(C) 2011 Ekaterina Shevtsova. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Wing interference patterns (WIPs) are shown to be an important tool for species recognition in the genus Achrysocharoides Girault (Hymenoptera: Eulophidae). This is demonstrated by combining information from two previously published papers, comprising two cases of cryptic species, and by new material including the description of two new species, Achrysocharoides maieri and Achrysocharoides serotinae from North America. The cryptic species were initially separated through their distinct male WIPs. Subsequent analyses of the external morphology uncovered additional morphological differences supporting the original findings through WIPs, and biological data further strengthened the identity of these species. The new species described here also differ in their WIPs but the WIPs are similar in both sexes. Thus they provide a strong link between male and female and demonstrate that WIPs can also be useful for species recognition when the sexes are otherwise difficult to associate. Both new species are from Connecticut, USA, and were reared from Phyllonorycter propinquinella (Braun) (Lepidoptera: Gracillariidae) on black cherry (Prunus serotina); Achrysocharoides maieri has also been reared from Ph. nr crataegella on pin cherry (Prunus pensylvanica). To facilitate the identification of the new species they are included in a previously published key to North American species of Achrysocharoides. As a supplement to colourful WIPs we also demonstrate that grey scale images of uncoated wings from scanning electron microscopy can be used for visualization of the thickness distribution pattern in wing membranes.
taxonomy, cryptic species, structural colours, sexual dimorphism, wing membrane thickness, Chalcidoidea, Entedoninae, leafminer parasitoids, Achrysocharoides acerianus, Achrysocharoides platanoidae, Achrysocharoides robiniae, Achrysocharoides robinicolus, Achrysocharoides butus, Achrysocharoides latreilleii, Achrysocharoides albiscapus, Achrysocharoides maieri, Achrysocharoides serotinae, Phyllonorycter propinquinella, Phyllonorycter nr crataegella, Prunus serotina, Prunus pensylvanica
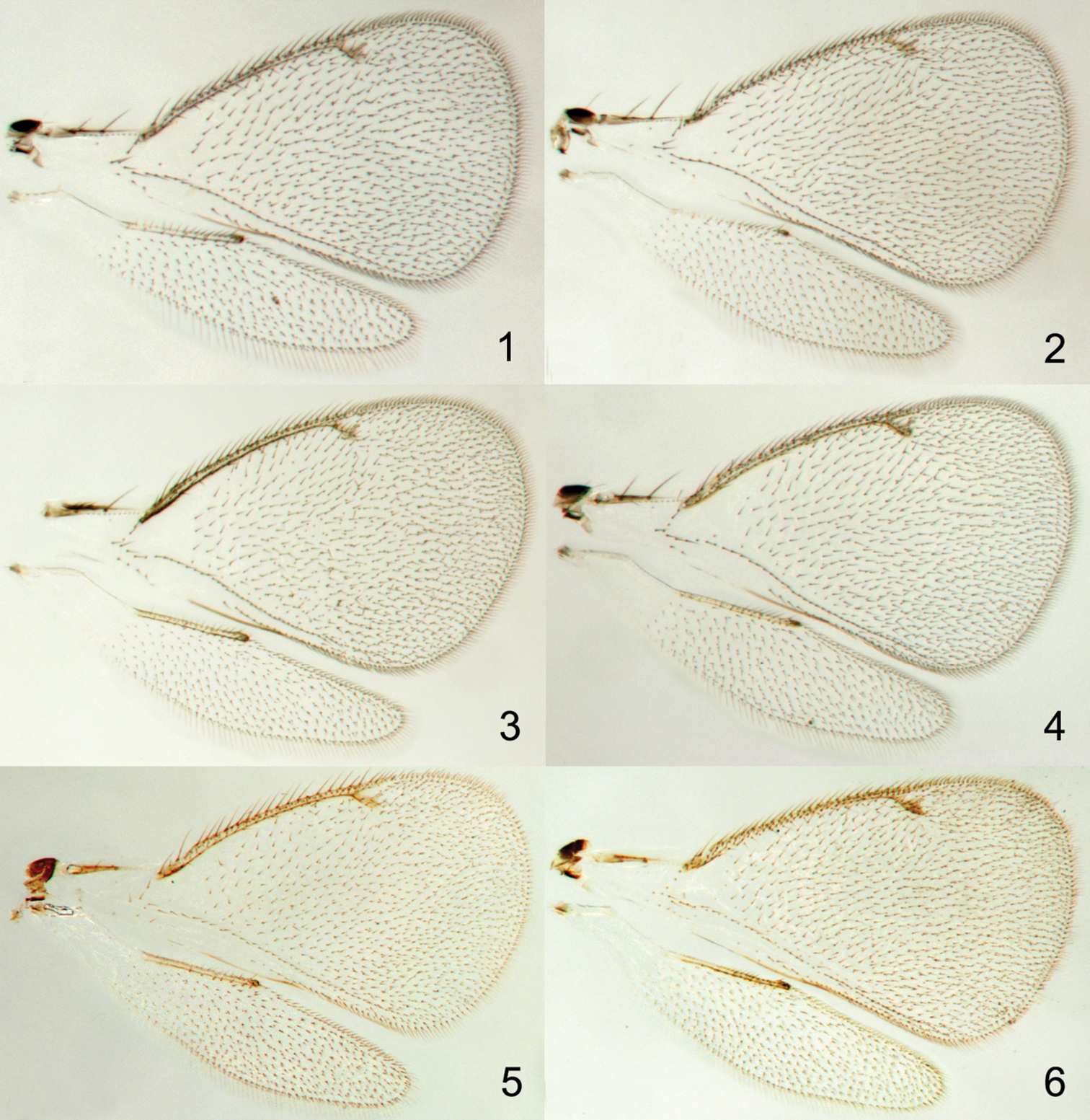
Species of Achrysocharoides Girault (Hymenoptera: Eulophidae) are small parasitic wasps with transparent non-pigmented wings (Figs 1–6, 9). The short postmarginal vein in the fore wing is characteristic for the genus and the shape of the fore wing can be used to distinguish males of some species, but otherwise wings have been disregarded as non-informative neutral entities in this genus (e.g.
WIPs as a morphological character are so new that very little is known about the significance of these patterns for their bearers or for entomologists studying them, although they have already proven useful for generic-level classification in Eulophidae (
The two new species of Achrysocharoides described here are from North America and the genus was initially recorded from this region by
Achrysocharoides spp., transparent wings: 1 Achrysocharoides acerianus (Askew), male 2 Ditto, female 3 Achrysocharoides platanoidae Hansson & Shevtsova, male 4 Ditto, female 5 Achrysocharoides butus (Walker), male 6 Ditto, female. Wings on Figs 1–4 from Sweden, Skåne, 2010 5–6 from Wales, 1976.
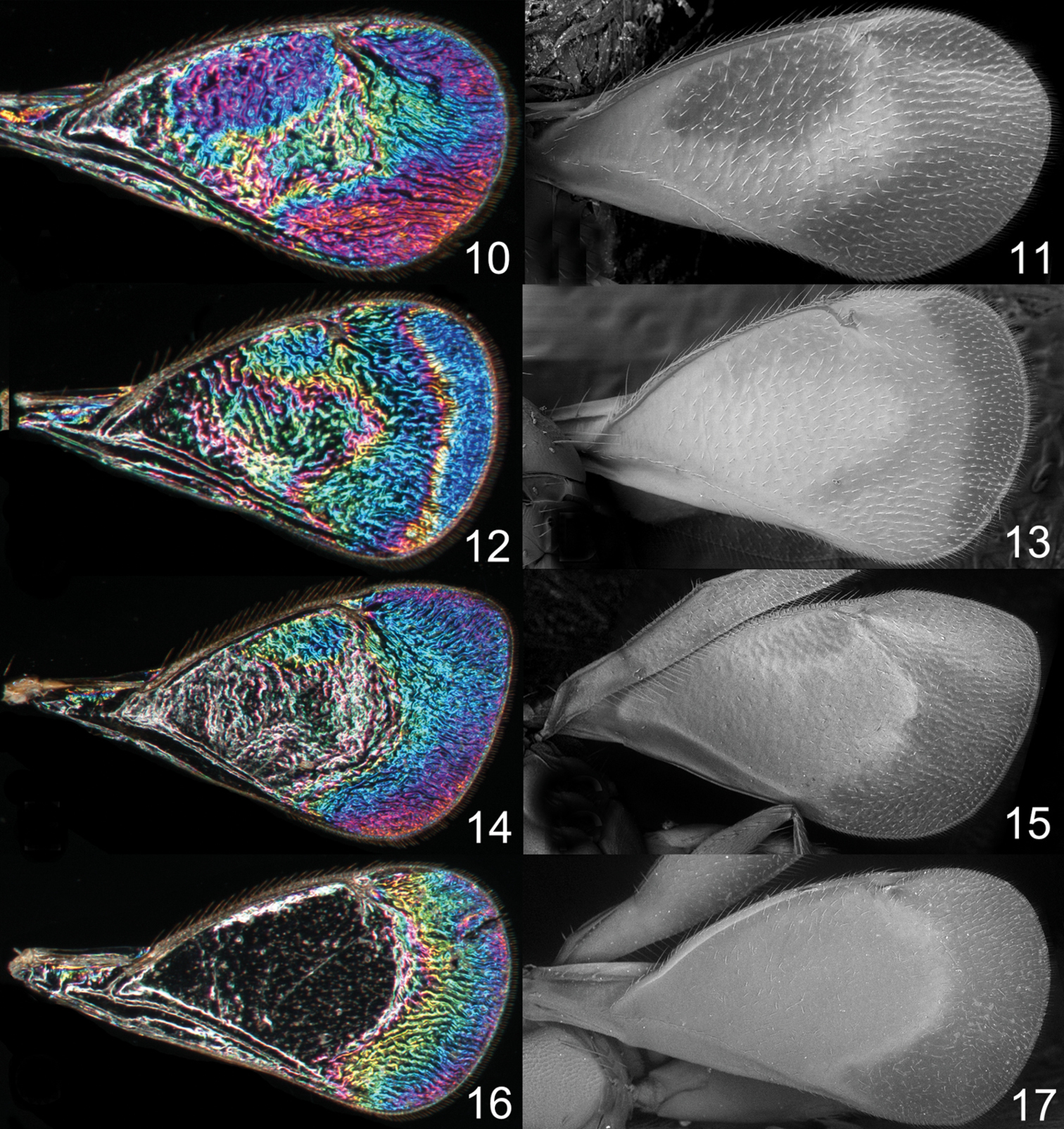
Achrysocharoides spp.: 7 Achrysocharoides zwoelferi (Delucchi), male, from Sweden, Blekinge, 1956 8 Undescribed species from USA, Arizona, 1982, male, wing interference pattern (WIP) 9 The same wings as in Fig. 8 in transparent mode.
The observation and documentation of WIPs do not require a special light source and can be done on any dry specimen with intact wings arranged against a dark background. However, to make the illustrations comparable all photos in this paper as well as in
Achrysocharoides spp., males, wing interference patterns (WIPs) to the left, scanning electron micrographs from uncoated wings to the right: 10–11 Achrysocharoides robiniae Hansson & Shevtsova 12–13 Achrysocharoides butus (Walker) 14–15 Achrysocharoides latreilleii (Curtis) 16–17 Achrysocharoides albiscapus (Delucchi).
HE = height of eye; HW = height of fore wing; LG = length of gaster; LM = length of marginal vein; LW = length of fore wing, measured from base of marginal vein to apex of wing; MM = length of mesosoma; MS = malar space; OOL = distance between one posterior ocellus and eye; PM = length of postmarginal vein; POL = distance between posterior ocelli; POO = distance between posterior ocelli and occipital margin; ST = length of stigmal vein; WH = width of head; WM = width of mouth; WT = width of thorax. For illustrations of the morphological terms see http://www.neotropicaleulophidae.com/.
Collection acronyms, for the deposition of type material: BMNH = Natural History Museum, London, England; CAES = Connecticut Agricultural Experiment Station, New Haven, U.S.A; CNC = Canadian National Collection of Insects, Ottawa, Canada.
Results and discussionThe paper by
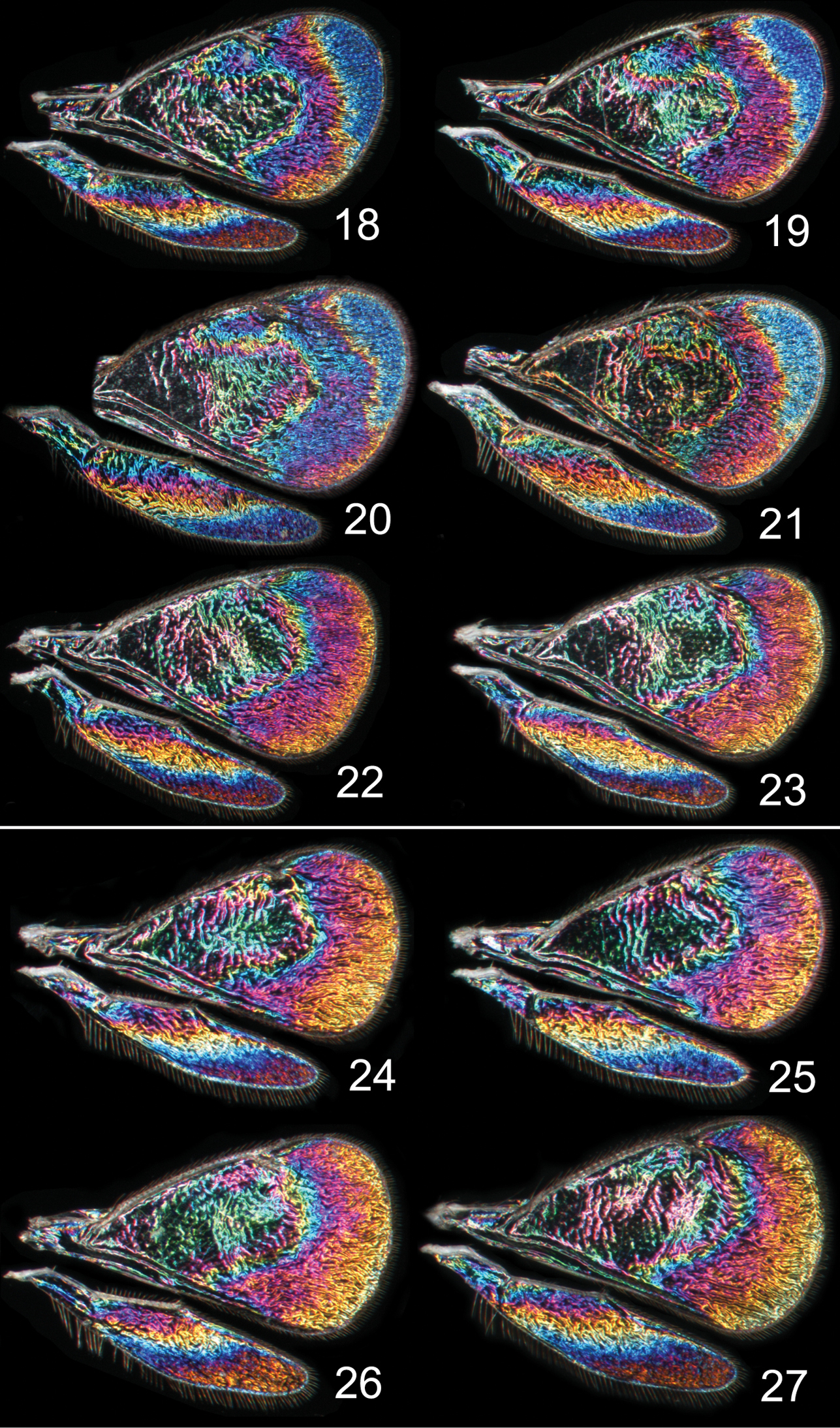
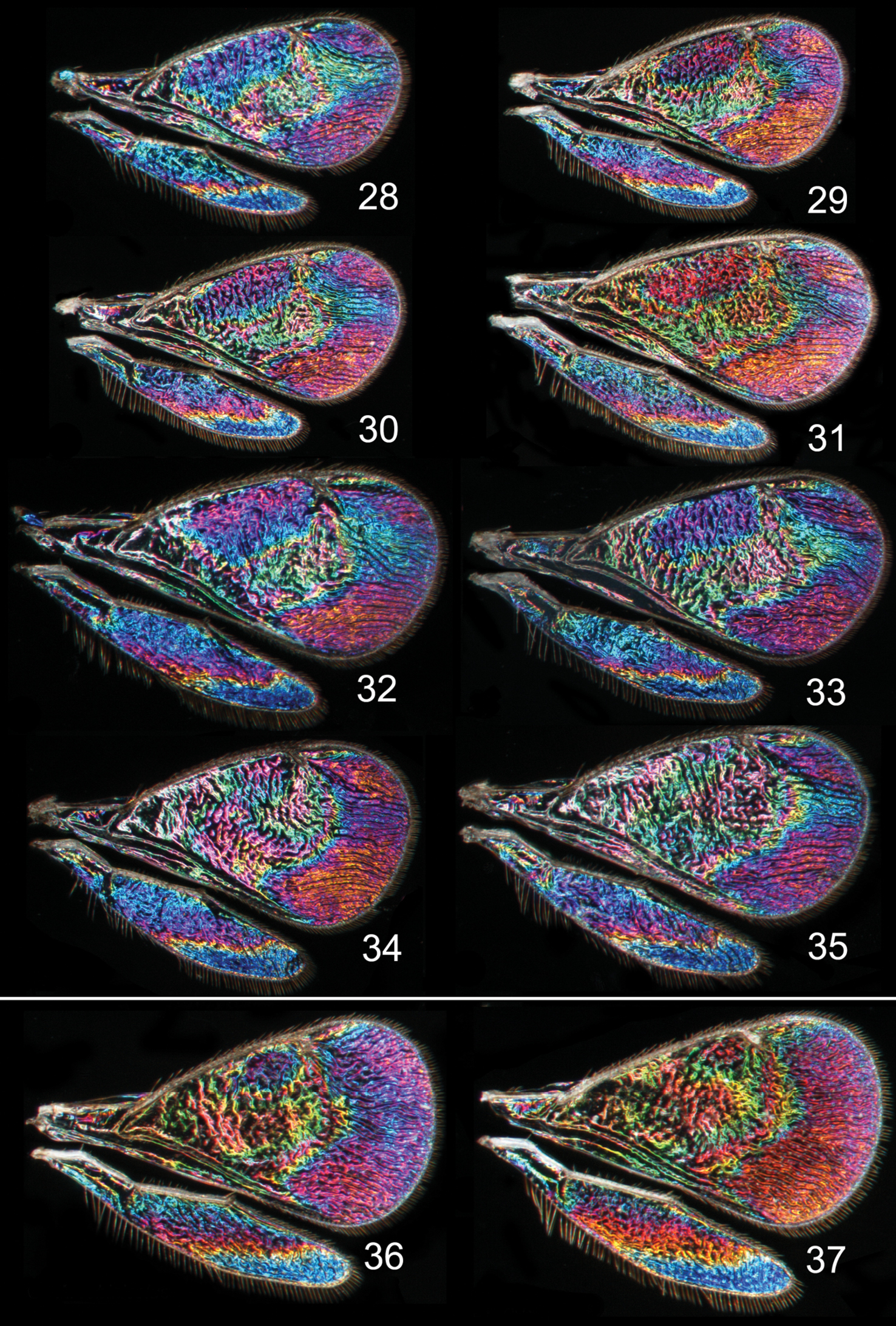
In both cryptic cases only one of the species displays a distinct species specific WIP, and in males only, while conspecific females and both sexes of the other cryptic species have similar WIPs. In the two Achrysocharoides species associated with Acer only males of Achrysocharoides platanoidae have a distinctive WIP with an eye-catching blue spot in the upper-apical corner of the fore wing (Figs 18–21). The female WIP of Achrysocharoides platanoidae displays no such spot (Figs 22–23) and is very similar to Achrysocharoides acerianus, which has the same WIP in both sexes (Figs 24–27). In the two other cryptic species, associated with Robinia pseudoacacia, only males of Achrysocharoides robiniae display a very characteristic WIP with a large ovate spot below the marginal vein. The male WIP also has an extended and usually green triangular area in the medio-apical part of the fore wing (Figs 28–33). In the female WIP the triangular area is usually less pronounced than in males and the submarginal ovate spot is significantly smaller (Figs 34–35). As the female does not display the characteristic features in these patterns as distinctly as the male, it can be confused with the female of Achrysocharoides robinicolus, which has the same WIP in both sexes (Figs 36, 37).
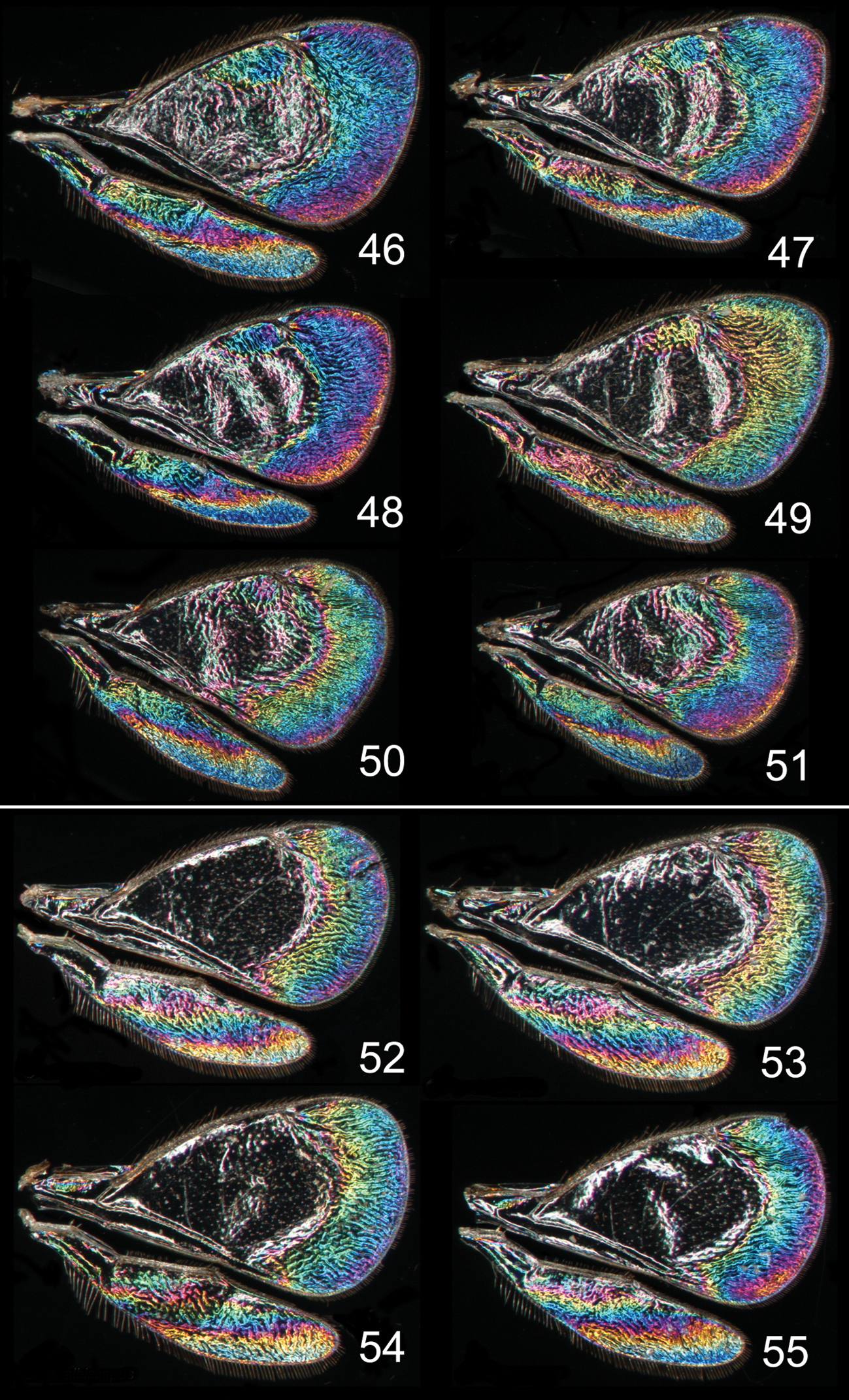
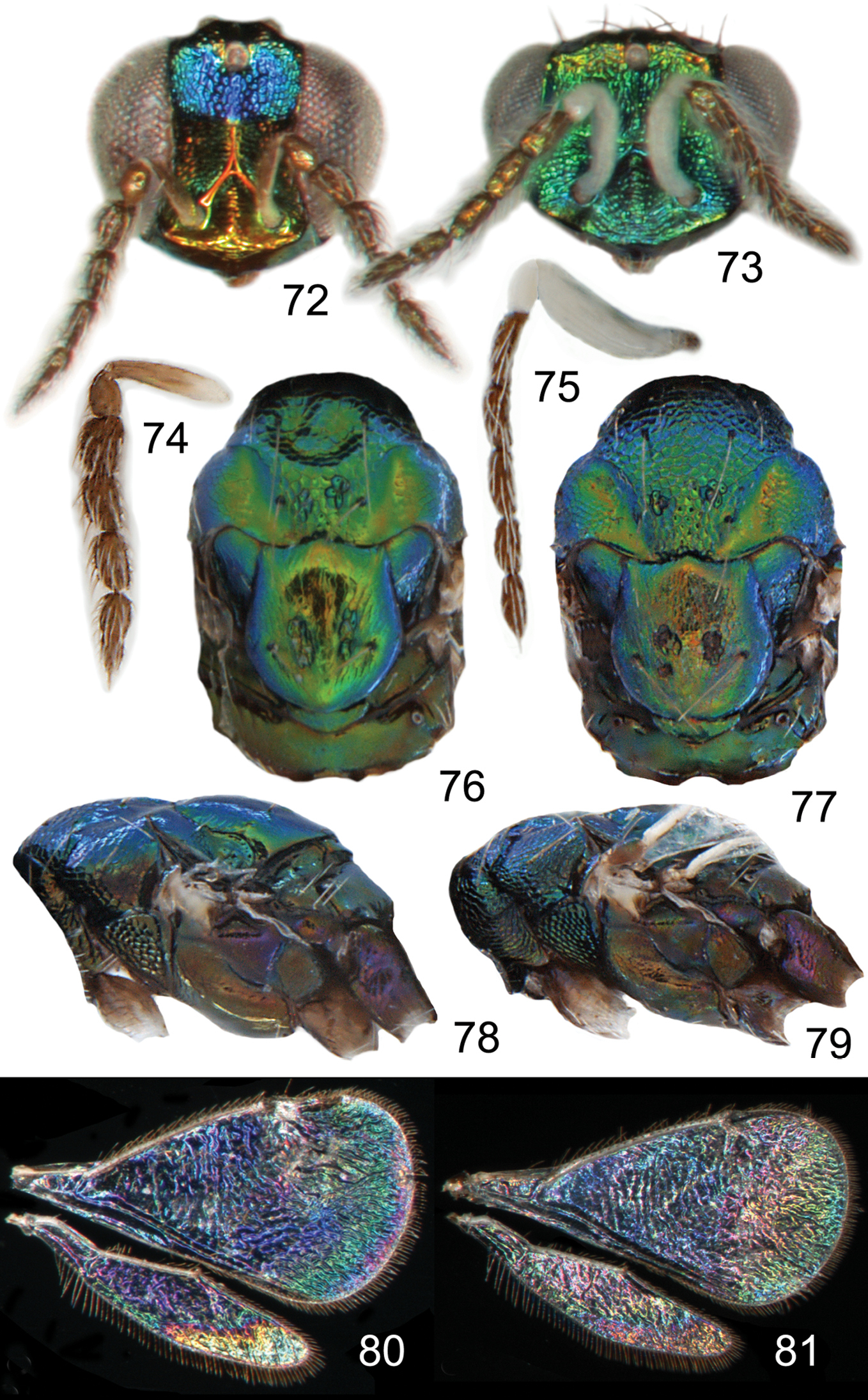
The two North American species described here, Achrysocharoides maieri and Achrysocharoides serotinae, are known only from, and are probably confined to, Phyllonorycter species on Prunus. Males can be distinguished through easy-to-see differences in the external morphology, e.g. the shape of the head (Figs 57, 67, 73, 83) but females are not so distinct and display less divergent characters (Figs 56, 66, 72, 82). Even though the wings of Achrysocharoides maieri and Achrysocharoides serotinae appear very similar in transparent mode (similar to Figs 1–6) the WIPs in these species are distinct and specific. Apart from being useful in the discrimination of the females, in this case WIPs are also useful for the association of otherwise dimorphic males and females of the same species. The external morphology in these species exhibits a pronounced sexual dimorphism and as they share the same host it is not obvious which females and males are conspecific. However, there is one important character they have in common – WIPs, which are identical in both sexes but different between the species. Achrysocharoides maieri has a WIP with wide coloured cross bands on the fore wing (Figs 64–65), and Achrysocharoides serotinae has a quite featureless almost unicoloured WIP (Figs 80–81).
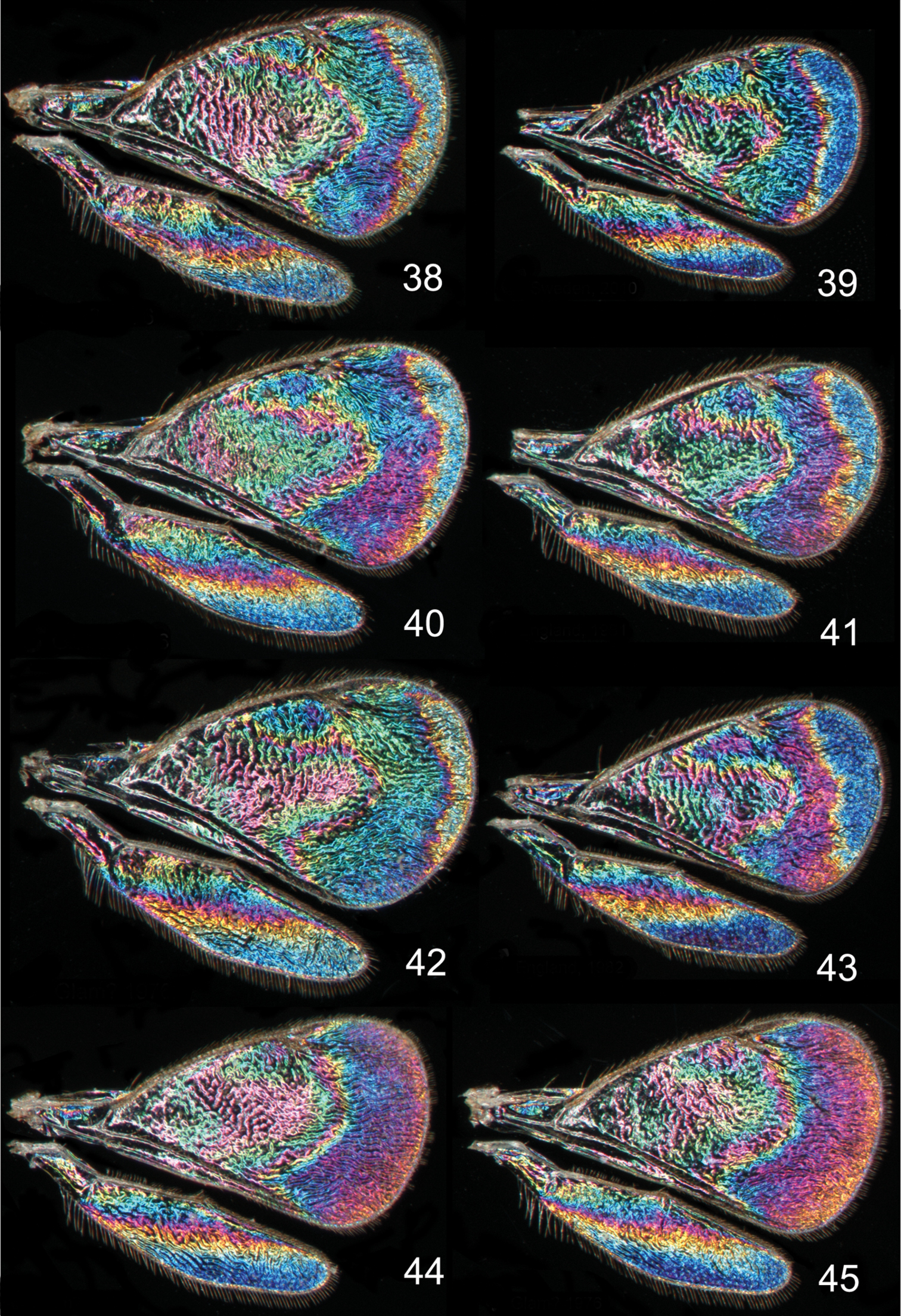
Additional examples of Achrysocharoides species with distinct and sexually dimorphic WIPs are Achrysocharoides butus (Walker) (Figs 38-43) and Achrysocharoides latreilleii (Curtis) (Figs 46-49) where characteristic and specific WIPs, again, are confined to males. Female WIPs of these two species are similar (Figs 44–45, 50–51), and as in females of Achrysocharoides platanoidae and Achrysocharoides acerianus (Figs 22–23, 26–27), and Achrysocharoides robiniae and Achrysocharoides robinicolus (Figs 34–35, 37), WIPs are not useful for species recognition. The WIP of male Achrysocharoides butus is similar to that of male Achrysocharoides platanoidae because the apical margin of the fore wing has a blue spot in both species. However, in Achrysocharoides butus this spot is prolonged and reaches along a major part of the apical margin (Figs 38–43) whereas in Achrysocharoides platanoidae the spot is short and confined to the upper-apical corner of the fore wing (Figs 18–21). The male of Achrysocharoides latreilleii is distinct not only in the truncate shape of the fore wing but also in its WIP (Figs 46–49). The basal 2/3 of the fore wing is the thickest part of the wing membrane and due to its micromorphology reflects very weak interference colours (
Similar to other morphological characters there is a certain intraspecific variation in WIPs (Figs 18–55), but the species specific traits nevertheless remain clearly recognizable and are reliable for species separation. The intraspecific variation in WIPs can be divided into two types, variation in colour and in shape of patterns. Variation in colour is basically size-dependent – the thickness of the wing membrane usually varies with the size of the specimen and there is a general shift of the hues in WIPs from larger to smaller specimens. Variation in the shapes of pattern outlines of conspecific WIPs is not apparently size dependent but reflects individual differences between specimens - the overall pattern nevertheless remains the same.
Wing interference patterns are due to structural organization patterns of the wing membrane where areas of different thickness reflect certain interference colours (
The clarification of the two cryptic species on Acer spp. (
Achrysocharoides spp., wing interference patterns (WIPs): 18–23 Achrysocharoides platanoidae Hansson & Shevtsova 18–21 Males 22–23 Females 24–27 Achrysocharoides acerianus (Askew) 24–25 Males 26–27 Females. All wings from specimens from Sweden, Skåne, 2010.
Achrysocharoides spp., wing interference patterns (WIPs): 28–35 Achrysocharoides robiniae Hansson & Shevtsova 28–33 Males 34–35 Females 36–37 Achrysocharoides robinicolus Hansson & Shevtsova 36 Male 37 Female. Wings on Figs 28–31, 34–37 from USA, Connecticut, 2002 32, 33, from Hungary, Vas Co., 2002.
Achrysocharoides butus (Walker), wing interference patterns (WIPs): 38–43 Males 44–45 Females. Wings on Figs 38, 40–45 from Wales, 1976 39 from Sweden, Skåne, 2010.
Achrysocharoides spp., wing interference patterns (WIPs): 46–51 Achrysocharoides latreilleii (Curtis) 46–49 Males 50–51 Females 52–55 Achrysocharoides albiscapus (Delucchi), males. Wings on Figs 46, 47, 49–51 from England, Surrey 1986–2004 48 from Sweden, Skåne, 2010; 52, 53, 55 from Greece, Crete, 1997 54 from France, 1984.
urn:lsid:zoobank.org:act:2E20B2E3-557F-413E-8729-D9ADD646FE3C
http://species-id.net/wiki/Achrysocharoides_maieri
Figures 56–71HOLOTYPE male (CNC) glued to a card, labeled “U.S.A.: Connecticut, New Haven Co., New Hamden, Lockwood Farm, 1.viii.1980, C.T. Maier”, “Tentiform mine of Phyllonorycter propinquinella on Prunus serotina, emerged in laboratory within 3 weeks”. PARATYPES: 1 female 3 males with same label data as holotype (BMNH, CAES, CNC)); 1 male labeled “U.S.A.: Connecticut, Tolland Co., Willington, 21.x.1981, Chris T. Maier”, “Mines of Phyllonorycter propinquinella on black cherry, Prunus serotina, on 21.x.1981, chilled outdoors, parasitoid emerged in laboratory in April 1982” (CNC); 3 females “U.S.A.: Connecticut, New Haven Co., North Haven, 1.vii.1981, C.T. Maier”, “Tentiform mine of Phyllonorycter nr crataegella on Prunus pensylvanica, emerged in laboratory within 3 weeks” (BMNH, CNC); 2 females 1 male from same locality and same host as previous but collected 2.vi.1986 (BMNH).
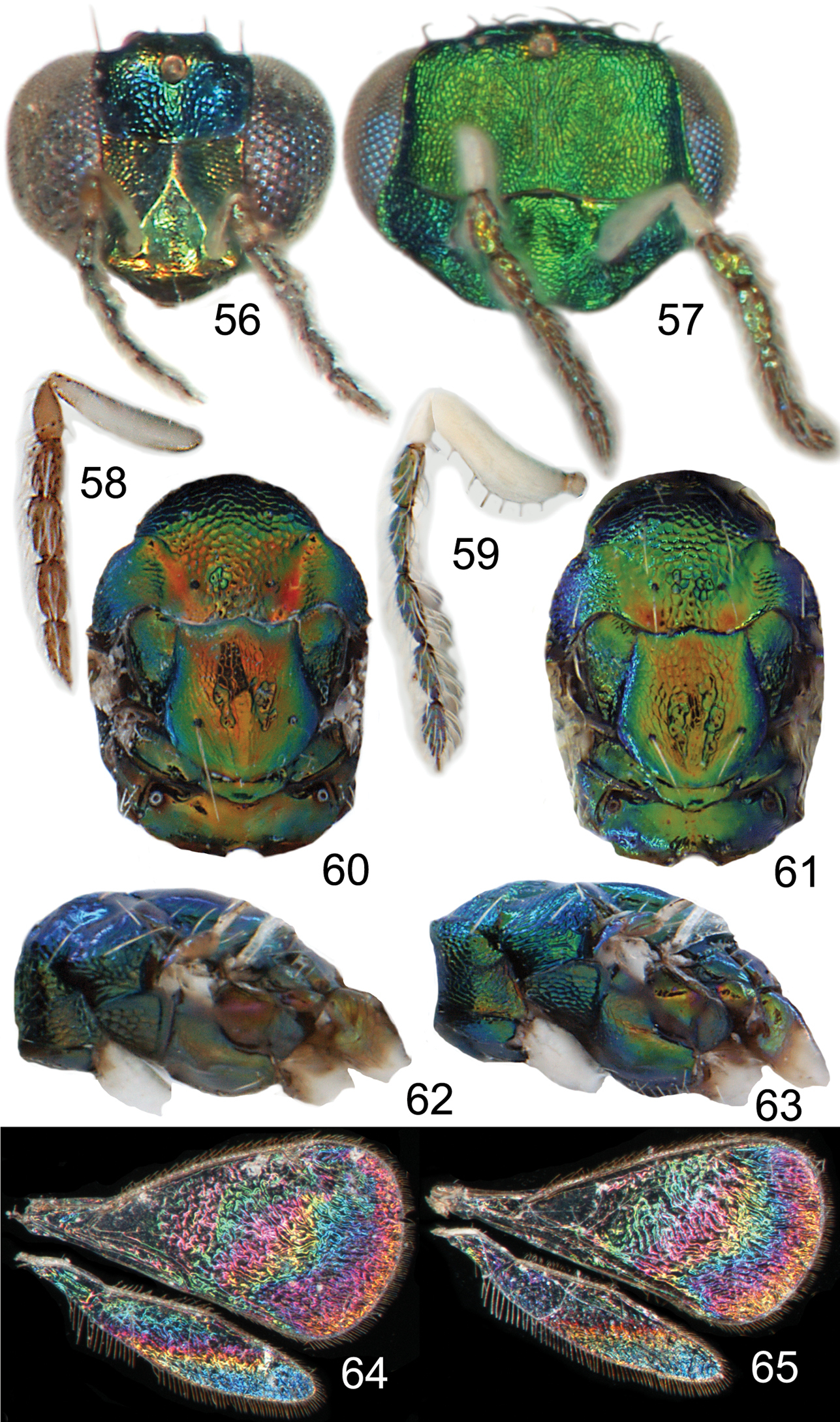
Both sexes: fore wing WIP with several distinct wide colourful cross-bands traversing the wing (Figs 64, 65), fore coxa white, hind coxa except apex golden green (Figs 62, 63); male: scape widest just below median part, with a single sparse row of setae along ventral margin (Fig. 59), antennal scrobes join frontal suture wide apart (Figs 67), vertex with long forward pointing setae (Fig. 69) – setae about as long as distance between posterior ocelli, upper frons without setae (Fig. 67), frons very large and wide (Fig. 67) - at its widest part 0.8X as wide as width of head; female: scape predominantly white and widest medially, with a single row of setae along ventral margin, propodeum smooth (Fig. 70), frons above frontal suture with raised and strong reticulation (Fig. 66).
Female. Length 1.1–1.5 mm. Scape white with inner apical tip infuscate; pedicel pale brown; flagellum dark brown (Fig. 58). Frons below frontal suture golden green to golden red, above frontal suture bluish green metallic (Fig. 56). Vertex inside ocellar triangle golden red, outside ocellar triangle golden green. Mesoscutum golden green with golden red areas – especially so in smooth posterior notaular depressions, to completely golden green (Fig. 60). Scutellum golden red with sides and posterior margin bluish green metallic, to completely golden green (Fig. 60). Propodeum golden red to golden green (Fig. 60). Fore coxa white, mid coxa dark brown with apical 1/3 white to completely dark brown, hind coxa golden green (Fig. 62); femora, tibiae and tarsi on all legs white. Wings without pigmented areas; WIP in fore wing with several distinct wide colourful cross-bands traversing the wing (Fig. 64). Gaster with first two tergites golden green, remaining tergites golden purple with green metallic tinges.
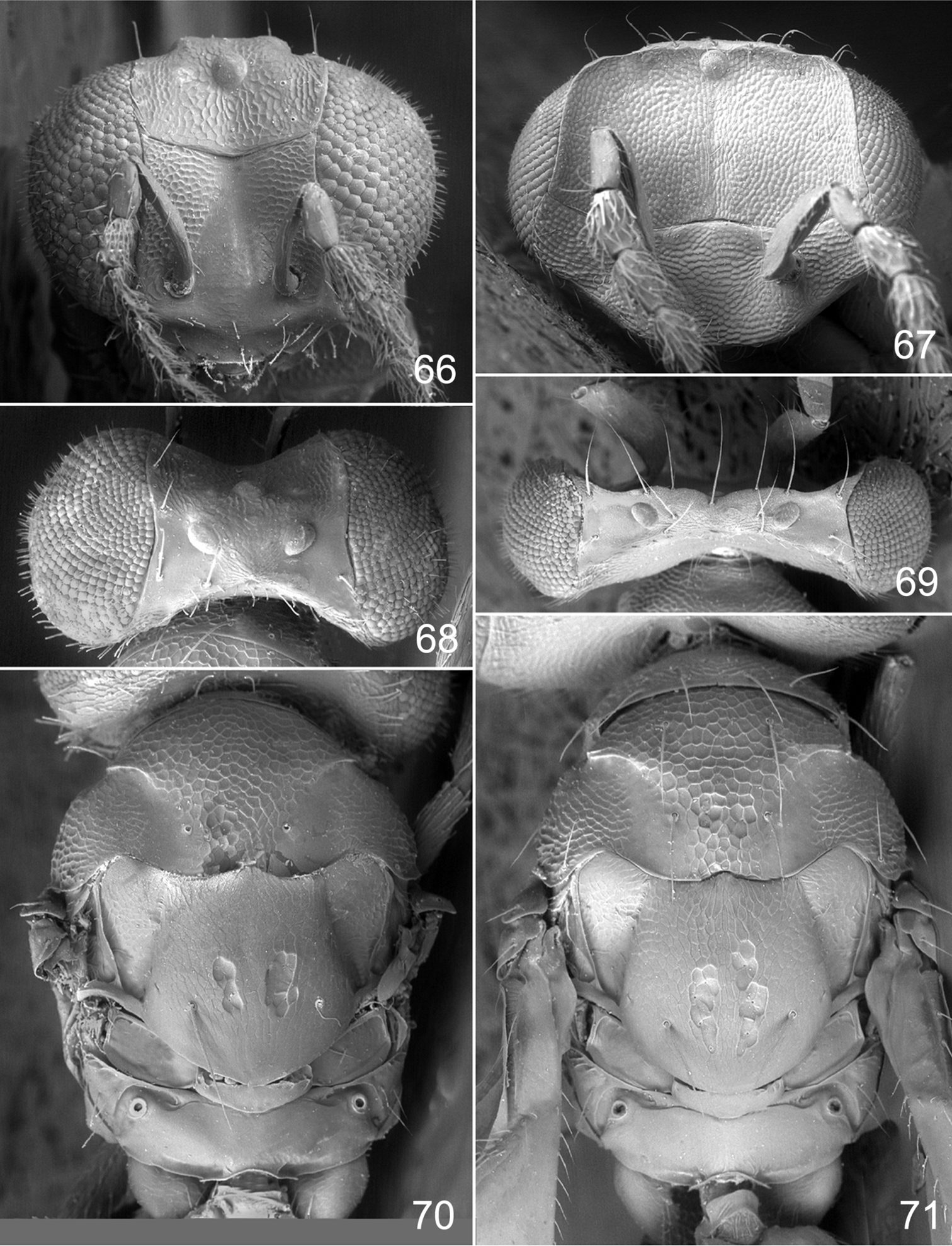
Antenna as in Fig. 58. Frons below level of toruli smooth and shiny (Fig. 66), between level of toruli and frontal suture with raised and strong reticulation lateral to antennal scrobes, between antennal scrobes with very weak reticulation, above frontal suture with raised and strong reticulation. Vertex inside ocellar triangle with engraved and weak reticulation, outside ocellar triangle smooth and shiny (Fig. 68). Occipital margin rounded.
Pronotal collar without transverse carina (Fig. 70). Mesoscutum with raised and strong reticulation (Fig. 70), meshes of reticulation smaller on sidelobes than on midlobe, midlobe with pits (i.e. with very strong reticulation) posteromedially; notauli as smooth impressions in posterior 2/3. Scutellum with very weak reticulation and shiny, smooth along posterior margin, with 3–4 pits medially on either side of imaginary median longitudinal line (Fig. 70). Dorsellum flat and smooth, anterolaterally with two foveae. Propodeum smooth and shiny (Fig. 70); propodeal callus with three setae. Fore wing speculum closed below. Petiole conical without shoulders.
Ratios. HE/MS/WM = 5.0/1.0/2.3; POL/OOL/POO = 2.6/1.1/1.0; WH/WT = 1.2; LW/LM/HW = 1.6/1.0/1.0; PM/ST = 1.0; MM/LG = 0.8–0.9.
Male. Length 1.4–1.5 mm. Scape and pedicel white; flagellum dark brown with golden green tinges (Fig. 59). Frons green metallic (Fig. 57). Vertex inside ocellar triangle golden red, outside ocellar triangle golden green. Mesoscutum golden green with posterior 1/3 of notaular depressions golden red (Fig. 61). Scutellum golden red with sides bluish green metallic (Fig. 61). Propodeum golden green (Fig. 61). Fore coxa white, mid coxa dark brown with apical 1/3 white to completely dark brown, hind coxa golden green with apical half white (Fig. 63); femora, tibiae and tarsi on all legs white. Wings without pigmented areas; WIP very similar to that of the female (Fig. 65). Gaster with tergites 1–2 golden green with a large white spot medially, remaining tergites dark brown with purple metallic tinges.
Antenna as in Fig. 59, i.e. scape widest just below middle. Frons with engraved and strong reticulation (Fig. 67); antennal scrobes reaching frontal suture wide apart; transverse ridge straight medially. Vertex inside ocellar triangle with engraved and very weak reticulation, outside ocellar triangle smooth and shiny (Fig. 69); anterior part with a row of seven long and proclinate setae. Occipital margin rounded.
Pronotal collar without transverse carina (Fig. 71). Mesoscutum with raised and strong reticulation (Fig. 71), meshes of reticulation smaller on sidelobes than on midlobe, midlobe with pits (i.e. with very strong reticulation) posteromedially; notauli as smooth impressions in posterior 2/3. Scutellum very weak reticulation and shiny, smooth along posterior margin, with 3–4 pits medially on either side of imaginary median longitudinal line (Fig. 71). Dorsellum flat and smooth, anterolaterally with two foveae. Propodeum smooth and shiny (Fig. 71); propodeal callus with three setae. Fore wing speculum closed below. Petiole conical without shoulders.
Ratios. HE/MS/WM = 2.3/1.0/1.3; POL/OOL/POO = 14.4/6.4/1.0; WH/WT = 1.4; LW/LM/HW = 1.5/1.0/1.0; PM/ST = 1.0; MM/LG = 1.0.
Named after Dr. Chris T. Maier, Entomologist at the Connecticut Agricultural Experiment Station, who collected all material of the two new species described here.
U.S.A. (Connecticut).
Phyllonorycter propinquinella (Braun) (Lepidoptera: Gracillariidae) on black cherry (Prunus serotina), and Phyllonorycter nr crataegella on pin cherry (Prunus pensylvanica).
Achrysocharoides maieri sp. nov.: 56 Head frontal, female 57 Ditto, male 58 Antenna lateral, female 59 Ditto, male 60 Mesosoma dorsal, female 61 Ditto, male 62 Mesosoma lateral, female 63 Ditto, male 64 Wing interference pattern (WIP), female 65 Ditto, male.
Achrysocharoides maieri sp. n.: 66 Head frontal, female 67 Ditto, male 68 Vertex, female 69 Ditto, male 70 Mesosoma dorsal, female. 71 Ditto, male.
urn:lsid:zoobank.org:act:C0EA95FF-793E-46BF-AF38-E300F345AB48
http://species-id.net/wiki/Achrysocharoides_serotinae
Figures 72–87HOLOTYPE male (CNC) glued to a card, labelled “U.S.A.: Connecticut, New Haven Co., North Haven, 30.ix.1981, Chris T. Maier”, “Adult parasitoid lab-reared from tentiform mine of Phyllonorycter propinquinella collected on black cherry, Prunus serotina on 30.ix.1981”. PARATYPES: 1 male with same label data as holotype (CNC); 2 females labeled “U.S.A.: Connecticut, Tolland Co., Union, 23.vi.1981, Chris T. Maier”, “Adult parasitoid lab-reared from tentiform mine of Phyllonorycter propinquinella collected on black cherry, Prunus serotina on 23.vi.1981” (CNC).
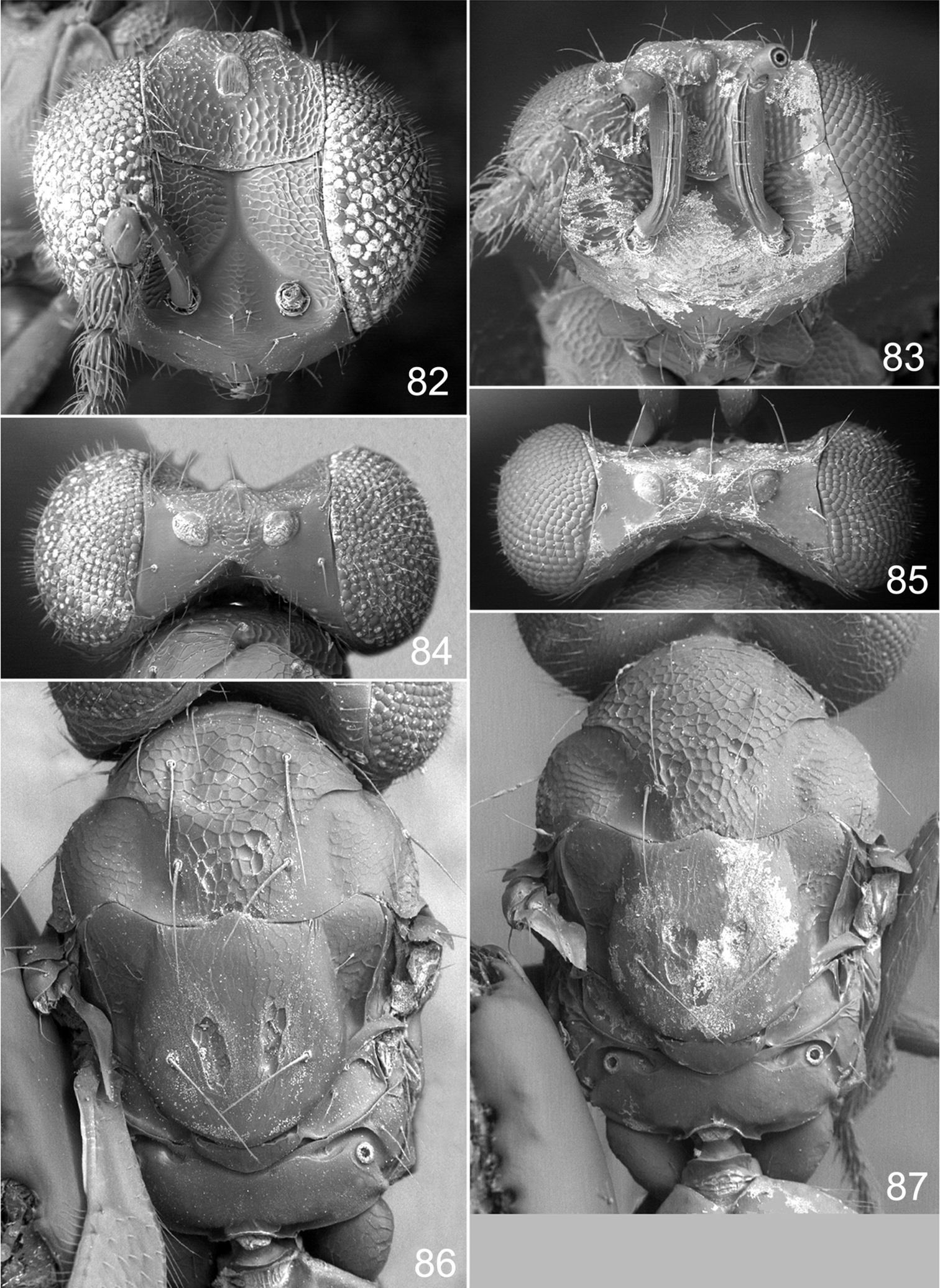
Both sexes: fore wing WIP almost unicoloured, gradually changing hue from purple to green towards the margin, without any distinct details such as lines or spots (Figs 80, 81), fore coxa predominantly dark brown, hind coxa golden green (Figs 78, 79); male: scape with about same width throughout, with a single sparse row of setae along ventral margin, antennal scrobes joining on frontal suture (Fig. 73, 83), vertex with long forward pointing setae (Fig. 85) – setae at most as long as distance between posterior ocelli, upper frons without setae (Fig. 83); female: scape pale brown and widest medially, with a single row of setae along ventral margin (Fig. 74), propodeum smooth (Fig. 86), frons above frontal suture with raised and strong reticulation (Fig. 82).
Female. Length 1.2–1.3 mm. Scape and pedicel pale brown; flagellum dark brown (Fig. 74). Frons below frontal suture golden red, above frontal suture bluish green metallic (Fig. 72). Vertex inside ocellar triangle golden red, outside ocellar triangle golden green. Mesoscutum green metallic with blue metallic tinges, smooth parts of notaular depression golden green (Fig. 74). Scutellum golden green with sides and posterior margin bluish green metallic (Fig. 74). Propodeum golden green with blue metallic tinges (Fig. 74). Fore coxa dark brown with apical 1/3 white, mid coxa dark brown, hind coxa purple metallic (Fig. 78); femora, tibiae and tarsi on all legs white. Wings without pigmented areas; WIP in fore wing almost unicoloured, gradually changing hue from blue to green towards the margin when the membrane becomes gradually thinner (Fig. 80). Gaster with first two tergites golden green, remaining tergites golden purple with green metallic tinges.
Antenna as in Fig. 74. Frons below level of toruli smooth and shiny (Fig. 82), between level of toruli and frontal suture with raised and strong reticulation with antennal scrobes smooth, above frontal suture with raised and strong reticulation. Vertex inside ocellar triangle with engraved and weak reticulation, outside ocellar triangle smooth and shiny (Fig. 84). Occipital margin rounded.
Pronotal collar without transverse carina (Fig. 86). Mesoscutum with raised and strong reticulation (Fig. 86), meshes of reticulation smaller on sidelobes than on midlobe, midlobe with singular pits (i.e. with very strong reticulation) posteromedially; notauli as smooth impressions in posterior 2/3. Scutellum with very weak reticulation and shiny, smooth along posterior margin, with 2–4 pits medially on either side of imaginary median longitudinal line (Fig. 86). Dorsellum flat and smooth, anterolaterally with two foveae. Propodeum smooth and shiny (Fig. 86); propodeal callus with three setae. Fore wing speculum closed below. Petiole conical without shoulders.
Ratios. HE/MS/WM = 3.7/1.0/1.6; POL/OOL/POO = 1.7/1.0/1.0; WH/WT = 1.2; LW/LM/HW = 1.6/1.0/1.0; PM/ST = 1.0; MM/LG = 0.8–0.9.
Male. Length 1.4 mm. Scape and pedicel white; flagellum dark brown (Fig. 75). Frons green metallic (Fig. 73B). Vertex inside ocellar triangle golden red, outside ocellar triangle golden green. Mesoscutum golden green with anterior part blue (Fig. 77). Scutellum golden green with golden red tinges and with lateral parts blue (Fig. 77). Propodeum golden green with golden red tinges (Fig. 77). Fore coxa dark brown with apical 1/3 white, mid coxa dark brown, hind coxa purple metallic (Fig. 79); femora, tibiae and tarsi on all legs white. Wings without pigmented areas; WIP very similar to that of the female (Fig. 81). Gaster with tergites 1–2 dark brown with golden green tinges, remaining tergites dark brown with weak metallic tinges, over tergites 1–3 with a large median white spot.
Antenna as in Fig. 75, i.e. scape with about same width throughout. Frons with raised and strong reticulation, some parts with transverse striation (Fig. 83); antennal scrobes joining on frontal suture; transverse ridge evenly curved. Vertex inside ocellar triangle with engraved and very weak reticulation (Fig. 85), outside ocellar triangle smooth and shiny; anterior part with a row of 3–5 long and forward directed setae. Occipital margin rounded.
Pronotal collar without transverse carina (Fig. 87). Mesoscutum with raised and strong reticulation (Fig. 87), meshes of reticulation smaller on sidelobes than on midlobe, midlobe with pits (i.e. with very strong reticulation) posteromedially; notauli as smooth impressions in posterior 2/3. Scutellum with weak reticulation, smooth along posterior and lateral margins, with 2–5 pits medially on either side of imaginary median longitudinal line (Fig. 87). Dorsellum flat and smooth, anterolaterally with two foveae. Propodeum smooth and shiny (Fig. 87); propodeal callus with three setae. Fore wing speculum closed below. Petiole conical without shoulders.
Ratios. HE/MS/WM = 2.5/1.0/1.3; POL/OOL/POO = 4.6/1.8/1.0; WH/WT = 1.1; LW/LM/HW = 1.6/1.0/1.0; PM/ST = 1.0; MM/LG = 0.9.
Named after black cherry (Prunus serotina), the host plant.
U.S.A. (Connecticut).
Phyllonorycter propinquinella (Braun) (Lepidoptera: Gracillariidae) on black cherry (Prunus serotina).
Achrysocharoides serotinae sp. n.: 72 Head frontal, female 73 Ditto, male 74 Antenna lateral, female 75 Ditto, male 76 Mesosoma dorsal, female 77 Ditto, male 78 Mesosoma lateral, female 79 Ditto, male 80 Wing interference pattern (WIP), female 81 Ditto, male.
Achrysocharoides serotinae sp. n.: 82 Head frontal, female 83 Ditto, male 84 Vertex, female 85 Ditto, male 86 Mesosoma dorsal, female 87 Ditto, male.
In the most recent key to North American Achrysocharoides by
Females of both species run to couplet 3, alternative 2 (where Achrysocharoides arienascapus falls out). The second alternative is changed to lead to 3a instead of Achrysocharoides arienascapus and then:
| 3a | Fore coxa and scape predominantly brown (Figs 74, 78) | Achrysocharoides serotinae sp. n. |
| – | Fore coxa and scape white | 3b |
| 3b | Entire frons above frontal suture with raised and strong reticulation (Fig. 66); scutellum with very weak and superficial reticulation (Fig. 70) | Achrysocharoides maieri sp. n. |
| – | Frons strongly reticulate medially and weakly reticulate laterally; scutellum with strong reticulation | Achrysocharoides arienascapus (Miller) |
| Males run to couplet 4: | ||
| 4 | Frons above frontal suture with many short and scattered setae (see fig. 5 in |
Achrysocharoides hirtiscapus (Miller) |
| – | Frons above frontal suture bare (Figs 67, 83); scape with a few short setae along ventral edge (Figs 59, 75) | 5a |
| 5a | Vertex with long setae about as long as distance between posterior ocelli (Figs 69, 85) | 5b |
| – | Vertex with long setae at least as long as width of vertex (see fig. 7 in |
5 |
| 5b | Scape widest close to base (Fig. 59); fore coxa white (Fig. 63) | Achrysocharoides maieri sp. n. |
| – | Scape with about same width throughout (Fig. 75); fore coxa predominantly brown (Fig. 79) | Achrysocharoides serotinae sp. n. |
Our foremost thanks are due to ArtDatabanken who through the Swedish Taxonomy Initiative funds the PhD project of Ekaterina Shevtsova. Thanks also to Chris T. Maier (at CAES) who generously sent us his material of Achrysocharoides, and to Gary A.P. Gibson and John T. Huber (both at CNC) for the loan of material. The help of Andrew Polaszek (BMNH) with obtaining molecular data for Achrysocharoides acerianus and A. platanoidae is highly appreciated. We thank the microscopy unit at the Biology Department, Lund University, for the use of their SEM. We also thank two anonymous reviewers for their useful suggestions.