(C) 2010 Zhiliang Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Omophorus Schoenherr, 1835 is recorded for the first time from the Oriental Region and a new subgenus and species, Omophorus (Sinomophorus subgen. n.) rongshu sp. n. is described from Yunnan province (P.R. China). The new subgenus differs from the subgenus Omophorus by the longer antennal club, the bifid vestiture of the ventral parts, the elongate subtrapezoidal scutellum, the very small size of sclerotizations in the endophallus, the absence of styli in the ovipositor and the absence of the spiculum ventrale on the VIII female sternite, and from the subgenus Pangomophorus Voss, 1960 by the developed metatibial uncus and the lack of a subhumeral tubercle. A detailed description and figures are provided to allow interpretation of characters in ongoing phylogenetic analyses.
Weevils, morphology, systematics, phylogeny, Sinomophorus, new species, Yunnan, China
The superfamily Curculionoidea Latreille, 1802 is a very speciose group of Coleoptera, and probably the family Curculionidae
Latreille, 1802 (in its different limits) the most speciose in the
Animal Kingdom; weevils, as they are known, currently comprise some
62, 000 known species and an approximate analysis ranks the number of
total species close to 220, 000 (
The tribe Metatygini was named by
The discovery of a new member of this tribe in the collections of the Institute of Zoology of the Chinese Academy of Sciences (IOZ-CAS), that we consider a new species of Omophorus, and a second representative of this tribe in China, leads us to the following description.
Materials and methodsSpecimens of a new species were found while sorting out
and identifying specimens in the collection of IOZ-CAS (Beijing, P.R.
China). Some of them were dissected after soaking them overnight in
lukewarm soapy water and later rinsed with de-ionized water. Lukewarm
10% potash solution overnight was used for digestion of soft tissues.
One defective male was completely dissected and mounted to study and
photograph notal areas, metendosternite, wings, terminalia and
genitalia. Description of these parts follows
The holotype of Omophorus rongshu has not been dissected to avoid damage, since there were enough specimens collected with it. The holotype and some paratypes have been unpinned and glued to bristol pointcards, their parts mounted in DMHF (5, 5-dimethyl hydantoin formaldehyde resin) in acetate cards pinned under each specimen. Other paratypes are as they were mounted after collecting, pinned through the right elytron base.
Descriptions were made using a binocular Zeiss Stemi SV11. Photographs were taken with a CCD Qimagine MircoPublisher 5.0 RTV mounted on a Zeiss SteREO Discovery V.12. Extended focus images were generated with Auto-Montage Pro 5.03.0061 and edited with Adobe Photoshop CS 5.0 if required. Microscopic slides were studied under a Leica DM 2500 microscope and photos were taken with a Nikon CoolPix 5400.
Label data are given as they are (in Chinese), with pinyin romanization and comments in square brackets; labels are separated by semicolons and lines by slashes.
Taxonomic treatmentOmophorus stomachosus Boheman, 1835. For a complete synonymy, check Appendix I. This genus includes two subgenera: Omophorus s.str., with five species showing an Afrotropical distribution and Pangomophorus Voss, 1960, with a single species described from Papua New Guinea. A new subgenus and species from China are described below:
urn:lsid:zoobank.org:act:D1F53C57-971E-40DF-9105-DE5096B54ADC
Omophorus rongshu sp. n.
It differs from subgenus Omophorus s.str. by the longer antennal club, the bifid vestiture of the ventral parts, the elongate subtrapezoidal scutellum, the very small size of sclerotizations in the endophallus, the absence of styli in the ovipositor and the absence of a spiculum ventrale on the VIII female sternite, and from subgenus Pangomophorus by the metatibial uncus developed and the lack of a subhumeral tubercle.
From Latin Sina (China) and genus Omophorus. Gender masculine, as Omophorus.
urn:lsid:zoobank.org:act:9173392E-0DAE-421C-A8CF-EF0E39C1C871
Figs 1–23(holotype, except where indicated). Measurements (in mm): Standard length: 4.95. Rostrum: length: 1.08, maximum width: 0.43. Pronotum: median length: 1.09, maximum width: 2.01. Elytra: median length: 4.10, maximum width: 3.70.
Integument. Reddish brown, base and apex of elytra, a broad sutural band, venter of femora, parts of meso- and metasternum and their pleurites, and part of the 1st ventrite dark brown; apical margin of 2nd-4th ventrites and the whole 5th, antennal scape and funicle testaceous.
Vestiture on elytra of scarce, short, very fine, pale piliform scales, most numerous on extreme base of 2nd-3tr interstriae, on head and pronotum formed by the same scales, but denser and thicker, creamy to tan, on disc directed cephalad, on sides directed to midpoint of pronotum; on legs as on head and rostrum, but larger and whiter; on ventral areas, coxae, trochanters and ventral part of femora, vestiture of bifid piliform scales, each scale with a common stalk hardly longer than basal width of each branch. Sclerolepidia absent. Remnants of waxy-pulverulent, creamy to tan exudate in some parts of elytra and pronotal base.
Rostrum (Figs 3–4) 0.99 × as long as pronotum, in dorsal view 2.51 × as long as wide, with apex slightly bisinuate, sides subparallel, densely punctate, except smooth and shiny apical tenth, metarostrum with a median sulcus prolongated basad to hind margin of eye, ending apically at antennal insertion level; in side view metarostrum dorsally depressed, prorostrum a little narrowed to apex, ventral margin straight, forming a soft curve with underhead. Scrobes dorsally visible at apex, in side view almost straight, deep, narrow, upper margin directed towards lower angle of eye, but not reaching it, becoming obsolete, lower margin convergent with upper and evanescent; scrobes in ventral view not convergent, obsolescent against under margin of eye. Underside of rostrum densely punctate, with three low keels, the lateral ones ending at insertion level, the median one distally fusing with the submentum. Labium ca. 1.5 × as long as wide, sides weakly rounded, medially longitudinally impressed, apically truncate to weakly emarginate, 1.5 × as long as labial peduncle, labial palps invisible; maxillary palp 3-segmented. Mandibles tridentate, overlapping. No postmandibular sensory setae.
Head in dorsal view subglobular, densely and subrugosely punctate, medially sulcate up to occiput, frons narrow, ca. 0.5 × as wide as rostral apex, in side view frons weakly convex; eyes moderately large, slightly convex, ca. 1.5 × higher than long, transversely elliptical, some 16 ommatidia in longitudinal diameter.
Antennae inserted at basal 0.49 of rostrum, with scape 5.58 × as long as wide and 1.09 × as long as funicle, slightly clubbed and bent at apex, glabrous except pubescent apical club, 1st desmomere 1.7 × as long as wide, obconical, well separated from 2nd, this also obconical and 1.56 × as long as wide, but slightly narrower than 1st and 0.82 × as long as it, desmomeres 3tr–6th 2.0 × as wide as long, tightly packed and increasing in width apicad, 7th annexed to club, 3.0 × as wide as long and covered with a denser vestiture than others; club of 3 segments, suture between 1st and second obliterated, almost invisible, that between 2nd and 3tr narrow but visible, so that apparently the club is bisegmented, last segment 1.3 × as long as remainder of club, whole club 2.54 × as long as wide, 1.69 × as long as funicle.
Pronotum in dorsal view 1.85 × as wide as long, its base 1.79 × as wide as apex, bisinuate, with marginal keel, median lobe weakly and widely emarginate, surface densely punctate at base and on disc, punctures oblong, ca. 60 μm long, separated half their diameter or less, on sides and apical third smaller, 30 μm or less, widely spaced, a small median smooth and shiny tubercle just behind the faint collar constriction. Basal angles rounded-subtruncate.
Mesonotum (Fig 18). Scutellum 0.82 × as long as wide, large, trapezoidal, wider at base, sides sinuate, apex subtruncate, slightly bituberculate, each tubercle with short setae, surface densely and rugosely punctate, punctures as small as those on apex of pronotum. Prephragma well developed, strongly prominent, antecostal sutures straight, meeting at middle in an obtuse V, mesoscutum smaller than scutellum. Axillary cord laterally prominent as a round lobe, joining in a soft curve the postero-lateral margin of mesonotum.
Metanotum (Figs 14–15). In general shape, anterior part forming a vertical wall with a very narrow, transversal opening. Anterior margin of prescutum with a pair of prominent teeth and a deep median notch. Antero-medial margin of allocrista rounded. Scutellar groove wide, its anterior margin widely open with a small elevation on each side, without anterior bridge, with a median longitudinal obtuse elevation, not keeled. Metascutum about as large as metascutellum, separated from it by a straight postero-medial margin. Postero-lateral margin of metanotum with a strong, laterally and slightly caudally pointing tooth, and a round lobe in front of it bearing two teeth. Postnotum small, strongly transverse, well separated from metascutum and metascutellum.
Elytra (Fig 1)
1.11 × as long as wide, base of interstriae 2–4 convergent and
prominent cephalad, covering base of pronotum, humeri moderately
convex, sides of elytra widening from under the calli, widest at
middle, uniformly rounded to apex, apices very narrowly rounded
separately, sutural angle small, subacute. Striae 10, widely sulcate,
with rows of small inner punctures, forming irregular aggregates
separated by bridges uniting interstriae, giving a moderately foveolate
aspect, each puncture with a subarcuate seta hardly as long as half
the interstrial ones, and finer; interstriae weakly convex, subequal
in width, with 2–3 irregular rows of extremely minute punctures (less
than 6 μm in diameter), surface almost smooth, weakly microreticulate,
first interstria strongly microreticulate, transversally rugose,
more convex behind scutellum. Striae at apex join 1+10, 2+9, 3+8,
4+5, 6+7, 10th marginal and obsolete in apical fourth, with a strong
outer keel in basal ¾, 11th interstria completely facing ventrally from
base up to level of abdominal suture III; at base, 1st shortened and
reaching the level of apical third of scutellum, 2nd-4th convergent
towards basal lobe of elytron. Internal submarginal fold well developed,
strongly curved. Sutural flanges dissimilar, that on the left elytron
wider, in basal part turned vertical, that on the right elytron
narrower, parallel to elytral surface in its whole length. Underside
with small, inconspicuous superficial file of stridulatory system type 1
(
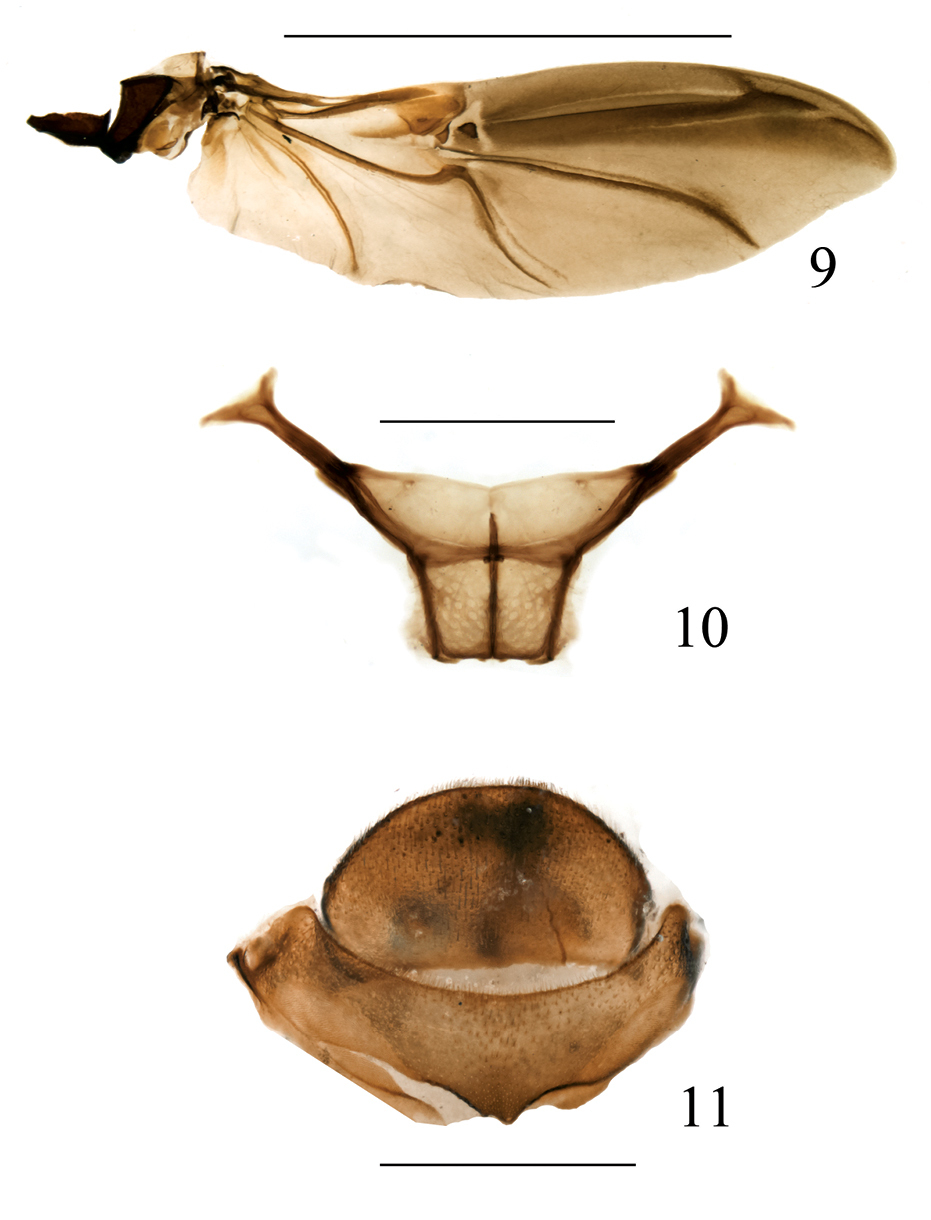
Metathoracic wing (based on 2 paratypes) (Fig. 9). A typical curculionid wing, ca. 3.1 × as long as wide, with the surface covered with microtrichia. C, Sc and R without special characters. Rr undifferentiated in the apical half, rm and rms appearing as a single unit, rfi, rcm and rc forming a single plate in which rcm is weakly distinguishable as a small elongate trait. 1rs and 2rs form separate plates, united posteriorly to rsc. R3 present, thin, incomplete; anterior stripe thinner than the postradial stripe. M1 thin, parallel to mst, and joining it near apical third of mst length. Cu uniting to Cu1 with a strongly arched loop, Cu1 following to near margin as af. 1A1 and 1A2 absent. A strong, weakly arched, not reaching margin, 3A very weak, stripe-like, not closing ac. Basal sclerites: Br and Bsc fused.
Ventral area. Procoxal cavities separated from front margin of prothorax a distance twice that separating them from hind margin. Mesosternum very short, vertical in front, forming a steep angle in side view; meso-metasternal median suture not evident, replaced with a weak transverse sulcus; mesepisternum fused to mesosternum, mesosternal suture indicated by a small depression; mesepimeron triangular, fused to mesepisternum, mesopleural suture visible, anteriorly deepened, not functional. Metasternum fused medially to mesosternum, suture not visible, replaced with a weak sulcus, this joined in middle by a median longitudinal sulcus running from base to apex of metasternum, deeper at apex; a prominent transverse tubercle in front of metacoxae, forming a vertical step; metepisternum elongate-trapezoidal, apical angles a little produced laterally, the upper angle fitting in a small notch on the elytral costal margin, basal margin rounded; metasternal suture visible, complete, functional; metepisternum much reduced, covered by elytron. Abdomen (Fig 12) with 2nd ventrite as long as 3tr medially, 4th shorter (0.9 ×), 5th 1.19 × as long than 4th and 4.97 × as wide as long, 1st ventrite 1.94 × as long as second in median length, 1.11 × as long as 2nd in postcoxal length. First ventrite strongly raised across basal margin, 5th weakly depressed across middle. Suture I deep, weakly bisinuate, sutures II-IV functional, straight, slightly curved backwards at outer angles.
Metendosternite (based on 1 paratype) (Fig 10): Stalk weakly trapezoidal and transverse, anterior part of longitudinal flange shorter than posterior, hemiductus robust, projection as long as wide, furcal arms long, apically clearly bifurcate, anteroventral branch shorter than laterodorsal one. Insertions of anterior tendons at the same distance as angles formed by margins of stalk and sheath.
Legs. Procoxae contiguous, subconical. Mesocoxae separated by a distance 1.4 × the mesocoxal diameter. Mesocoxae and metacoxae separated 0.8 × the mesocoxal diameter. Metacoxae separated 0.5 × the distance between mesocoxae. Metacoxae transversely elongate, reaching the outer front angles of 1st ventrite, outer part covered by meeting lobes of metasternum and 1st ventrite, thus apparently very narrow. Trochanteral seta present. Femora edentate, robust (profemur 3.38 × as long as wide). Tibiae robust (protibia 4.06 × as long as wide), sides subparallel, inner margin very weakly bisinuate, not crenulate. Tibial unci raising from middle part of talus, leaving an outer angle and an inner praemucro, metatibial uncus shorter than others. Tibial comb complete, on almost transverse margin of talus. Front tibiae with a patch of dense golden setae near front inner apical angle, above comb, a grooming area also present in apical inner margin. Tarsomeres moderately robust, 1st protarsomere 1.67 × as long as wide, 2nd 0.73 ×, 3tr 0.65 ×, with complete ventral sole, except for narrow glabrous midline, onychium 3.7 ×, surpassing lobes of 3tr by 0.38 × length of onychium. Claws free, simple, divaricate, short and robust, as long as onychium height in side view.
Abdominal tergites (based on 1 paratype) (Fig 13).
Tergites I-III weakly sclerotized, submembranous; spiracular sclerites
a little more sclerotized, round; tergites IV-VI more sclerotized,
mostly in two marginal fasciae on each side of front margin; tergite VII
(prepygidium) trapezoidal-semilunate, front median area with a
transverse keel, the keel triangularly prominent at middle and apex of
prominence bearing 2 small tubercles (plectra of stridulatory system
type 1 of
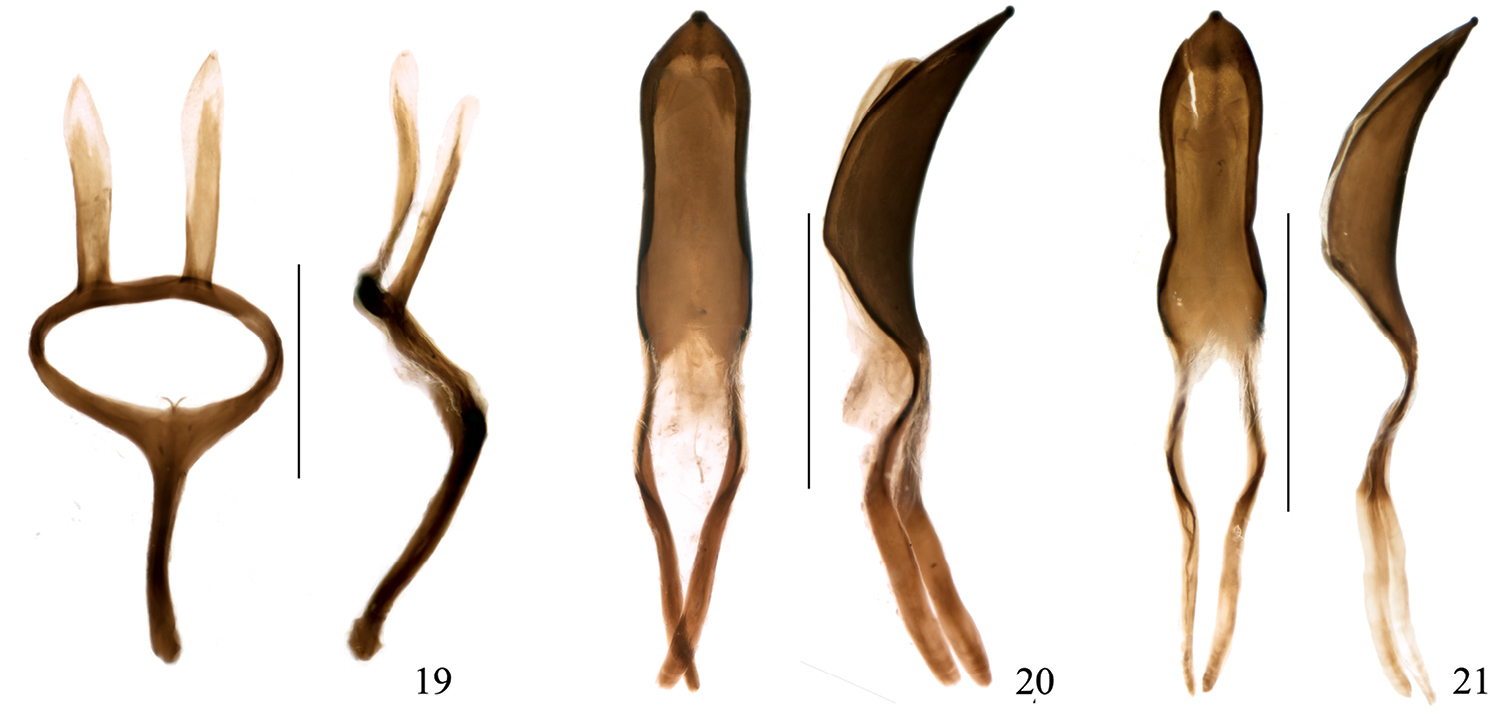
Male genitalia and terminalia (based on 1 paratype). Sternite VIII (Fig 17) undivided, semilunar, apex widely emarginate, more sclerotized basally, the membrane between this and the sternite IX with 2 very small, dot-like sclerotizations. Sternite IX with hemisternites fused between them and to the front margin of the spiculum gastrale to form an irregular subpentagonal plate, widely notched caudad; spiculum robust, directed towards left side of abdomen, almost straight, apex weakly hooked. Penis (Fig. 20) in dorsal view with tube 1.32 mm in length and temones 1.17 mm, tube ca. 3.2 × as long as wide, apex ogival, subacute, sides rounded, with a small apical peg, widest at basal 4th, temones fused; in lateral view moderately and uniformly curved at sides, highest at basal 3tr, apical peg rounded; a subtobtuse ventral longitudinal keel present. Endophallus without large sclerotized structures, covered with very dense, minute (2.5 μm long) teeth, some longer teeth forming several diffuse longitudinal streams. Tegmen (Fig. 19) with ring narrow, manubrium about as long as parameroid lobes, these widely spaced at base, lanceolate, slightly asymmetrical, apex and inner margin desclerotized, covered with minute translucid microchaetae, those at apex ca. 25 μm long.
Omophorus (Sinomophorus) rongshu sp. n., male holotype, habitus. 1 dorsal view 2 lateral view. Scale: 1–2: 2 mm.
Omophorus (Sinomophorus) rongshu sp. n., head and rostrum. 3 male holotype, dorsal view 4 male holotype, lateral view 5 male from Xīnpíng, dorsal view 6 male from Xīnpíng, lateral view 7 female paratype, dorsal view 8 female paratype, lateral view. Scale: 3–8: 1 mm.
Omophorus (Sinomophorus) rongshu sp. n. 9 paratype, metathoracic wing 10 male paratype, metendosternite 11 female paratype, VII-VIII tergite, dorsal view. Scales: 9: 5 mm; 10–11: 1 mm.
Measurements (in mm) (n=7): Standard length: 4.40–5.30 (mean= 4.85). Rostrum: length: 1.08–1.22 (mean= 1.15), maximum width: 0.38–0.46 (mean= 0.42). Pronotum: median length: 0.91–1.20 (mean= 1.06), maximum width: 1.90–2.20 (mean= 2.05). Elytra: median length: 3.70–4.30 (mean= 4.00), maximum width: 3.05–3.95 (mean= 3.50).
Rostrum 0.98–1.11 × as long as pronotum, in dorsal view 2.57–2.88 × as long as wide, sides varying from subparallel to visibly but weakly widened towards apex in an almost straight line. Pronotum 1.79–2.09 × as wide as long in dorsal view. Scutellum with sides more or less straight and apex more or less rounder to weakly notched, the apical tubercles more or less separated and variably prominent. Elytra 1.08–1.25 × as long as wide.
Measurements (in mm) (n=7): Standard length: 4.60–5.30 (mean= 4.95). Rostrum: length: 1.42–1.56 (mean= 1.49), maximum width: 0.38–0.44 (mean= 0.41). Pronotum: median length: 1.04–1.28 (mean= 1.16), maximum width: 1.95–2.30 (mean= 2.13). Elytra: median length: 3.70–4.55 (mean= 4.13), maximum width: 3.20–3.90 (mean= 3.55).
As males, but rostrum (Figs 7–8) 1.21–1.37 × as long as pronotum, in dorsal view 3.55–3.74 × as long as wide, in dorsal view prorostrum glabrous, with sides gently curved, constricted at middle, minutely and sparsely punctate, punctures weak, metarostrum with sides weakly converging to mesorostrum, punctate and pubescent as in male; in side view straight, dorsal and ventral margins of prorostrum parallel; underside almost impunctate, subglabrous. Head with frons ca. 0.63 × as wide as rostral apex. Antennae inserted at basal 0.42 of rostrum, shorter, scape about as long as funicle, club 1.25 × as long as funicle.
Pronotum 1.79–1.95 × as wide as long in dorsal view. Elytra 1.05–1.25 × as long as wide.
Tergites VII and VIII (Fig 11) similar to those of male, tergite VIII less convex, semicircular, rather flat, with one small (functional?) spiracle on each side.
Female genitalia and terminalia (based on 2 paratypes) (Fig 22): Sternite VIII heart-shaped, with a wide apical sinus, most of it membranous and thin, laterally sclerotized, setose and with multiple sensilla, medially broadly membranose to base; basal convergent margins a little folded and sclerotized; spiculum absent. Ovipositor with gonocoxites robust (ca. 3.5 × as long as maximum width), moderately curved, weakly constricted at middle, rounded at apex, without styli, a small apicomedial fovea carrying a short, thick tuft of setae; dorsal surface with a membranous elongate triangular area, pointing apicad; ventral surface with a rounded longitudinal finlike flap; outer surface covered with moderately dense sensilla. Bursa irregular, large, wrinkled, with some small irregular sclerotized areas near the point of union of the spermathecal duct and of the oviduct. Spermatheca C-shaped, large (ca. 0.43 × as long as the 8th sternum wide), with a long, apically rounded cornu not clearly differentiated from the spermathecal body (nodulus), except for the dorsal gibbosity, collum about as long as nodulus, ramus absent. Spermathecal gland ca. 3.2 × as long as maximum length of spermatheca.
Omophorus (Sinomophorus) rongshu sp. n., male paratype. 12 ventrites, ventral view 13 tergites II-VII, dorsal view 14 metanotum, dorsal view 15 metanotum, frontal view (dorsum below) 16 tergite VIII, dorsal view 17 sternites VIII and IX, dorsal view 18 mesonotum, dorsal view. Scales: 12–15, 17: 1 mm; 16, 18: 0.5 mm.
Omophorus (Sinomophorus) rongshu n.sp , male paratype. 19 tegmen, dorsal and lateral views 20 penis, dorsal and lateral views 21 male from Xīnpíng, penis, dorsal and lateral views. Scales: 19: 0.5 mm; 20–21: 1 mm.
Omophorus (Sinomophorus) rongshu sp. n., female genitalia. Scale: 1 mm.
The specimens show some variation in colour, mostly a lesser presence of the dark brown colouration on elytra, restricted to some basal spots and an apical patch, or to a lining of the bottom of the elytral striae, the apical margin of the ventrites, the sides of pronotum and the basal two thirds of the rostrum, the other areas remaining reddish brown, or all the head and rostrum may become also of the same colour. Legs may also show different variations. No variation has been noticed in vestiture, but the pronotal median tubercle may be more or less covered with punctures in different individuals.
Holotype: ♂: (white, handwritten): 云南路西 [Yúnnán Lùxī] / 1958.6.26; (white, handwritten): 中国科学院,榕树 [Zhōngguó Kēxuéyuàn, róngshù] 575; (white, printed): IOZ (E) 909962. Paratypes (12♂, 7♀): same data as holotype: 2♂ labeled IOZ(E) 909960, IOZ(E) 909961 and 1♀ labeled IOZ(E); 1♂, same data as holotype except date 1958.6.27 and IOZ(E) 909962 with same data as holotype except for collecting date: 1958.VI.27; 8♂, same data as holotype except date 1958.6.30 and IOZ(E) 909948, IOZ(E) 909949, IOZ(E) 909950, IOZ(E) 909954, IOZ(E) 909957, IOZ(E) 909958, IOZ(E) 909959, IOZ(E) 909963; 6♀, same data as holotype except date 1958.6.30 and IOZ(E) 909946, IOZ(E) 909951, IOZ(E) 909955, IOZ(E) 909956, labeled IOZ(E)1799147, IOZ(E)1799150; 1♂: (white, handwritten): 云南瑞丽 [Yúnnán Ruìlì] 1958.7.6; (white, handwritten): 中国科学院,榕树 [Zhōngguó Kēxuéyuàn, róngshù] 688; (white, printed): IOZ(E) 909964.
Holotype and all paratypes to be conserved in IOZ-CAS, except one male and one female paratype to be deposited in MNCN-CSIC (Madrid) and another paratype couple in the NHM (London).
The first character used to write the second place name (Lùxī) is incorrect. It should have been 潞.The only Yunnanese locality named 路西 is found at more than 2, 000 m height.
The specific epithet is a pinyin transliteration (without diacritics) of the indication of the host plant on the labels, róngshù (榕树), which is applied to Ficus microcarpa L.f. (Moraceae) (Zhou and Gilbert, 2003). It is used as a noun in apposition.
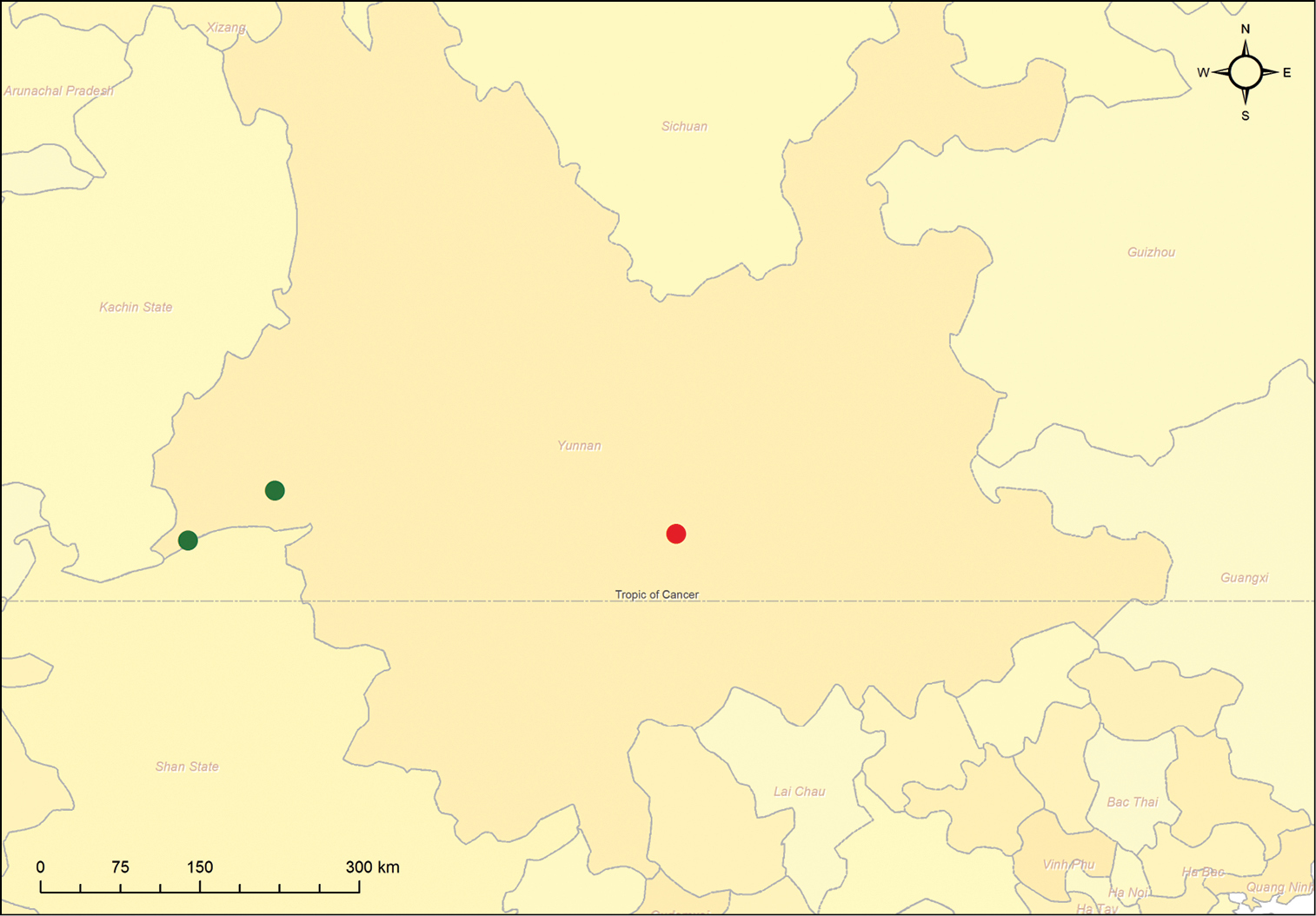
The species is known from two close localities
in Yunnan province (P.R. China): Lùxī (575 m) and Ruìlì (688 m),
separated by 87 km, but see below (Fig 23).
No exact data about the biology of this species are known, except the
identity of the host plant on which the specimens were captured, a
common tree below 1900 m in the area, and widely planted as a shade
tree (
Omophorus (Sinomophorus) rongshu sp. n., distribution map. Red dot is Xīnpíng.
One male has not been included in the type series, because it differs from the specimens collected in the two above mentioned localities as follows: Rostrum (Figs 5–6) more visibly widened towards apex, more robust (2.49 × as long as wide, 0.93 × as long as pronotum). Scape more robust (5.30 × as long as wide) but longer (1.23 × as long as funicle), 2nd desmomere more robust (1.36 × as long as wide), 3tr-6th desmomeres 2.7–3.7 × as wide as long, club more robust (2.39 × as long as wide) and shorter (1.42 × as long as funicle). Pronotum narrower, in dorsal view 1.67 × as wide as long. Scutellum as long as wide. Abdomen with 2nd ventrite 1.2 × as long as 3tr, 4th as long as 2nd, 5th 4.65 × as wide as long, 1st ventrite 1.66 × as long as 2nd in median length, 0.89 × as long as 2nd in postcoxal length. Mesocoxae separated by a distance 1.2 × the mesocoxal diameter. Profemur more robust, 3.0 × as long as wide, protibia more slender (5.43 × as long as wide. Onychium surpassing lobes of 3tr tarsomere by 0.5 × length of onychium. Penis (Fig 21) similar, but more constricted medially in dorsal view, and ventral angle sharper (in cross-section).
Label data for this specimen are as follows: (white, two marginal faded red lines, pencil handwritten): 黑尾棕象甲 [Hēi Wěi Zōng Xiàng Jiǎ] / 新平 [Xīnpíng]; (white, faded red lined, field names printed, handwritten): 新平 [Xīnpíng];,80年; (white, printed): IOZ(E)1799122. The first label corresponds to an intent of identification in Chinese (the brown weevil with black tail) and the locality; the second repeats the locality Xīnpíng (Yunnan, China) and includes only the year of collection [19]80. This locality is separated from the closest one, Lùxī, by ca. 347 km (Fig 23).
Although some of the differences, notably those of the proportions of the ventrites and of the antennomeres, could be taken as enough to describe a different species, we prefer to wait until a series from the latter locality can be studied. These differences may be individual or populational, and the erection of a new taxon unjustified.
The genus Omophorus was placed in its original description between Tylomus Schoenherr, 1835 and Rhinaria
W. Kirby, 1819, in Schoenherr’s Divisio Erirhinides, a conglomerate
of unrelated long-nosed weevils with contiguous procoxae. The first
genus is now a member of the tribe Sternechini Lacordaire, 1863 (Curculionidae: Molytinae) and the second is in Aterpini Lacordaire, 1863 (Curculionidae: Cyclominae) (
Neither of the above mentioned authors made reference to H.
There are no known detailed descriptions of the members of the genus Omophorus or of any other Metatygini. The most modern description is that of Omophorus boxi by
Among the characters that can be of phylogenetic use, we want to emphasize the wing, the meso- and metanotal structures and the peculiar conformation of the female tergite VII.
The wing of Omophorus rongshu is very similar to that figured for Trigonocolus curvipes by
The meso- and metanota present similarities to some other taxa presented by Davis (l.c.). The broadly developed mesonotal axillary cord is a character commonly found in Baridinae, Trigonocolus and some Conoderinae, while a reduced axillary cord is found in some Curculioninae, Cossoninae, Ceutorhynchinae and some Conoderinae (the latter having been already considered probably paraphyletic in a phylogenetic analysis by
The dorsally uncovered VIII tergite of females (similar
to that of male) is a distinctive character. It is exposed by the VII
tergite being widely notched apically, an uncommon feature. So far, an
uncovered female VIII tergite has been recorded only in Brachyceropseini, Ulomascini, Ectemnorhinini and Sitonini (
The distribution of some characters used in the systematics of Curculionidae
is, so to speak, erratic and there is no certainty that their
presence or absence is a proof of monophyly. Unci on the three pairs of
tibiae are a common feature in several tribes in Curculioninae and they have been lost in some other groups (Cossoninae, Molytinae)
in at least one pair (usually the hind one). Thus the presence of unci
alone is a questionable synapomorphy to delimit monophyletic taxa (Curculioninae vs. Molytinae). The same can be said of the type 1 of stridulatory system (
We want to thank here Drs. Rolf Oberprieler (CSIRO), Christopher H.C. Lyal (NHM) and Hiroaki Kojima (Tokyo University of Agriculture) for their help during this research and Ms Arabia Sánchez, Pilar Rodríguez and Isabel Morón (MNCN) for help with documentation. Dr. Jens Prena (USDA) and one anonymous reviewer improved this manuscript with their suggestions. This research has been supported by an Invited Professor Award (2009) of the Chinese Academy of Sciences to the senior author and by project grant CGL2010-15786 (Ministerio de Ciencia e Innovación, Spain).
Checklist of tribe Metatygini. The countries are in bold, the provinces in small capitals, general localities in inverted commas.
| Genus Omophorus Schoenherr, 1835: 479 [type species: Omophorus stomachosus Boheman, 1835, by original designation] | ||
| Metatyges Pascoe, 1865: 424 [type species: Metatyges turritus Pascoe, 1865 by subsequent designation by Pascoe, 1870: 444; syn. Marshall, 1917: 195) | ||
(Omophorus) | ||
| Omophorus (Omophorus) boxi Marshall 1944: 350 | Kenya: Eastern: Kabete; Nairobi: Nairobi | |
| Omophorus (Omophorus) cupreus Pascoe 1870: 443 (Metatyges) | Benin; Ghana; “widely distributed in West Africa” [Marshall 1944: 351] | |
| Omophorus (Omophorus) nicodi Hustache 1925: 22 (syn.: Marshall 1944: 351) | ||
| Omophorus (Omophorus) hacquardi Chevrolat 1881: 89 (Metatyges) comb. n. (1) | Tanzania: Morogoro: Mhonda | |
| Omophorus (Omophorus) indispositus Boheman 1845: 447 | Angola: Benguela: Benguela; D.R. Congo: Bas-Congo: Boma, Banana-Boma; Sud-Ubangi: Zambi | |
| Omophorus (Omophorus) parvus Faust 1899: 416 (Metatyges) (syn.: Marshall, 1917: 195) | ||
| Omophorus (Omophorus) occidentalis Fairmaire 1902: 135 (syn.: Marshall 1944: 351) | ||
| Omophorus (Omophorus) stomachosus Boheman 1835: 480 | South Africa: “Caffraria, Caffernland” | |
| Omophorus (Omophorus) turritus Pascoe 1865: 424 (Metatyges) (syn.: Marshall 1917: 195) | ||
(Pangomophorus) Voss 1960: 344 [type species: Omophorus biroi Voss, 1960, by original designation] | ||
| Omophorus (Pangomophorus) biroi Voss 1960: 344 | Papua New Guinea: Morobe: Sattelburg (= Sattelberg) | |
(Sinomophorus) Alonso-Zarazaga, Wang, Ren & Zhang, h.o. | ||
| Omophorus (Sinomophorus) rongshu Alonso-Zarazaga, Wang, Ren & Zhang, h.o. | China: Yunnan: 潞西 (Lùxī), 瑞丽 (Ruìlì), 新平 (Xīnpíng) (?) | |
Genus Physarchus Pascoe 1865: 425 [type species: Physarchus pyramidalis Pascoe, 1865, by monotypy] | ||
| Physarchus castaneipennis Faust 1891: 285 | India: Sikkim | |
| Physarchus conspicillatus Fairmaire 1878: 286 | “Polynesia” | |
| Physarchus pyramidalis Pascoe, 1865: 425 | Fiji | |
Genus Sternechosomus Voss 1958: 46 [type species: Sternechosomus octotuberculatus Voss, 1958, by original designation] | ||
| Sternechosomus octotuberculatus Voss 1958: 46 | China: Fujian: Guàdūn 挂墩 (= Kuatun) | |