(C) 2010 Suphan Karaytug. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Male and female of Odaginiceps korykosensis sp. n. (Copepoda, Harpacticoida, Tetragonicipitidae), collected in the intertidal zone of Kızkalesi beach along the Mediterranean coast of Turkey (Mersin Province), are described. The new species is the fifth member of the genus and can easily be distinguished from the other species by the presence of four setae/spines on the second endopodal segment of P4 and by the structure of the caudal rami. Previously, representatives of the genus Odaginiceps have been reported from Gulf of Mexico, off Bermuda and Kenya. Odaginiceps korykosensis sp. n. is the first record of the genus in the Mediterranean Sea.
Harpacticoida, Tetragonicipitidae, Odaginiceps, taxonomy, new species
The genus Odaginiceps Fiers, 1995 is one of the 12 genera currently recognized in the family Tetragonicipitidae and now comprises four species (
Samples were collected using the Karaman-Chappuis method (
Some physical and chemical parameters of interstitial water on different sampling dates at the type locality.
| Date | 09 April 2007 | 27 July 2007 | 26 November 2007 |
|---|---|---|---|
| pH | 8.16 | 7.29 | 8.14 |
| Temperature (°C) | 18.3 | 29.8 | 19.1 |
| Conductivity (ms) | 45.7 | 57.1 | 54.0 |
| Salinity (ppt) | 34.5 | 37.8 | 35.5 |
| Oxygen (mg/L) | 2.78 | 1.11 | 1.71 |
Order Harpacticoida Sars, 1903
Family Tetragonicipitidae Lang, 1944
Genus Odaginiceps Fiers, 1995
urn:lsid:zoobank.org:act:8EF0600A-D85B-4032-9661-26A514C0ADDD
Figs 1– 6Turkey, Mediterranean coast, Mersin Province; intertidal zone of Kızkalesi beach (36°27.473'N; 34°08.647'E). The type locality is fine sand beach.
: Holotype ♀ dissected on seven slides. Allotype ♂ dissected on six slides. Legs. S. Karaytuğ, S. Sak, A. Alper and S. Sönmez.
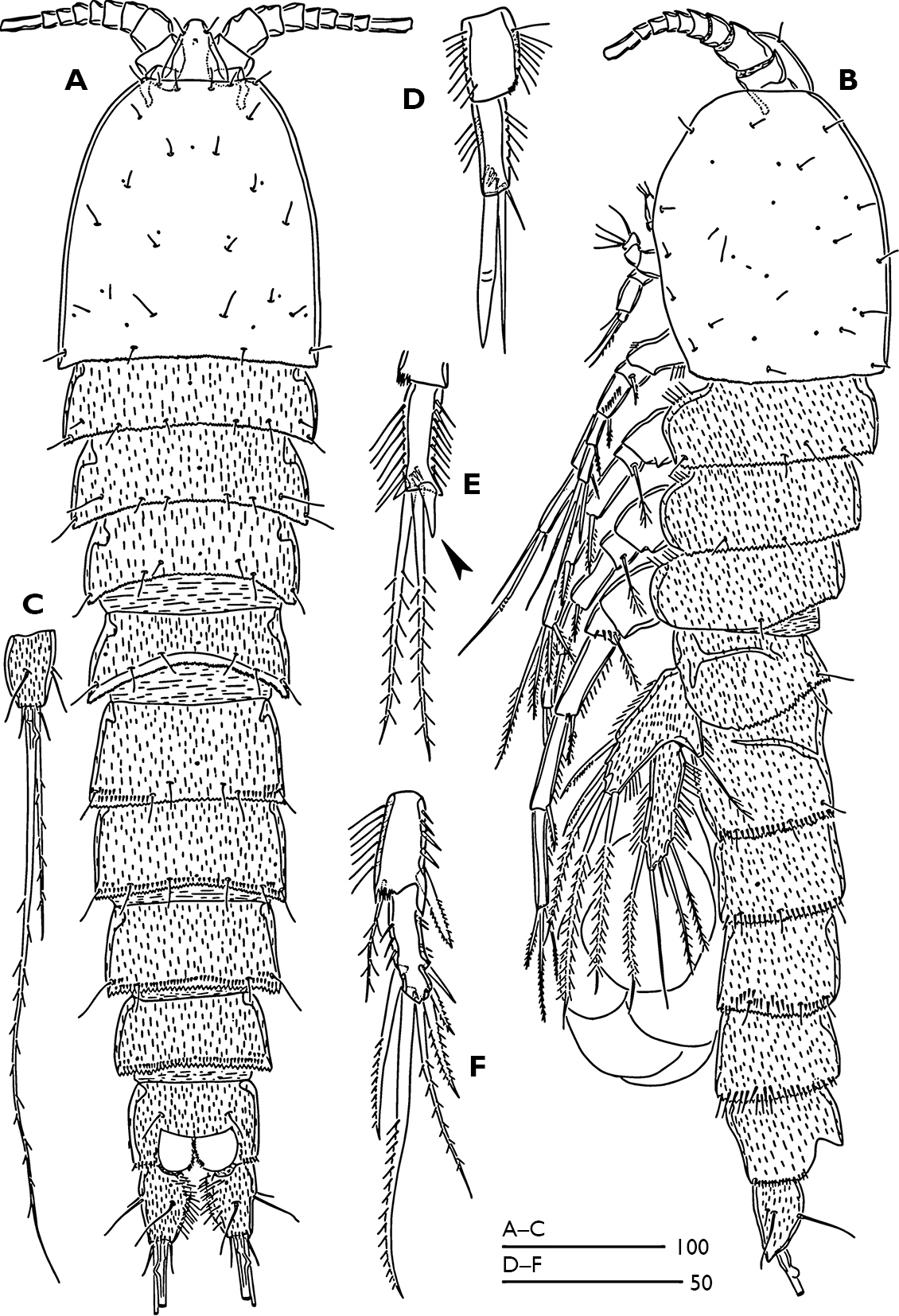
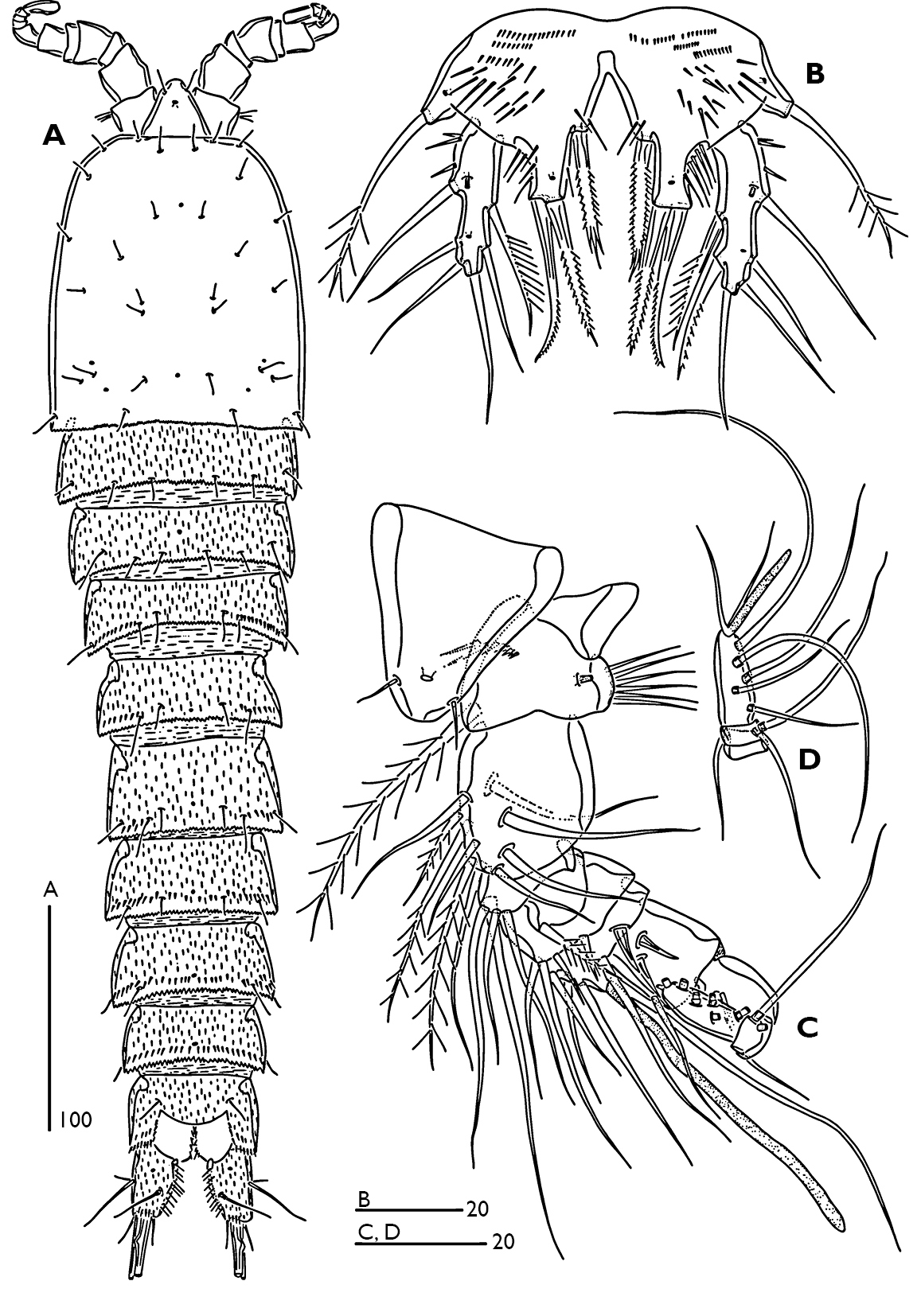
. Female (Fig. 1A, B).Total body length 770 µm, with largest width measured at cephalothorax. Integument of cephalic shield smooth, of all other somites ornamented with irregular pattern of hardly visible spinules. Body surface with sensilla pattern as figured. Posterior margin of the body somites with serrate hyaline frills.
Rostrum (Fig. 1A) large, widest at base, extending halfway along second antennular segment; with two delicate sensillae and a mid-dorsal pore.
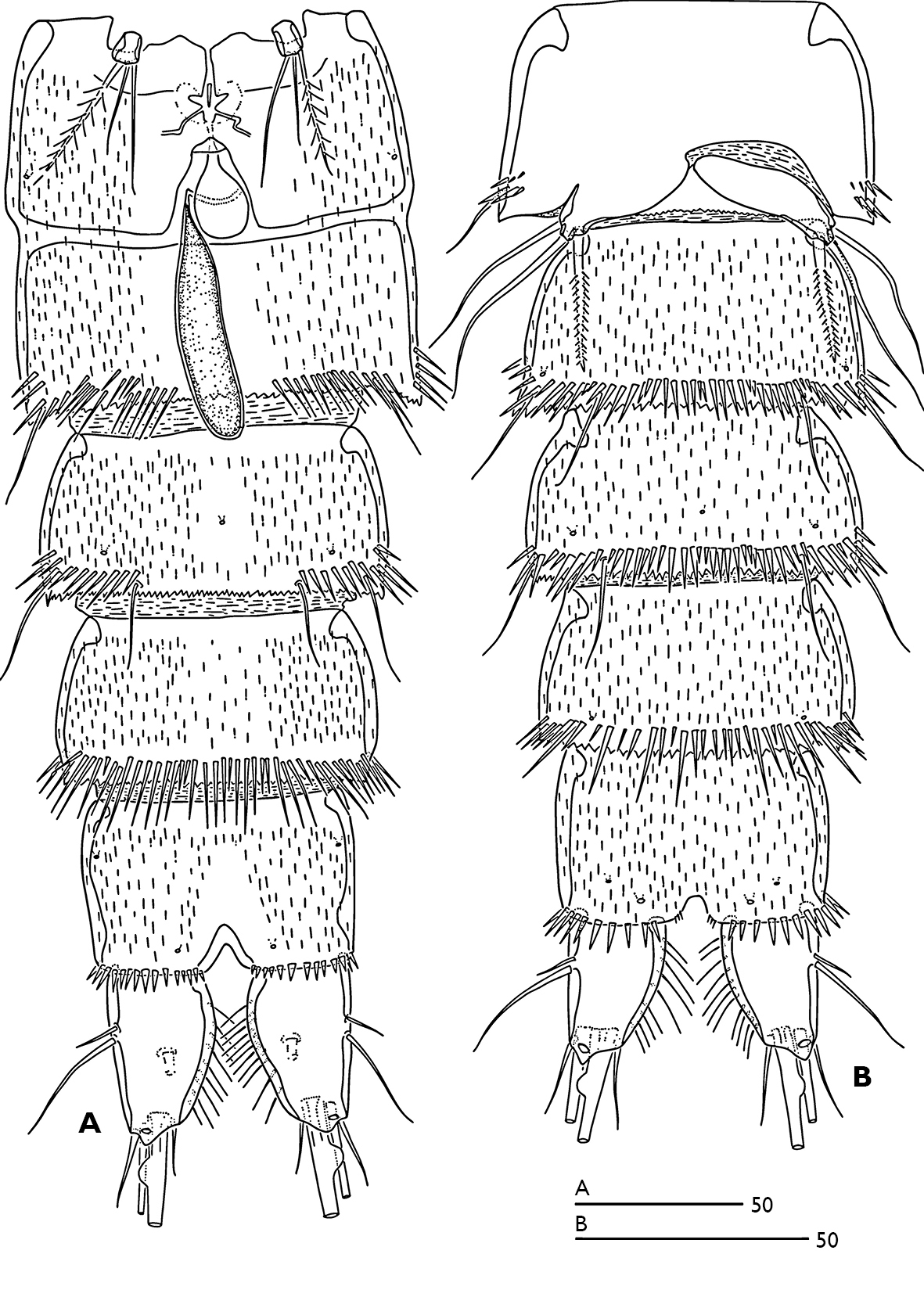
Urosome (Figs 1A, B) 5-segmented, comprising P5-bearing somite, genital double somite and three free abdominal somites. Genital double-somite longer than wide; with transverse surface ridge dorsally and laterally (Fig. 1B) extending ventrally (Figs 1A; 2A), indicating original segmentation. Genital field (Fig. 2A) with small copulatory pore located in median triangular depression. A spermatophore attached to the copulatory pore. First and second somite of genital double-somite and second and third abdominal somites with continuous spinules near distal margin dorsally (spinules of first somite of genital double-somite interrupted midway) (Fig. 1A); genital double somite and second abdominal somite with spinular row near distal margin ventrally, interrupted midway; third abdominal somite with continuous spinular row near distal margin ventrally (Fig. 2A). Anal somite (Fig. 1A) with distal spinular row extending dorsally to the either side of anal operculum; operculum smooth, slightly convex.
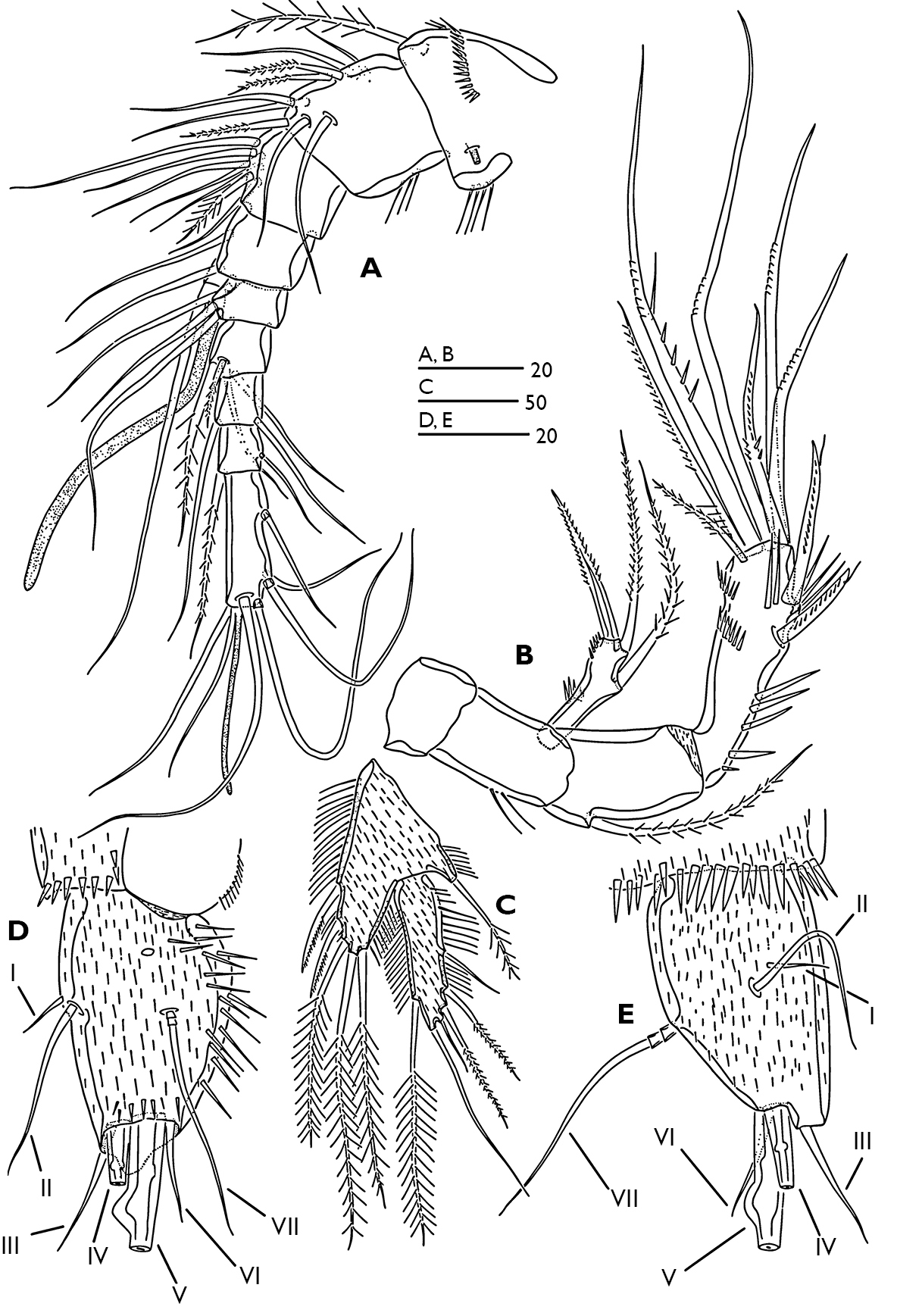
Caudal rami (Figs 1C; 3D, E) tapering posteriorly with 4–5 dorsal spinules distally near the base of seta V; 1.7 times longer than wide; inner margin ornamented with spinules (Fig. 3D); with a pore on proximal third of dorsal surface (Fig. 3D), another pore present on ventral surface near the base of seta III (Fig. 2A); with seven setae (Fig. 3D, E), seta I minute located near the base of seta II; setae II–III bare; setae IV–V strongly developed and bipinnate; seta V with swollen sinuate base; seta VI short and as long as seta III; seta VII tri-articulated.
Antennule (Fig. 3A) 9-segmented; segment 1 with a long plumose seta at anterodistal margin, a spinular row on anterior surface, long spinules along inner margin, small tube-pore on dorsal surface near inner margin. Segment 2 with long spinules on caudal margin. Segment 4 with long aesthetasc fused basally to seta. Segment 9 longest, bears an apical acrothek consisting of a short aesthetasc and two setae. Armature formula 1-[1 plumose], 2-[6+3 plumose], 3-[6+1 plumose], 4-[3+(1+ae)], 5-[2], 6-[2+2 plumose], 7-[2], 8-[1+1 plumose], 9-[5+acrothek].
Antenna (Fig. 3B). Coxa small and smooth. Basis with 2 long spinules at outer margin. Exopod 1-segmented with one lateral pinnate seta, apical armature consists of one pinnate seta and one pinnate spine; a few spinules present around outer distal corner and midway along outer margin. Endopod 2-segmented; first endopod segment with one plumose seta at proximal third of outer margin. Distal endopod segment with various spinular rows as figured and with two abexopodal unipinnate spines laterally (both spines with subapical tubular extension). Apical armature of enp-2 consisting of two pinnate setae, and five geniculate setae; longest geniculate seta with large spinules and fused at base to long pinnate seta.
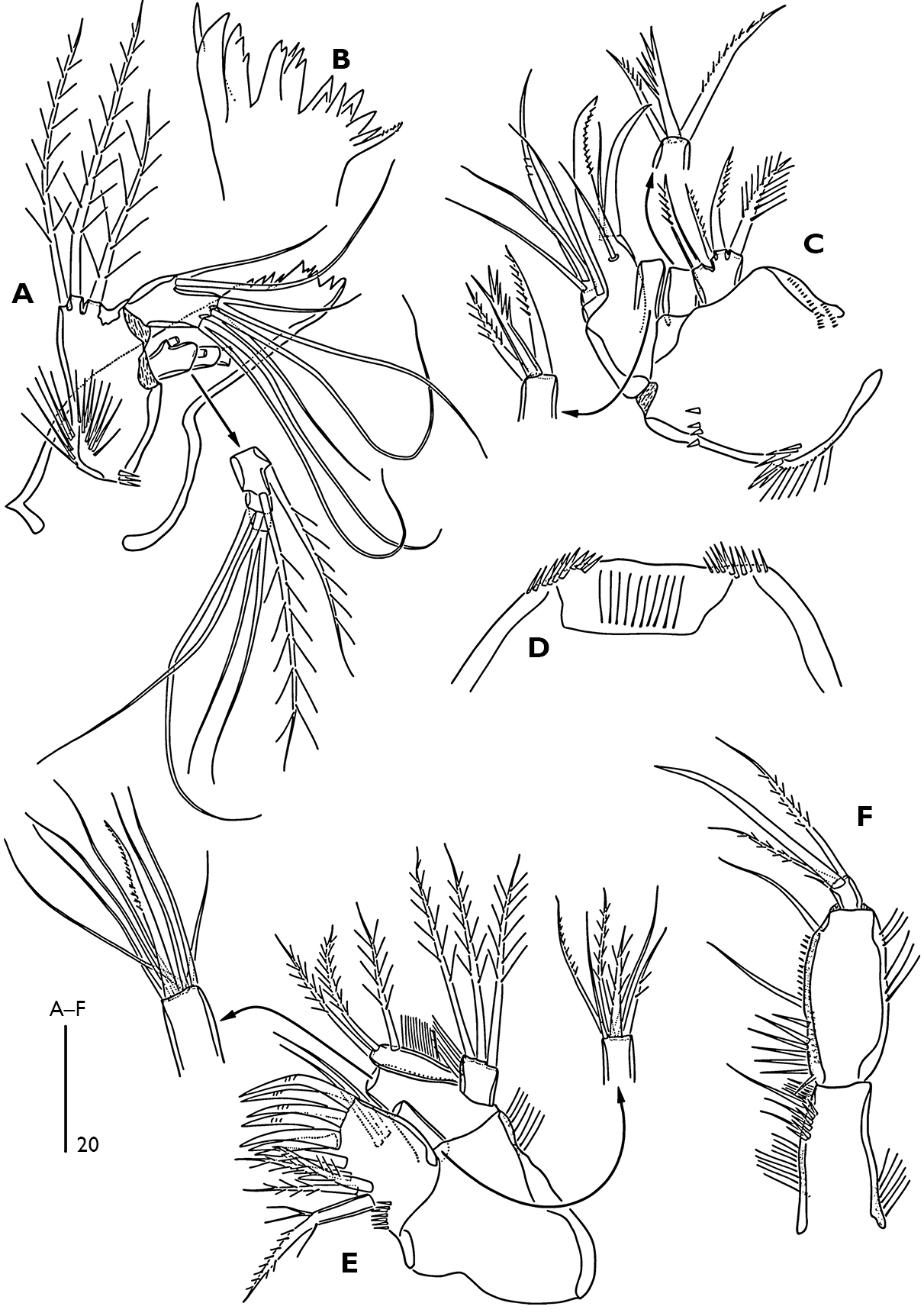
Labrum (Fig. 4D). Free margin straight, with spinular row at distal corners and fine spinular row subdistally on ventral surface.
Mandible (Fig. 4A, B). Coxa robust, gnathobase with one pinnate seta at dorsal corner and several blunt multicuspidate teeth along distal margin. Palp biramous; basis strong, with three plumose setae along inner margin, ornamented with a group of long spinules proximally. Exopod 3-segmented, first segment with two plumose setae, second segment with two bare setae and third segment with two bare setae fused at base. Endopod 1-segmented with two lateral and six distal bare setae (two inner distal setae and two outer distal setae fused at base).
Maxillule (Fig. 4E). Praecoxal arthrite with seven spines around distal margin, with spinules as figured; anterior surface with two bare setae; posterior surface with three plumose and one pinnate setae. Coxal endite with two smooth and four plumose setae. Basis with seven bare and one unipinnate setae. Long endopod segment and square exopod segment each with three plumose setae. Endopod and exopod with a row of fine marginal setules.
Maxilla (Fig. 4C). Syncoxa ornamented with spinules as figured; with three endites. Proximal endite with one plumose and three unipinnate setae; middle endite with three unipinnate setae; distal endite with two unipinnate and one plumose setae. Allobasis drawn out into pinnate claw; accessory armature consisting of two bare setae and one curved spine. Endopod 2-segmented; proximal segment with one bare seta, distal segment with one geniculate and two bare setae.
Maxilliped (Fig. 4F). Subchelate and ornamented with spinular rows as figured. Syncoxa with three inner bare setae subdistally. Basis with two bare setae along inner margin. Endopod with one small accessory seta, one bare and two plumose setae.
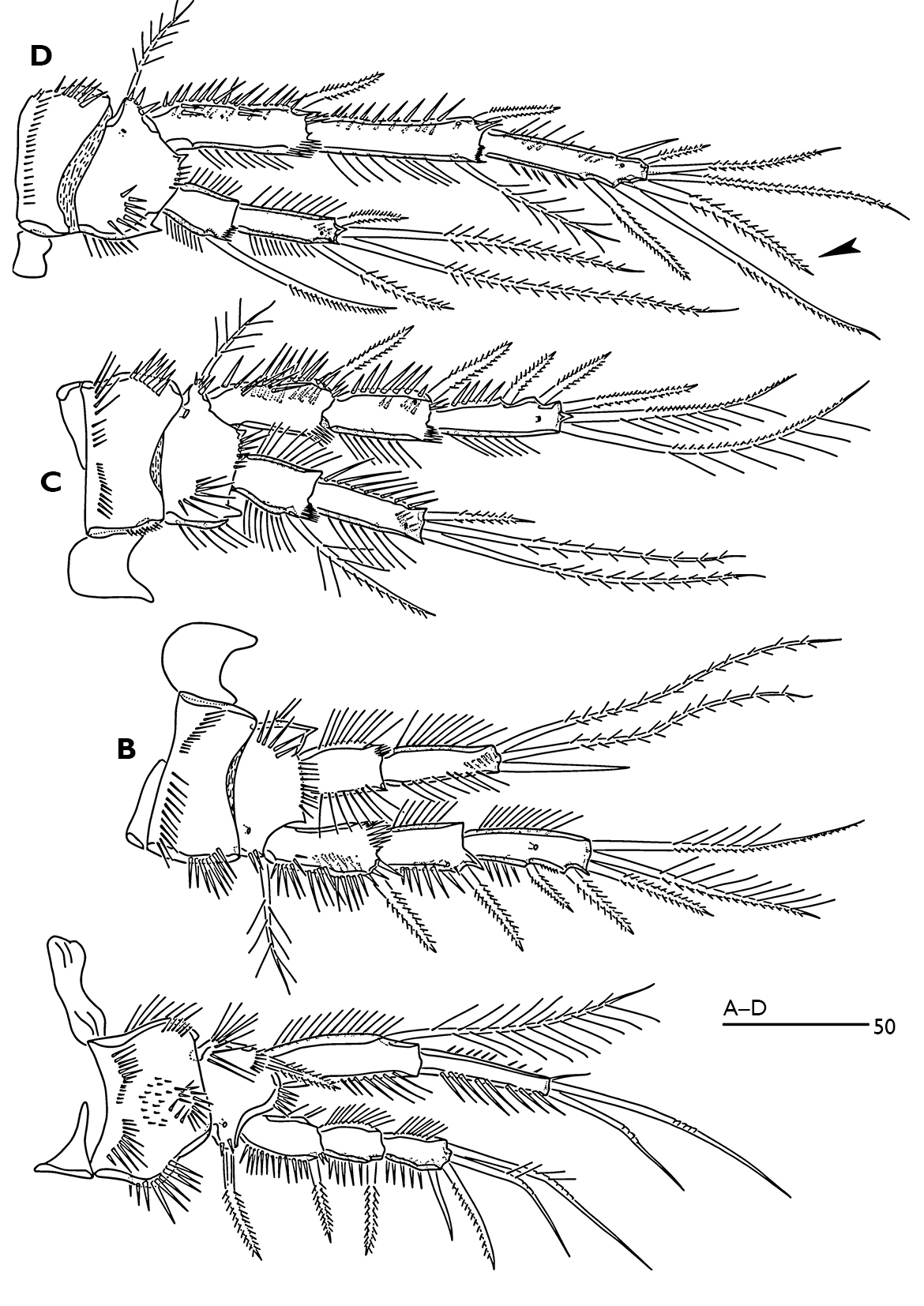
P1 (Fig. 5A). Intercoxal sclerite rectangular and smooth. Small praecoxa triangular and bare. Coxa with complex spinular ornamentation anteriorly as figured. Basis narrower than coxa; anterior surface with pore near the base of outer bipinnate spine; inner side with long slender spinules. Exopod 3-segmented, segments with spinular rows along inner and outer margins. Endopod 2-segmented; enp-1 reaching almost middle of exp-3, with spinular row along inner and outer margins; long inner seta plumose and located subdistally; enp-2 slightly shorter than enp-1, with spinules along inner and outer margins and with two long articulated setae and one small inner apical seta.
P2–P4 (Fig. 5B–D). Intercoxal sclerite unornamented. Coxa and basis with complex spinular ornamentation as figured. Exopod 3-segmented. Endopod 2-segmented. Endopodal and exopodal segments with spinular rows along inner and outer margins. Exp-1 of P2-P3 without spinules along inner margin. Enp-1 of P2 without inner seta. Terminal outer seta of P2 enp-2 bare. P4 exp-2 with one plumose inner seta. With a pore on anterior surface of enp-2 and anterior surface of exp-2 and -3. Exp-1 and -2 (and -3 in P4) with a posterior spinule patches. Enp-2 with a posterior spinular row subdistally.
Armature formula of swimming legs:
| P1 | P2 | P3 | P4 | ||||
|---|---|---|---|---|---|---|---|
| Exp. | Enp. | Exp. | Enp. | Exp. | Enp. | Exp. | Enp. |
| 0.0.022 | 0.120 | 0.0.023 | 0.021 | 0.0.023 | 1.021 | 0.1.322 (♀) | 1.121 |
| 0.1.222 (♂) | |||||||
P5 (Fig. 3C). Baseoendopod and exopod covered with fine spinules on anterior surface, with long slender spinules along inner and outer margins. Exopod 3.6 times longer than wide, with 6 setae; seta 1 longest and plumose; seta 3 smallest and bare. Baseoendopod longer than wide; with 2 unipinnate and 3 plumose setae.
P6 (Fig. 2A) represented by a small segment with one outer plumose seta and two slender bare setae.
Odaginiceps korykosensissp. n. Female. A habitus, dorsal B habitus, lateral C right caudal ramus with terminal complement, dorsal. Male. D P2 endopod, anterior E P3 terminal endopod segment, anterior F P4 endopod, anterior.
Odaginiceps korykosensis sp. n. A female, abdomen, ventral B male, abdomen, ventral.
Odaginiceps korykosensis sp. n. Female. A antennule, dorsal B antenna C P5, anterior D left caudal ramus, dorsal E right caudal ramus, lateral.
Odaginiceps korykosensis sp. n. Female. A mandible B distal margin of gnathobase C maxilla, posterior D labrum, ventral E maxillule, anterior F maxilliped, posterior.
Odaginiceps korykosensis sp. n. Female. A P1, dorsal B P2, anterior C P3, anterior D P4, anterior.
Odaginiceps korykosensis sp. n. Male. A habitus, dorsal B P5, anterior C rostrum and antennule, dorsal D terminal segments of antennule.
Male (Fig. 6A). Total body length 510 µm. Body smaller and more slender than female, largest width measured at midway of cephalothorax. Body ornamentation generally as in female. Sexual dimorphism observed in antennule, P2-P6 and genital segmentation.
Antennule (Fig. 6C, D) indistinctly 10-segmented, sub-chirocer. Segment 1 short, with small tube-pore on dorsal surface and with long spinules along caudal margin. Segment 2 longest. Segment 4 with partial suture line dorsally. Segment 5 with long aesthetasc fused basally to seta. Segment 10 bears an apical acrothek consisting of a short aesthetasc and two slender setae. Armature formula 1-[1 plumose], 2-[7+3 plumose], 3-[4], 4-[4+1 plumose], 5-[4+1 spine+(1+ae)], 6-[1+2 spines], 7-[2], 8-[1], 9-[2], 10-[5+acrothek].
P2 enp-2 (Fig. 1D); outer terminal spine more robust than female; middle terminal seta bare, shorter than female and as long as outer spine; inner terminal seta minute. P3 enp-2 (Fig. 1E); inner terminal seta modified to a short spine (arrowed in fig. 1E). P4 exp-3 (Fig. 1F) with 6 setae, inner terminal seta of female (arrowed in fig. 5D) absent in the male.
P5 biramous (Fig. 6B), fused medially. Baseoendopod ornamented with patch of spinules as figured; with two pores (one near the base of outer basal seta and the other near the base of inner terminal spine of endopodal lobe); endopodal lobe with one lateral and two distal spines. Exopod with three outer bare setae, 1 terminal bare seta and two inner unipinnate setae; with two pores (one tube pore near the base of outer proximal bare seta and the other near the base of outer median bare seta). P6 vestiges asymmetrical (Fig. 2B); each P6 with one plumose inner seta and two long bare setae.
The specific name refers to “korykos” which is the historical name of Kızkalesi province (Mersin, TURKEY).
The new species can be attributable to the genus Odaginiceps
by the absence of inner seta on the proximal segments of P2-P4 exopod
and P2 endopod, the short second antennulary segment, the large
prominent rostrum, the presence of pinnate setae on the second and
third antennulary segments, and the two-segmented P1 endopod with three
armature elements on the second segment (
The new species lacks a seta (arrowed in the Fig. 5D) on the third exopodal segment of the P4 in the male. The absence of this seta in the male supports the assumption of
Note on the ecology and distribution. Examination of the extensive interstitial samples
taken from almost all sandy beaches along the Turkish coasts revealed
that the new species occurs at the type locality only. On the other hand
three samples taken from the type locality in different seasons
revealed no true interstitial forms. The absence of true interstitial
forms but the presence of Odaginiceps korykosensis sp. n. in the type locality can be explained by the very low oxygen levels measured at the type locality (Table 1).
Most harpacticoids are sensitive to reduced oxygen supply, which
restricts their occurrence to the upper sediment layers and favours
epibenthic life. It can be discerned from the general body shape of Odaginiceps spp. (see Fig. 1A, B;
This study was funded by TÜBİTAK under the project number 106T590. We also would like to thank Serdar Sönmez for his help in collecting the material.