(C) 2011 Monique Nguyen Duy–Jacquemin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
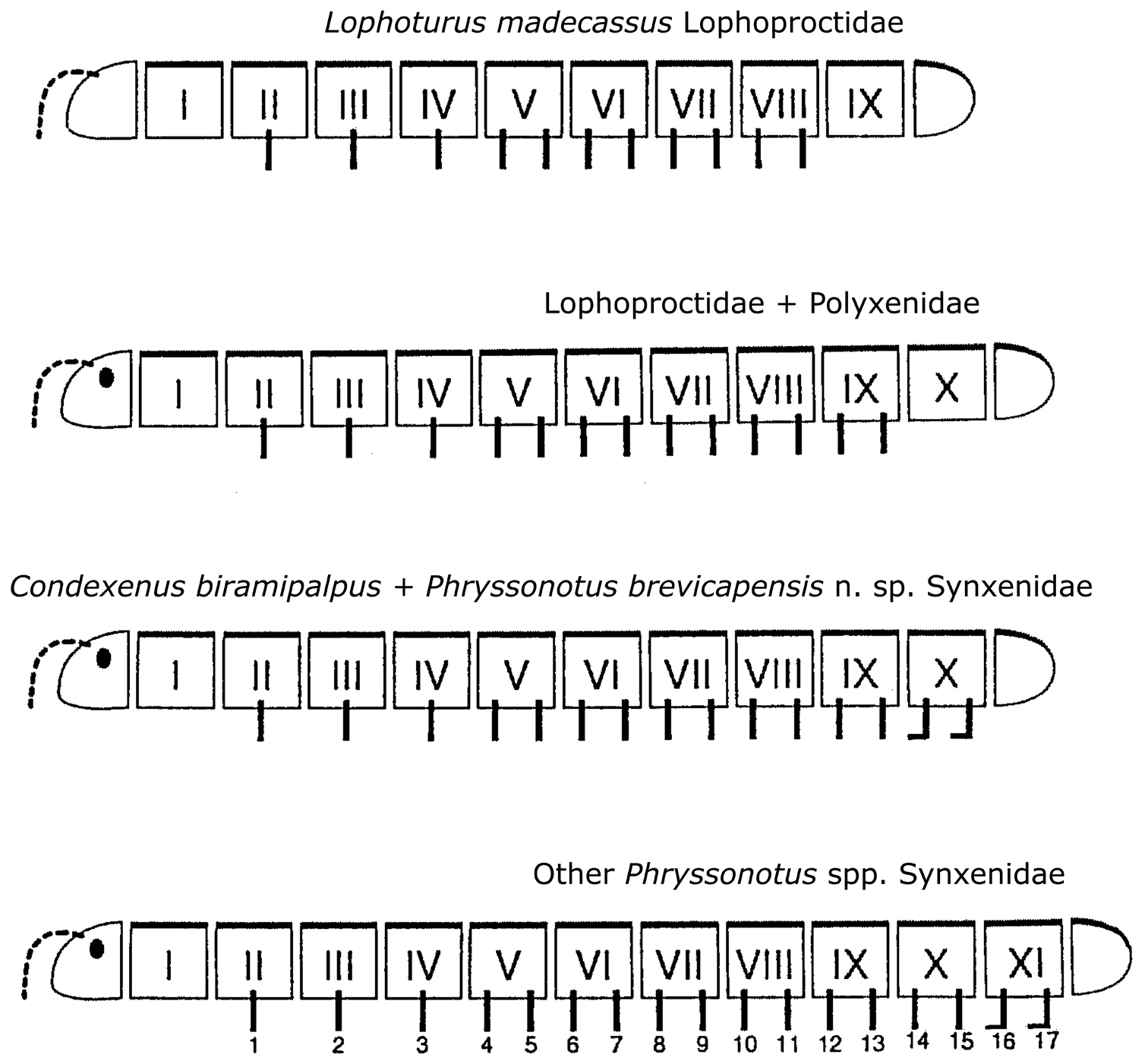
Two new species of the families Polyxenidae and Synxenidae, are described from Table Mountain National Park, South Africa. Propolyxenus squamatus sp. n. (Polyxenidae) has tergites I–X mostly covered by scale–shaped trichomes directed caudally, a character previously known only in Synxenidae. The structure of scale–shaped dorsal trichomes is different to that of the scales in Phryssonotus and Condexenus species. Phryssonotus brevicapensis sp. n. (Synxenidae) is the only known species of the genus Phryssonotus having 11 tergites, (including collum and telson) and 15 pairs of legs, as in Condexenus biramipalpus Nguyen Duy–Jacquemin, 2006. These two species therefore appear to occupy an intermediate position between Phryssonotus (12 tergites) and Polyxenoidea (maximum of 11 tergites).
Diplopoda, Polyxenidae, Synxenidae, Propolyxenus, Phryssonotus, new species, scales, barbate trichomes, postembryonic development, South Africa
Two new species of Penicillata from Table Mountain National Park (near Cape Town), South Africa, belonging to two different families, were collected in the same biotope: leaf litter of felled pine and fynbos.
The first species, represented by five specimens, belongs to the family Polyxenidae and the genus Propolyxenus Silvestri, 1948, created for Propolyxenus aegeus Silvestri, 1948 from Rhodes (Pelecano) (
The second species, represented by 20 specimens, belongs to the family Synxenidae and the genus Phryssonotus Scudder, 1885 (a replacement for the preoccupied name Lophonotus Menge, 1854), whose type species is Lophonotus hystrix, a fossil found in Eocene Baltic amber (Menge in
Abbreviations used:
MNHN Muséum National d’Histoire Naturelle, Paris, France
SEM Scanning Electron Microscopy
pl pairs of legs
Material and methodsThe material serving as the basis for the present work was obtained by hand collecting, pitfall trapping and litter sampling in pine and fynbos areas in Table Mountain National Park, South Africa (by Charmaine Uys). The material is preserved in 70% ethanol. The bulk of this material, including the holotypes and several paratypes, has been deposited in MNHN.
For light microscopy, the specimens are mounted on slides in “Baume de Marc André”. SEM micrographs were taken using a scanning electron microscope at the Zoology Department, University of Cape Town.
Systematics Class Diplopoda de Blainville in Gervais, 1844Subclass Penicillata Latreille, 1831
Order Polyxenida Verhoeff, 1934
Superfamily Polyxenoidea Lucas, 1840
Family Polyxenidae Lucas, 1840
Subfamily Polyxeninae Lucas, 1840
The genus is typical of the subfamily Polyxeninae, due to the structure of the telson, but shows more than 2 transverse rows of barbate trichomes on each tergite.
urn:lsid:zoobank.org:act:B4809CFA-3D81-413E-8F07-ADEEE3398883
http://species-id.net/wiki/Propolyxenus_squamatus
Figs 1–5South Africa, Cape Town, Table Mountain National Park. Cecilia, Rooikat, site 12, felled pine, altitude 300 m, 33°59'43S, 18°25'22E, 4/X/2008, holotype adult female (no. 4); Cecilia, Rooikat, site 9, Afrotemperate forest, altitude 400 m, 33°59'34S, 18°25'12E, 4/X/2008, paratype adult female (no. 3); other paratypes: Kirstenbosch, Afrotemperate forest, site 5, altitude 400 m, 33°58'55S, 18°25'25E, 12/IX/2008, subadult female (12 pl) (no. 2); Cecilia, Spilhaus, Afrotemperate forest, site 13, altitude 400 m, 33°59'43S, 18°25'05E, 18/X/2008, larva with 8 pl (no. 5), all collected from leaf litter by Charmaine Uys and mounted on slides (MNHN).
Cecilia, Spilhaus, Fynbos, leaf litter, site 14, 33°59'53"S, 18°24'52"E, altitude 520 m, 18/X/2008, subadult male (12 pl) (no. 6), used for SEM.
The specific name refers to the scale–shaped tergal trichomes.
Differs from all other congeners by the position and structure of the tergal trichomes: these flat scale–shaped trichomes cover the tergites and are different from the barbate trichomes of the lateral tufts, pleurites and head. They are observed for the first time in the family Polyxenidae. As in the family Synxenidae, they lie close to the tergites and are all directed caudally, but differ from those of Synxenidae in their shape and structure.
Measurements. Body length (without caudal penicil): 2.50 mm (holotype). Tarsus II length of 13th leg: 100 µm (holotype) and 105 µm (paratype).
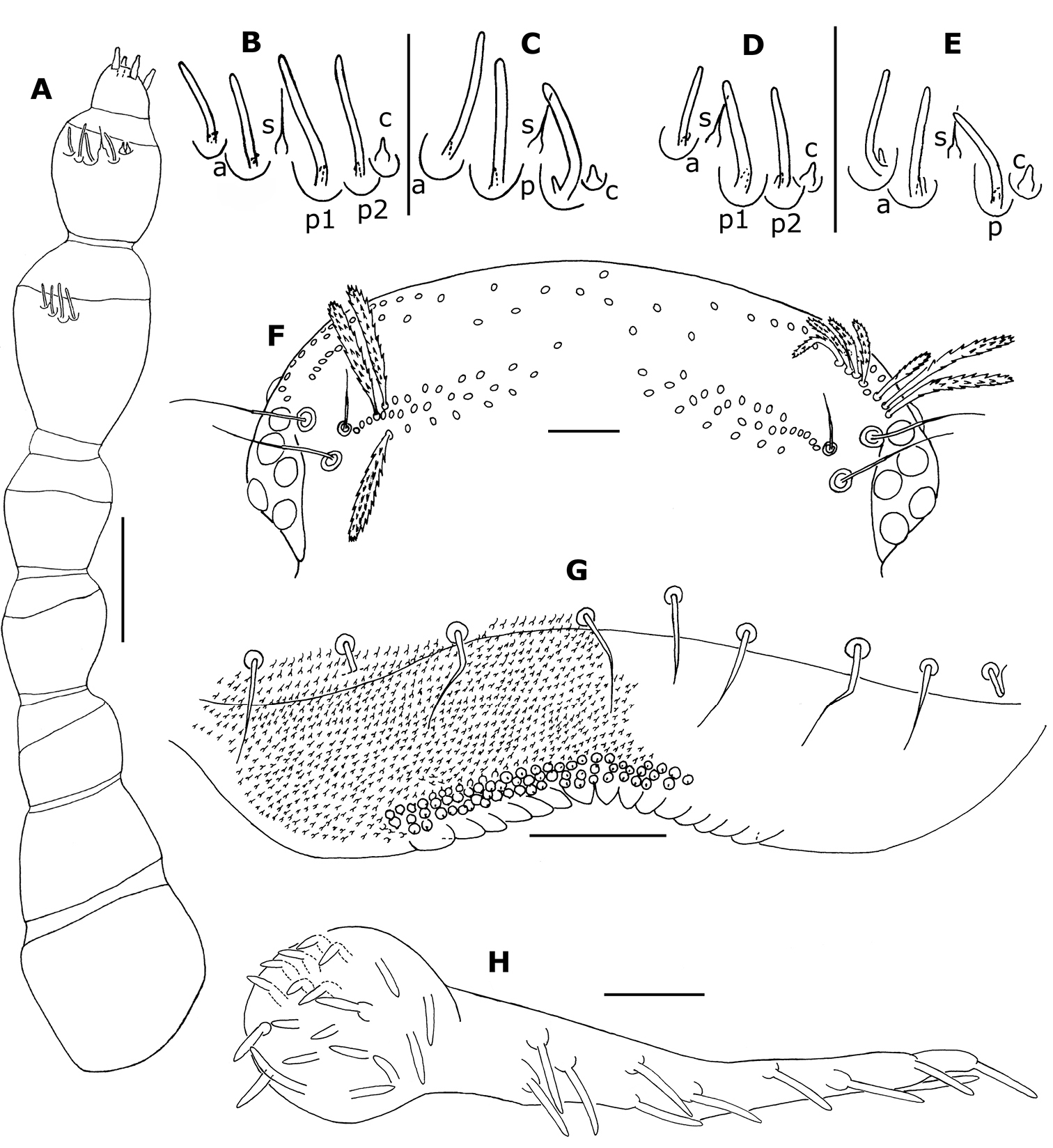
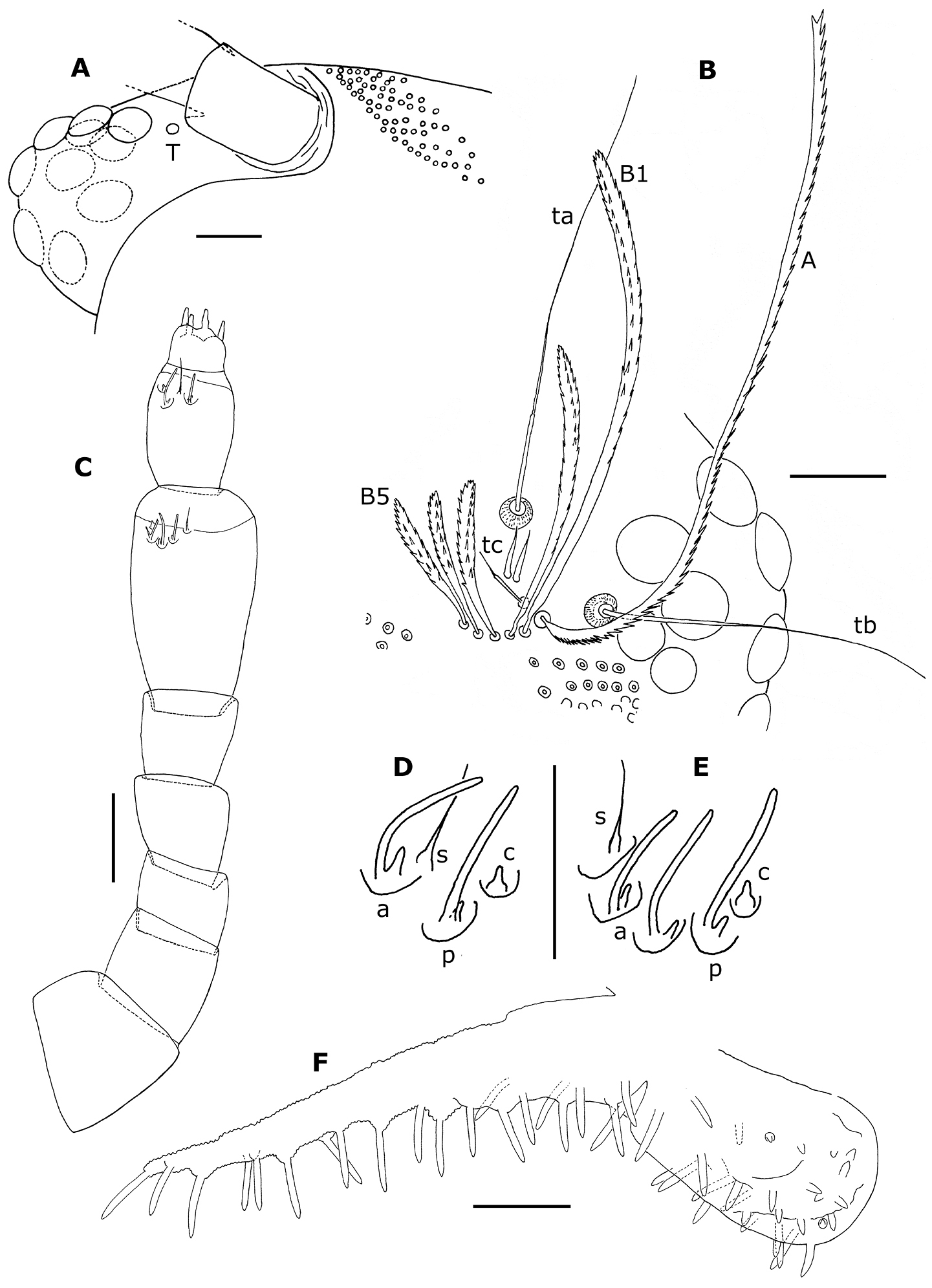
Head (Fig. 2C). 6 ocelli on each side of which 1 antero–sternal (Figs 2D, 3F). Vertex with 1 pair of posterior tufts of 27+27 (holotype) and 24+25 trichomes (paratype), consisting of 3 rows, middle row with 12–13 trichomes (Fig. 3F); the distance between each tuft is about half their length.
Proportions of antennal articles as in Fig. 3A. Antennal article VI with 4 basiconic sensilla (Figs 2E, F, 3A): 2 anterior (a)shorter and thinner than the 2 posterior ones; the more posterior (p2)slightly thinner than the (p1)(Fig. 3B); 1 setiform sensillum (s) between anterior and posterior ones and 1 posterior coeloconic sensillum (c); antennal article VII with 3 basiconic sensilla, the anterior (a) slightly thinner than the others (Fig. 3C), 1 setifom sensillum (s) between the 2 posterior basiconic sensilla and 1 posterior coeloconic sensillum (c). The right antennal article VII of the holotype has 4 basiconic sensilla and 2 coeloconic sensilla, but this is recognizable as a regenerated antenna (as shown by
3 trichobothria, arranged in a triangle, with the most internal (near posterior tufts of vertex) smaller than the 2 others (Figs 2C, D, 3F). Surface of labrum (Fig. 3G) with numerous small short cuspidate papillae; papillae of anterior 2 to 3 rows larger; 7+8 lamellate teeth on anterior margin (holotype: Fig. 3G), 8+8 (paratype); clypeo–labrum with 9 setae along posterior margin (Fig. 3G). Outer palp of gnathochilarium with 11 or 12 sensilla; middle palp with 19 or 20 sensilla (Fig. 3H).
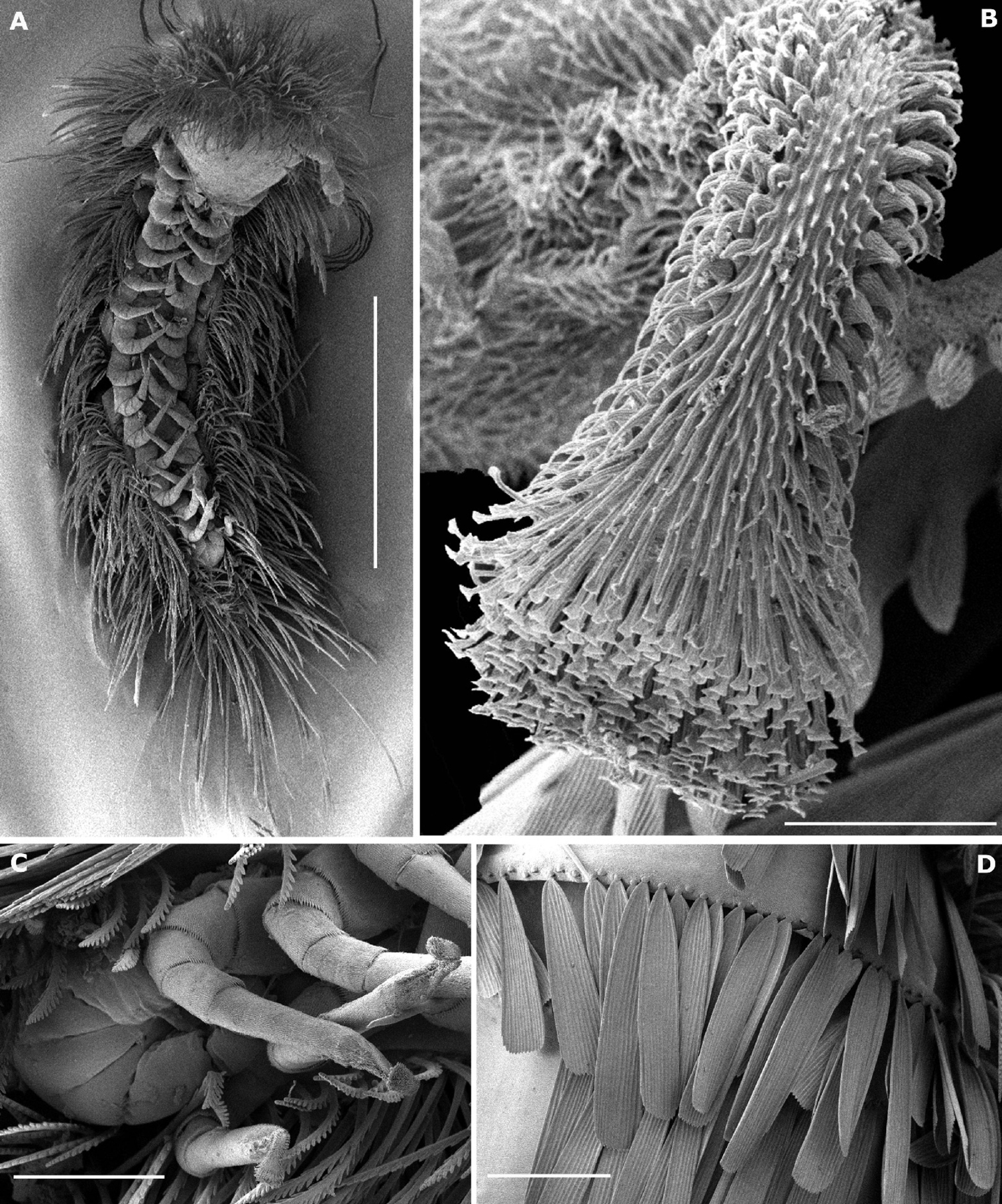
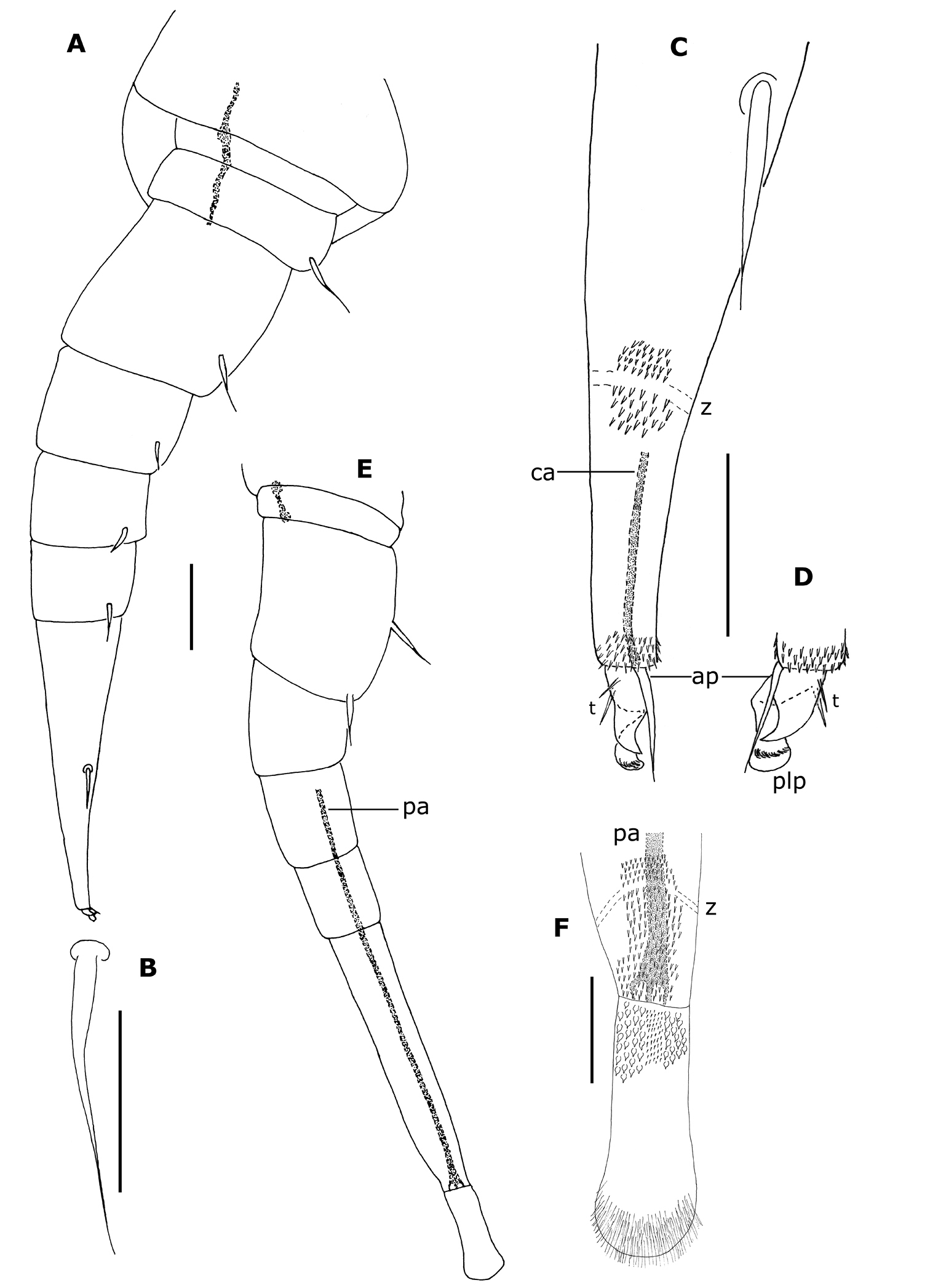
Trunk (Fig. 2A): On each tergite (except collum, tergite X and telson) the trichomes are arranged in 3 rows and 2 lateral tufts (Figs 2B, 4A, B); each paired tuft connected by posterior and anterior rows of trichomes; middle row with more spaced trichomes (Fig. 4A). There are 2 types of trichomes. The flat trichomes, referred to as scales (sc) of the 3 rows are wider than barbate trichomes of lateral tufts (bt) and their shape and structure are different (Figs 2B, 4B, 5A–C); they look like the scales of Synxenidae by their position: all are directed caudally and cover the tergites I (with only a posterior row) to X (Figs 4A, B). Their structure is different from scales of Synxenidae (Figs 5A–E). The trichomes of the lateral tufts are longer and arranged in a bunch (Figs 2B, 4A, B (bt), C). Lateral protuberance of tergite I with 3 barbate trichomes (Fig. 4A).
Legs (Figs 4D–H): Naming of leg segments is after
Telson (Figs 1, 2A): typical of genera Propolyxenus, Polyxenus, Typhloxenus (subfamily Polyxeninae). 21 (holotype) and 25 (paratype) dorsomedian barbate trichomes on caudal penicil. Hooked trichomes with 3 or 4 hooks.
Propolyxenus squamatus sp. n. subadult male, habitus, dorsal view, body length: 2.50 mm. (Photograph by M. Judson).
Propolyxenus squamatus sp. n. subadult male. A habitus, dorsal view B collum and tergites II–V, latero–dorsal view C right part of head with 2 trichobothria and antenna D ocelli and trichobothria E 4 last articles of right antenna F antennal sensilla on articles VI and VII, apical cones on article VIII. Scale bars: A 500 µm B 200 µm C 100 µm D 40 µm E 50 µm F 20 µm.
Propolyxenus squamatus sp. n. A right antenna of holotype female B, C sensilla of right antennal articles VI and VII of female paratype (no. 3) D, E sensilla of articles VI and VII right antenna of larva with 8 pl (no. 5) F vertex of holotype female G labrum of holotype female, papillae only represented on right part H left palp of gnathochilarium female paratype (no. 3). Abbreviations: a anterior basiconic sensillum c coeloconic sensillum p, p1, p2 posterior basiconic sensillum s setiform sensillum. Scale bars: A, F 50 µm; others, 25 µm.
Propolyxenus squamatus sp. n. A Collum and tergite II of holotype female B tergite X of holotype female C barbate trichome of the right lateral tuft of tergite VII of holotype female D left leg 12 of holotype female E, F details of tibial and prefemora setae of the left leg 12 G, H telotarsus and tarsal II spine of right leg 13 of female paratype (no. 3). Abbreviations: ap anterior process bt barbate trichomes plp posterior lamellate process sc scale t latero–anterior and posterior teeth. Scale bars: C, E–H 25 µm; others, 50 µm.
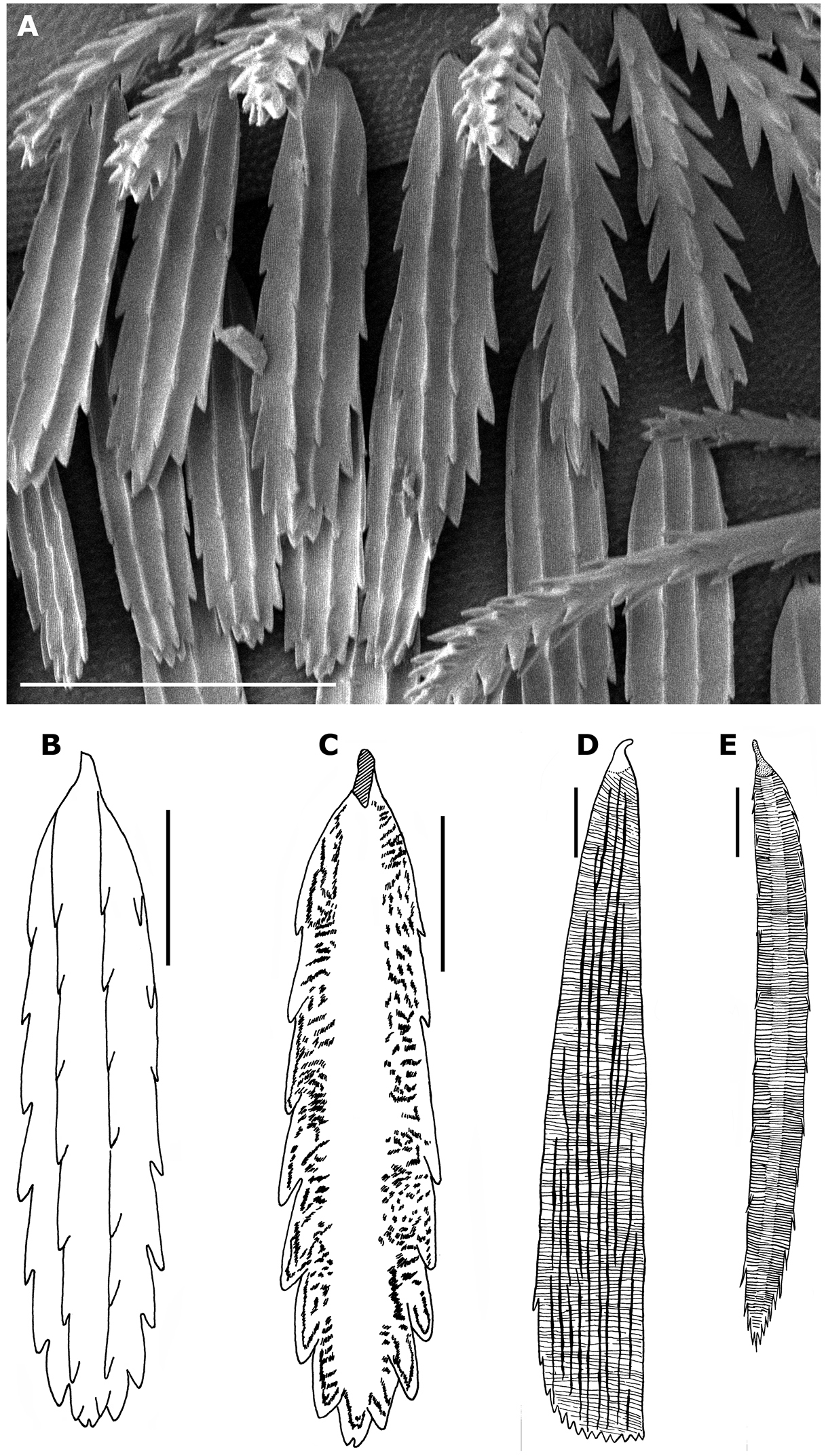
Propolyxenus squamatus sp. n. A tergal scales B, C scale of tergite VII of paratype female. external and internal views respectively D scale of tergite VIII of Phryssonotus capensis male with 12 pl from Mtuzini, Natal E scale of posterior row of tergite VIII of the holotype Condexenus biramipalpus. D and E modified after
Measurements. Body length (without caudal penicil): 2.40 mm. Caudal penicil length: 0.60 mm. Tarsus II length of 12th leg: 112 µm.
Head: 6 ocelli on each side. Antennal article VI with 3 basiconic sensilla (Fig. 3D); antennal article VII with 3 basiconic sensilla (Fig. 3E). Surface of labrum with numerous small short cuspidate papillae; papillae of anterior 2 rows larger; 9+9 lamellate teeth at anterior margin. Outer palp of gnathochilarium with 11 sensilla; middle palp with 19 sensilla.
Trunk: Scales on tergites I-IX. Lateral protuberances of tergite I with 3 barbate trichomes.
Legs: Coxae I with 1 seta and coxae II with 3 setae; all other coxae without setae. All trochanters and prefemora with 1 seta. All tibias (except 11 and 12) have 1 small seta tapered apically; other articles without seta. Telotarsus bearing an anterior process with a spinous projection longer than claw, 2 latero–anterior and posterior spiniform processes; posterior lamellar process thickened and basally pleated.
Telson: 22 dorsomedian barbate trichomes of caudal penicil. Hooked trichomes with 3 or 4 hooks.
Measurements. Body length (without caudal penicil): 1.80 mm. Tarsus II length of 8th leg: 110 µm.
Head: 6 ocelli on each side. Vertex with 1 pair of posterior tufts of 20+19 trichomes consisting of 3 rows, the middle row with 10 trichomes. Antennal article VI with 3 basiconic sensilla: 1 anterior shorter and thinner than the 2 posterior ones; the more posterior slightly thinner than the other; 1 setiform sensillum between anterior and posterior basiconic sensilla and 1 posterior coeloconic sensillum; antennal article VII with 3 basiconic sensilla, the anterior slightly thinner than the others, 1 setiform sensillum between the 2 posterior basiconic sensilla and 1 posterior coeloconic sensillum. 3 trichobothria, arranged in a triangle, with the most internal smaller than the 2 others. Surface of labrum as in adult females; clypeo–labrum with 10 setae along posterior margin. Outer palp of gnathochilarium with 12 sensilla.
Trunk: Trichomes arranged in 2 lateral tufts with 19 to 25 barbate trichomes connected by 3 rows of scales on tergites III–V and only 2 on other tergites. The tergites II–V have 22 to 31 scales, the collum 12 and the tergite VII 18. Lateral protuberance of tergite I with 3 (left) and 2 (right) barbate trichomes.
Telson: 18 dorsomedian barbate trichomes of caudal penicil. Hooked trichomes with 3 hooks (rarely 2 and 4).
Propolyxenus squamatus sp. n. is strongly distinguished from other species of the genus by the shape of the trichomes covering the tergites. Compared with the most closely related species Propolyxenus lawrencei Condé, 1949, from Natal (Champagne Castle, Drakensberg Mountains, alt. 6000 ft.), Propolyxenus squamatus sp. n. shares the following characters: 6 ocelli; internal trichobothrium shorter than the other 2; number and shape of sensilla on antennal articles VI and VII (
The new species shows the following important differences from Propolyxenus lawrencei:
Position and structure of trichomes on tergites: on each tergite (except collum and telson) the trichomes are arranged in 3 rows and 2 lateral tufts; each paired tuft being connected by posterior and anterior rows of trichomes; the middle row has more spaced trichomes. In Propolyxenus lawrencei, the trichomes are arranged in 3 or 4 irregular rows, forming 2 elongated lateral areas, slightly separated by a narrow medial space.
There are two types of trichomes: the trichomes of the three rows are wider and flatter than the trichomes of the lateral tufts, pleurites and head, and their shape and structure are different, being observed in the family Polyxenidae for the first time. They can be compared to the scale–shaped trichomes of Synxenidae: the trichomes of the rows are all directed caudally and cover the posterior half of tergites II–X and their internal structure is reinforced by differently distributed chitinous elements (Figs 5C–E). The lateral trichomes are longer and arranged in lateral tufts. It is remarkable that the barbate trichomes of Propolyxenus squamatus sp. n. show a progressive transformation into scale–shaped trichomes in the posterior row of the tergite, representing a transition between the two types of trichomes as if, during the course of evolution, the former trichomes had changed into scale–shaped trichomes. These scale–shaped trichomes are thought to protect the animals from desiccation, abundant rain or other environmental disturbances.
In a key of the genus Propolyxenus, Propolyxenus squamatus sp. n. would be easily distinguished from all other congeners as is only species with scale-like trichomes. The other species of Propolyxenus are more difficult to identify using morphological characters such as the number of ornamental trichomes or coxal glands of the males. For instance, both Propolyxenus patagonicus (Silvestri, 1903) and Propolyxenus australis Short and Huynh, 2010, bear four pairs of coxal glands (cf.
The genus Phryssonotus is characterised by the tergites having dark striated, scale-shaped trichomes directed caudally, all the others being long, dark barbate trichomes; trichomes A and B on head close to trichobothria, one of them being shorter and different from the two others.
urn:lsid:zoobank.org:act:DADD9CDA-BB36-491F-85BB-FE609759F2E9
http://species-id.net/wiki/Phryssonotus_brevicapensis
Figs 6–9South Africa, Cape Town, Table Mountain National Park. Tokai S, site 30, Fynbos, altitude 310 m, 34°04'01S, 18°24'03E, leaf litter, 24/XI/2008, holotype adult male (no. 16a) and 1 paratype adult male (no. 16b) (MNHN). Other paratypes: adult male (no. 1) (MNHN), Newlands, site 4, pine plantation, altitude 260 m, 33°58'24S, 18°26'27E, sugar–baited ant trap, 15/I/2009; adult female (no. 20) (MNHN), Tokai S, site 31, pine plantation, altitude 300 m, 34°03'54S, 18°24'10E, decaying log, 19/I/2009; adult female (no. 11) (MNHN), Constantia Nek, site 19, felled pine, altitude 330 m, 34°00'20S, 18°24'45E, pitfall trap, 02/II/2009; male with 14 pl (subadult) (no. 8) (MNHN), Cecilia, Spilhaus, site 16, felled pine, altitude 470 m, 34°00'04S, 18°24'46E, pitfall trap, 23/0I/2009; female with 14 pl (subadult) (no. 13) (MNHN) and female with 12 pl (no. 15) (MNHN), Tokai N, site 27, pine plantation, altitude 330 m, 34°02'23S, 18°23'53E, leaf litter, 21/XI/2008; 2 larvae with 10 pl and 8 pl (no. 12) (MNHN), Orange Kloof, site 22, pine plantation, altitude 240 m, 34°00'23S, 18°24'02E, leaf litter, 18/XI/2008. All specimens collected by Charmaine Uys.
1 male with 14 pl (no. 6b) was collected at the same site as a male with 12 pl of Propolyxenus squamatus sp. n. (no. 6, used for SEM).
Refers to the shorter body length and development compared to the most closely related species, Phryssonotus capensis.
10 ocelli; 5 trichomes B close to the smallest trichobothrium (tc) as in Phryssonotus capensis. Differing from Phryssonotus capensis with 10 rings in adults instead of 11 (without telson), 15 leg pairs instead of 17, and the attendant shorter post–embryonic development. Males with 3 pairs of coxal glands on legs 7–9.
Measurements. Body length (without caudal penicil): 4.00–4.50 mm; caudal penicil length: 0.90–1.00 mm (Figs 6, 7A).
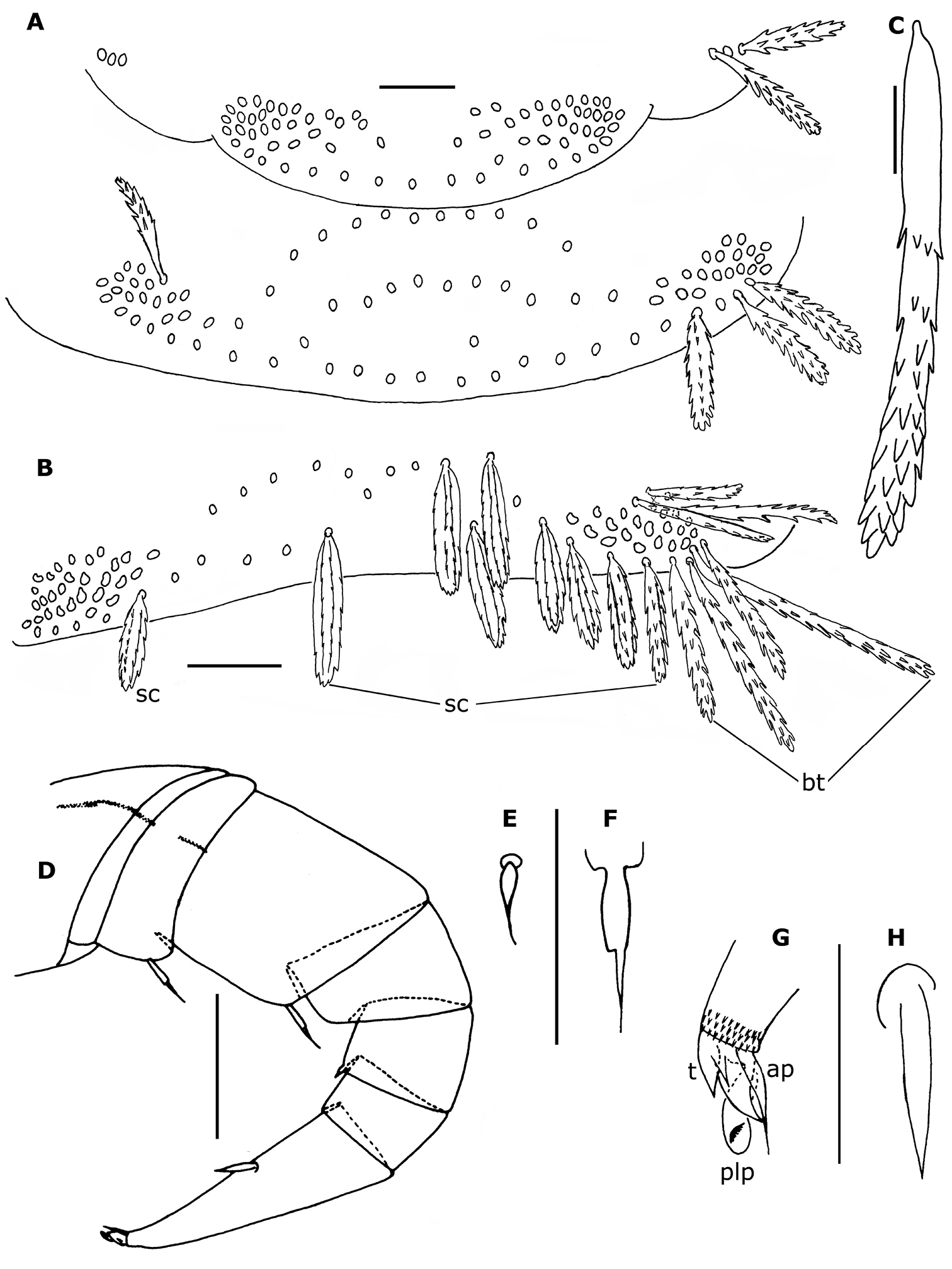
Head with 10 ocelli on each side (Fig. 8A); 3 trichobothria with the anterior 1 (tc) possessing a much shorter sensory hair than the other 2 (ta and tb). 5 short frontal trichomes B1–B5 and 1 long, curving trichome A (Fig. 8B).
Proportions of antennal articles as in Fig. 8C. Antennal article VI with 3 basiconic sensilla (Fig. 8C, E): 2 anterior (a), which are shorter and slightly thinner than the posterior one (p); 1 anterior setiform sensillum (s) and 1 posterior coeloconic sensillum (c); antennal article VII (Fig. 8D) with 2 basiconic sensilla, the anterior (a) slightly shorter than the posterior one (p), 1 setifom sensillum (s) between the 2 basiconic sensilla and 1 posterior coeloconic sensillum (c).
Surface of labrum with numerous, small, short cuspidate papillae; papillae of anterior 3 or 4 rows larger, the size of the following papillae decreasing progressively, the smaller ones in the posterior third; about 30 lamellate teeth at anterior margin. Clypeo–labrum with ca. 10 setae, about 3/4 maximum width of labrum. Lateral expansions of gnathochilarium about twice as long as diameter of middle palp, with 21–25 sensilla, middle palp with 26–29 sensilla, of which antero–medial sensilla shorter than the others (Fig. 8F).
Trunk of adults with 11 tergites (including collum and telson) and 15 pairs of legs (Fig. 7A). Collum with 2 medial, separate oval clusters comprising 80–90 barbate trichomes and a lateral group of 8–14 barbate trichomes. Tergites II–X with submedial and posterior rows of scale–shaped trichomes directed caudally (Fig. 7D), the posterior row arranged along the posterior margin of the tergite; 1 area of aligned barbate trichomes at end of each row, except on tergite II where are 35–40 barbate trichomes arranged, on each side, in 2 diagonal lines above the first scale–row; 2 short rows of barbate trichomes at end of submedial scale–row and 4 (sometimes 5 on tergite II and X) short rows of barbate trichomes at end of posterior scale–row. The number of scales by row ranges from 29–62 on tergites II–X.
Legs short (Fig. 9A), with 8 articles except on legs 1, 14 and 15; last 2 pairs (14–15) without telotarsus, tarsus II terminated in palettes (Figs 7B, C; 9E, F); palettes covered by numerous cuticular setae of different types (Figs 7B; 9F); apodeme of palettes (pa) and claws (ca) extending into distal part of trochanter and linked to the flexor unguiculi muscle, which allows a great flexibility of the palette of the leg pairs 14 and 15, as well as to the claws of legs 1–13, in accordance with the description given by
Female: large vulval sacs elongated, reaching as far as fourth pair of legs and bearing numerous small setae inserted in parallel circles and sparse longer setae.
Male: all areas of penis with usual thin cuticular setae and about 15 longer setae (holotype). Coxal glands on legs 7–9.
Conical telson with a transverse row of 15 (male no. 1), 10 (male no. 16b) or 14 (female no. 20) scale–shaped trichomes with each lateral end prolonged by barbate trichomes; long barbate trichomes on distal part.
Phryssonotus brevicapensis sp. n, habitus, dorsal view, body length: 5 mm. (Photograph by M. Judson).
Phryssonotus brevicapensis sp. n. A habitus, adult male, ventral view showing 15 pl B detail of a palette of left leg 15 C postero–ventral view showing leg pairs 14 and 15 terminating in palettes D detail of scales arranged along posterior margin of tergite. Scale bars: A 1 mm B 10 µm C, D 100 µm.
Phryssonotus brevicapensis sp. n. A right ventral part of head of female adult (no. 20) showing the 10 ocelli, dorsal ones shown with dotted lines B right part of the head of holotype showing position of trichobothria ta, tb and tc, long frontal trichome A and short trichomes B1–B5 (only some ocelli drawn) C left antenna of holotype; the posterior sensillum is abnormally bifurcated on article VI D, E antennal sensilla on articles VII and VI of right antenna of holotype F right palp of gnathochilarium of holotype. Abbreviations: a anterior basiconic sensillum c coeloconic sensillum p posterior basiconic sensillum s setiform sensillum; T, Tömösvary’s organ. Scale bars: A, B, C 50 µm; others, 25 µm.
Phryssonotus brevicapensis sp. n., adult male (no. 1). A right leg 12 B seta of prefemur of right leg 12 C distal part of tarsus II of the right leg 12 D telotarsus of left leg 12 E right leg 15 F distal part of tarsus II and palette of right leg 15. Abbreviations: ap anterior process ca apodeme of claw pa apodeme of palette plp posterior lamellate process t latero–anterior and posterior teeth z smooth area. Scale bars: A, E 50 µm; others, 25 µm.
1 male, 1 female: Measurements: Body length (without caudal penicil): 3.20 mm (male no. 8) and 3.90 mm (female no. 13); caudal penicil length: 0.90 mm. 14 pairs of legs, the 14th terminating in a palette; 1 pair of appendage–buds on lateral side of anal valves, from which 15th pair of legs will develop, the future adult stadia having the leg pair 15 terminating in palettes. Other characters as in adults, except no scale–row on telson.
Male: Coxal glands on legs 7–9.
Female: large vulval sacs elongated, reaching as far as fourth pair of legs.
1 female (no. 15) with 12 pl; body length (without caudal penicil): 3.80 mm; caudal penicil length: 0.80 mm; 10 ocelli, vulval sacs elongated, reaching as far as third pair of legs. 2 pairs of external buds.
1 juvenile male with 10 pl; body length (without caudal penicil): 3.30 mm; 10 ocelli; rudimentary coxal glands on legs 7 and 8. 2 pairs of external buds.
1 larva with 8 pl; body length (without caudal penicil): 2.70 mm; 9 ocelli. 2 pairs of external buds.
Phryssonotus brevicapensis sp. n. exhibits all the general characters usually present in the family Synxenidae: long and thin dark barbate trichomes all along the body, tergites covered by tergal scale–shaped trichomes that are striated and arranged in 2 transverse rows along all the tergites except the collum; telson subconical; elongated vulvae; and last 2 leg pairs terminating in palettes instead of claws. It also shows the typical structure of the scale–shaped trichomes found in the genus Phryssonotus. Phryssonotus brevicapensis sp. n. differs from other members of the genus in having 11 tergites and 15 pl; the last 2 pairs (14th and 15th) terminating in palettes; and males with 3 pairs of coxal glands on legs 7–9. These differences are strongly related to biology and development, and justify the creation of a new species. All other species of Phryssonotus have 12 tergites and 17 pairs of legs with the last 2 pairs (16th and 17th) terminating in palettes, and males with 3 pairs of coxal glands on legs 9–11. Due to its shorter length and the position of the coxal glands on legs 7–9, it is similar to Condexenus biramipalpus. The elongated vulvae (ovipositors) of the females also resemble those of Condexenus species in reaching as far as the fourth pair of legs, as opposed to sixth at most in other Phryssonotus species.
Phryssonotus brevicapensis sp. n. is most closely related to Phryssonotus capensis, in having 10 ocelli and 5 short frontal trichomes B1 to B5, Phryssonotus capensis has 5–6 trichomes B (
Following the discovery of Condexenus biramipalpus from Namibia, it is of great interest to add Phryssonotus brevicapensis sp. n. as the second example of reduction of ring and leg number in the family Synxenidae, whose representatives bear the largest number of segments among the Penicillata. This supports the hypothesis of a trend towards a shortened postembryonic development during the course of evolution of Polyxenida (
Comparison of segmentation in Polyxenida, corrected and improved after
We are very grateful to Mike Picker [UCT Cape Town] for support, to Miranda Waldron [UCT Cape Town] for scanning electron micrographs, to Dr Mark Judson [MNHN Paris] for the colour photographs, comments and linguistic improvement of the text, and to Megan Short and Hans Reip for very careful reviewing.