(C) 2011 A. S. Konstantinov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new genus (Borinken) and five new species (Borinken elyunque, Distigmoptera chamorrae, Kiskeya elyunque, Ulrica eltoro, and Ulrica iviei) from Puerto Rico are described and illustrated. A keyto all West Indian Monoplatini genera is provided, as are keys to all speciesof Kiskeya and to the speciesof Ulrica from Puerto Rico. A list of the flea beetle genera, along with the number of species and some of the faunal features is presented and discussed for the West Indies.
Leaf beetles, species diversity, moss, West Indies, Puerto Rico
The West Indies Islands are one of the World’s biodiversity hotspots (
The flea beetles of the West Indies are comparatively well studied. Extensive collecting and publications during the first half of the 20th and early 21st century reported many unusual and endemic flea beetle taxa (
Recent collecting efforts in Puerto Rico revealed unique flea beetles with several features rarely observed among flea beetle genera.
Flea beetle species diversity in the West Indies (we grouped some islands in a few columns because there are just a few species known to occur there. Names in bold indicate that taxon is endemic for West Indies. To make this table current, we include two undescribed species of Monotalla from Dominica and St. Lucia, manuscript describing them is in preparation).
| Genus Author | Cuba | Hispa-niola | Jamaica | Puerto Rico | Grenada | Baha-mas | Antigua & VirginIslands Barbuda St. Lucia Montserrat | Guade-loupe | Dominica | St. Croix St. Thomas | St. Vincent | Total species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acallepitrix Bechyné | 3 | 3 | ||||||||||
| Acrocyum Jacoby | 1 | 1 | ||||||||||
| Aedmon Clark | 21 | 8 | 2 | 6 | 36 | |||||||
| Alagoasa Bechyné | 1 | 4 | 1 | 6 | ||||||||
| Altica Geoffroy | 5 | 1 | 1 | 6 | ||||||||
| Apleuraltica Bechyné | 1 | 1 | ||||||||||
| Apraea Jacoby | 8 | 4 | 4 | 3 | 1 | 19 | ||||||
| Argopistes Motschulsky | 1 | 1 | 1 | 1 | 4 | |||||||
| Asphaera Chevrolat | 1 | 1 | ||||||||||
| Blepharida Chevrolat | 1 | 1 | ||||||||||
| Bonfilsus Scherer | 1 | 1 | 2 | |||||||||
| BorinkenKonstantinov & Konstantinova | 1 | 1 | ||||||||||
| Centralaphthona Bechyné | 5 | 3 | 1 | 6 | 2 | 2 | 16 | |||||
| Chaetocnema Stephens | 5 | 5 | 4 | 5 | 2 | 1 | 2 | 1 | 1 | 16 | ||
| Cyrsylus Jacoby | 1 | 1 | 1 | 1 | 1 | 2 | 5 | |||||
| Diphaulaca Chevrolat | ? | ? | ? | 1 | 2 | |||||||
| Disonycha Chevrolat | 7 | 3 | 4 | 4 | 3 | 13 | ||||||
| Distigmoptera Blake | 1 | 1 | 2 | |||||||||
| Epitrix Foudras | 3 | 3 | 3 | 1 | 5 | |||||||
| Exoceras Jacoby | 1 | 1 | 2 | |||||||||
| Gioia Bechyne | 1 | 3 | 3 | 1 | 8 | |||||||
| Glyptina LeConte | 1 | 1 | ||||||||||
| Guadeloupena Bechyné | 1 | 1 | ||||||||||
| Heikertingerella Csiki | 2 | 2 | 1 | 1 | 1 | 5 | 2 | 1 | 13 | |||
| Hemilactica Blake | 7 | 1 | 1 | 9 | ||||||||
| Hirtiasphaera Medvedev | 1 | 1 | ||||||||||
| Homoschema Blake | 6 | 5 | 4 | 5 | 2 | 1 | 2 | 1 | 26 | |||
| Homotyphus Clark | 1 | 1 | ||||||||||
| Hypolampsis Clark | 1 | 1 | 1 | 2 | ||||||||
| Kiskeya Konstantinov & Chamorro-Lacayo | 2 | 1 | 3 | |||||||||
| Kuschelina Bechyné | 1 | 1 | ||||||||||
| Leptophysa Baly | 3 | 4 | 2 | 1 | 1 | 11 | ||||||
| Longitarsus Latreille | 7 | 2 | 1 | 6 | 1 | 3 | 2 | 1 | 1 | 21 | ||
| Lupraea Jacoby | 1 | 1 | 2 | |||||||||
| Lysathia Bechyné | 1 | 1 | 1 | 2 | 2 | 1 | 2 | |||||
| Macrohaltica Bechyné | 1 | 2 | 1 | 1 | 2 | |||||||
| Megasus Jacoby | 1 | 1 | ||||||||||
| Megistops Boheman | 4 | 2 | 2 | 3 | 1 | 1 | 1 | 12 | ||||
| Monomacra Chevrolat | 3 | 1 | 6 | 1 | 2 | 3 | 2 | 3 | 2 | 22 | ||
| Monotalla Bechyné | 1 | 1 | 1 | 3 | ||||||||
| Neothona Bechyné | 2 | 1 | 2 | 1 | ||||||||
| Nesaecrepida Blake | 2 | 2 | 1 | 2 | ||||||||
| Normaltica Konstantinov | 1 | 1 | 2 | |||||||||
| Oedionychus Berthold | 12 | 1 | 2 | 1 | 1 | 17 | ||||||
| Omophoita Chevrolat | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 5 | ||
| Phyllotreta Chevrolat | 1 | 1 | 2 | 3 | ||||||||
| Physimerus Clark | 1 | 1 | 1 | |||||||||
| Platiprosopus Chevrolat | ? | ? | ? | 2 | 2 | |||||||
| Pseudodisonycha Blake | 2 | 1 | 1 | 4 | ||||||||
| Strabala Chevrolat | 5 | 1 | 2 | 2 | 7 | |||||||
| Syphraea Baly | 1 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 14 | |||
| Systena Chevrolat | 3 | 1 | 2 | 2 | 2 | 2 | 7 | |||||
| Ulrica Scherer | 2 | 2 | ||||||||||
| Number of genera/species | 30/102 | 32/79 | 23/51 | 33/74 | 16/24 | 9/14 | 5/7 | 20/32 | 7/16 | 5/7 | 15/18 | 53/351 |
Various collecting methods were used in Puerto Rico. Among them, beetles from sifted and unsifted moss samples by Berlese extraction represent most of the new taxa described here. Collecting in moss cushions was one of the most effective methods for uncovering a previously unknown fauna.
Dissecting techniques and terminology for most internal and external structures follow
urn:lsid:zoobank.org:act:B5FCB199-B009-47CD-960D-76ACAE936BFD
http://species-id.net/wiki/Borinken
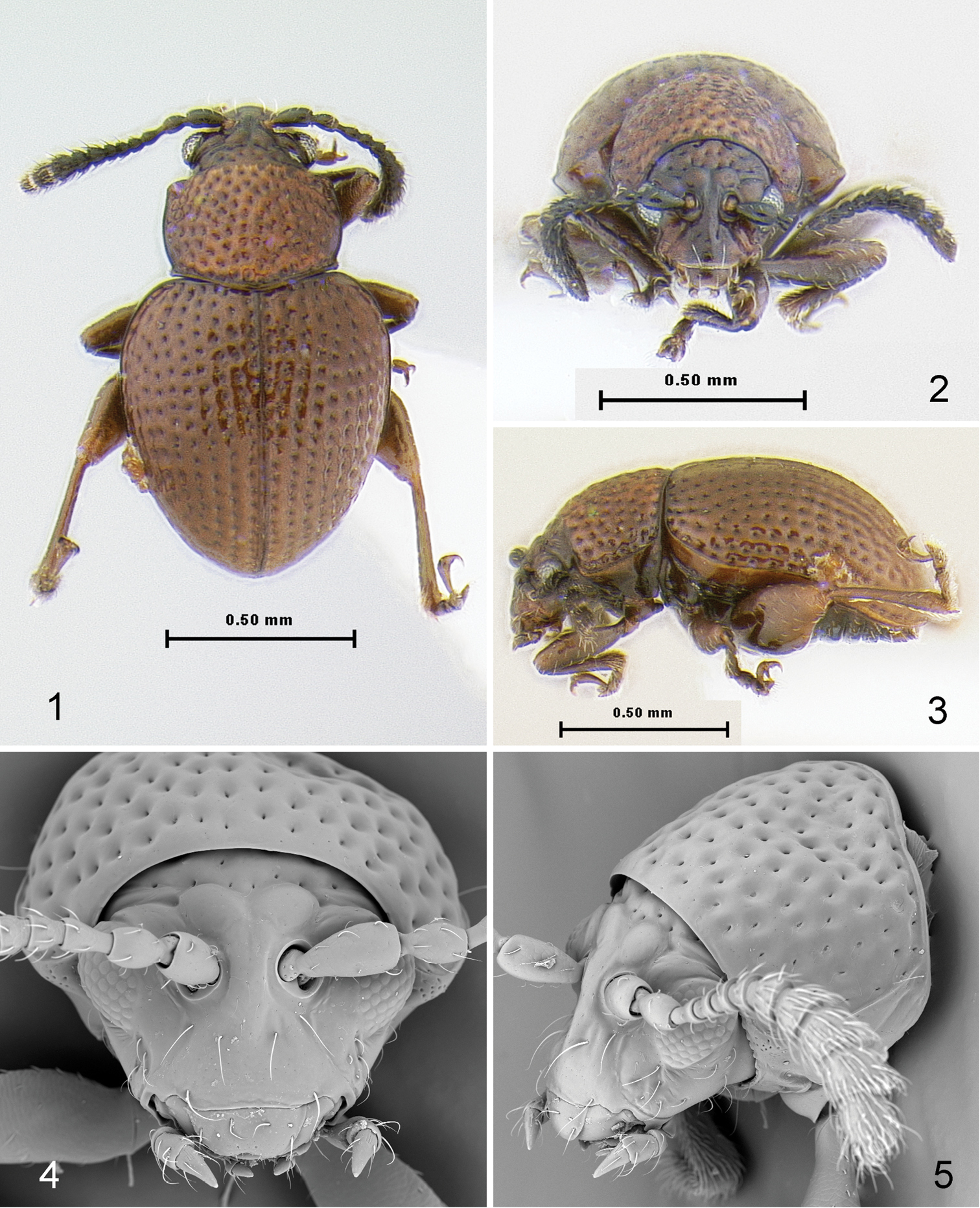
Figs 1–14Body length 1.08–1.18 mm, width 0.70–0.81 mm, elongate, relatively flat in lateral view (2.18 times as long as thick). Color brown without metallic luster, legs slightly lighter and antennae, except last antennomere, darker, almost black.
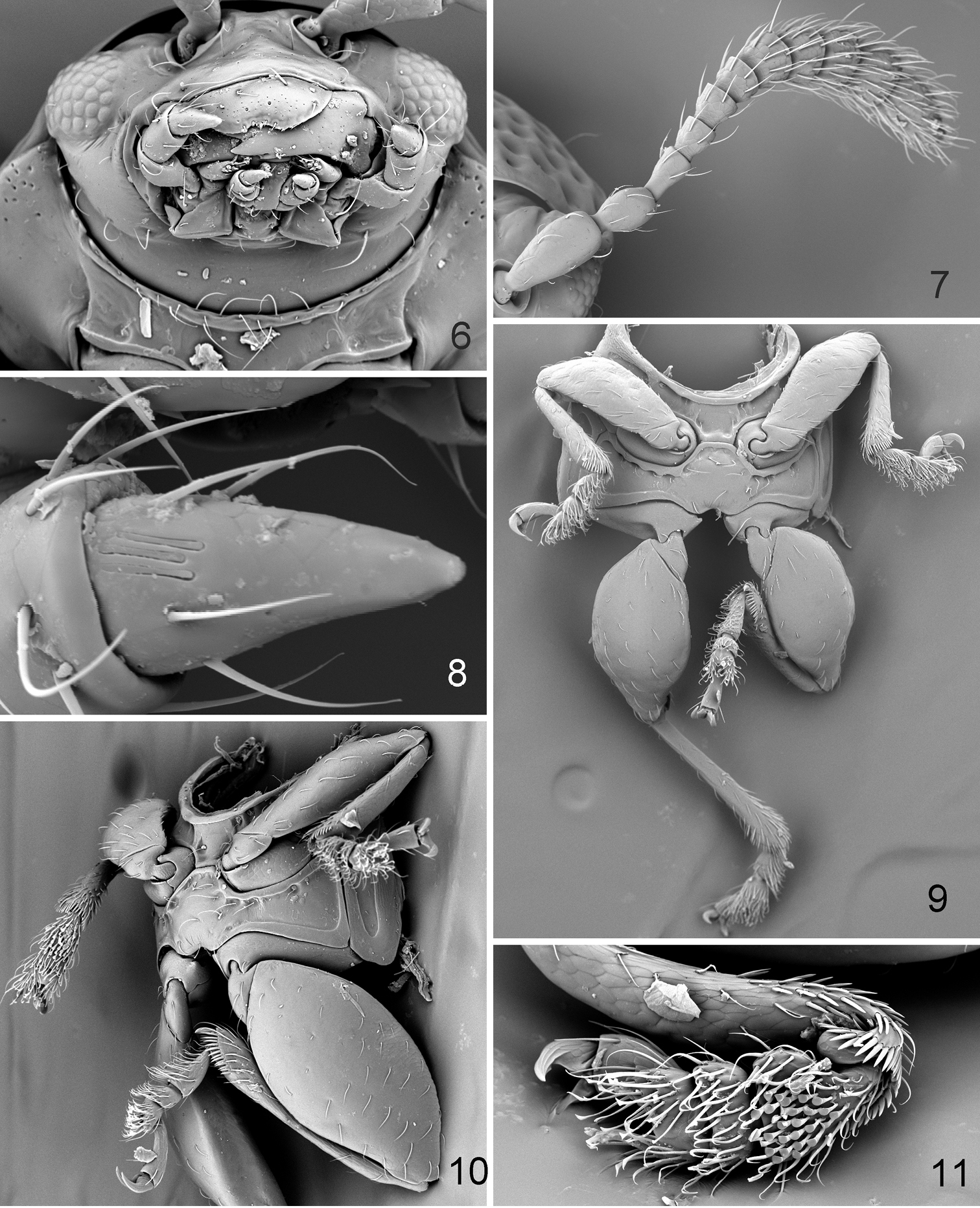
Head (Figs 4, 5) flat in lateral view. Frons and vertex forming nearly straight line (Fig. 5) in lateral view. Facial part of head elongate. Supraorbital pore situated near outer corner of antennal callus, poorly visible. Antennal calli well developed, slightly longer than wide, oblique, separated from each other by wide midfrontal sulcus. Supracallinal sulcus deep, convex. Suprafrontal and supraantennal sulci well developed, deep. Supraorbital sulcus slightly impressed. Orbit as wide as transverse diameter of eye. Interantennal space nearly as wide as transverse diameter of eye and as transverse diameter of antennal socket. Frontal ridge narrow, lowering in front of anterofrontal ridge. Anterofrontal ridge not separated from frontal ridge, long and swollen. Two ridges situated laterally of frontal ridge from lower margin of antennal socket to outer corner of mouth. Long seta situated at beginning of each ridge under antennal socket. Another long seta located on both sides of frontal ridge. Eyes small, slightly protruding laterally, 0.72 times as wide as long. Vertex covered with few large and deep punctures. Labrum with six setiferous pores, apically slightly incised. Labium with three palpomeres per palpus, distal palpomere longer than wide (Fig. 6). Maxillary palpus with four palpomeres, distal palpomere conical, slightly longer than preapical, sensilla patch with three setae (Fig. 8). Antenna with 11 antennomeres. First antennomere slightly wider and much longer than second and rest of antennomeres separately. Third and fourth antennomeres much thinner than second. Antennomeres gradually widening distally (Fig. 7).
Pronotum (Fig. 1) 1.34 times wider than long (measured in middle), without impressions, covered with large, deeply impressed punctures. Sides weakly rounded and relatively narrowly explanate, with maximum width in front of middle. Marginal anterolateral callosity situated perpendicularly to midline of body, 3.71 times shorter than lateral margin. Posterolateral callosity protruding laterally. Basal margin evenly convex, slightly extends posteriorly, with distinct border. Procoxal cavity open. Intercoxal prosternal process relatively narrow and parallel-sided in middle, with longitudinal ridge bordered by two deep grooves laterally, abruptly expanding beyond procoxae. Scutellum flat, wider than long, apex sharply triangular, sides straight. Mesocoxae separated by both meso- and metasterna. Mesosternum not covered by metasternum, horizontal (Fig. 9). Metasternum (Fig. 9) protruding anteriorly between mesocoxae, wide, nearly flat at apex.
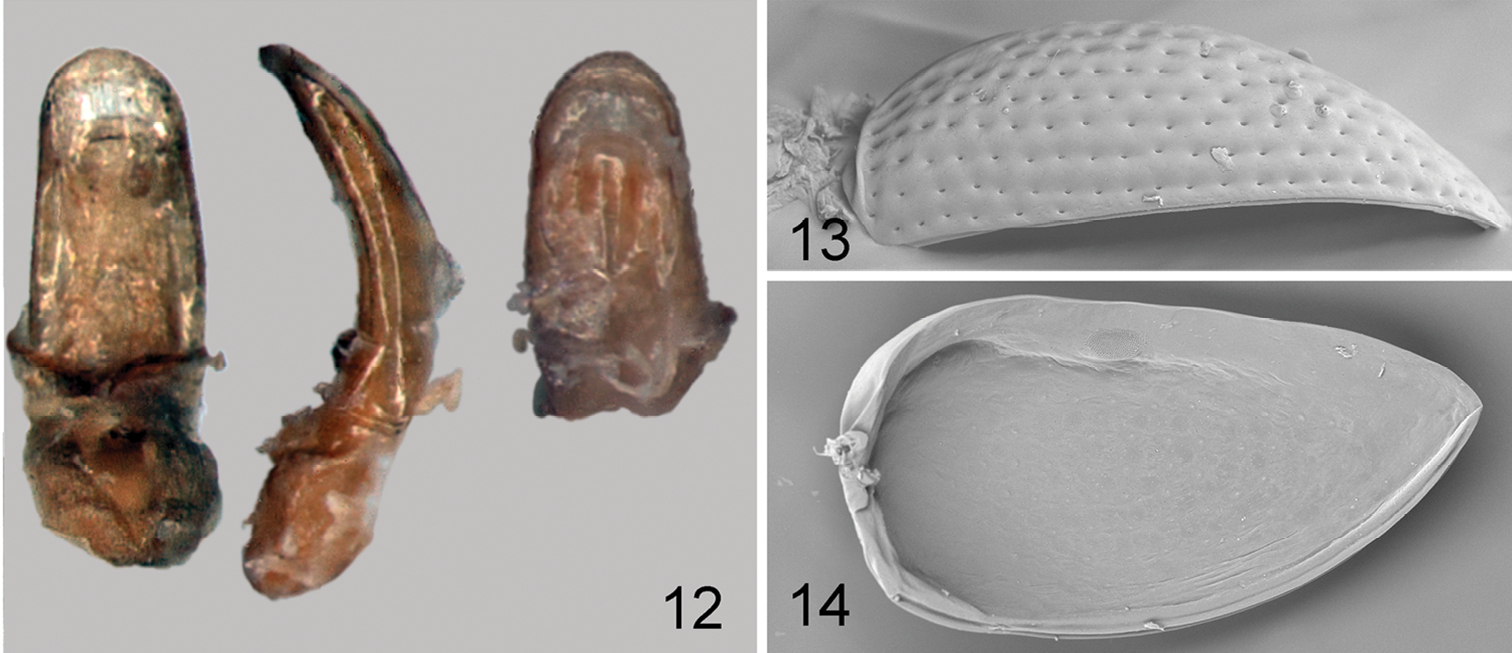
Elytron (Fig. 1) widest near mid-length. Humeral callus absent. Elytral punctures arranged in nine rows not counting scutellar row. Punctures large, about as large as space between rows. Elytral apex narrowly rounded, surrounded by distinct border. Epipleura broad, slightly oblique, gradually narrowing posteriorly, not attaining sutural margin of elytron. Elytron with sensilla distributed evenly throughout surface, others concentrated in single sensilla patches (Fig. 14). Elytra fused. Elytral lock consists of longitudinal groove along its suture (Fig. 14). Wings absent.
Pro- and mesofemora normally round, only slightly flat dorsoventrally. Metafemur robust, flat dorsoventrally, fairly symmetrical (Fig. 10), 2.15 times as long as wide. Pro- and mesotibiae cylindrical, slightly wider in distal 1/3, without spurs apically. Metatibia (Figs 1, 9) straight in dorsal and lateral views, generally cylindrical, gradually widening distally (in dorsal view), dorsal surface convex nearly to apex. Apical spur long, slightly shorter than tarsal claw. Claw appendiculate near base. Third tarsomere deeply incised (Fig. 11). First metatarsomere as long as two following tarsomeres together.
Abdomen with five distinctly visible sternites. Apical sternite shorter than three preceding sternites combined, without appendages basally. Basal sternite longer than four following sternites together.
Median lobe of aedeagus (Fig. 12) simple, robust, slightly and evenly curved in lateral view, without any sculpture ventrally.
Borinken elyunque Konstantinov & Konstantinova, new species.
This genus is based on the native Taino Indian name for the Island of Puerto Rico, Borinquen. The name is masculine.
Borinken is very different from other flea beetle genera that are known to occur in mosses in the New World (Kiskeya in the West Indies, Nicaltica Konstantinov, Chamorro-Lacayo and Savini in Nicaragua, and Ulrica Scherer in the West Indies and Central and South America). Based on the general shape of the body, shape of the base of the pronotum without a lobe extending posteriorly, general shape of the metatibia and tarsal claw, Borinken is similar to Benedictus Scherer, which inhabits mosses in Asia and does not occur in the New World (
Borinken is also very different from any other West Indian or New World flea beetle genera. Among New World genera it is somewhat similar to Centralaphthona Bechyné based on the presence of antennal calli, lack of the prebasal groove on the pronotum, regular elytral striae and open procoxal cavities. Borinken can be easily differentiated from Centralaphthona by the following features: elongate facial part of head (normally short in Centralaphthona); antennal calli longer than wide (usually shorter than wide in Centralaphthona); apical antennomeres much wider than basal (about same width in Centralaphthona); vertex, pronotum, and elytra strongly punctured (punctation normally small in Centralaphthona; overall, this kind of coarse punctation is rare among flea beetles); apex of metatibia convex up to tarsomere (flat in Centralaphthona).
Borinken elyunque: 1 habitus, dorsal view 2 habitus, frontalview 3 habitus, lateral view 4 head and pronotum, frontal view 5 head and pronotum, lateral view.
Borinken elyunque: 6 head, anteroventral view 7 antenna 8 last maxillary palpomere 9 thoracic sternites with mid- and hind legs, ventral view 10 thoracic sternites with mid- and hind legs, lateral view 11 protibia and protarsus.
Borinken elyunque: 12 median lobe of aedeagus, ventral, lateral and dorsal views 13 right elytron, lateral view 14 left elytron, ventral view.
urn:lsid:zoobank.org:act:30A86614-5362-4AE0-9372-80B07A98BBD4
http://species-id.net/wiki/Borinken_elyunque
Figs 1–14Body length 1.08–1.18 mm, width 0.70–0.81 mm, elongate, relatively flat in lateral view (2.18 times as long as thick). Color brown without metallic luster, legs slightly lighter and antennae, except last antennomere, darker, almost black. Vertex covered with large punctures, shiny, without wrinkles. Oblique fold situated between orbit and antennal callus. Proportions of antennomere lengths in male: 14:9:6:6:6:4:5:5:5:7:10. Antennomeres widened abruptly beginning from antennomere 7 (it is 0.71 times as long as wide). Pronotum evenly covered with large punctures, their diameter much larger than distance between them. Ventral side of body without many setae. Elytron with nine complete rows of punctures. Additional scutellar row incomplete. Punctures large, about as large as space between rows. Interspaces shiny with wrinkles or punctures. Proportions of tarsomere lengths of male as follows: protarsomeres 5:4:4:11; mesotarsomeres 5:4:4:11; metatarsomeres 9:4:5:11.
Median lobe of aedeagus (Fig. 12) robust in ventral view, with apex evenly convex without apical denticle. Apex slightly swollen in lateral view. Ventral side flat, without membranous window.
The specific epithet is a noun in apposition based on the type locality.
Unidentified moss samples that contained Borinken elyunque were collected in the forest from a variety of substrates (rocks, tree stumps, trunks and branches) (Figs 59, 60).
Holotype: ♂, Puerto Rico: El Yunque, El Toro trail, 18°16.850'N, 65°49.753W, 1066m, 14.VI.2008, moss (unsifted) leg. A. Konstantinov (USNM). Paratype ♂, same label as holotype (USNM). Paratypes 2 ♂, same label as holotype except the date, 16.VI.2008 and moss being “unsifted” (USNM).
http://species-id.net/wiki/Distigmoptera
Figs 15–27Distigmoptera was first recorded in the West Indies by
Distigmoptera chamorrae: 15 habitus, dorsal view, male 16 habitus, frontalview, female 17 habitus, lateral view, male 18 habitus, dorsal view, female 19 metatibia and metatarsus, lateral view 20 claw, male 21 claw, female.
Distigmoptera chamorrae: 22 median lobe of aedeagus, ventral, lateral and dorsal views 23 spermatheca 24 vaginal palpi 25 tignum 26 abdominal sternites, female 27 last abdominal tergite.
urn:lsid:zoobank.org:act:3C9F8E9B-A3C3-4264-BC7D-A0CBD4108508
http://species-id.net/wiki/Distigmoptera_chamorrae
Figs 15–27Body (Figs 15, 18) length 1.72–2.27 mm, width 0.91–1.14 mm, pubescent, oval and moderately flat in lateral view. Head, except mouthparts, antenna, except antennomere five in males and five and six in females, pronotum, base of elytron, and metafemur dark brown to blackish. Mouthparts, antennomere five in male and five and six in female, front and middle legs and metatibia yellowish to very light brown. Apex of protibia and apical part of elytron (larger in female than in male) slightly darker. Lighter part of elytron with two transverse dark bands, narrower and better separated in female and wider and poorly visible in male. Lighter parts of elytron covered with setae lighter in color, darker parts covered with setae darker in color.
Head (Figs 16, 17) slightly convex in lateral view, evenly and strongly rugose and pubescent. Frons and vertex forming slightly convex line in lateral view. Supraorbital pore indistinguishable. Antennal callus clearly visible, nearly quadrate, its surface situated above surface of vertex. Midfrontal sulcus wide and deep. Supracallinal and supraorbital sulci poorly visible. Suprafrontal and supraantennal sulci shallow. Orbit relatively narrow, 2.50 times narrower than transverse diameter of eye. Interantennal space as wide as transverse diameter of eye. Antennal socket rounded. Frontal ridge wide, parallel sided. Anterofrontal ridge merged with frontal ridge forming denticle in middle. Eyes small, slightly protruding laterally, inner margin curved. Labrum deeply notched in middle, with six setiferous pores. Apical maxillary palpomere as wide as long, conical, much smaller than preceding palpomere. Labial palpomeres of equal length, apical conical. Antenna with 11 antennomeres, antennomeres widening apically. Antennomere four thinnest (Fig. 18). Proportions of antennomere lengths in male: 12:4:6:5:5:5:7:7:7:6:9; in female: 12:6:5:5:5:6:6:6:6:7:9.
Pronotum (Figs 15, 18) 1.47 times wider than long. Pronotal disc anteriorly raised in two wide ridges separated by shallow and wide impressions. Anterior margin straight, with distinct border. Lateral margins subparallel, very slightly convex, without explanation. Posterior margin nearly straight, without distinct border. Anterolateral callosity globular and evenly rounded, bearing seta and not forming denticle posteriorly. Posterolateral callosity absent. Pronotal surface covered with large closely placed punctures and a few yellow, whitish and black setae. Scutellum triangular, densely covered with yellow setae. Prosternal surface densely covered with irregular punctures. Prosternal intercoxal process extended posteriorly beyond coxa and truncate posteriorly. Posterior end about twice as wide as middle. Procoxal cavities closed posteriorly. Mesosternum shorter than prosternal process, quadrate, rugose and pilose. Metasternum smooth and pilose, convex in lateral view, as long as pro- and mesosterna together. Posterior margin with sharp notch.
Elytral surface punctate (Figs 15, 18), with punctures forming nine striae (not counting marginal and short scutellar striae), densely pilose with black setae near base and yellow setae in posterior half. Interspaces between puncture rows two and three, four and five, six and seven form convex ridges. Humeral callus absent. Base of elytron with callus situated between suture and humeral corner. Epipleura wide, nearly vertical, narrowing abruptly at elytral apex but not reaching it. Elytral apex narrowly truncate.
Pro- and mesofemora slightly dilated and tibiae subcylindrical, somewhat enlarged towards apical edge (Fig. 17); pubescence sparsely distributed. Metafemur greatly enlarged, 1.82 times longer than wide and 1.72 times longer than metatibia. Pro- and mesotibiae without apical spurs. Metatibia straight in lateral view, slightly curved in dorsal view. Outer and inner lateral dorsal ridges more or less straight with apical third with numerous denticles. Metatibial spur well developed. First metatarsomere inserted preapically and about as long as two subsequent tarsomeres together. Claw tarsomere swollen. Claw split in male and appendiculate in female.
Abdomen pubescent, with five visible sternites. Apical sternite shorter than three preceding sternites combined, without appendages basally (Fig. 26). Basal sternite shorter than three following sternites together. Last abdominal tergite of female without groove in middle, evenly covered with long setae.
Median lobe simple, slightly curved in lateral view with more abrupt curvature near middle; in ventral view, with lateral margins almost parallel, apex subtriangular without denticle (Fig. 22). Ventral side apically flatter than basally.
In female genitalia, posterior part of sternite eight sclerotized along its entire margin (Fig. 25). Tignum with rounded anterior margin, evenly sclerotized, bearing many moderately long setae (Fig. 25). Vaginal palpi (Fig. 24) elongate, anteriorly and along middle strongly sclerotized and merged anteriorly for more than half of their length, each with about eight setae at apex, with posterior sclerotization shorter than anterior (Fig. 24). Spermatheca curved (Fig. 23), with receptacle and pump not differentiated from each other (pump about as wide as receptacle). Apex of pump with flattened projection. Spermathecal duct long, forming “S” coils.
Distigmoptera chamorrae can be easily differentiated from all continental speciesof Distigmoptera by the bicolorous antennae with antennomeres five in the male and five and six in the female being yellowish, much lighter than the rest of the antennae. It can be distinguished from the only other West Indian species (Distigmoptera antennata Medvedev) by the absence of wings (Distigmoptera antennata is winged).
The specific epithet is a patronym dedicated to Lourdes Chamorro who collected the only known specimens.
Holotype: ♂, Puerto Rico: Toro Negro, 18°11.850'N, 66°29.533'W, 904 m, 20.VI.2008, leg. M. L. Chamorro (USNM). Paratype ♀, same label as holotype (USNM).
http://species-id.net/wiki/Kiskeya
Discovery of about a hundred specimens of a third species of Kiskeya in Puerto Rico provided additional material that allowed us to observe structures that were not available for study at the time of the description of the genus (
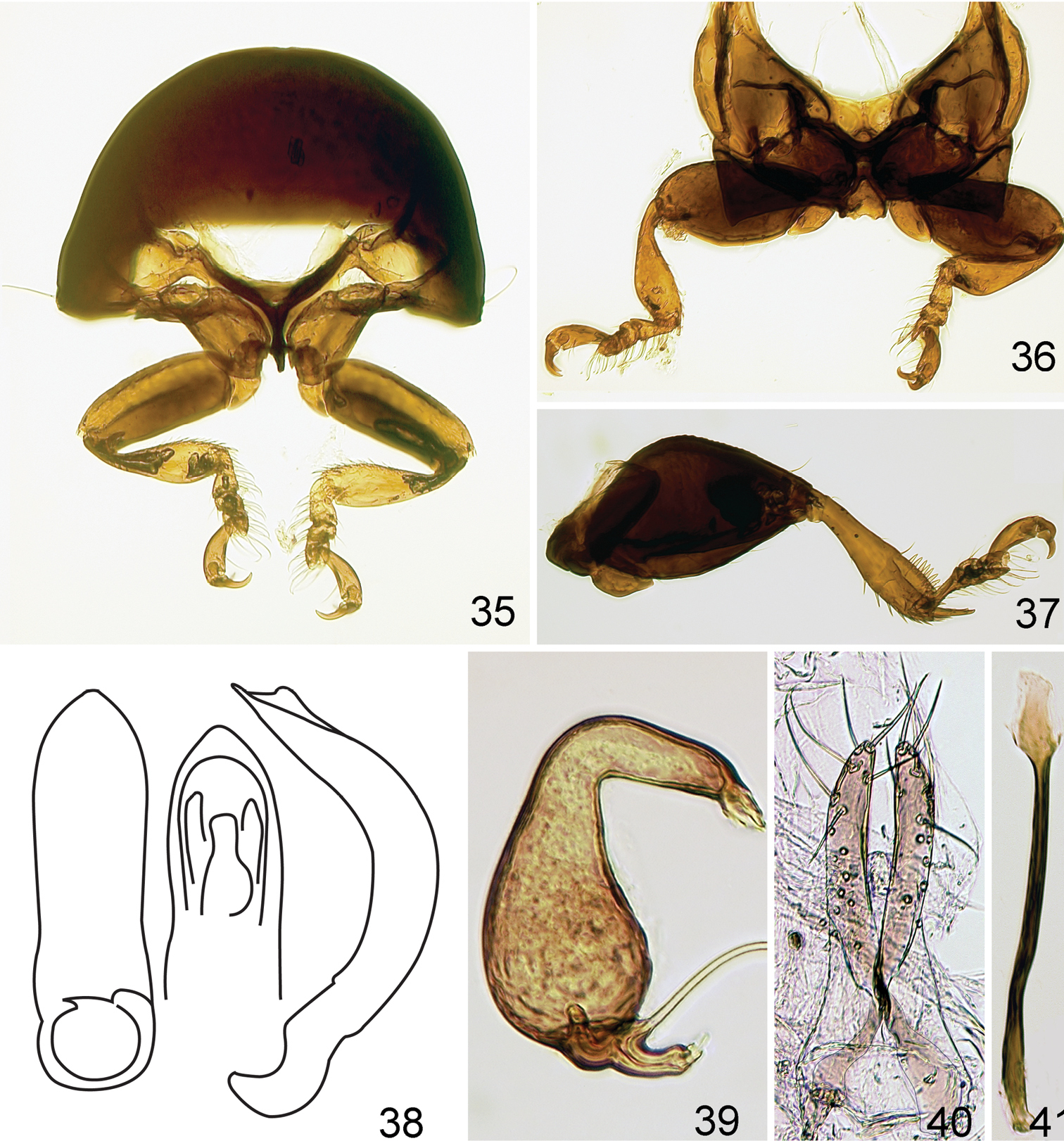
Labrum 1.54 times as long as wide and 0.57 times longer than thorma (Fig. 31). Labium as long as wide. Apical labial palpomere conical, longer than wide, slightly longer than palpomeres two and three separately (Fig. 30). Mandible with 4 denticles and well developed prostheca (Fig. 33). Pro- and mesotibia flat, widening apically and abruptly narrowing from apical one-third to apex (Figs 35, 36). Metendosternite typical to one of flightless flea beetles with short stalk and relatively long arms with poorly developed tendons (Fig. 34).
Kiskeya elyunque: 28 habitus, frontalview 29 habitus, lateral view 30 labium and maxilla 31 labrum 32 last seven antennomeres 33 mandible 34 metendosternite.
urn:lsid:zoobank.org:act:77AC612D-625B-4C57-9798-8144A0751C1E
http://species-id.net/wiki/Kiskeya_elyunque
Figs 28–41Body length 0.81–0.92 mm, width 0.52–0.65 mm. Color black with light greenish luster. Femur dark brown, rest of legs and antenna dark yellow. Vertex smooth, without punctures or wrinkles. Supraantennal sulcus absent. Pronotum with tiny, sparse, sharp punctures. Antennal club with 3 antennomeres (Fig. 32). Elytron (Fig. 29) convex in lateral view [length (from apex to connection with pronotum) nearly equal to height], with tiny, sparse, barely visible punctures. Proportions of protarsomeres of female (starting with first) 4:2:3:10; mesotarsomeres 4:2:3:10; metatarsomeres 11:2:3:10. In male, proportions as follows: protarsomeres 5:2:3:10; mesotarsomeres 5:2:3:10; metatarsomeres 10:2:2:10. Apex of median lobe of aedeagus without acute denticle in ventral view (Fig. 38). Vaginal palpi with eight long setae posteriorly (Fig. 40), curved towards middle. Anterior sclerotizations widening anteriorly, strongly diverging. Spermatheca (Fig. 39) with receptacle longer and wider than pump. Outer and inner sides of receptacle nearly equally convex. Tignum curved laterally. Posterior sclerotization of tignum wider than middle, arrow shaped (Fig. 41).
The specific epithet is a noun in apposition based on the type locality.
Kiskeya elyunque is the only species of Kiskeya known to occur in Puerto Rico. It can be easily differentiated from the other two known species of the genus based on the key below.
Unidentified moss samples which contained Kiskeya elyunque were collected in the forest from a variety of substrates (rocks, tree stumps, trunks and branches) (Fig. 59, 60).
Holotype: ♂, Puerto Rico: El Yunque, El Toro trail, 18°11.850’N, 66°29.533'W, 1066m, 14.VI.2008, moss (unsifted) leg. A. Konstantinov (USNM). Paratypes: 45 specimens, same label as holotype (USNM); 37 specimens, same label as holotype except the date, 16.VI.2008 and moss being “unsifted” (USNM).
Key to speciesof Kiskeya| 1 | Supraantennal sulcus present.Apex of median lobe of aedeagus thick and straight in lateral view with narrowly rounded denticle (Fig. 46) | Kiskeya neibae Konstantinov & Chamorro-Lacayo |
| – | Supraantennal sulcus absent | 2 |
| 2 (1) | Apex of median lobe of aedeagus with acute denticle in ventral view (Fig. 45). Posterior sclerotization of tignum not wider than middle, shapeless (Fig. 44). Internal side of spermathecal receptacle nearly straight (Fig. 42). Posterior ends of vaginal palpi directed laterally (Fig. 43) | Kiskeya baorucae Konstantinov & Chamorro-Lacayo |
| – | Apex of median lobe of aedeagus without acute denticle in ventral view (Fig. 38). Posterior sclerotization of tignum wider than middle, arrow shaped (Fig. 41). Internal side of spermathecal receptacle convex (Fig. 39). Posterior ends of vaginal palpi directed medially (Fig. 40) | Kiskeya elyunque sp. n. |
Kiskeya elyunque: 35 prothorax, frontal view 36 mesosternum, ventral view 37 hind leg 38 median lobe of aedeagus, ventral, dorsal and lateral views 39 spermatheca 40 vaginal palpi 41 tignum.
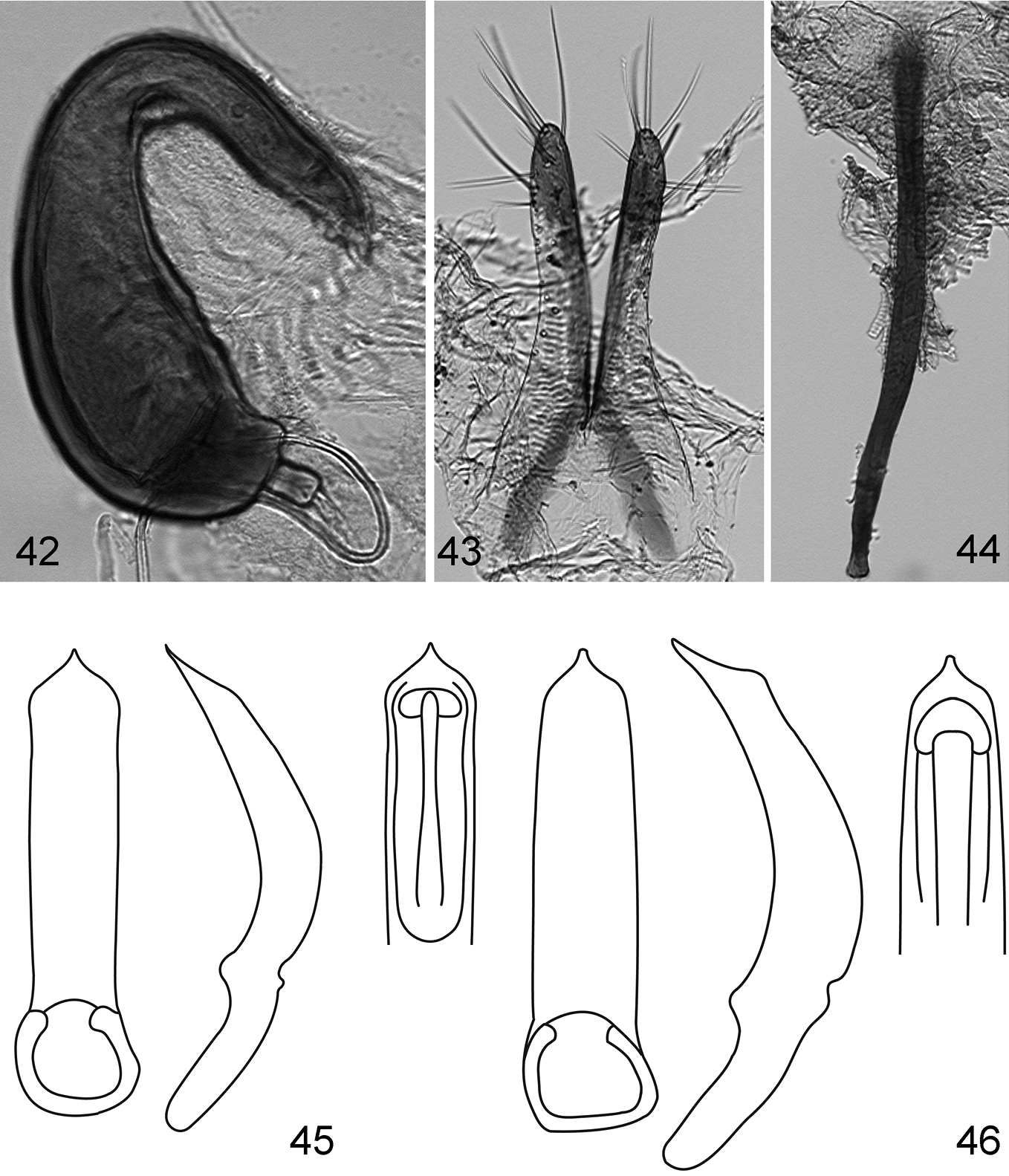
Kiskeya species:Kiskeya baorucae:42 spermatheca 43 vaginal palpi 44 tignum 45 Kiskeya baorucae median lobe of aedeagus, ventral, lateral and dorsal views 46 Kiskeya neibae median lobe of aedeagus, ventral, lateral and dorsal views.
http://species-id.net/wiki/Ulrica
The only three previously described species of Ulrica are known from Venezuela. There are about 20 more species that remain undescribed in various collections, but they were also collected from Venezuela. Among West Indian genera, Ulrica can be recognized based on the following key. The five specimens of Ulrica that were collected in Puerto Rico come from the same mountain region, El Yunque. Based on the labels, the substrate that they came from differs for two species: it is moss for Ulrica eltoro and leaf litter for Ulrica iviei. The exact collecting locations for Ulrica ivieiare unknown.
urn:lsid:zoobank.org:act:F5BD9D1D-4F73-4F1B-894B-6640B4C652F1
http://species-id.net/wiki/Ulrica_eltoro
Figs 47–50Body length 1.94–2.16 mm, width 1.18–1.29 mm. Color chestnut brown with appendages lighter (Fig. 47). Head surface shiny with few small punctures (Fig. 48), supraorbital pore much larger than a few small punctures on vertex. Supracallinal sulcus clearly separates antennal calli and vertex medially. Frontal ridge wide, longer than antennal calli. Anterofrontal ridge making long denticle about as long as half clypeus length. Pronotum and elytron with fine punctures. Interspaces of elytron flat. Proportions of tarsomeres of male as follows: protarsomeres 7:4:4:9; mesotarsomeres 7:4:4:9; metatarsomeres 10:4:4:9. Median lobe of aedeagus more or less parallel sided in ventral, median lobe view, with ridge in middle being wider at base, narrowing towards middle and widening and disappearing towards apex. In lateral, median lobe view slightly curved with bump on ventral side beyond middle (Fig. 50).
The specific epithet is a noun in apposition based on the type locality.
Ulrica eltoro can be easily differentiated from Ulrica ivieibased on the key below.
Unidentified moss samples that contained Ulrica eltoro were collected in the forest from a variety of substrates (rocks, tree stumps, trunks and branches) (Figs 59, 60).
Holotype: ♂, Puerto Rico: El Yunque, El Toro trail, 18°16.850'N, 65°49.753'W, 1066m, 14.VI.2008, moss (unsifted) leg. A. Konstantinov (USNM). Paratype ♂, same label as holotype (USNM).
Ulrica eltoro: 47 habitus, dorsal view 48 habitus, frontalview 49 habitus, lateral view 50 median lobe of aedeagus, ventral, lateral and dorsal views.
urn:lsid:zoobank.org:act:1DCF15CA-E09E-4B16-9F13-FBDD84421895
http://species-id.net/wiki/Ulrica_iviei
Figs 51–58Body length 1.89–2.05 mm, width 1.08–1.29 mm. Color chestnut brown to almost black with appendages lighter (Figs 51, 52). Head surface dorsally shiny, ventrally with some wrinkles (Fig. 53). Vertex with several large punctures, supraorbital pore as large as a few punctures on vertex near it. Supracallinal sulcus not separating antennal calli and vertex medially. Frontal ridge wide, about as long as antennal calli (Fig. 53). Anterofrontal ridge making long denticle about as long as entire clypeus. Pronotum and elytron with coarse punctures. Interspaces of elytron flat on disc, slightly convex apically. Proportions of tarsomeres of male as follows: protarsomeres 5:4:5:9; mesotarsomeres 5:4:5:9; metatarsomeres 10:5:5:9. Median lobe of aedeagus with more or less curved sides in ventral view, with ridge in middle being wider at base, narrowing towards middle and widening towards apex. In lateral view slightly curved without bump on ventral side beyond middle (Fig. 55). Spermatheca with pump at base wider than receptacle and duct making coils (Fig. 56). Sternite eight nearly fully sclerotized with tignum sharply bent anteriorly (Fig. 58). Vaginal palpi merged at about apical one third (Fig. 57).
The specific epithet is a patronym dedicated to Mike Ivie who collected the holotype.
Ulrica ivieican be easily differentiated from Ulrica eltoro based on the key below.
Holotype: ♂, Puerto Rico: Caribbean Nat. For. Pico El Yunque, El Toro trail, 975 m, 23 Sept. 1987, leg. M. A. Ivie, dwarf forest litter (WIBF). Paratypes ♂ and ♀, Puerto Rico El Yunque, Mt. Britton Tr. VIII.11.1999, C. W. O’Brien, P. Kovarik (MLBU, USNM).
Key to species of Ulrica from Puerto Rico| 1 | Head with supraorbital pore much larger than a few small punctures on vertex. Supracallinal sulcus separating antennal calli and vertex medially. Pronotum and elytron with fine punctures (Fig. 47) | Ulrica eltoro sp. n. |
| – | Head with supraorbital pore as large as a few punctures on vertex near it. Supracallinal sulcus not separating antennal calli and vertex medially. Pronotum and elytron with coarse punctures (Fig. 51) | Ulrica iviei sp. n. |
Ulrica iviei: 51 habitus, dorsal view 52 habitus, lateral view 53 head, frontalview 54 abdominal sternites, female 55 median lobe of aedeagus, ventral, lateral and dorsal views.
Ulrica iviei: 56 spermatheca 57 vaginal palpi 58 tignum.
El Yunque, Puerto Rico: 59 moss on tree trunks 60 forest along El Toro trail where moss occurs.
In addition to Distigmoptera and Ulrica, six other Monoplatini genera are reported in the West Indies: Aedmon Clark, Apleuraltica, Bonfilsus Scherer, Homotyphus Clark, Hypolampsis Clark, and Physimerus Clark (
| 1 | Apical spur of metatibia as long as second metatarsomere. Elytron generally bare, with just a few long setae | Ulrica |
| – | Apical spur of metatibia much shorter than second metatarsomere. Elytron, densely covered with numerous short setae | 2 |
| 2(1) | Supracallinal sulcus absent, antennal calli merged with vertex | Bonfilsus |
| – | Supracallinal sulcus present, antennal calli separated from vertex | 3 |
| 3(2) | Antennal calli much longer than wide. Basal antennomeres about same width as apical. | 4 |
| – | Antennal calli about as long as wide. Basal antennomeres much narrower than apical | 7 |
| 4(3) | Pronotal surface more or less even, without two protuberances separated by relatively deep furrow | 5 |
| – | Pronotal surface uneven, with two protuberances separated by relatively deep furrow | 6 |
| 5(4) | Pronotum about as long as wide | Physimerus |
| – | Pronotum significantly wider than long | Aedmon |
| 6(4) | Pronotal protuberances very well developed, tall. Two denticles before metatibial apex symmetrically developed | Homotyphus |
| – | Pronotal protuberances poorly developed, low. Two denticles before metatibial apex asymmetrically developed, one on lateral side much wider than one on medial side | Hypolampsis |
| 7(3) | Elytron with deep impression posterior to basal callus. Metatibia with transverse ridge above insertion of tarsus. Metatarsal claw in male simple | Apleuraltica |
| – | Elytron without deep impression posterior to basal callus. Metatibia without transverse ridge above insertion of tarsus. Metatarsal claw in male split | Distigmoptera |
We thank M. L. Chamorro, J. Micheli family and A. Segarra for companionship and hospitality during collecting trip to Puerto Rico. M. Ivie (WIBF) and S. Clark (MLBU) provided specimens of Ulrica iviei. We thank M. L. Chamorro, R. Kula, and A. Norrbom (Systematic Entomology Laboratory, Washington DC, USA), and C. Staines (Department of Entomology, Smithsonian Institution, Washington DC, USA) for thoroughly reviewing earlier versions of this manuscript and for their valuable suggestions. USDA is an equal opportunity provider and employer.