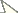(C) 2010 Silvio Shigueo Nihei. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Diaugia angusta Perty, 1833 is a Neotropical species of Tachinidae (Diptera) reported here as a parasitoid of Metamasius ensirostris (Germar, 1824) and Metamasius hemipterus (Linnaeus, 1758) (Coleoptera: Dryophthoridae) in Brazil. Several species of Dryophthoridae and Curculionidae cause damage to bromeliad and palm species, and most are regarded as pests. In the present study, the male and female of Diaugia angusta are morphologically characterized and illustrated to provide a means for the identification of this parasitoid. Data obtained from preliminary field research show that natural parasitism of Metamasius pupae by Diaugia angusta varies by year but can reach nearly 30%. A network of parasitoid-host interactions among tachinid parasitoids and coleopteran hosts reported as bromeliad and palm pests (Dryophthoridae and Curculionidae) in the Americas indicates that the species of the tribe Dexiini sensu lato (including Diaugia angusta) might be promising as biological control agents of these pests.
Dexiinae, taxonomy, host record, biological control, palm, bromeliad, Brazil, Neotropical Region
The Neotropical genus Diaugia (Tachinidae: Dexiinae: Dexiini) was described by
Metamasius Horn (Coleoptera: Dryophthoridae)
is a Neotropical weevil genus comprising about 110 species, mostly
associated damaging bromeliads, sugarcane, bananas and palms (
Recently, great efforts have been applied in order to
find ways to control the bromeliad and palm weevils, such as the field
exploration for parasitoids (
In the present study, comprehensive material of Diaugia from several museums was studied in detail. Within the material, there was one specimen reared on Metamasius ensirostris from the state of Santa Catarina (southern Brazil), and several specimens reared on Metamasius hemipterus from the state of São Paulo (southeastern Brazil). Additionally, there were some specimens from São Paulo reared from an undetermined Metamasius.
This paper aims to provide taxonomic support for the identification of this parasitoid and for its potential use as a biological control agent of Metamasius species. We provide a detailed morphological characterization of Diaugia angusta Perty. Both male and female specimens are characterized, the male terminalia are described and illustrated for the first time, and photographs and distribution map are presented. In addition, based on preliminary field research performed in the state of São Paulo (Brazil), some biological data on the interaction between Diaugia and Metamasius is presented. Finally, a parasitoid-host network is elaborated showing the interactions between these parasitoid flies (Tachinidae) and coleopteran hosts reported as bromeliad and palm pests (Dryophthoridae and Curculionidae) in the Americas.
Material and methodsThe examined material is deposited at the following
institutions: Museu Nacional, Universidade Federal do Rio de Janeiro,
Rio de Janeiro, Brazil (MNRJ); and Museu de Zoologia, Universidade de
São Paulo, São Paulo, Brazil (MZSP). The morphological terminology
follows mainly
The subfamily and tribal classification of Tachinidae followed here is that used in the latest Nearctic Catalogue by
To elaborate the network of parasitoid-host interactions, we included all the species of Tachinidae recorded as parasitoids of species of Dryophthoridae and Curculionidae
which are reported in the literature as pests of bromeliads and palms
in the Americas. However, the network is complete only in that all the
hosts of “Zeliini” and all the parasitoids of Dryophthoridae and Curculionidae
are presented. For the other taxa in the network, neither all
parasitoids nor all hosts are presented. For complementary information
about them, please refer to
Diaugia differs from other South American Dexiini by the following combination of characters: eye bare; arista densely long plumose; no facial carina; parafacial bare; proepisternum bare; intrapostalar seta absent; 2 katepisternal setae; katepimeron (barette) setulose anteriorly; costal spine undeveloped; R1 without setulae; base of R4+5 setulose dorsally and ventrally; abdominal syntergite 1+2 and tergite 3 without median marginal setae (but females have a pair of median marginal setae on tergite 3) and all tergites without discal setae (but some ground setulae rather developed middorsally); male abdomen conspicuously elongate (although not caudate as in Uramya Robineau-Desvoidy and Trichodura Macquart).
Among Dexiini, Diaugia undoubtedly resembles the other genera formerly included in the extinct tribe Zeliini (sensu
Figures 1–11
Male: Body length: 13.5 mm (11.5–15.0), wing length: 11.0 mm (10.0–12.0) (n=20).
Colouration (Figures 1–7): Frontal vitta dark-brown to black (Figure 3); head silver or light-golden pruinose but gena with reddish dark brown area from the eye lower margin to vibrissal angle (Figures 1). Antenna dark-brown. Palpus yellowish brown; proboscis dark-brown. Thorax dark-brown to black with silver or light-golden pruinosity (Figure 6); the scutum with 4 dark stripes not reaching the scutellum, and the remaining scutum silver pruinose. Wing hyaline, the membrane tinged with light-brown or at least along the veins (Figure 5); calypteres and halter brown. Legs dark brown with silver pruinosity on coxae and femora. Abdomen (Figures 5 and 7) dark brown to black with silver pruinosity on anterior margin of tergites 3 to 5, and with extensive yellow areas on sides of syntergite 1+2 and tergites 3 and 4, both dorsally and ventrally.
Head (Figures 1, 3): Eye apparently bare, with very short and sparse setulae. About 14 pairs of frontal setae. Two minute proclinate fronto-orbital setulae; fronto-orbital plate bare and narrower than frontal vitta and parafacial. Parafacial bare. Facial ridge with few weak setulae near vibrissa. Postpedicel slender, 4x the length of pedicel; arista long plumose, with 2–3 dorsal and 2 ventral rows (but one single ventral row on basal half), with about same length of postpedicel, and the length of longest cilia about 5x the basal width of arista. Vibrissa fine and long, inserted at level of lower facial margin. Genal dilation covered by fine pale setulae. Palpus filiform; labella developed, slightly shorter than prementum, which is as long as palpus.
Diaugia angusta: 1 male head, lateral view 2 female head, lateral view (head partially collapsed) 3 male head, frontal view 4 female head, frontal view. (Scale bar: 1 mm)
Thorax (Figure 6): Acrostichals 3+1, but a weak pair before the postsutural pair. Dorsocentrals 3+4, but seems 3+3 as the second postsutural is weakly developed or indistinct from ground setulae in some specimens. Posthumerals 2, aligned with the intralar row, the posterior seta stronger and the anterior located lateral to the postpronotum. Presuturals 2, the posterior stronger. Postpronotals 3. Notopleurals 2. Postutural intra-alars 2, the anterior weak; intra-postalar absent. Postsutural supra-alars 3, the anteriormost (prealar) weakly developed, about 1/3 the length of the strongest supra-alar and shorter than the first postsutural intra-alar and dorsocentral. Prosternum and proepisternum bare. Six strong anepisternal setae. Katepisternals 2, but some specimens with a reduced lower anterior seta is present. Katepimeron (barette) setulose anteriorly. Scutellum with one basal, one lateral, one apical and one discal pairs of setae.
Wing (Figure 5): costal spine undeveloped; base of R4+5 setulose dorsally and ventrally; M vein bent forward to R4+5, and convex after bend.
Legs: Fore tibia with 2 posterior setae. Mid femur with 2 anterior setae on median third, 2 dorsal preapical setae, and one posterodorsal preapical setae. Mid tibia with 1 submedian anterodorsal seta, 2 posterior setae on apical and basal third (the basal seta weak), and one ventral seta on apical third. Hind tibia with an anterodorsal row of irregularly sized and spaced setae but one strong submedian seta; with one submedian anteroventral and two posterodorsal seta (the submedian stronger).
Abdomen (Figure 7): Abdomen elongate and tapering to apex in dorsal view. Syntergite 1+2 and tergite 3 each with one lateral marginal seta. Tergite 4 with a marginal row of setae, the ventral setae reduced. Tergite 5 with marginal row of setae, with the ventral setae reduced; no discal setae but the ground setulae rather developed dorsally.
Diaugia angusta: 5 male wing, lateral view 6 male thorax, dorsal view 7 male abdomen, dorsal view. (Scale bar: 1 mm)
Terminalia (Figures 8–10): Cercal plate enlarged at base and tapering to the apex in posterior view (Figure 9), with long setulae mostly on base, the tip long and narrow and slightly curved inwards, ending before apex of surstylus (Figure 8). Surstylus broad and the apex rounded, with subparallel margins in lateral view (Figure 9), while in posterior view tapering slightly to the subtruncate apex, the inner surface of surstylus concave. Pregonite and postgonite widely fused as a single piece on each side and firmly connected to the hypandrium (Figure 10); pregonite+postgonite somewhat stout at basal 2/3, strongly curved downwards at middle and tapering to the apex. Aedeagal apodeme straight, with subparallel margins, and elongate, longer than hypandrium (Figure 10). Epiphallus enlarged at base but uniformly narrow until a subtruncate apex, a little longer than half length of aedeagal apodeme. Distiphallus composed of a dorsal sclerite, long, straight and quite narrow, and anteriorly to it, a ventral membrane narrow and extremely elongate. The latter curved at apical half and bearing spinulae all along, these spinulae with their points upwardly oriented and becoming tinier towards the apex (Figure 10).
Diaugia angusta: 8 male terminalia, lateral view 9 male terminalia, posterior view 10 aedeagus in lateral view and the detail showing the tip of aedeagal apodeme in dorsal view. (Scale bar: 0.5 mm)
Female: Body length: 11.0 mm (11.5–15.0), wing length: 9.0 mm (7.5–10.5) (n=12). Differs from male in the following: two strong proclinate fronto-orbital setae (Figures 2, 4); fronto-orbital plate as wide as both frontal vitta and parafacial; fore tibia with 1 posterior seta; abdomen oblong in dorsal view, not elongate as in males, with the yellow areas on sides of syntergite 1+2 and tergites 3 and 4 less extensive than in male; tergite 3 with a median marginal pair; and tergite 5 with the ground setulae weakly developed.
Within the examined material, the specimens showed some significant intraspecific variation in colouration: body colouration varied from dark brown to black (compare Figures 3 and 4); head and thorax with silver to light-golden pruinosity (compare Figures 1 and 2, and 3 and 4); wing always hyaline but the membrane tinged throughout with light-brown (Figure 5) or infuscated at least along the veins, and in some specimens with no noticeable infuscation.
BRAZIL: Goiás, Anápolis, 1 male, 3.iii.1937, 1 male, vii.1934, Serviço Febre Amarela M.E.S. leg. (MZSP); Rio de Janeiro: Itatiaia, 700m, 1 male, 28.iv.1941, J. F. Zikán leg. (MZSP); Rio de Janeiro, Corcovado (Paineiras), 2 males (MZSP), 1 male (MNRJ), iii.1934, L.T. [Travassos] leg. (MZSP); idem, 1 male, xi.1935, L. Travassos leg. (MNRJ); idem, 1 male, 19.i.1938, Oiticica leg. (MZSP); Rio de Janeiro, Jardim Botânico, 1 male, vi.1935[? year hardly readable], H. S. Lopes leg. (MZSP); São Paulo: Barueri, 1 male, 3.iv.1957, 1 male, 20.iv.1957, 1 male, 20.vi.1957, 1male, 28.xii.1965, 2 males, 15.i.1966, 1 male, vii.1966, K. Lenko leg. (MZSP); Juquiá, 3 males and 3 females, i.2006, R. Pavarini leg. (ex. Metamasius sp. in “pupunha” crop [Bactris gasipaes Kunth]) (MZSP); Pariquera-Açu, 8 males and 11 females, iv-xii.2007, P.H. Silva & R. Pavarini leg. (ex. Metamasius hemipterus in “pupunha” crop – Bactris gasipaes) (MZSP); Araçatuba, Sítio Santo Amaro, 1 male, 6.i.1963, Rabello leg. (MZSP); Salesópolis, Boracéia, 1 male, 10–14.xi.1947, L. Trav. F., G. Ramalho & E. Rabello leg. (MZSP); idem, 2 males, 14.viii.1947, E. Rabello, Trav. F. & J. Lane leg. (MZSP); São Paulo, Ipiranga, 1 male, i.1932, R. Spitz leg. (MZSP); Paraná, Rio Negro, 1 male, 7.i.1929, no collector (MZSP); Santa Catarina: Blumenau, 1 male, xii.1924, Luederwaldt leg. (ex. Metamasius ensirostris) (MZSP); Nova Teutônia, 1 male, iv.1964, 1 male, viii.1967, F. Plaumann leg. (MZSP).
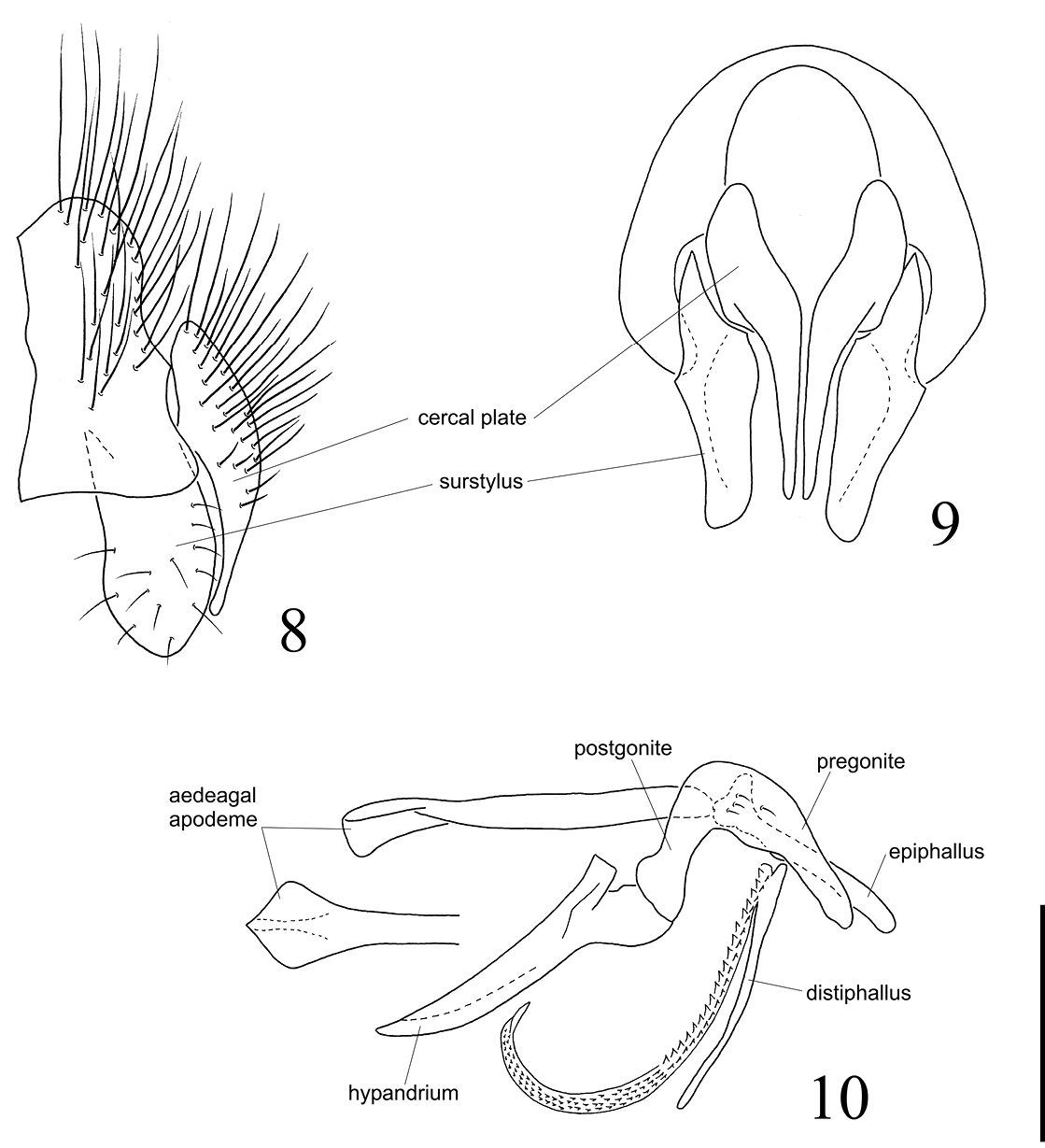
BRAZIL (states of Goiás, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina) (Figure 11).
Based on geographical data of the examined material, Diaugia angusta has been recorded in areas covered by Atlantic Forest (Figure 11). Most areas are characterized by dense evergreen ombrophilous tropical forests, whereas there is a single record in semi-deciduous forest (Brazil: São Paulo: Araçatuba). Outside the Atlantic Forest, this species also occurs in drier biomes such as Cerrado (Brazil: Goiás: Anápólis), however, it is very likely that it actually inhabits the gallery forests (humid forests accompanying riverine systems). Additionally, based on the known records, the altitude ranges from sea-level to 900 meters.
Distribution map of Diaugia angusta (black dots).
i) Metamasius ensirostris (Germar, 1824) (Coleoptera: Dryophthoridae) (new record) from Blumenau, state of Santa Catarina;
ii) Metamasius hemipterus (Linnaeus, 1758) (new record) from Pariquera-Açu, state of São Paulo;
iii) Metamasius sp. (new record) from Juquiá, state of São Paulo.
The junior author is developing a long-term research project searching for natural enemies of boring weevils attacking peach-palm crops (“pupunha”, Bactris gasipaes). In a non-commercial crop of peach-palms in the municipality of Pariquera-Açu (southernmost of state of São Paulo), larvae of Metamasius sp. (undetermined species) were observed attacking the stems. Population peaks of the weevil were recorded during the months with higher temperatures and higher levels of precipitation.
Between April and December
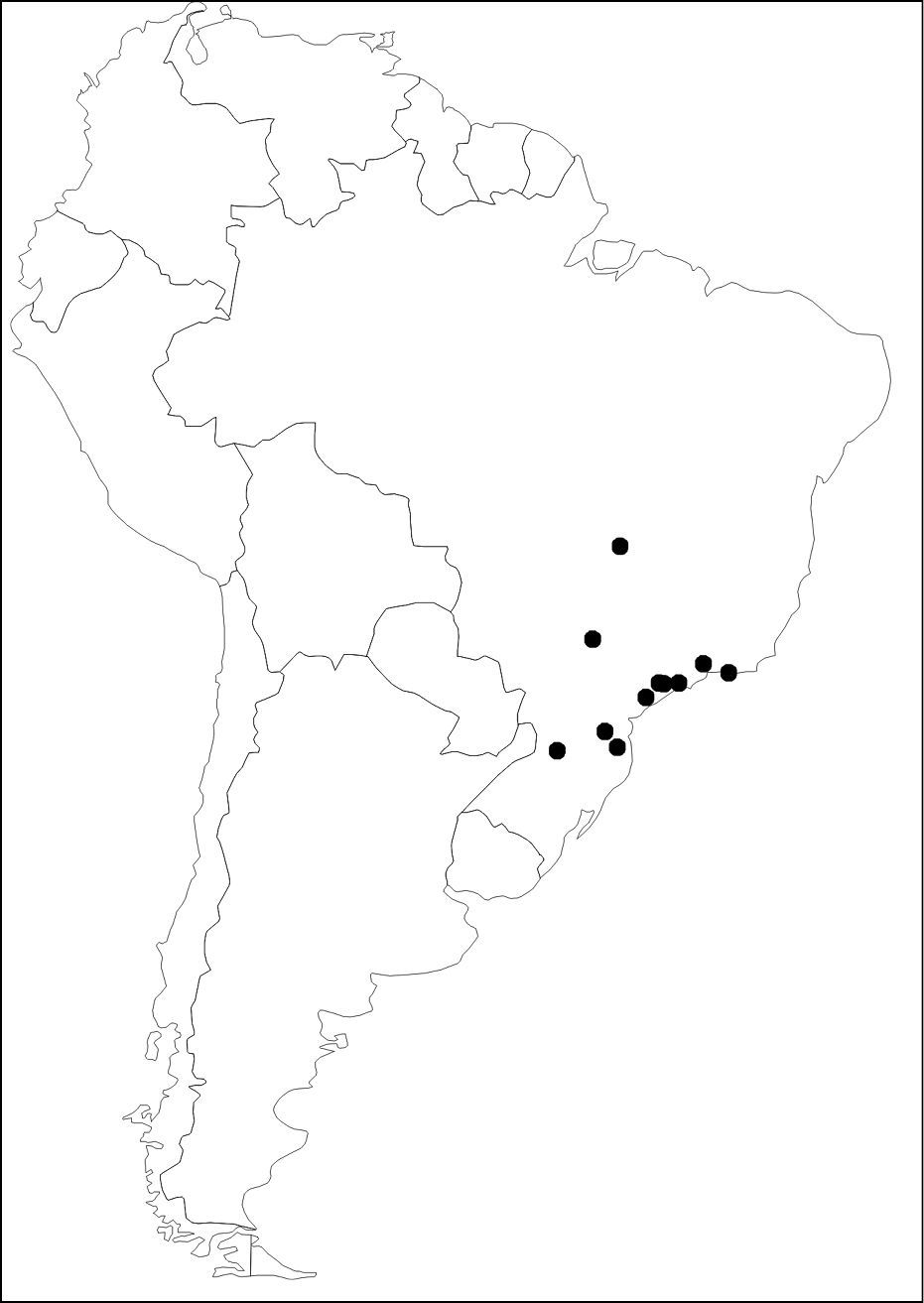
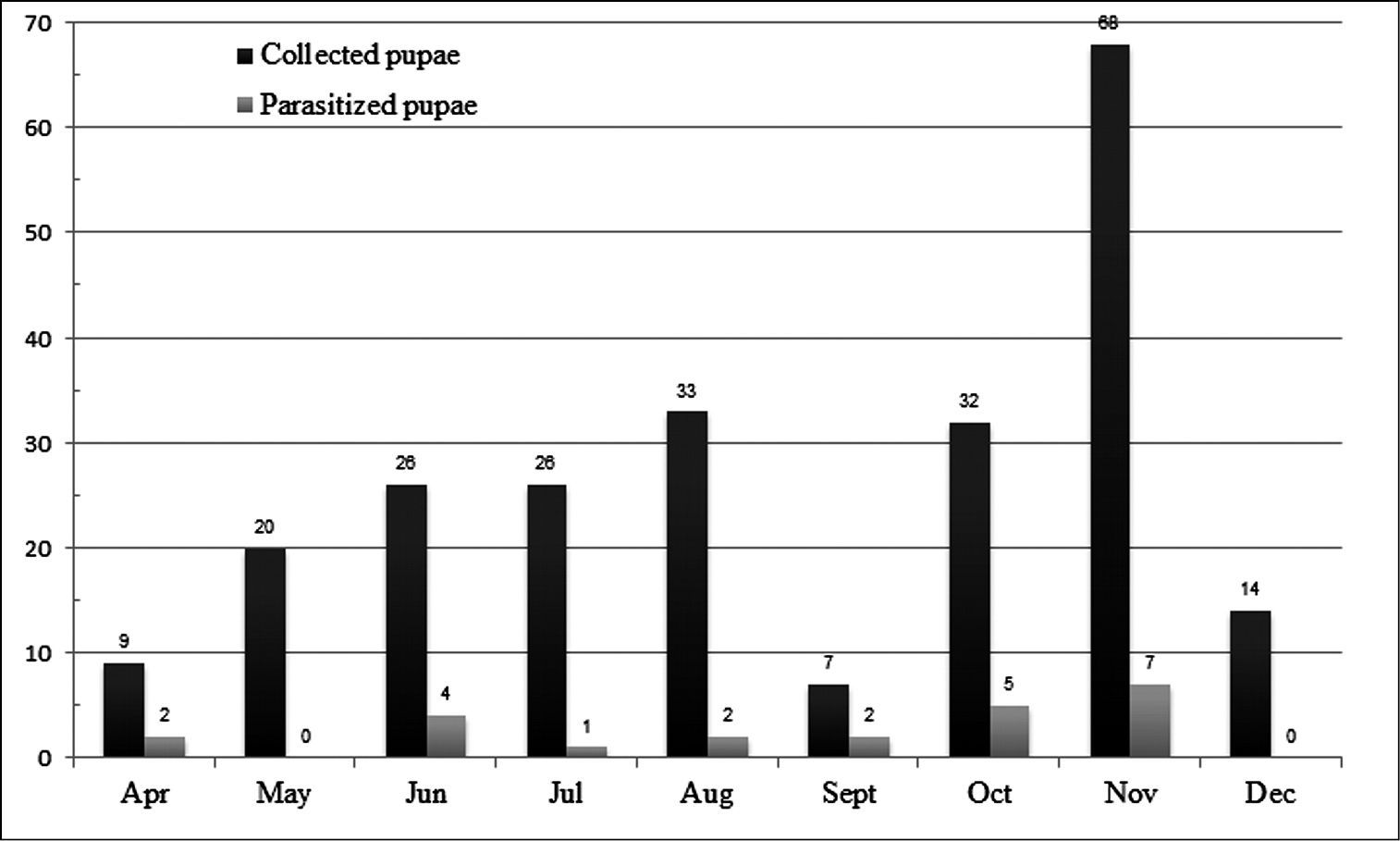
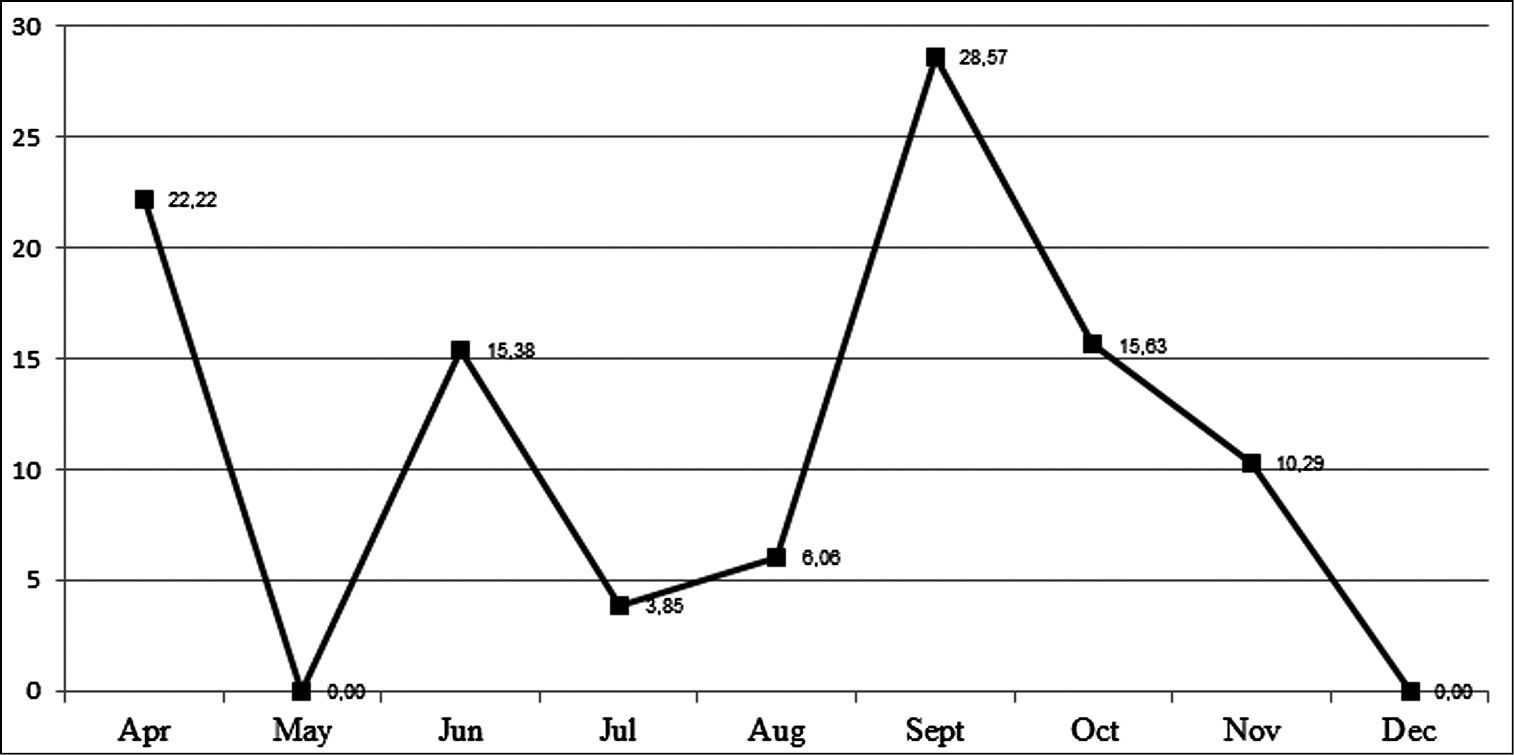
Within the 235 field-collected pupae, 23 were parasitized by the tachinid Diaugia angusta. This parasitism varied along the time (Figure 12), ranging from 28.57% in September to 0.00% in May and December (Figure 13). Although the samples were higher in May (20 pupae) and December (14 pupae) than in September (9 pupae), there was no tachinid emergence in May and December. The largest sample was obtained in November with 68 pupae, but only 7 parasitized pupae (10.28%).
We do not have data available for January, February and March, which are usually the months with the highest levels of precipitation and temperature in the study area. Furthermore, additional field studies are required to obtain reliable and statistically significant data on this interaction. Nevertheless, this preliminary field research indicates that Diaugia angusta has a great potential for use in the biological control of Metamasius species in peach-palm crops and, perhaps, in other crops.
Monthly parasitism of Metamasius sp. by Diaugia angusta (total number of collected pupae and total number of parasitized pupae) from April to December
Monthly percentages of parasitism of Diaugia angusta on pupae of Metamasius sp. from April to December
Parasitoidism of tachinid flies on Dryophthoridae and Curculionidae bromeliad and palm pests
The present study reveals important reports of Diaugia angusta parasitizing Metamasius ensirostris and Metamasius hemipterus.These
are the first host records for this tachinid species, and absolutely
nothing is known about a potential host specialization over Metamasius ensirostris and/or Metamasius hemipterus. The geographical distribution known for Diaugia angusta ranges from central to southeastern and southern Brazil (Figure 11), while the host Metamasius ensirostris occurs from northern South America (Colombia, Venezuela) southwards to Paraguay, southern Brazil and northern Argentina (
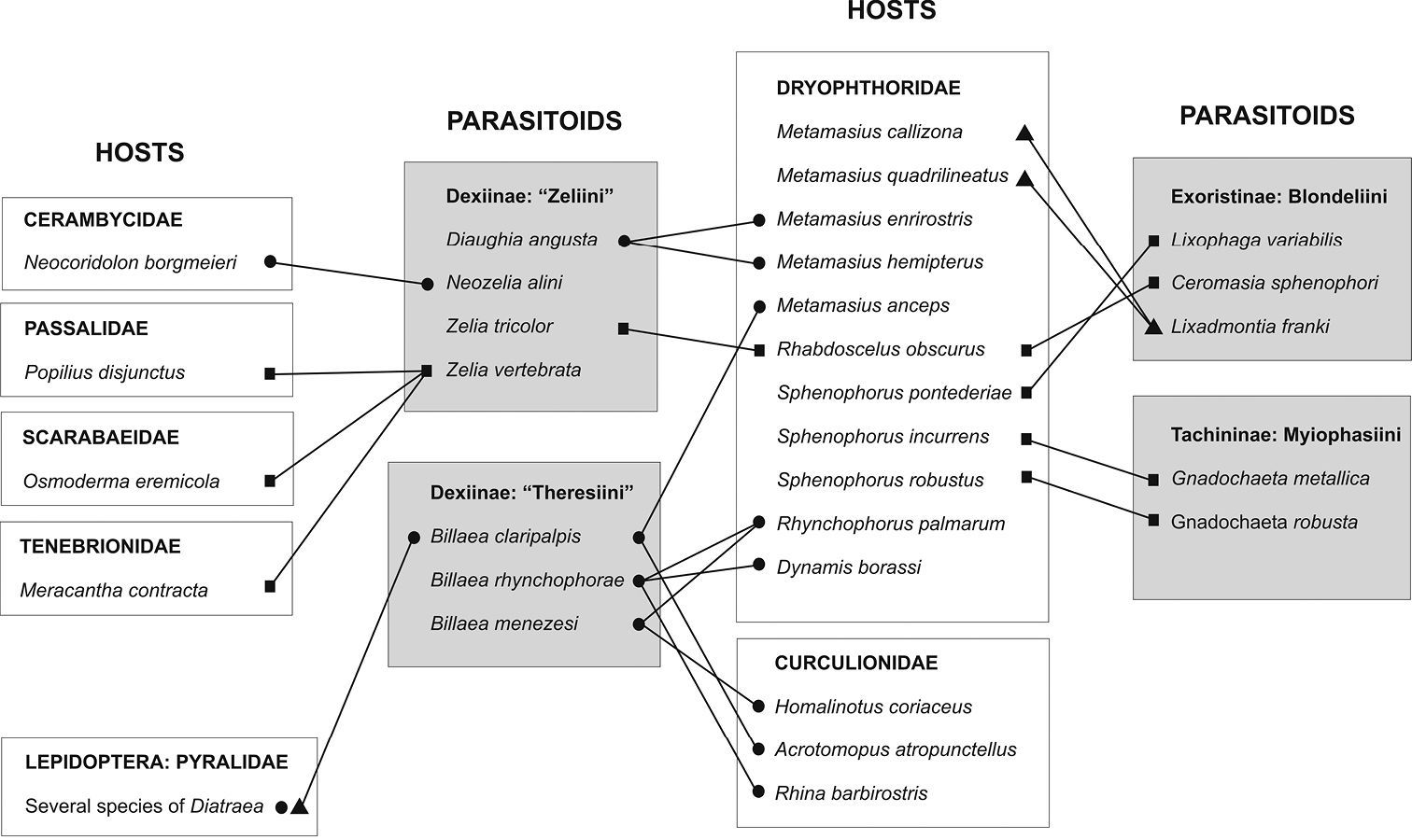
Host-Parasitoid network for Tachinidae, Dryophthoridae
and other taxa. Legends: grey boxes = parasitoid taxa; white boxes =
host taxa; circles = records in South America; squares = records in
North America; triangles = records in Central America. (Sources:
The host-parasitoid network presented in Figure 14 is focused on the interaction between Tachinidae parasitoids and Dryophthoridae and Curculionidae species reported as pest of bromeliads and palms in the Americas. The network is not complete for every species included, as only “Zeliini” has all its hosts and only Dryophthoridae and Curculionidae have all their parasitoids depicted (see Material and Methods for details).
The network (Figure 14) shows that the parasitoid-host relationship is not very well established in terms of species-specific associations. Dryophthoridae species are parasitized by a great variety of tachinids, and these tachinid species develop on several hosts, not only Dryophthoridae.
There are only 10 tachinid species connected to 11 dryophthorid
species, on the other hand, these 10 tachinids have several other
connections (most not shown) to species of Coleoptera and Lepidoptera (
The present study records Diaugia angusta to Metamasius ensirostris and Metamasius hemipterus in southeastern and southern Brazil. Before that, the only host record for a South American “Zeliini” was the one reporting Neozelia alini Guimarães, 1975 on a cerambycid species in southeastern Brazil (
Members of “Zeliini” has been recorded on several different and not closely related branches of Coleoptera. On this sense, the Dryophthoridae species are also parasitized by a disparity of tachinid species, belonging to three different subfamilies: Exoristinae (Blondeliini), Tachininae (Myiophasiini) and Dexiinae (Dexiini, “Zeliini”, “Theresiini”) (
Although the interactions between Dryophthoridae species and the tachinids Lixophaga Townsend, Ceromasia Rondani and Gnadochaeta
Macquart seem to be specific, this is an artefact as the network does
not show all the host associations recorded to these tachinid genera.
North American species of Gnadochaeta parasitize Sphenophorus spp. (Dryophthoridae), but there are also records to several other host species among Curculionidae (Coleoptera), and among lepidopterans Arctiidae, Pyralidae and Noctuidae (
However, unlike other Blondeliini, Lixadmontia franki Wood and Cave, 2006 (Tachinidae: Exoristinae: Blondeliini) has some specificity in attacking only species of Metamasius.
This tachinid has been considered a potential biological control agent
of two species of meristem-boring weevils of bromeliads: Metamasius callizona (
Great efforts have been done recently in order to find
parasitoids of bromeliad and palm weevils, by searching for parasitoids
for the biological control of Metamasius callizona in Florida (
Although still premature, the use of the tachinid Diaugia angusta as an alternative for the biological control of Metamasius species may be promising, either in its native geographical range or in Central America and tropical North America. On this respect, further studies are needed to test the efficiency of this species under laboratory and natural conditions (through mass hearing and field release). We encourage the community to focus their research on “Zeliini” and “Theresiini” flies while aiming to search for parasitoids of Dryophthoridae and Curculionidae bromeliad and palm pests. Unfortunately, the host records and present knowledge for these tachinids are scattered in the literature. The neotropical “Zeliini” comprises about eleven genera and 18 species, but only four species have some knowledge about their host associations. As for the neotropical “Theresiini”, there are about 14 genera and 23 species, but host records are available to only three species. At present, both “Zeliini” and “Theresiini” are included into the large tribe Dexiini.
AcknowledgementsThanks to Fábio Gaiger and Sérgio Vanin (IB-USP) for the identification of Metamasius
specimens, to Marcia Couri (MNRJ) and Carlos Lamas (MZSP) for the loan
of material. James O’Hara and Pierfilippo Cerretti provided suggestions
and comments which improved the manuscript. Finnancial support from
FAPESP (proc. n. 2004/13663-9 and