(C) 2010 Kadir Boğaç Kunt. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species, Hygrocrates deelemanus Kunt & Yağmur sp. n., is described on the basis of both sexes from the Mediterranean region of Turkey. Detailed morphological descriptions, diagnosis and figures of the copulatory organs of both Turkish species are presented. An identification key is presented for all the currently known species of Hygrocrates.
Dysderidae, Hygrocrates, new species, systematic key, Turkey
Hygrocrates Deeleman-Reinhold, 1988 is a small dysderid genus which contains only three previously described species: Hygrocrates caucasicus Dunin, 1992; Hygrocrates georgicus (Mcheidze, 1972) and Hygrocrates lycaoniae (Brignoli, 1978). Hygrocrates caucasicus and Hygrocrates georgicus are endemic to Georgia, while Hygrocrates lycaoniae is known from Rhodes and Turkey. Hygrocrates lycaoniae was originally ascribed to Harpactocrates Simon, 1914. In their revision of Dysderinae spiders of the Mediterranean region,
To date, only Hygrocrates lycaoniae
has been recorded from Turkey based on only one male in a sample
collected from outside Körükini Cave (Konya province, Beyşehir
District, Çamlık town). After the original description by
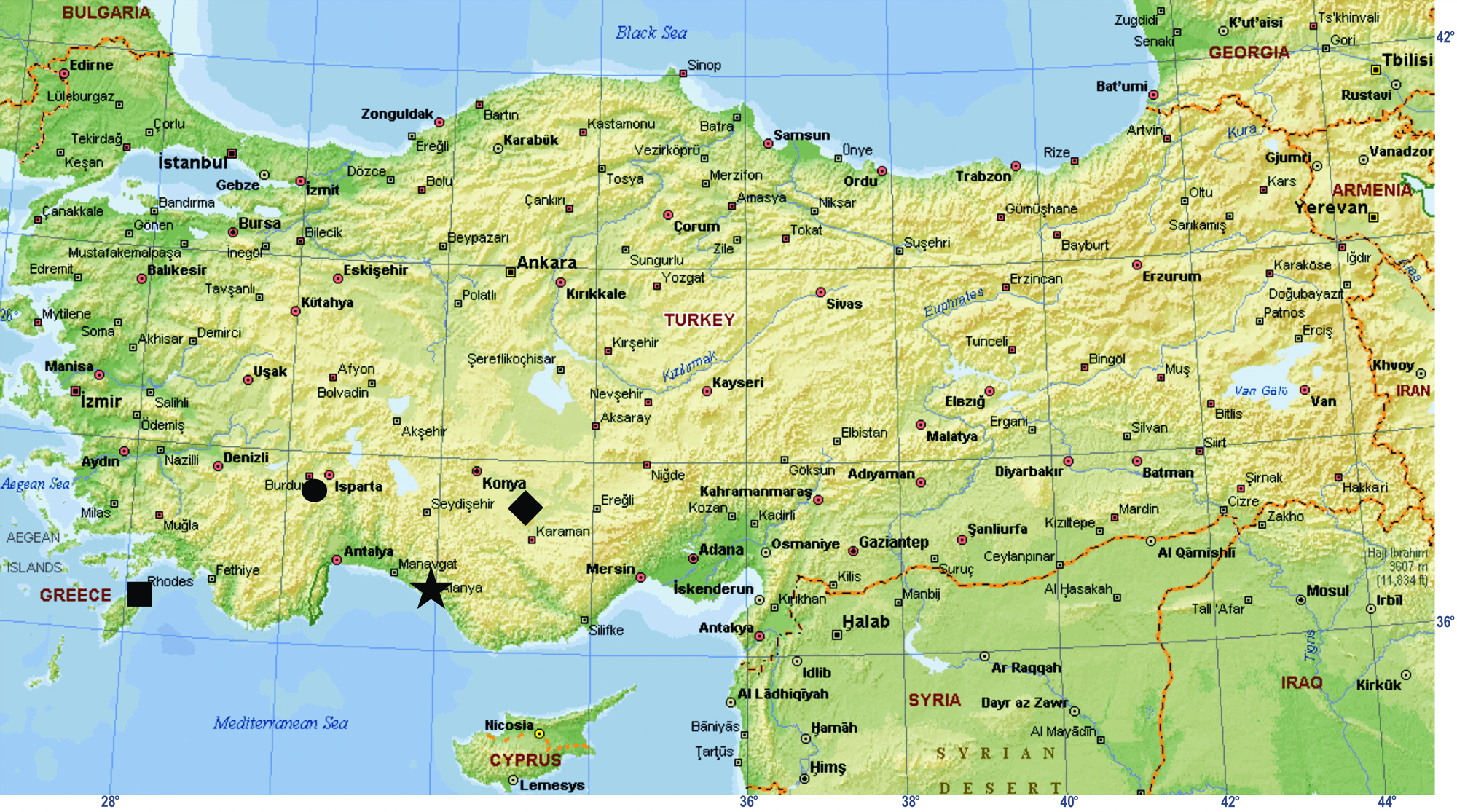
All specimens were collected from two different localities in Turkey (Fig. 1).
The specimens were collected by sifting of leaf litter and preserved in
70% ethanol. Digital images of the pedipalp and vulva were taken with a
Leica DFC295 digital camera attached to a Leica S8AP0 stereomicroscope
and 5–15 photographs were taken in different focal planes and combined.
Photographic images were edited using Photoshop CS2 and Corel-DRAW X3
was used to create the plates. All measurements are in mm. Terminology
for the body measurements and copulatory organ structures follows
Material treated herein is deposited in the personal collection of Kadir Boğaç Kunt (cKBK, Ankara, Turkey) and in the Senckenberg Museum (SMF, Frankfurt am Main, Germany). The following abbreviations are used in the text: AL, abdominal length; CL, carapace length; CWmax, maximum carapace width; CWmin, minimum carapace width; AME, anterior median eyes; PLE, posterior lateral eyes; PME, posterior median eyes; AMEd, diameter of anterior median eyes; PLEd, diameter of posterior lateral eyes; PMEd, diameter of posterior median eyes; ChF, length of cheliceral fang; ChG, length of cheliceral groove; ChL, total length of chelicera (lateral external view); Ta, tarsus; Me, metatarsus, Ti, tibia; Pa, patella; Fe, femur; Tr, trochanter; C, coxa; D, dorsal; Pl, prolateral; Rl, retrolateral; V, ventral.
Map showing the localities from which Hygrocrates specimens have been recorded. ★ the type locality of Hygrocrates deelemanus sp. n.; ♦ the type locality of Hygrocrates lycaoniae; ● locality of Hygrocrates lycaoniae from Turkey; ■ locality of Hygrocrates lycaoniae from Rhodos island (Greece).
| 1 | Male | 2 |
| – | Female | 4 |
| 2 | Bulbus straight, cylindrical; embolus lobe-shaped; apophysisa and apophysisb nearly same size | Hygrocrates caucasicus |
| – | Bulbus pyriform; embolus hook-shaped; apophysisa and apophysisb smaller than embolus | 3 |
| 3 | Transition between bulbus and distal continuation is gradual (Figs 30–33, 36) | Hygrocrates lycaoniae |
| – | Transition between bulbus and distal continuation is abrupt, clearly curved over 90° (Figs 16–19, 35) | Hygrocrates deelemanus sp. n. |
| 4 | Distalmost part of spermathecae linear | Hygrocrates georgicus |
| – | Distalmost part of spermathecae oval | 5 |
| 5 | Proximalmost part of spermathecae oval (Fig. 20) | Hygrocrates deelemanus sp. n. |
| – | Proximalmost part of spermathecae circular (Fig. 34) | Hygrocrates lycaoniae |
Hygrocrates Deeleman-Reinhold, 1988
Hygrocrates Deeleman-Reinhold, in Deeleman-Reinhold and Deeleman, 1988: 240, type Harpactocrates lycaoniae Brignoli, 1978.
urn:lsid:zoobank.org:act:3EBEFEC5-1A65-480F-BAB1-954463DCFC5C
Figs 2–21, 35, 37Holotype ♂ (SMF) Antalya Province, Alanya District, Taşatan Plateau [36°38'33.20"N, 32°4'44.40"E], 09.I.2010, leg. K.B.Kunt. Paratypes: 1♀ (abdomen heavily damaged during dissection) (SMF), 1♂ (cKBK) same data as holotype.
The male palp of Hygrocrates deelemanus sp. n. is most similar to that of Hygrocrates lycaoniae. Bulbal apophyses are shorter than the embolus in both species. However, the transition between the bulbus and distal continuation is more abrupt in Hygrocrates deelemanus sp. n., clearly curved over 90°, whereas it is more gradual in Hygrocrates lycaoniae. In the vulva, the proximalmost part of the spermathecae is larger and wider than in Hygrocrates lycaoniae.
Hygrocrates deelemanus sp. n. (Holotype ♂); scale line: 1 mm.
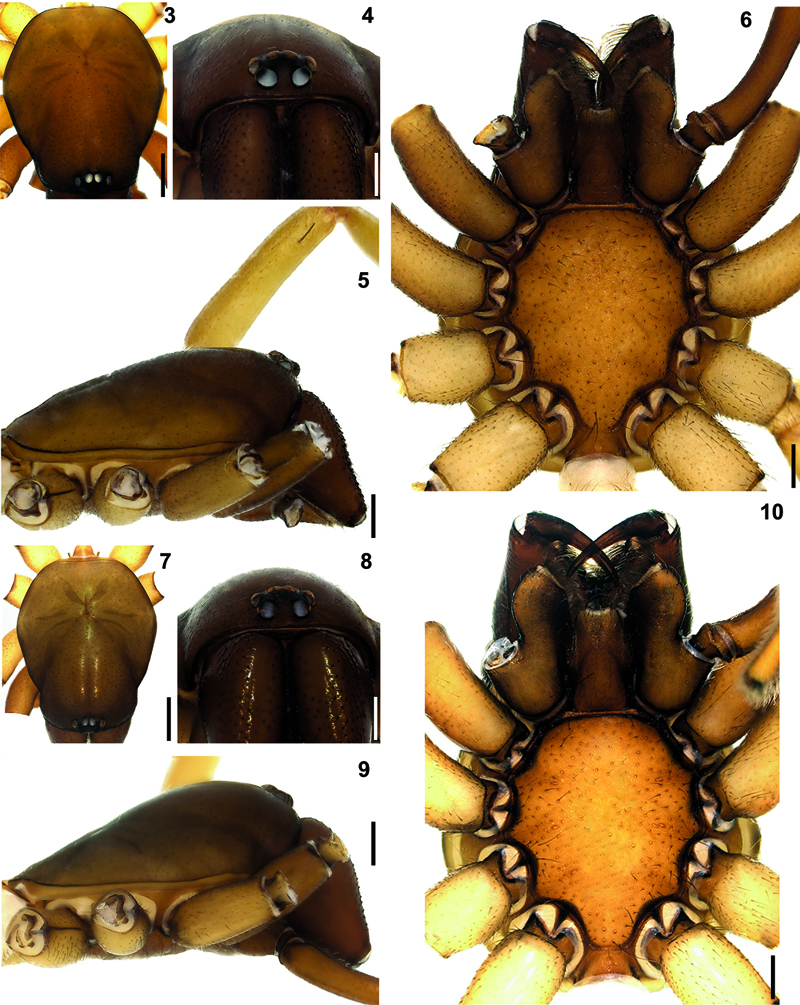
Hygrocrates deelemanus sp. n. 3 (♂), 7 (♀) carapace, dorsal view 4 (♂), 8 (♀) ditto, anterior view 5 (♂), 9 (♀) ditto, lateral view 6 (♂), 10 (♀) ditto, ventral view. Scale lines: (3, 5, 6, 7, 9, 10) 0.5 mm; (4, 8) 0.25 mm.
The specific name is given in honour of Dr. Christa L. Deeleman-Reinhold, a prominent Netherlander arachnologist who described the genus Hygrocrates.
AL 3.84 / ?; CL 2.76 / 3.04; CWmax 2.28 / 2.40; CWmin 1.40 / 1.76; AMEd 0.16 / 0.19; PLEd 0.12 / 0.14; PMEd 0.10 / 0.11; ChF 0.77 / 0.82; ChG 0.46 / 0.52; ChL 1.20 / 1.50. Leg measurements are given in Table 1.
Carapace hexagonal-shaped, reddish brown, surface of carapace with small dark shallow depressions. Cephalic region dark brownish, narrow and clearly higher than thoracic region (Figs 2, 3, 5, 7, 9). Chilum triangular-shaped, distinct, coloured as carapace and chelicerae and broader in females (Figs 4, 8). Fovea short, straight and longitudinal (Figs 2, 3, 7). AME, PLE and PME closely grouped. Distance of AME-PLE shorter than PLE-PME. AME separated (Figs 4, 8). Labium, gnathocoxae and chelicerae brown. Labium and gnathocoxae covered with dark hairs; more densely so on gnathocoxae. Labium wider at the base. Gnathocoxae rounded laterally, with inwardly notched tips and blackish along the margins. Sternal border of gnathocoxae pentagonal-shaped, cheliceral region arrow-shaped with blunt tip (Figs 6, 10).
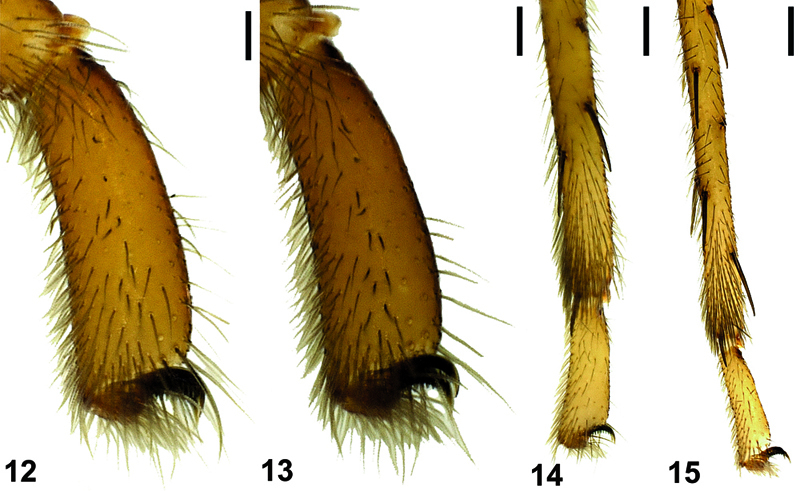
Chelicerae brownish with darker tubercules; basally broader in females and laterally swollen (Figs 4, 8). Cheliceral groove with four teeth: retromargin with four teeth, including one small and one large tooth at the base of the groove (Fig. 11). Sternum and abdomen yellowish brown, with thin blackish hairs over the entire surfaces. Legs yellowish brown. Palps and legs I and II darker than legs III and IV. Leg length formula: Leg I > Leg IV > Leg II > Leg III. Tarsi with two claws and claw tufts. All tarsi with fine tarsal scopulae. Legs III and IV with metatarsal scopulae (Figs 12–15). Coxae without spines. Details of leg spination are given in Table 2.
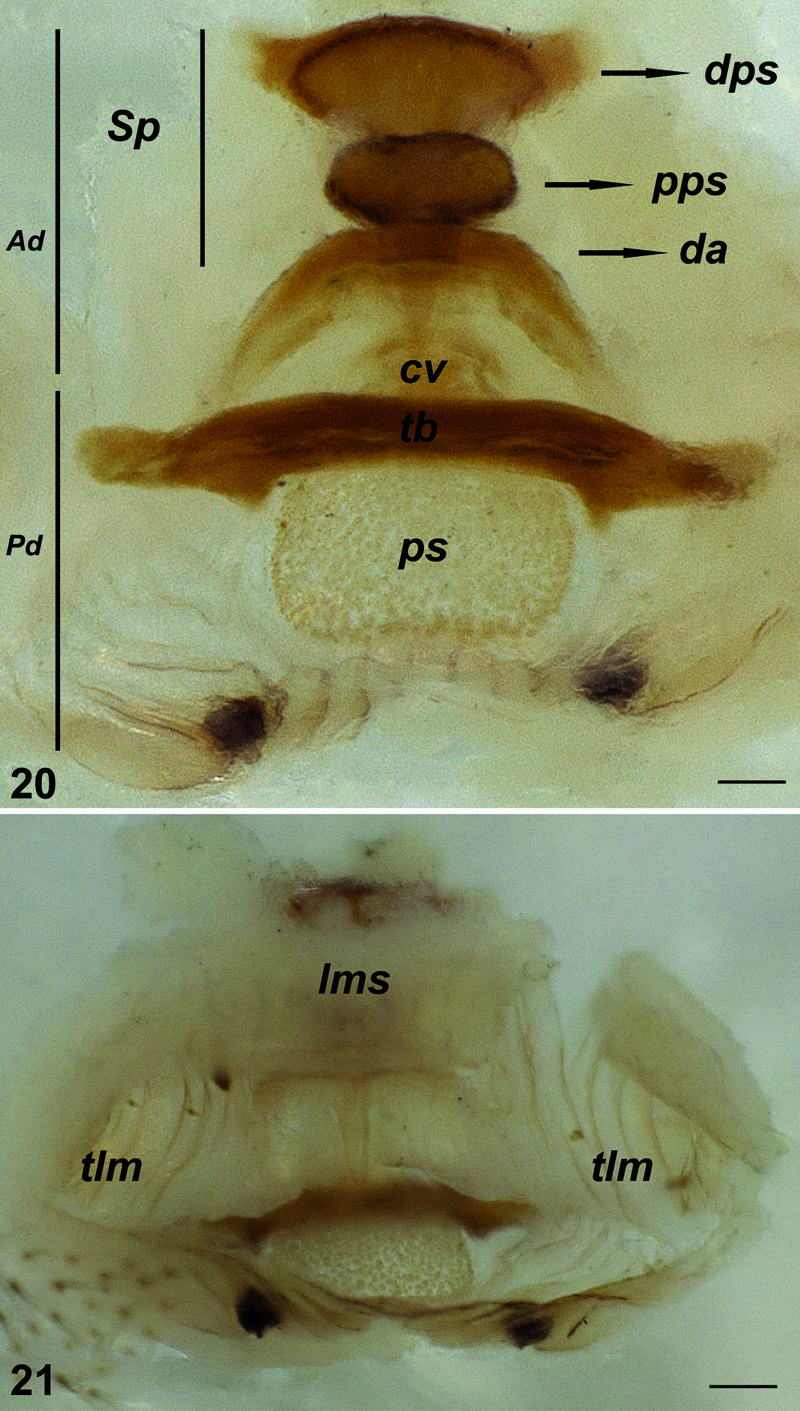
Palpal organ with pyriform bulbus and hook-shaped (tapering towards the tip) embolus. Bulbal apophyses are more strongly sclerotized than the embolus (Figs 16–19). Vulva with two parts: anterior diverticulum and posterior diverticulum (Fig. 20). Anterior diverticulum consists of a dorsal arch with spermathecae which have two parts (distalmost and proximalmost parts), a large membranous sac (clearly visible in dorsal view) and widened twisted lateral membranes (Fig. 21). Posterior diverticulum consists of a central valve with a transverse bar, a wide membranous sac and a couple of small lateral membranous pockets (Fig. 20).
Cheliceral teeth of Hygrocrates deelemanus sp. n.
Tarsal and metatarsal scopulae of Hygrocrates deelemanus sp. n. (♂), 12 leg I, 13 leg II, 14 leg III, 15 leg IV. Scale lines: 0.25 mm
Male palp of Hygrocrates deelemanus sp. n. Abbreviations: Apa apophysisa Apb apophysisb E embolus. Scale line: 0.1 mm.
Vulva of Hygrocrates deelemanus sp. n. 20 dorsal view 21 ventral view. Abbreviations: Ad anterior diverticulum Pd posterior diverticulum Sp spermatheca dps distalmost part of spermatheca pps proximalmost part of spermatheca da dorsal arch cv central valve tb transverse bar ps membranous sac lms large membranous sac tlm twisted lateral membranes. Scale lines: 0.1 mm.
Leg measurements of Hygrocrates deelemanus sp. n.
| (Holotype ♂ / Paratype ♀) | Fe | Pa | Ti | Me | Ta |
|---|---|---|---|---|---|
| Leg I | 2.32 / 2.52 | 1.60 / 1.66 | 2.00 / 2.43 | 2.24 / 2.30 | 0.64 / 0.63 |
| Leg II | 2.08 / 2.32 | 1.48 / 1.44 | 1.80 / 1.84 | 2.08 / 1.96 | 0.56 / 0.60 |
| Leg III | 1.76 / 1.92 | 0.88 / 1.12 | 1.20 / 1.40 | 1.60 / 1.88 | 0.56 / 0.60 |
| Leg IV | 2.40 / 2.48 | 1.36 / 1.36 | 1.88 / 2.08 | 2.20 / 2.60 | 0.59 / 0.61 |
Figs 22–34, 36, 38
1 ♂, 1 ♀ (SMF) (abdomen heavily damaged during dissection) Burdur Province, Yeşilova District, side of Salda Lake [37°30'32.78"N, 29°41'56.66"E], 14.VII.2010, leg. E.A.Yağmur & M. Elverici.
Hygrocrates lycaoniae can be distinguished from Hygrocrates caucasicus by the pyriform shape of the bulbus (bulbus smooth and cylindrical in Hygrocrates caucasicus) (see
Hygrocrates caucasicus was originally described on the basis of two males by
AL 3.36 / ?; CL 2.40 / 3.00; CWmax 2.20 / 2.44; CWmin 1.52 / 1.76; AMEd 0.14 / 0.16; PLEd 0.13 / 0.15; PMEd 0.11 / 0.12; ChF 0.77 / 0.79; ChG 0.45 / 0.50; ChL 1.30 / 1.46. Leg measurements are given in Table 3.
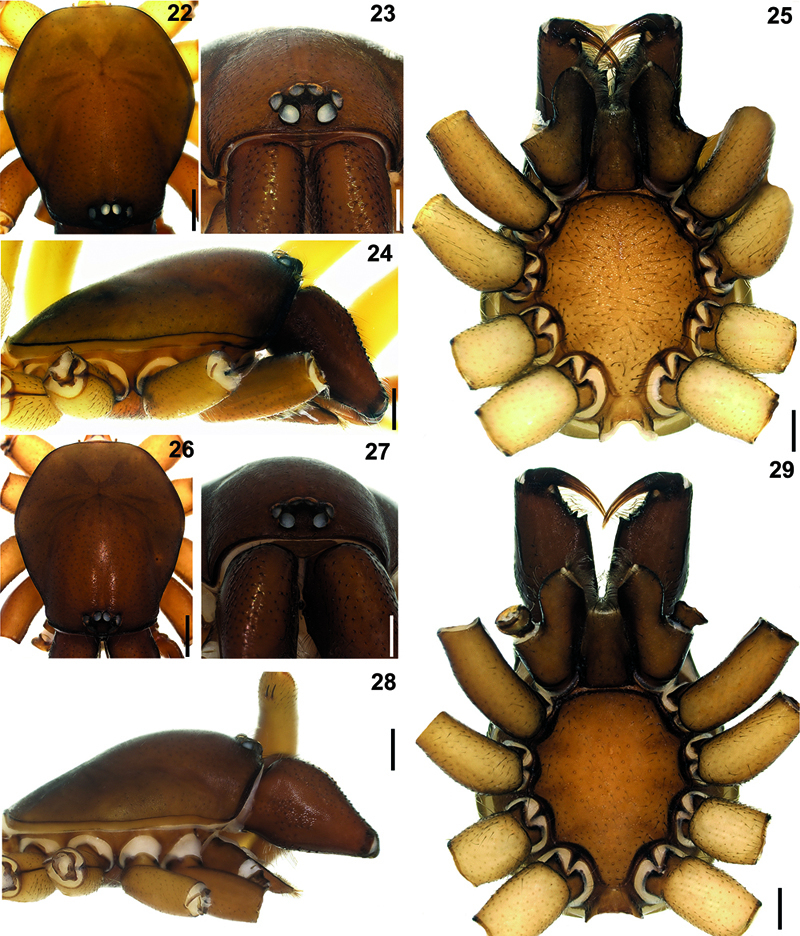
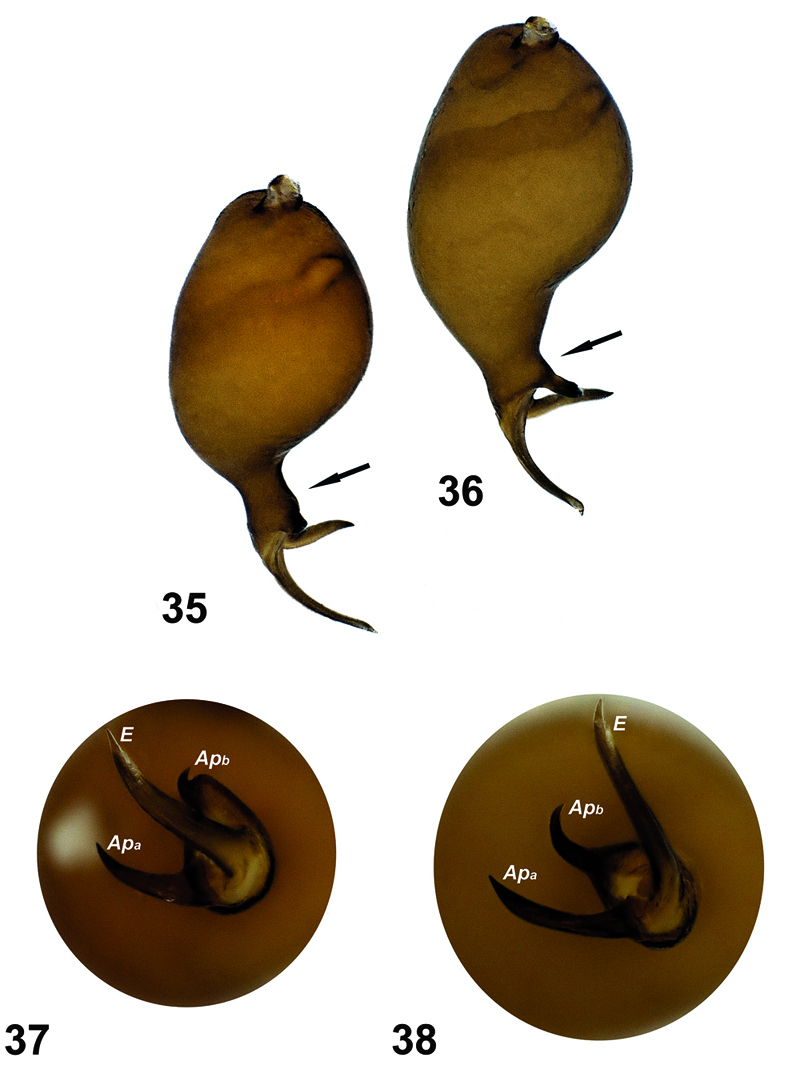
General features of the body of Hygrocrates lycaoniae closely resemble the new species (Figs 22–29), Hygrocrates deelemanus sp. n., but the two are easily differentiated by their different body sizes and by structures of the male and female genitalia (Figs 30–33, 35–38). The males of the two species are easily distinguished in ventral view (90° angle) by the terminal part of the bulbus having the following characteristics:
1. Embolic base is pear-shaped in the two species, but the tip of the embolic base located at 12 o’clock in Hygrocrates deelemanus sp. n. (Fig. 37) and at 10 o’clock in Hygrocrates lycaoniae (Fig. 38).
2. apophysisa and apophysisb are short and blunt in Hygrocrates deelemanus sp. n. (Fig. 37), but long in Hygrocrates lycaoniae (Fig. 38).
3. apophysisb originates near the tip of the embolic base in Hygrocrates deelemanus sp. n. (Fig. 37), but originates from the central part of the tip of the embolic base in Hygrocrates lycaoniae (Fig. 38).
4.Embolus is curved between apophysisa and apophysisb in Hygrocrates deelemanus sp. n. (Fig. 37), but is raised from the embolic base and separated from apophysisa and apophysisb in Hygrocrates lycaoniae (Fig. 38).
The females of the two species are easily distinguished by the form of the proximalmost part of the spermathecae which is oval in Hygrocrates deelemanus sp. n. (Figs 20–21) and circular in Hygrocrates lycaoniae (Fig. 34). Details of leg spination are given in Table 4.
Hygrocrates lycaoniae 22 (♂), 26 (♀) carapace, dorsal view 23 (♂), 27 (♀) ditto, anterior view 24 (♂), 28 (♀) ditto, lateral view 25 (♂), 29 (♀) ditto, ventral view. Scale lines: (22, 24, 25, 26, 28, 29) 0.5 mm; (23, 27) 0.25 mm
Male palp of Hygrocrates lycaoniae. Abbreviations: Apa apophysisa Apb apophysisb E embolus. Scale line: 0.1 mm.
Vulva of Hygrocrates lycaoniae, dorsal view. Abbreviations: Ad anterior diverticulum Pd posterior diverticulum Sp spermatheca dps distalmost part of spermatheca pps proximalmost part of spermatheca da dorsal arch cv central valve tb transverse bar ps membranous sac tlm twisted lateral membranes. Scale line: 0.1 mm
Leg spination of Hygrocrates deelemanus sp. n.
| ♂ (Holotype) | Leg I | Leg II | Leg III | Leg IV |
|---|---|---|---|---|
| C | 0 | 0 | 0 | 0 |
| Tr | 0 | 0 | 0 | 0 |
| Fe | Pl 1 | Pl 0–1 | D 1 | D 1, 1 |
| Pa | 0 | 0 | 0 | 0 |
| Ti | 0 | 0 | Pl 1 Rl 1, 2 V 1, 2 | Pl 2, 2 Rl 2, 1 V 1, 1 |
| Me | 0 | 0 | Pl 1, 1 D 2, 2 Rl 1, 2, 1, 1 V 2, 2 | Pl 5 Rl 5 V 2 |
| Ta | 0 | 0 | 0 | 0 |
| ♀ (Paratype) | ||||
| C | 0 | 0 | 0 | 0 |
| Tr | 0 | 0 | 0 | 0 |
| Fe | Pl 2 | Pl 1 | 0 | D 1, 1 |
| Pa | 0 | 0 | 0 | 0 |
| Ti | 0 | 0 | Pl 1 Rl 1, 2 V 1, 2 | Pl 2, 2 Rl 1, 1 V 1, 2 |
| Me | 0 | 0 | Pl 1, 1 Rl 1, 1, 1, 1 V 1, 2 | Pl 5 Rl 5 V2 |
| Ta | 0 | 0 | 0 | 0 |
Leg measurements of Hygrocrates lycaoniae
| (♂ / ♀) | Fe | Pa | Ti | Mt | Ta |
|---|---|---|---|---|---|
| Leg I | 2.28 / 2.24 | 1.40 / 1.44 | 1.84 / 1.96 | 2.36 / 1.92 | 0.53 / 0.60 |
| Leg II | 2.08 / 2.00 | 1.44 / 1.40 | 1.85 / 1.72 | 1.92 / 1.80 | 0.50 / 0.56 |
| Leg III | 1.73 / 1.68 | 1.10 / 0.92 | 1.29 / 1.16 | 1.58 / 1.52 | 0.50 / 0.48 |
| Leg IV | 2.19 / 2.20 | 1.20 / 1.20 | 1.83 / 1.76 | 2.20 / 2.12 | 0.60 / 0.56 |
Comparison of the palps of the two species. 35, 37 Hygrocrates deelemanus sp. n. 36, 38 Hygrocrates lycaoniae. Abbreviations:Apa apophysisa Apb apophysisb E embolus.
The holotype of Hygrocrates lycaoniae
was collected from outside of Körükini Cave, which is located in
Çamlık Village (Beyşehir District, Konya Province). The vegetation
surrounding the cave is the mixed forest composed of Taurus fir,
juniper, oak and maple species. Annual and perennial herbaceous plants
also grow very densely around the cave (Fig. 39). Although several field trips were conducted to the type locality, we failed to find any specimensof Hygrocrates lycaoniae. However, our male and female specimens of Hygrocrates lycaoniae were collected near to Salda Lake (Burdur Province), which is approximately 170 km apart from the type locality (Figs 40, 41). The specimens were collected in the leaf-litter of shrublands surrounding the Salda Lake.
Leg spination of Hygrocrates lycaoniae
| Leg I | Leg II | Leg III | Leg IV | |
|---|---|---|---|---|
| ♂ | ||||
| C | 0 | 0 | 0 | 0 |
| Tr | 0 | 0 | 0 | 0 |
| Fe | Pl 2 | Pl 1 | D 1 | D 2 |
| Pa | 0 | 0 | 0 | 0 |
| Ti | 0 | 0 | Pl 1 Rl 1, 2 V 1, 2 | Pl 2, 2 Rl 1, 1, 1 V 1, 2 |
| Me | 0 | 0 | Pl 1, 1, 1 Rl 1, 1, 1 V 2, 2 | Pl 1, 1, 1, 1 Rl 5 V 2 |
| Ta | 0 | 0 | 0 | 0 |
| ♀ | ||||
| C | 0 | 0 | 0 | 0 |
| Tr | 0 | 0 | 0 | 0 |
| Fe | Pl 1–2 | Pl 1 | 0 | D 2 |
| Pa | 0 | 0 | 0 | 0 |
| Ti | 0 | 0 | Pl 1 Rl 1, 2 V 1, 2 | Pl 2, 2 Rl 2, 1 V 1, 2 |
| Me | 0 | 0 | Pl 1 Rl 1, 1, 1 V 2, 2 | Pl 1, 1, 1 Rl 1, 1, 1, 1 V 2, 2 |
| Ta | 0 | 0 | 0 | 0 |
Habitats ofHygrocrates species. 39 The type locality of Hygrocrates lycaoniae in Konya province40, 41 Localities of Hygrocrates lycaoniae in Burdur province 42 The type locality of Hygrocrates deelemanus sp. n.
In general, the taxonomy of the subfamily Dysderinae is well defined (see
This work was supported by the Research Foundation of Anadolu University (Project Number: 1001F31). We are very grateful to Dr. Fulvio Gasparo (Italy), Dr. Christa Deeleman-Reinhold (Netherlands) and Dr. Christo Deltshev (Bulgaria) for their valuable comments regarding the new species. We would like to thank Dr. Yuri Marusik (Russian Federation) for translate Russian texts; Dr. Stefan Otto (Germany) and Dr. Vera Pkhakadze (Georgia) for giving information on holotype of H. georgicus. We also thank Mr. Savaş Kunt (Turkey) who is the lovely father of the first author and our colleague Mr. Mert Elverici (Turkey) for their important help during field trips. The English of the final draft was kindly checked by Dr. David Penney (United Kingdom).