Additional moults into ‘elongatus’ males in laboratory-reared
Polydesmus angustus Latzel, 1884 (Diplopoda, Polydesmida, Polydesmidae) – implications for taxonomy
Introduction
In millipedes (Diplopoda), post-embryonic development occurs basically by anamorphosis, i.e. the number of body rings is small at birth (e.g., four podous rings bearing three pairs of legs, two apodous rings, plus the telson) and increases progressively at each moult. However, the relationships between developmental stages (= stadia) and sexual maturity vary greatly depending on the order (Enghoff et al. 1993). In most millipede orders, adults occur in several stadia within a species, either because the stadium at which maturity is reached varies among individuals, or because adults undergo further moults. In addition, adults in a given stadium often have a variable number of body rings (e.g. in Julida, Polyzoniida, Spirobolida, Spirostreptida). By contrast, development is much more canalized in the order Polydesmida, in which there is generally only one adult stadium in each species, with a fixed number of body rings (Mesibov 2011). In most polydesmidan species, adults are in stadium VIII, with the head, 18 podous rings, 1 apodous ring and the telson (in abbreviated form: 18+1+T); in other species, adults are in stadium VII (17+1+T); in a few species, the stadium and ring number of adults differ between the sexes, but with no intra-sex variation. In spite of obvious exceptions, e.g. in the genus Devillea Brölemann, 1902 (Xystodesmidae), in which adults probably occur in several stadia (Enghoff et al. 1993), the number of body rings in the adult is considered as fixed in most Polydesmida and is sometimes used in taxonomy (e.g. Djursvoll et al. 2000).
Surprisingly, Verhoeff (1916, 1928) reported the occurrence of an additional moult in a number of male and female Polydesmus complanatus (Linnaeus, 1761) (Polydesmidae) reared in the laboratory. Whereas adults of this species are normally in stadium VIII (18+1+T), some individuals were observed in stadium IX with a further podous ring (19+1+T). Verhoeff (1928) was convinced that the existence of these ‘elongatus’ specimens shed light on the evolution of ring numbers in millipedes and used it as an argument in his controversy with Brolemann (1921) over elongation vs. contraction. However, this additional moult in a polydesmid has sometimes been regarded sceptically, and Enghoff et al. (1993: p. 153) did not exclude the possibility of Verhoeff’s mistakes in the counting of rings. More recently, intraspecific variability in the ring number of adult males was demonstrated in another polydesmidan millipede, the pyrgodesmid Muyudesmus obliteratus Kraus, 1960 (Adis et al. 2000).
Herein, the reality of the phenomenon observed by Verhoeff in Polydesmus is confirmed for the first time since 1928. During laboratory studies on another, closely related species, Polydesmus angustus Latzel, 1884, two cases of moult into stadium IX were observed in males. In the present paper, we first describe the conditions under which these moults occurred and some characteristics of the males before and after moulting, we briefly discuss possible underlying mechanisms, and then highlight implications of intraspecific variability in ring number for the taxonomy of Polydesmida.
Material and methods
Study species
The flat-backed millipede Polydesmus angustus is widespread in north-western Europe, west of the range of Polydesmus complanatus (Kime and Enghoff 2011). Its post-embryonic development consists of eight stadia, which can be determined by counting the number of body rings, and the sexes can be distinguished from stadium IV onwards (Enghoff et al. 1993). Towards the end of each immature stadium, millipedes build a chamber made of earthy faecal material, in which they coil during moulting. The last stadium is the adult (18+1+T), which dies after the breeding season. The life cycle is completed in either one or two years depending on the individuals. Under mean seasonal conditions, development time from egg hatch to adult emergence lasts about 10 months for annual individuals, plus a further 3 months in aestivation for biennial individuals (David et al. 1999).
Laboratory rearing
During experimental studies on the biology and ecology of Polydesmus angustus, hundreds of specimens were reared throughout their life cycle in the laboratory. Broods that were produced by adults from a field population living at Brunoy, 20 km south-east of Paris, were kept in lidded, transparent plastic boxes containing 1 cm of sieved soil and moist leaf litter. The boxes were placed in incubators fitted with a glass door and exposed to natural daylight. Temperature followed the mean monthly temperatures of the region of origin, with a daily thermoperiod of 4°C (David et al. 1999). The young from each brood were reared in groups up to stadia IV–V, sexed and then kept at a low population density to be monitored individually. Some individuals were fed on leaf litter alone throughout development, while others received a monthly pinch of dry yeast in addition to leaf litter, which greatly improves growth and female fecundity (David and Celerier 1997). Under those laboratory conditions, additional moults were observed in two males, one of which was reared with yeast and the other without.
Results
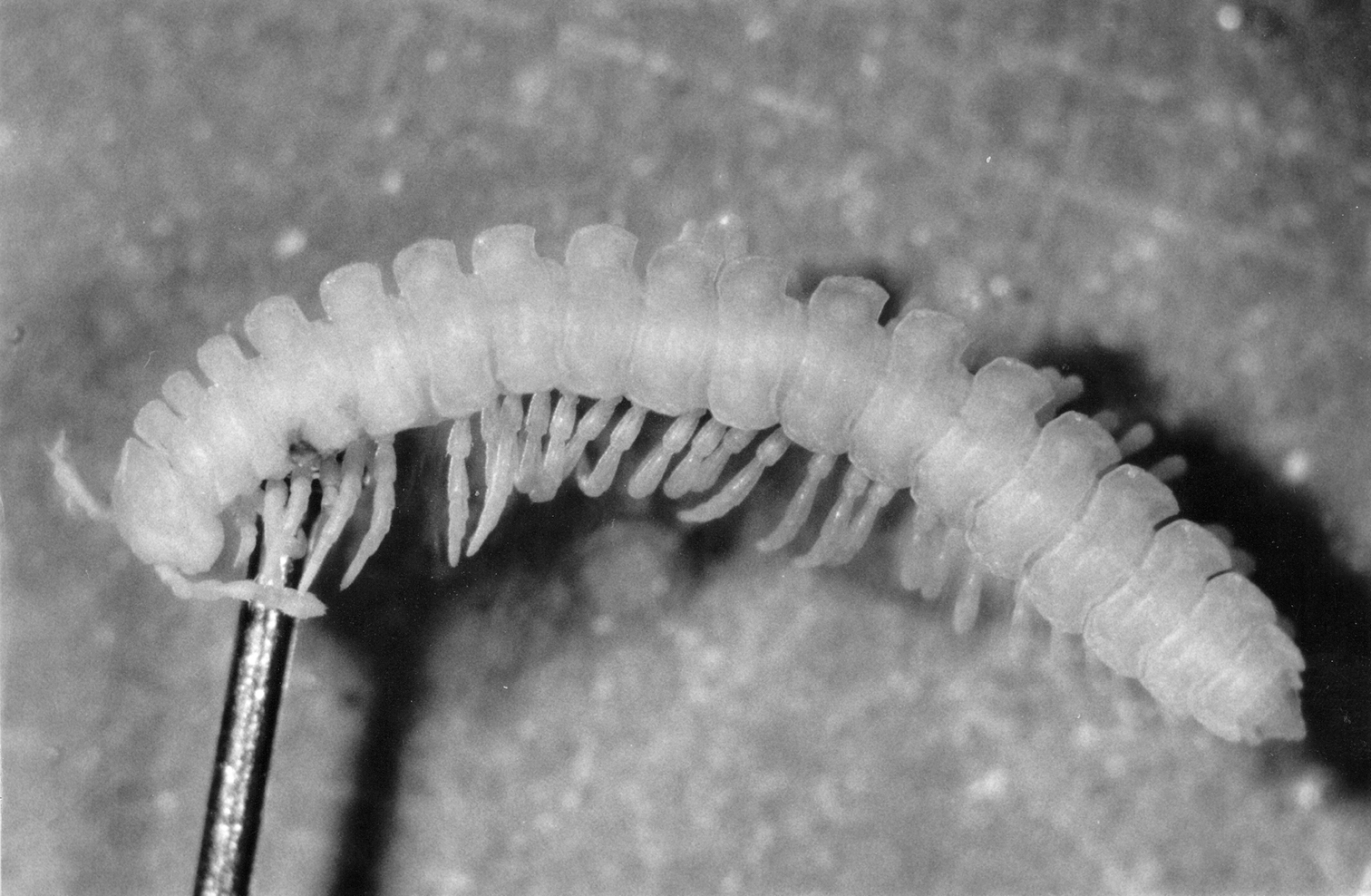
The first ‘elongatus’male hatched in late August from eggs produced by field-collected parents (first generation in the laboratory). It was fed on leaf litter without dry yeast and emerged as a small adult (stadium VIII) in mid-September, at the age of 12 months. Its live weight was 15.3 mg, which is usually the weight of a stadium VII specimen in the field. The 8th leg-pair was transformed into apparently normal, well-developed gonopods. The male was left unmated and received a pinch of yeast in early October. It coiled into a moulting chamber on October 27th and then emerged as stadium IX (19+1+T) on November 18th. Its live weight was 22.4 mg. Although the examination of the exuvia confirmed that its gonopods were fully formed in stadium VIII, with all the characteristics of Polydesmus angustus, they were transformed regressively at stadium IX. The coxites were strongly developed, the coxal hooks clearly visible, but the telopodites were even more simplified and atrophied than in the figures shown by Verhoeff (1928: p. 691). This type of additional moult with regression of gonopods is similar to the ‘regressionis’ form described by Verhoeff (1916). This ‘elongatus’ male overwintered a second time and died in late March (Fig. 1).
The second male hatched in June from eggs produced by laboratory-reared parents (second generation in the laboratory). It was fed on leaf litter supplemented with yeast and emerged as stadium VIII on May 3rd, at the age of 10 months. It had the normal number of rings (18+1+T) but was extremely small. Its live weight was 9.2 mg, which is usually the weight of a stadium VI specimen in the field. It had no gonopods, the 8th leg-pair being replaced by small buds, as is usually the case in immature males of stadium VII. This male coiled into a moulting chamber in mid-May. Although it was accidentally frozen before emergence due to an incubator failure, the moulting process had already reached an advanced stage. An examination of the specimen dead before emergence clearly showed that it was about to give a male in stadium IX (19+1+T) with 32 leg-pairs and developed gonopods. This type of additional moult with appearance of gonopods is similar to the ‘progressionis’ form described by Verhoeff (1916).
Figure 1.
Dorsal view of a Polydesmus angustus male of stadium IX after its death at the age of 19 months. The trunk consists of 19 podous rings – bearing 32 pairs of legs plus regressed gonopods (8th leg-pair) – and one apodous ring anterior to the telson.
Discussion
It is clear that the number of stadia is not completely fixed in millipede species of the family Polydesmidae. Additional moults described by Verhoeff (1916, 1928) in Polydesmus complanatus have been confirmed in adult males of Polydesmus angustus. In the latter species, it is a very rare phenomenon, which was observed in only two males and no females. Nevertheless, two modalities corresponding to Verhoeff’s ‘regressionis’ and ‘progressionis’ forms have been confirmed, which lends considerable credibility to all the results reported by Verhoeff (1928). This also suggests that the additional moults briefly mentioned by Stephenson (1961) in a number of males of Brachydesmus superus Latzel, 1884 from the field, may not necessarily be due to confusion of species.
Possible underlying mechanisms
In this study, the two ‘elongatus’ males with 19 podous rings were obtained under controlled laboratory conditions and did not experience environmental stress in terms of temperature, humidity and photoperiod. However, an obvious difference between these males and normally developing animals was their small body size at stadium VIII. Similarly, the first ‘elongatus’ specimens obtained by Verhoeff (1916) were from a poorly-fed brood that yielded very small adults (the so-called ‘forma nana’), and most of those he obtained later were less than 20 mm long at stadium VIII (Verhoeff 1928). Body size may thus be critical for the appearance of ‘elongatus’ specimens. A small size can result from poor quality food (David and Celerier 1997), although this is not a valid explanation for the smaller ‘elongatus’ male obtained in Polydesmus angustus, which was reared on leaf litter supplemented with yeast. In an experiment with a species of the order Spirobolida in which the mode of anamorphosis is very different from that of Polydesmida, Berns and Keeton (1968) showed that semi-starvation resulted in smaller juveniles, and these underwent a greater number of moults before maturity than well-fed individuals.
Hormone imbalance is undoubtedly involved in the occurrence of additional moults, and body size may play a role in this respect. Although little is known about the hormonal control of development in millipedes (Descamps et al. 1990; Hopkin and Read 1992), hypotheses based on knowledge from other arthropod classes can be put forward. In insect species with variable numbers of instars, such as the Lepidoptera Manduca sexta (Linnaeus, 1763) (Sphingidae) and Malacosoma disstria Hübner, 1820 (Lasiocampidae) (Kingsolver 2007; Etile and Despland 2008), further larval moults occur only in small individuals, because attainment of a critical weight is the signal that stops juvenile hormone production (Davidowitz et al. 2003). A similar mechanism can be hypothesized for the additional moult in the ‘progressionis’ form of Polydesmus, which is typically the occurrence of a further immature stadium during development.
The ‘regressionis’ form in males is more difficult to interpret. In this case, small males reach stadium VIII with the normal number of rings and well-developed gonopods. However, according to Verhoeff (1928), these males are unable to breed because other adult characters are missing, especially the male openings on the coxae of the second pair of legs. These characters appear in stadium IX, while the gonopods are transformed regressively. A mixture of development and regression of secondary sexual characters during the additional moult is difficult to interpret in terms of hormonal control.
It is clear, however, that the additional moult with regression of gonopods in Polydesmus is not akin to periodomorphosis (Verhoeff 1923). This phenomenon, which is well known in the order Julida, involves the appearance of intercalary males with regressed sexual characters between two adult stadia (Enghoff et al. 1993). If Polydesmus males in stadium VIII are not sexually mature despite their well-developed gonopods, their moult to stadium IX is by no means the first stage in periodomorphosis (Verhoeff 1928; Sahli 1968). While some secondary sexual characters regress, others develop in stadium IX, so that ‘regressionis’ males are quite different from true intercalary males. Moreover, the next stage in periodomorphis, i.e. a further moult into mature males of stadium X, has never been observed in Polydesmus.
Taxonomic implications
The confirmation that additional moults can occur in Polydesmida, the post-embryonic development of which is generally assumed to consist of a fixed number of stadia, has implications for taxonomy. In Polydesmus, adults with a further body ring are quite capable of surviving and Verhoeff (1928) even showed that ‘elongatus’ females of Polydesmus complanatus were able to breed. Although such individuals have not been reported so far in field populations of Polydesmus, they might be encountered besides normally developing adults under specific ecological conditions, especially in populations composed of small individuals. Clearly, it is biologically possible. Therefore polydesmidan millipedes that have very similar characteristics, particularly the male gonopods, but different numbers of rings in the adult, might belong to the same species. They should not be automatically classified as distinct genera. For example, Demange (1970) stressed that the polydesmids Brachydesmus proximus Latzel, 1889 (17+1+T) and Polydesmus geochromus Attems, 1952 (18+1+T) have virtually identical gonopods; the paradoxosomatids Paradoxosoma granulatum Daday, 1889 (17+1+T) and Trachydesmus simonii Daday, 1889 (18+1+T) also have virtually identical gonopods. Distinctions at the generic level for such closely related organisms were criticized (Jeekel 1968; Demange 1970), and the latter author correctly argued that these differences in ring number could reflect environmentally induced variation within a single species. In the recent scientific literature, Shelley (2000) chose the option to classify adult males with 17 and 18 podous rings in the same sphaeriodesmid species, Desmonus pudicus (Bollman, 1888). At most, such differences could reflect speciation, assuming that each ring number has already been fixed in reproductively isolated populations. But the number of rings as such should no longer be viewed as a character of generic importance in Polydesmida. Its use in phylogenetic analyses, working on the assumption that this character shows no intraspecific variation (Djursvoll et al. 2000), perpetuates divisions which may be artificial, such as the genera Brachydesmus (17+1+T) and Polydesmus (18+1+T).
Conclusion
Enghoff et al. (1993) coined the term teloanamorphosis to describe a mode of anamorphosis in which the number of moults and the number of rings added at each moult are fixed within each species (or each sex of a given species). In millipedes, this type of post-embryonic development is characteristic of the orders Chordeumatida and Polydesmida, with some interspecific variation in the number of stadia to maturity. However, there appears to be a difference between the two orders. Whereas no additional moults have ever been mentioned in the Chordeumatida, the data available to date show that there are various degrees of intraspecific variability for the stadium number in Polydesmida, at least in Devillea, Muyudesmus and Polydesmus, and possibly in other genera mentioned above. The number of body rings in the adult appears therefore to be of more limited taxonomic value in this order.