(C) 2010 Bingbing Hu. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species and new record of gracillariid moths from China are reported: Conopomorpha flueggella Li, sp. n. and Epicephala relictella Kuznetzov, 1979. Specimens were collected on flowers or leaves of Flueggea suffruticosa (Pall.) Baill. (Euphorbiaceae) at night, and reared from fruits in captivity. Larvae of both species feed on the seeds of Flueggea suffruticosa, but they can be differentiated externally by the position of the red pattern on the thorax and abdomen. Morphology of the eggs, larvae, pupae and the life history of the two species are described and compared. Images of the life history and figures of the genital structures are provided.
Lepidoptera, Gracillariidae, Conopomorpha, Epicephala, Flueggea suffruticosa, new species, biology
Most species of Gracillariidae are leaf-miners, although some are stem-, fruit- or peel-miners or feed on flower buds (
In this paper, we describe the morphology and biology of adults and larvae of Conopomorpha flueggella Li, sp. n. and Epicephala relictella Kuznetzov, 1979. Both species are seed–parasites of Flueggea suffruticosa in the Baxian Mountain State Nature Reserves in Tianjin, China. The hostplant of Epicephala relictella is here recorded for the first time, and the biology of the two gracillariid species is described and compared. Images of the adults and genitalia are provided.
Material and methodsField studies were conducted from 2007 to 2009 in the
Baxian Mountain State Nature Reserves (40°7'24"–40°13'53"N,
117°30'35"–117°36'24"E) in Tianjin, China (Fig. 1),
at an altitude ranging from 500 to 800 m. The area covers 5360 hm2,
with 1583 hm2 as the core region. It is characteristic of warm temperate
deciduous broad-leaved forest, and belongs to the warm and humid
continental monsoon climate. The annual average rainfall amounts to
968.5 mm, and the annual average temperature is 8–10 °C (
Flueggea suffruticosa (Pall.) Baill. (Fig. 3) occurs in scrubby slopes, forest margins and at road sides (Fig. 2)
at an altitude of 500 to 2500 m. It is distributed in China (except in
Gansu, Qinghai, Xinjiang and Tibet), Japan, Korea, Mongolia and
Russia (
The biology of Conopomorpha flueggella Li, sp. n. and Epicephala relictella Kuznetzov was observed and studied during August–October 2007 and May–October of 2008 and 2009. Life history observations were made during flowering and fruiting seasons. The developing and mature fruits were collected from different individuals and dissected to examine the feeding habit with a light microscope. In addition, the developing fruits were collected in a cylindrical box (10 cm × 10 cm2) to rear mature larvae and braconid wasps, and the behaviors of the mature larvae were observed.
Specimens examined in this study were collected on flowers or leaves of Flueggea suffruticosa
at night, and reared from fruits in captivity, and a few specimens
were collected by using light traps. Genitalia dissection and mounting
follow
The type specimens are deposited in the Insect Collection, College of Life Sciences, Nankai University, Tianjin, China.
Habitats and host plants of two gracillariidspecies in Baxian Mountain State Nature Reserves. 1 general habitat 2 habitat of Flueggea suffruticosa, arrow pointing to host plant 3 female individual of Flueggea suffruticosa 4 fruits of Flueggea suffruticosa.
Conopomorpha
currently consists of 13 species worldwide: eight species in the
Australian Region, three in the Oriental and Afrotropical regions
respectively, and one in the Palearctic Region (
Epicephala
includes 40 described species: 18 in the Oriental Region, 15 in the
Australian Region, six in the Afrotropical Region, and one in the
Palearctic Region (Russian Far East). Two species were recorded to occur
in China prior to this study: Epicephala venenata Meyrick, 1935 and Epicephala albifrons (Stainton, 1859) (
In 2007, we discovered Conopomorpha flueggella Li, sp. n. and Epicephala relictella Kuznetzov, 1979 in the Baxian Mountain State Nature Reserves in Tianjin, China, whose larvae feed on Flueggea suffruticosa (Pall.) Baill. (Euphorbiaceae). Epicephala relictella is the only species of the genus distributed in the Palearctic Region, and is newly recorded for China. Its hostplant and biology were unknown previously.
Resultsurn:lsid:zoobank.org:act:66017EDE-6984-4D06-B309-9414D0BE3C19
Figs 5–10, 11, 13Holotype ♂ – China, [1] Tianjin: Baxian Mountain [40°11'03"N, 117°32'55"E], | Ji County, 600 m, 23.VII.2009, | Bingbing Hu reared [from fruit of Flueggea suffruticosa (Pall.) Baill.]. [2] Conopomorpha | flueggella | Li, sp. nov.Holotype ♂. Paratypes – 82 ♂♂, 172 ♀♀, same data as for holotype except date and altitude: 19–24.VIII.2007, 10.V.–26.VII.2008, 16.V.–30.VIII.2009, 290–600 m; 1 ♂, Limutai (40°11'17"N, 117°33'23"E), Ji County, 360 m, 24.VI.2009, coll. Bingbing Hu.
This species is similar to Conopomorpha litchiella, but distinguishable by the uniformly greyish brown to dark brown forewing with three pairs of stripes (more conspicuous when moths alive); the valva without protuberance on ventral margin distally and the saccus long linguiform in the male genitalia; the corpus bursae shorter than twice the length of the ductus bursae in the female genitalia; and the larva red-coloured. In Conopomorpha litchiella, the forewing is whitish yellow in distal portion; the valva has one large and one small protuberance on ventral margin distally, and the saccus is very short and small; the corpus bursae is twice as long as the ductus bursae; and the larva is yellowish green.
Adult (Figs 5–6). Wing expanse 8.0–15.5 mm. Head grey to greyish brown, frons greyish white. Compound eye dark brown. Labial palpus white, second segment with outer surface and distal tuft of ventral surface fuscous, third segment porrect or obliquely upward. Maxillary palpus greyish brown to dark brown. Antenna with scape greyish brown, flagellum brown to dark brown ringed with greyish white basally. Thorax and tegula dark brown. Forewing narrow, costal and dorsal margins nearly parallel; ground color greyish brown to dark brown; costal and dorsal margins with three oblique greyish white stripes respectively, first costal stripe from near middle extending obliquely to end of cell; dorsal margin with black speck at basal 1/3; bluish grey fascia with metallic reflection extending from near costal 5/6 to dorsum and along termen, respectively, between them set a large black spot; cilia pale greyish brown except fuscous apically. Hindwing and cilia greyish brown. Fore and mid legs brown; hind leg greyish white, distal half of tibia dark fuscous on outer surface. Abdomen grey, with first two segments shining white; ventral surface with five pairs of dark brown stripes along lateral sides.
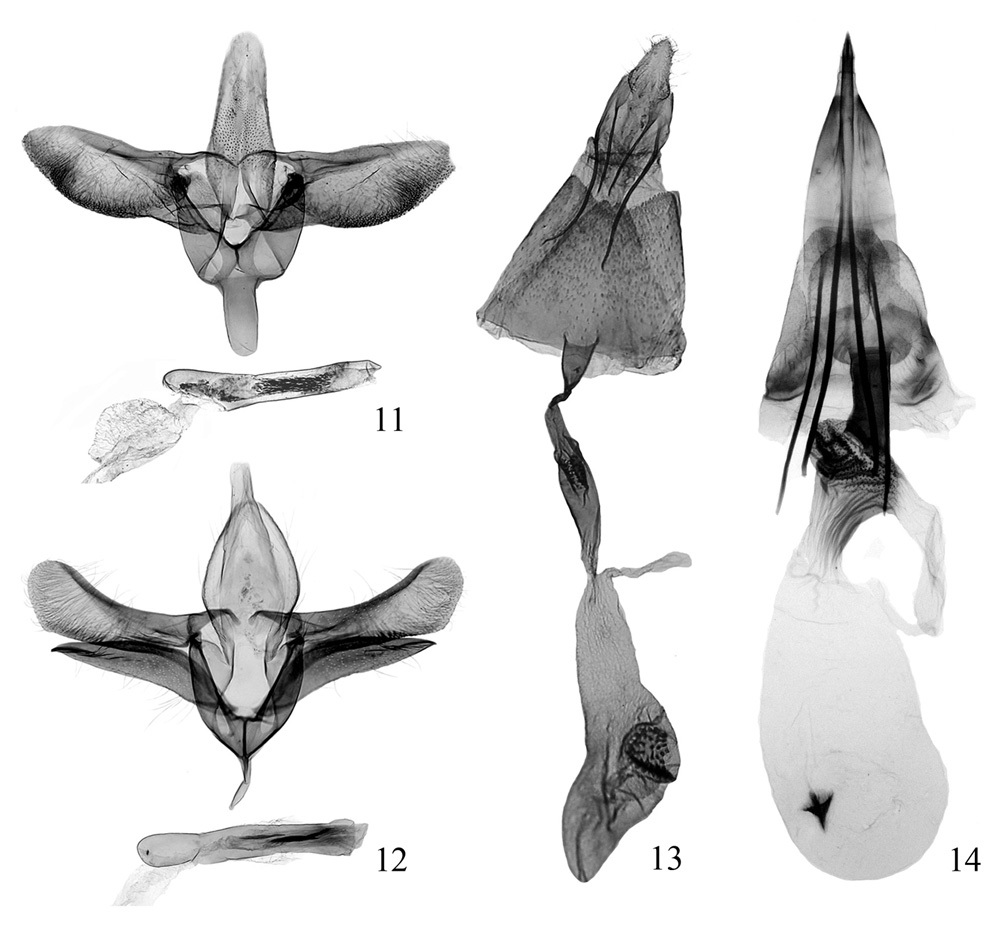
Male genitalia (Fig. 11). Tegumen narrowed gradually to rounded caudal margin, with lateral side straight. Tuba analis indistinct. Valva broad, slightly longer than tegumen; costa nearly straight, basal half slightly sinuate, apex rounded; ventral margin of valva roundly protruded medially, densely with fine hairs; sacculus narrow and short, about 1/4 length of valva. Vinculum broad and short, nearly quadrate. Saccus long linguiform, about half length of tegumen, rounded at apex. Phallus tubular, nearly straight, as long as valva, medially with dense small spines inside.
Female genitalia (Fig. 13). Papillae anales short and small, sparsely with setae. Apophysis anterioris thicker than and 1.6 × as long as apophysis posterioris. Antrum long funnel-shaped. Ductus bursae longer than apophysis anterioris, membranous except posterior 1/3 sclerotized and narrowed, medially expanded slightly and with longitudinal carinae. Corpus bursae membranous, prolonged pyriform, about 1.5 × as long as ductus bursae, with one side concave; signum large, rounded, situated at middle, covered with spines.
Egg. Flat, elliptic, 0.3 mm in length and 0.2 mm in width. Transparent membrane in surface, irregular meshy stripe on egg shell. Milky white, semitransparent; straw yellow when close to hatch.
Larva (Fig. 7). Young instar larva flat, yellowish white, semitransparent, segments distinct, with sparse setae, anterior end wider than posterior. Head capsule semicircular, brown; mandible strong, protruded like pincers. Mature larva 5.5–7.0 mm; head deep brown, anterior 1/2–2/3 of each segment on thorax and abdomen red, posterior 1/3–1/2 white. Body with sparse setae. Three pairs abdominal legs on segment 3, 4 and 5 respectively; anal leg protruded backward.
Pupa (Fig. 8). 4.0–6.0 mm, fusiform. Greenish yellow in early pupal stage, changing gradually to yellowish brown, blackish brown before eclosion. A corniform cocoon breaker on forehead. Forelegs to third abdominal segment, midlegs to fourth abdominal segment, hindlegs to seventh or eighth abdominal segment, wings to fifth abdominal segment, antenna to or slightly exceeding end of abdomen.
Cocoon (Fig. 9). 7.0–9.0 mm, white, flat elliptic, with some white grains attached on surface.
Life history of Conopomorpha flueggella. 5 adult, holotype, male 6 female moth resting on a female flower at night 7 mature larva weaving pupal cocoon on a host leaf 8 pupa 9 pupal cocoon on a host leaf 10 infested fruit.
Genitalia of two gracillariidspecies. 11 Conopomorpha flueggella, male, paratype, slide No. BHY07239 12 Epicephala relictella, male, slide No. BHY07296 13 Conopomorpha flueggella, female, paratype, slide No. HBB09034 14 Epicephala relictella, female, slide No. BHY08143.
Euphorbiaceae: Flueggea suffruticosa (Pall.) Baill.
Conopomorpha flueggella has two generations annually in Tianjin, China (Table 1). The larvae feed on the seeds of Flueggea suffruticosa (Fig. 10). Mature larvae quit the fruits before they are ripe and pupate on leaves or leaf litter. The pupal stage lasts from 9 to 12 days. Adults of the second generation hibernate. Adults occur from May to the first ten days of June, and from the last ten days of June to the first ten days of August. Adults can emerge during the whole day, but the peak occurs in the morning. The mating occurs usually in the morning. At night, the moths are actively drinking nectar and ovipositing. Adults come sometimes at light. A parasitic Ichneumonid species was reared from pupae collected on leaves of Flueggea suffruticosa in the field.
Annual life history of Conopomorpha flueggella in Tianjin, China.
| Months/Generations | 1–4 | 5 | 6 | 7 | 8 | 9 | 10–12 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | L | F | M | L | F | M | L | F | M | L | F | M | L | F | M | L | F | M | L | |
| Second generation | (+) | (+) | (+) | + | + | + | + | ||||||||||||||
| First generation | ● | ● | ● | ● | |||||||||||||||||
| – | – | – | – | – | |||||||||||||||||
| □ | □ | □ | □ | ||||||||||||||||||
| + | + | + | + | + | |||||||||||||||||
| Second generation (hibernating) | ● | ● | ● | ● | ● | ||||||||||||||||
| – | – | – | – | – | |||||||||||||||||
| □ | □ | □ | □ | □ | |||||||||||||||||
| (+) | (+) | (+) | (+) | (+) | (+) | (+) | |||||||||||||||
● egg, ― larva, □ pupa, + adult, (+) adult hibernating.F: First ten days, M: Middle ten days, L: Last ten days.
China (Tianjin).
The species name is derived from the larval host plant, Flueggea.
Figs 12, 14, 15–20
Russia: Holotype ♂, – Southern Maritime Territory, Gornotayezhnaya Station, 12.VII.1978, coll. V. I. Kuznetzov [in Russian]. Paratypes – 2 ♂♂, 1 ♀, same data as for holotype but dated 3.VII.1978. China, Tianjin: 20 ♂♂, 9 ♀♀, Mt. Jiulong, Ji County, 130–200 m, 9–28. VI.2004; Limutai, Ji County, 300 m, 11.VI.2004, coll. Houhun Li et al., 6 ♂♂, 1 ♀, 24.VI.2009, coll. Bingbing Hu; 1 ♂, 2 ♀♀, Baxian Mountain, Ji County, 550 m, 15.VII.2007, coll. Mingfeng Cao & Bingbing Hu; 74 ♂♂, 60 ♀♀, 290–480 m, 8.V.–1.VII.2008, 19.V.–30.VIII.2009, coll. Bingbing Hu; Hebei Province: 1 ♂, Shangsi, Xiaowutai, Wei County, 1200 m, 25.VII.2000, coll. Yanli Du & Zhendong Li; Heilongjiang Province: 1 ♀, Haerbin, 150 m, 22.VII.1997, coll. Houhun Li; Gansu Province: 1 ♂, 2 ♀♀, Bifenggou, Wenxian, 860 m, 10–12.VII.2005, coll. Haili Yu.
Life history of Epicephala relictella. 15 adult 16 moth resting on a leaf of host 17 mature larva resting on host leaf 18 pupa 19 pupal cocoon on stone nearby host 20 infested fruit.
Adult (Figs 15–16). Wing expanse 9.0–13.0 mm. Head white, tufted. Labial palpus white except outer surface grey. Antenna with scape pale grey dorsally, white ventrally; flagellum dark brown dorsally, copper-colored ventrally. Thorax white. Tegula and forewing greyish brown; white stripes at costal 2/5, 3/5, 4/5 and near apex as well as at dorsal 2/5 and 3/5 respectively, concentrated obliquely outward to 2/3 length and outside of cell, outmost one shortest, between first three stripes sometimes with short white strigulae; thin bluish white fascia with metallic reflection extending from costal 5/6 to dorsum; large black spot near apex; dorsal margin white tinged with ocherous yellow, longitudinally forming a broad band; termen dark brown; cilia white except fuscous distally from costal 5/6 along termen to before tornus, greyish brown along dorsal margin. Hindwing and cilia greyish brown. Fore and mid legs brown, hind leg greyish white, tibiae and tarsi with white rings. Abdomen greying brown on dorsal surface except first two segments grey; vental surface grey, with five pairs of oblique dark brown stripes along lateral sides.
Male genitalia (Fig. 12). Tegumen broadly elliptic, caudal margin rounded. Tuba analis broad, distinct. Valva narrow, slightly longer than tegumen, expanded subapically, rounded at apex; costa sclerotized, gently concave; ventral margin nearly straight except basal 1/4 oblique, with dense fine hairs. Sacculus sclerotized, separated from valva, elongate lanceolate, about 4/5 length of valva; dosal margin gently arched, ventral margin slightly concave medially; distal portion longitudinally with sclerotized carina, apex spiculate. Vinculum broad, rounded anteriorly. Saccus slender, tapering, about 1/3 length of tegumen. Phallus tubular, straight, as long as valva, apex truncate; cornuti composed of dense small spines, compacted into one to three bundles.
Female genitalia (Fig. 14). Ovipositor sclerotized to a strong spine, extensible. Apophysis very strong, apophysis posterioris slightly longer than apophysis anterioris. Lamella antevaginalis nearly trapezoid, caudal margin concave medially. Antrum strongly sclerotized, about half length of apophysis anterioris, oblique anteriorly. Ductus bursae thick and short, weakly sclerotized, slightly longer than antrum, expanded with irregular sclerotized carinae posteriorly, narrowed gradually towards corpus bursae, with sclerotized longitudinal pleats. Corpus bursae membranous, elongate elliptic, about same length as apophysis posterioris; signum small, coniform or stelliform, placed anteriorly.
Egg. Oval, diameter about 0.15–0.20 mm. Surface smooth, shiny. Egg first yellowish white, nearly transparent, then becoming straw yellow before hatching.
Larva (Fig. 17). Young instar larva very similar to that of Conopomorpha flueggella. Mature larva 5.0–6.5 mm; head capsule brownish yellow, median 2/3 of each segment on thorax and abdomen dark red, anterior and posterior ends white; thoracic segments slightly blue, abdominal segments with blue spots. Body with sparse white setae. Three pairs abdominal legs on segment 3, 4 and 5 respectively; anal leg protruded backward.
Pupa (Fig. 18). 4.0–5.5 mm, fusiform. Greenish yellow in early pupal stage, changing gradually to dark brown. A corniform coccon breaker on forehead. Forelegs to third abdominal segment, midlegs to fourth abdominal segment, hindlegs to eighth abdominal segment, wings to fifth abdominal segment, antenna obviously exceeding end of abdomen.
Cocoon (Fig. 19). 6.0–8.0 mm; white, flat elliptic, with some white grains attached on surface.
Euphorbiaceae: Flueggea suffruticosa (Pall.) Baill., recorded for the first time herein.
Epicephala relictella has one generation annually in Tianjin, China (Table 2). The larvae feed on the seeds of Flueggea suffruticosa (Fig. 20). The larval stage is completed within one fruit. When completing larval development, the mature larvae quit the fruits and pupate on the leaves, and overwinter under leaf litter or stones.
Adults appear from June to July. They can emerge during the whole day, but the peak occurs in the morning. The moths are most active at night, drinking nectar and ovipositing. During the daytime they rest on leaves or branches. Adult longevity is 3–10 days, but adults generally live for 5–7 days. Adults hardly come to light.
China (Tianjin, Hebei, Heilongjiang, Gansu), Korea, Russia.
Annual life history of Epicephala relictella in Tianjin, China.
| Months/Generation | 1–5 | 6 | 7 | 8 | 9 | 10–12 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | L | F | M | L | F | M | L | F | M | L | F | M | L | F | M | L | |
| First generation | (□) | (□) | (□) | (□) | (□) | (□) | (□) | |||||||||||
| + | + | + | + | + | ||||||||||||||
| ● | ● | ● | ● | ● | ||||||||||||||
| – | – | – | – | – | – | – | – | – | ||||||||||
| (□) | (□) | (□) | (□) | (□) | (□) | (□) | (□) | (□) | (□) | |||||||||
● egg, – larva, □ pupa, (□) pupa through the winter, + adult F: First ten days, M: Middle ten days, L: Last ten days.
Life history comparisons of Conopomorpha flueggella and Epicephala relictella
| Characteristics | Conopomorpha flueggella | Epicephala relictella | |
|---|---|---|---|
| Similarities | Feeding habits | Seed parasite | |
| Pupation site | Boring an exit hole to escape from the fruit to pupate on the leaves or litter | ||
| Mating site | On the leaves of host | ||
| Do adults feed ? | Yes | ||
| Differences | Flight period | In early May, slightly earlier than flowering season | In early June, keeping pace with early fruiting season |
| Overwintering | Adult | Pupa | |
| Generation | Two generations annually | One generation annually | |
| Phototaxy | Feeble | Hardly any | |
Calybites securinella (Ermolaev, 1986) was the only species in Gracillariidae known to be associated with Flueggea suffruticosa. It occurs in Russia (Primorye) and Korea (
Similar to Conopomorpha flueggella, Epicephala relictella also feeds on the seeds of Flueggea suffruticosa. They are very similar in morphology and biology, and hard to distinguish. Table 3 compares the life histories of the two species.
Most gracillariid species are leaf-miners, and the seed-parasitic habit is infrequent. Epicephala is noteworthy for its obligate pollination habit, which involves a mutualistic relationship with trees of Euphorbiaceae (
We are grateful to Mr. Xiubai Ren and Mr. Tiejian Zhao (Baxian Mountain State Nature Reserves, Tianjin) for their assistance given in the fieldwork, to Dr. Roger C. Kendrick (Fauna Conservation Department, Kadoorie Farm & Botanic Garden, Hong Kong) for providing useful literature. Special thanks are given to Dr. Svetlana Baryshnikova (Zoological Institute, Russian Academy of Sciences, St. Petersburg) for her kind assistance given to the corresponding author in checking the types of Epicephala relictella Kuznetzov, and to Dr. Erik J. van Nieukerken (Netherlands Centre for Biodiversity Naturalis, Leiden) and Jurate De Prins (Royal Museum for Central Africa, Belgium) for reviewing the manuscript and giving valuable suggestions in improving the quality of the paper. This research was supported by the National Natural Science Foundation of China (No. 30930014).