kerala's homestead gardens, as the fruits (Fig. 19) are used in various ways (
Study of fecal coat formation. Nine laboratory-reared second and third instar larvae were washed under a very light stream of tap water and lightly brushed with a soft camel-hair brush to remove the fecal cover. Larvae thus cleaned were observed for the formation of a new fecal cover. The fecal thread was removed from the live animal and immersed in water on a slide for microscopic examination.
Tables 1 and 2. For host plants of the Blepharida-group taxa (Table 1) we incorporated many little-known articles from Indian journals and assembled host records from an extensive primary literature to collate a list that could be most valuable to the widest community of users. We assembled data on enemies for Podontia only, to aid agriculturists dealing with the defoliating effects of these species in Asia. We suspect that there may be obscure agricultural records for other Blepharida-group taxa where they are pests (e.g., Crimissa is a pest of cashew in Brazil) but such a literature survey will need collaborators involved at the local level.
Specimens. The identity of Podontia congregata was determined by examining the holotype deposited in the Natural History Museum, London, UK, with four labels: Type HT, Baly coll., Podontia congregata Baly, examined K. Prathapan, 2005. Specimen vouchers of our study are deposited in the Travancore Insect Collection, Kerala Agricultural University, Vellayani, India, and in the Snow Entomology Collection (SEMC), University of Kansas, Lawrence, U.S.A. (Voucher codes IMcsc00385–IMcsc00390). Vouchers of the bug predator, Eucanthecona parva (Distant) (Heteroptera: Pentatomidae), are deposited in the University of Agricultural Sciences, Bangalore, India, and in SEMC. Vouchers of Ooencyrtus are deposited in the Aligarh Muslim University, India, and in SEMC. Plant vouchers are deposited in the Calicut University Herbarium, Calicut, India (Accession no. 6394).
The host plant, Garcinia gummi-gutta (L.) N. Robson (Clusiaceae; kodampuli) in India. 17. Tree. 18. Flower. 19. Fruit. (Photos by D. Prathapan).
Eggs of Podontia congregata are deposited in masses (Fig. 20), usually laid in two layers at Vellayani, egg masses were observed in the field on both abaxial and adaxial surfaces of leaves. In the laboratory, the egg masses comprise 4–20 eggs, and were attached mostly on the adaxial surface. Each orange-yellow egg is oriented vertically. Eggs measure 1.82–1.92 mm long and 0.94–1.03 mm wide. About 6–7 days after oviposition, the egg coloration changes to grey brown just before hatching.
The neonate larva (Fig. 21) is lemon yellow with a dark head. Young larvae feed by scraping on the adaxial surface of the lamina (Fig. 21). Older larvae feed by cutting the leaf lamina while positioning themselves on the abaxial side of the leaf. Older larvae were observed singly on leaves, indicating a solitary nature (Figs 22–24). Larvae that are old enough to cut the leaf tend to remain on the abaxial side of the leaf. The larva with its fecal coat resembles bird droppings (Figs 22–23). The larval period varied from 18–25 days.
The larval fecal coat is formed with feces being excreted as a single thread, which is then transversely folded over the back to cover the dorsum of each larva (Fig. 25). Convulsive movements of the dorsum move it forward. The fecal thread is extruded with a glue-like, transparent material that binds the particles together (Fig. 25). When the fecal coats were removed, larvae took about 6–8.5 hours to refurbish a new coat. The coat color depends on the maturity of the leaf eaten by the larva; larvae feeding on tender leaves have a light colored, wet fecal cover, while those feeding on mature leaves have a rather dark green, apparently drier fecal coat.
Formation of pupae (Figs 27–29) was observed in the laboratory. Full fed final instar larvae shed the fecal coat and remained motionless for about 1–2 days and then assumed a C-shape with concave venter. Prior to pupation, they wriggle on wet soil that was provided in the rearing cage, creating a small depression on the surface and then gathering soil particles from around the body and manipulating these with the legs and mouthparts to form a layer covering the body. Ultimately this layer becomes an earthen cocoon roughly globular in shape (Figs 27–28). The larva never dug into soil, but always constructed the cocoon on the surface.
The adult emerged through a nearly circular exit hole. Construction of the cocoon to adult emergence took 21–24 days. The total life cycle was completed in 49–53 days. Adults (Fig. 30) lived in captivity for about 3–4 months. They feed by cutting the leaf lamina. Adults feign death and fall down (= thanatosis) or reluctantly jump when disturbed. Laboratory-reared adults released on naturally growing host plants at Vellayani were found to be less mobile. Some adults remained on the same branch for weeks and oviposited. The color pattern of adults appears to mimic bird droppings. Like larvae, adults too preferred to remain on the abaxial side of leaves.
At Vallamkulam, the insect was active throughout the year except during the dry summer months. Adult and larval presence was noticed after the onset of monsoon rains in May-June in 2008, and larvae were observed until early January 2009. Neither larvae nor adults were observed during the harsh, dry, summer months. Vellayani received the first summer rain of 9.8 mm on 13 March in 2009, and a single newly emerged adult was noticed on 15 March in the field. Two third instar larvae were observed on 11 April indicating sustenance and possible establishment of Podontia congregata at Vellayani where it was newly introduced. Six adults and several larvae were noticed on this tree during the last week of May, 2009. Two adults and three final instar larvae could be spotted after thorough checking of 14 host trees on 14 April at two spots in Vallamkulam. This indicates a similar seasonality and pre-monsoon buildup of the population in both the localities. Interestingly, the introduced Podontia congregata at Vellayani was confined to the single tree on which it was introduced, till the last quarter of 2009. There are 11 other host trees in its vicinity, with the nearest one at a distance of 19 m. Grown-up larvae were observed during December, 2009 on a second tree about 22 m away from the tree on which the beetle was first introduced. This indicates extremely slow dispersal of the insect.
At Vellayani, in 2010, the host trees put forth new flushes during the harsh summer, and all stages of the insect were active throughout the summer, without a break in activity. Diapause in Podontia congregata is probably correlated with flushing of the host tree rather than the harsh dry summer. However, the entire population mysteriously disappeared in May, indicating a probable local extinction of the species.
Nymphs of a pentatomid, Eucanthecona parva (Distant) (Heteroptera), were observed feeding on the larvae of Podontia congregata. A parasitoid was reared from the beetle eggs at Vellayani and is described as a new species, Ooencyrtus keralensis Hayat and Prathapan (Hymenoptera: Encyrtidae;
Life stages of Podontia congregata Baly in India. 20. Egg mass. 21. Gregarious instar I larva scraping leaf. 22. Instar II covered with green fecal pellets, being attacked by a juvenile predatory bug, Eucanthecona parva (Distant) (Heteroptera: Pentatomidae: Asopini). 23. Instar III larva with incomplete fecal cover and under attack by the juvenile bugs. 24. Mature larva with long fecal strands. 25. Fecal strand, immersed in water. 26. Mature larva, prior to construction of pupation chamber. 27. Pupation chamber. 28. Prepupa within pupation chamber. 29. Pupa. 30. Adult and chewing damage on leaf. (Beetle adult < 2 cm long; Photos by D. Prathapan, N. Anith).
The occurrence of Podontia congregata at Vallamkulam and Pandanad extends its range beyond the Western Ghats Mountains to the southwest plains. The absence of Podontia congregata at Vellayani in Trivandrum District, in spite of the presence of the host plant, is curious. Vellayani is only at a linear distance of about 37 km away from Ponmudi, the nearest locality where Podontia congregata was collected. There is no significant difference in altitude, vegetation, or climate between Vellayani and Pandanad or Vallamkulam, except that the rainfall is low at Vellayani (average annual rainfall of about 1833 mm) compared to Vallamkulam (average annual rainfall recorded at Thiruvalla, about 4 km north of Vallamkulam, is 2912 mm) (M. C. Kiran, pers. comm.). Low rainfall, low abundance of the host plant population, competition or poor rate of dispersal could probably explain its past absence in Vellayani.
Members of the Blepharida-group have been reported on many plant families (Table 1), but some records are questionable as they are singleton reports lacking further confirmation. For example,
At least three Podontia species are regarded as serious pests— Podontia affinis on Spondias dulcis in Indonesia, Podontia lutea on Toxicodendron vernicifluum in China, and Podontia quatuordecimpunctata on Spondias spp. At this time, Podontia congregata is a minor pest of Garcinia gummi-gutta, causing damage of little economic significance. The large size and fecundity of these species may contribute to their defoliating impacts. Documenting natural enemies as in Table 2 may be useful in finding biocontrol agents.
Species in six Blepharida-group genera are now documented with fecal retention—Blepharida (
Larvae may reduce enemy attack in several ways. Larvae which are large enough to feed by cutting the lamina position themselves on the abaxial side of the leaf and thus probably evade pouring rains as well as secure some cover from natural enemies. Young larvae prefer to feed on young, tender leaves. Older larvae feed on both light green tender leaves as well as tougher, darker green mature leaves. Fecal cover of larvae feeding on tender leaves is light green while that of those feeding on tougher mature leaves is dark green-grey, which may enhance any background camouflage effect. The fecal coats may further act as physical barriers against some predators and parasitoids. However, bugs may be specialist predators by virtue of their propensity to insert their beaks into the vulnerable ventro-lateral area of the body not covered by the fecal coat (Figs 15, 23). Host specific parasitoids, like Ooencyrtus podontiae, are also known to attack Podontia affinis (
Pupation within hard earthen cocoons is widespread among flea beetles and may reduce vulnerability to predators and parasites.
Podontia adults escape by thanatosis, whereby they fall from the foliage, remain motionless and thus disappear into the undergrowth. This defensive tactic is a widespread escape response among Chrysomelidae. Larvae appear to use an “anal extremity"to adhere to leaves (
Chrysomelids are well known for their chemical defenses (e.g.,
The monophyly of the Blepharida-group is supported by characters from host plants, beetle morphology, and behavior of all life stages (
The host plant choices of Blepharida-group species are interesting to agriculturists, foresters, anthropologists, and chemists. In Brazil, India and Thailand, the pest species on economically important plants attract agricultural interests. In China, forestry officials are concerned about damage to forests and trees used in traditional medicine. Southern African species are the source of the San's indigenous arrow poisons. The Blepharida-group is a model for research on diverse questions.
We are indebted to many individuals for their help: A.K. Pradeep, Calicut University Herbarium, Calicut, Kerala for confirming the identity of G. gummi-gutta; M.C. Kiran, Asoka Trust for Research in Ecology and the Environment, Bangalore for providing rainfall data of Thiruvalla and the map of Western Ghats; C.A. Viraktamath, University of Agricultural Sciences, Bangalore for identifying E. parva and alerting us to the case of the idiocerine leafhoppers; M. Hayat, Aligarh Muslim University for identifying the new Ooencyrtus; K.N. Anith for help with photographs of P. congregata; S.-H. Su for translating Chinese references; D. Furth for answering questions; D. Little for advice on plant nomenclature; and C.W. Shin for taking some adult habitus photographs. We are grateful to S.T Damrongsiri, V. Flinte and T. M. Tavares for permission to use their photographs, and S. Shute, Collections Manager, Natural History Museum, London for access to specimens in her care. Nithin Pradeep and Mithun Pradeep enthusiastically helped KDP in field work at Vallamkulam. We are grateful to S. Clark, F. Vencl, and two anonymous reviewers for suggestions that improved the manuscript. KDP's work on flea beetles is supported by the Kerala State Council for Science, Technology and Environment, Trivandrum. KDP is indebted to C.A. Viraktamath for guidance and support. CSC's research is supported by the University of Kansas and NSF-EPSCoR First Awards Grant # 66928.