(C) 2010 Pérez Hidalgo Nicolás. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The oriental aphid Schizaphis piricola (Matsumura) is recorded for the first time in Europe, on the ornamental pear tree Pyrus calleryana in landscaped areas in Madrid (Spain). Data on the morphology of the forms on primary host (apterous and alate fundatrigeniae and fundatrices), and their biology and distribution are given. The keys for identifying species of Schizaphis (Schizaphis) in the Iberian Peninsula are updated. Two species of aphids are also recorded for the first time on Pyrus calleryana: Schizaphis piricola and Aphis pomi.
Schizaphis piricola, aphids, adventive species, Spain
The genus Schizaphis Börner contains approximately 36 Palearctic species and 6 Nearctic ones. Is a genus resembling Rhopalosiphum Koch with little differences between both genus, and for this reason require further taxonomical and molecular study (
Twenty-seven species have been recorded in Europe (
A photograph of a colony of aphids on the pear tree of oriental origin Pyrus calleryana Decne in “Juan Carlos I park”, Barajas (Madrid, Spain) (Fig. 1)
taken on 26th April, 2009 and posted on the “Biodiversidad Virtual”
portal (http://www.biodiversidadvirtual.org/) enabled the oriental
species Schizaphis piricola
(Matsumura) to be detected for the first time in Europe. Its presence
was confirmed in a study of samples collected the following spring on
the same host and in the same place. Its route of entry into Europe is
probably linked to when the host plant was imported, as is the case of
many other species introduced into Europe (
This finding is yet another example of how social
networks play an important role in our knowledge of biodiversity and the
detection and/or monitoring of invasive or endangered species (
Samples containing several apterae (3 fundatrices and 15 fundatrigeniae, measured) and alatae (15 measured) were collected between 11th April and 4th May, 2010 in “Juan Carlos I park”, Barajas (Madrid, Spain) [40°28'12.77"N, 3°35'6.22"W] (reference M-222). Several populations were also located on the same host on 7th May, 2010 in Torrejón de Ardoz (Madrid, Spain) [40°27'23.17"N, 3°28'3.02"W] (M-224) and at the “Vallecas Villa” railway station (Madrid, Spain) [40°22"6.23"N, 3°37'1.15"W] (M-225) on 17th May, 2010. These samples are deposited in the aphid collection of the University of León, Spain and the samples of associated ants in the Collection of the Universidad Autónoma de Barcelona, Spain (Dr. Xavier Espadaler).
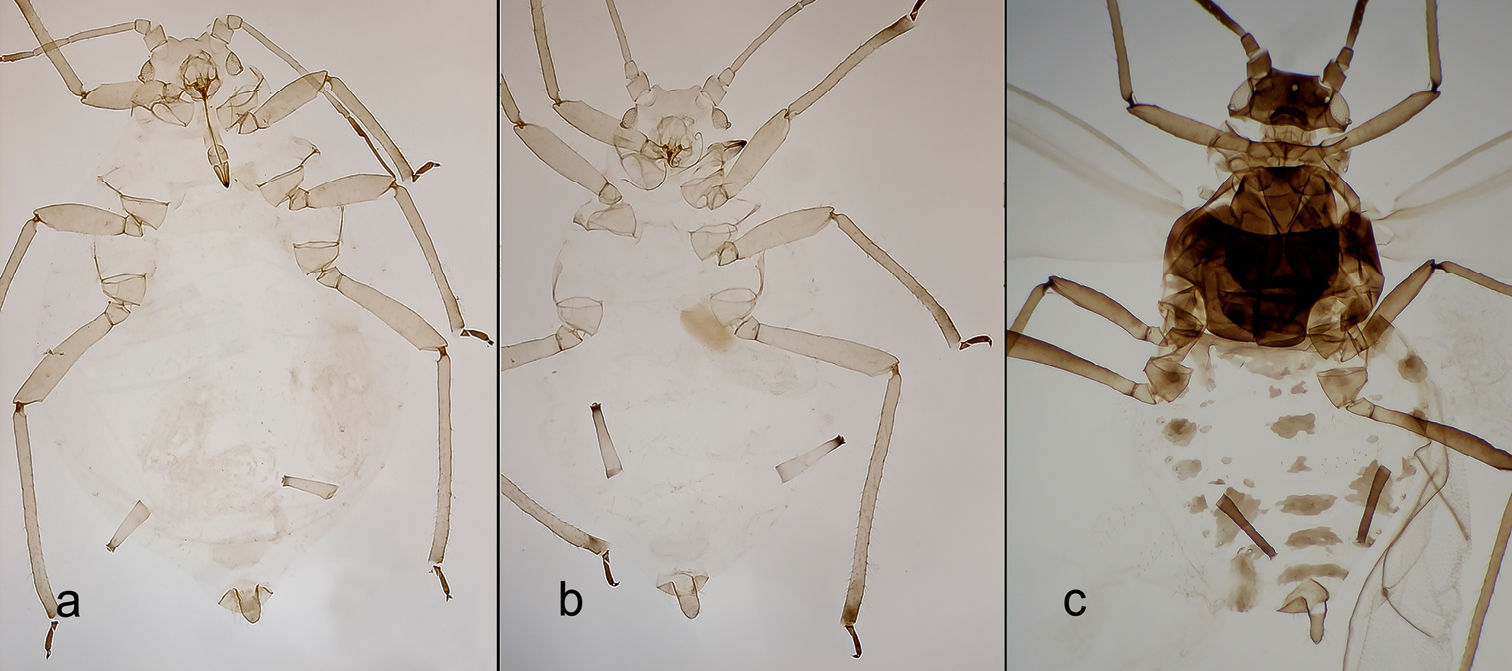
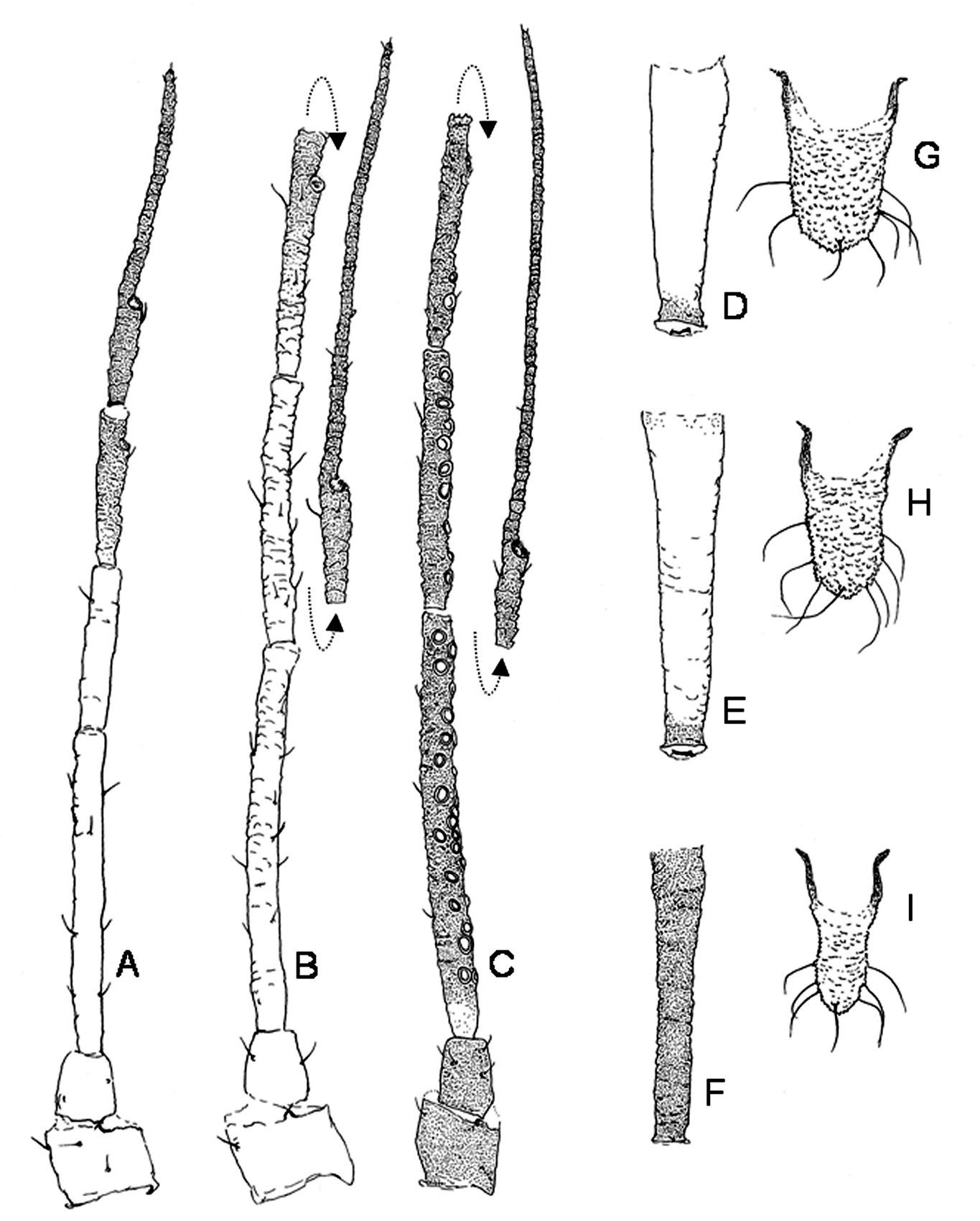
Description of the forms of Schizaphis piricola on primary hostThe apterous fundatrigeniae (Figs 1C, 2B, 3B, E, H) are between 1.47 and 2.50 mm long and yellowish green to green, with pale antennae and siphunculi bearing dark tips and dark tarsi. Antennae 0.65 to 0.90 times the body; processus terminalis of antennal segment VI 3.13 to 3.92 times its base, and 0.97 to 1.13 times antennal segment III. Antennae without secondary rhinaria. Apical rostral segment 0.90 to 1.09 times second segment of posterior tarsi and usually with 2 accessory setae. Dorsal sclerotization absent. Marginal papillae on abdominal segments I to VII, absent on III to V in some specimens. Dorsoabdominal setae of anterior terguites shorter (17 to 30 μm) than those of posterior (55 to 90 μm), ventral setae intermediate in size (45 to 60 μm). Siphunculi cylindrical, with weakly defined subapical constriction, 0.12 to 0.19 times body and 1.84 to 2.12 times cauda. Cauda 0.17 to 0.19 mm, 1.21 to 1.46 times basal width, bearing 7 to 9 setae. Tibiae of posterior legs 0.44 to 0.50 times body.
The fundatrices (Figs 2A, 3A, D, G) resemble the apterous fundatrigeniae except for the characteristics of the “fundatrix facies” (
Alatae fundatrigeniae (Figs 1D, 2C, 3C, F, I) 2.10 to 2.52 mm, green, antennae and siphunculi dark, cauda lighter in colour. Well-pigmented marginal sclerites in terguites II to IV, postsiphuncular sclerites fully developed and spinopleural pigmented bands always present on abdominal segments VI to VIII and also occasionally on III to V. Antennae 0.71 to 0.80 times body; processus terminalis of antennal segment VI 3.41 to 5.18 times base; antennal segments III, IV and V bearing 15–32, 7–18, 0–6 secondary rhinaria, respectively. Siphunculi 1.68 to 1.80 times cauda. The remaining characters are similar to those of the apterae.
Pyrus calleryana with fruit (A), malformation caused by Schizaphis piricola (Matsumura) on Pyrus calleryana leaves (B), apterous fundatrigeniae (C) and alate fundatrigeniae (D) of Schizaphis piricola, the arrows indicate the tips of the two branches of the medial vein.
Fundatrix (A), apterous fundatrigenia (B) and alate fundatrigenia (C).
Antennae (A, B, C), siphunculi (D, E, F) and cauda (G, H, I) of fundatrix (A, D, G), apterous fundatrigenia (B, E, H) and alate fundatrigenia (C, F, I) of Schizaphis piricola (Matsumura).
Schizaphis piricola (Matsumura) is an aphid of oriental origin which, according to
Nevertheless, it is possible that Schizaphis piricola is now more widely distributed because Pyrus calleryana
is a very commonly planted ornamental tree species. For example, in
the United States there is evidence that this tree species is rapidly
becoming invasive in much of its horticultural range (
It is a holocyclic dioecious species with species of pear tree (Pyrus sp.) as its primary host and Cyperaceae (Carex spp. and Cyperus rotundus L.) as secondary host (
In spring in Spain, colonies of this species cause the leaves of Pyrus calleryana to curl (Fig. 1B) as occurs in Pyrus pyrifolia in Japan, Korea and China (
The direct action of sucking by the aphids (clearly seen in the curling of the leaves), and indirect damage caused by the honeydew they excrete, which covers the leaves, can affect the normal growth of the trees, all the more so if other aphid species (Aphis pomi De Geer 1773 and Dysaphis sp.) sometimes forming mixed colonies with Schizaphis piricola, are present.
The trees of Pyrus calleryana in “Juan Carlos I park” (Barajas, Madrid, Spain), which have been monitored more carefully, were planted two years ago and do not seem to have reached the height and size expected for this species. In any case, a more in-depth study of the population dynamics and auxiliary fauna (coccinellidae, syrphids, etc…) is necessary, taking into account other variables (humidity, temperature, etc…), to be able to reach conclusions on possible damage.
Identification keysThe following keys enable all the species in the subgenus Schizaphis recorded in the Iberian Peninsula to be separated.
Species key (apterae viviparae females)| 1 | Siphunculi pale with pigmented apex (Figs 2A, B, 3D, E) | 2 |
| – | Siphunculi entirely dark | 4 |
| 2 | Siphunculi 0.8 times cauda at most. Usually on Phalaris arundinacea | Schizaphis (Schizaphis) longicaudata |
| – | Siphunculi at least 0.9 times cauda. On many species of Gramineae and on Pyrus calleryana | 3 |
| 3 | Siphunculi 1.1 to 1.6 times cauda. On many species of Gramineae | Schizaphis (Schizaphis) graminum |
| – | Siphunculi 1.82 to 2.1 times cauda in apterous fundatrigeniae (Figs 2B, 3E) and 1.47–1.53 in fundatrices (Figs 2A, 3D). On Pyrus calleryana (primary host) | Schizaphis (Schizaphis) piricola |
| 4 | Processus terminalis of antennal segment VI 3.6 to 4.5 times its base. Siphunculi 2.5 times cauda at most. Marginal papillae on abdominal segments I, VI and VII. On Pyrus communis (primary host) or Ciperaceae (secondary host) | Schizaphis (Schizaphis) pyri |
| – | Processus terminalis of antennal segment VI 4.7 to 6.8 times its base. Siphunculi at least 2.5 times cauda. Marginal papillae on abdominal segments I and VII only. On Cyperus and seldom other plants | Schizaphis (Schizaphis) rotundiventris |
| 1 | Siphunculi pale with pigmented apex | 2 |
| – | Siphunculi entirely dark (Figs 2C, 3F) | 3 |
| 2 | Siphunculi 0.8 times cauda at most. Normally on Phalaris arundinacea | Schizaphis (Schizaphis) longicaudata |
| – | Siphunculi at least 0.9 times cauda. On many species of Gramineae and on Pyrus calleryana | 3 |
| 3 | Siphunculi same size as cauda. Bearing 4–10, 0–4 and 0–1 secondary rhynaria on antennal segments III, IV and V, respectively. On many species of Gramineae | Schizaphis (Schizaphis) graminum |
| – | Siphunculi (Figs 2C, 3F) 1.6 to 1.8 times cauda. Bearing 18 to 32, 7–18 and 2–6 secondary rhynaria on antennal segments III, IV and V, respectively. On Pyrus calleryana (primary host) | Schizaphis (Schizaphis) piricola |
| 4 | Siphunculi 0.1 times body at most and 1.5 to 1.8 times cauda. Processus terminalis of antennal segment VI 3.6 to 5.0 times its base and 1.1 to 1.6 times antennal segment III, and approximately 2.0 times siphunculi. On Pyrus communis (primary host) and Ciperaceae (primary host) | Schizaphis (Schizaphis) pyri |
| – | Siphunculi at least 0.1 times body and 1.1 to 3.0 times cauda. Processus terminalis of antennal segment VI 4.7 to 6.0 times its base and 1.3–1.8 times antennal segment III. On Cyperus | Schizaphis (Schizaphis) rotundiventris |
The authors would like to express their gratitude to Xavier Espadaler (Unidad de Ecología y CREAF, Universidad Autónoma de Barcelona) for identifying the ants associated to this species. This study was carried out as part of the “Proyecto Fauna Ibérica IX” (CGL2007-66786-C08-03).