(C) 2010 Charles R. Bartlett. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The number of species of planthoppers (excluding Delphacidae) known from Delaware is updated from 7 (in 4 families) to 62 species (in 9 families). Specimen abundance is tallied by county and seasonally by two week intervals. The Chao1 abundance estimator suggests that the true fauna may be 74 species, although species incidence tallied from adjacent states (MD, NJ, PA and DC) suggests that a total fauna of approximately 100 species may be possible. An artificial key is presented to genus and select species with photos of most included taxa.
Auchenorrhyncha, Fulgoromorpha, Acanaloniidae, Achilidae, Caliscelidae, Cixiidae, Derbidae, Dictyopharidae, Flatidae, Issidae, species inventory, generic key
The distribution of planthoppers (Hemiptera: Auchenorrhyncha: Fulgoroidea) in the eastern United States was most recently summarized by
Our objectives were to provide an abundance-based list of planthopper species found in Delaware (excluding Delphacidae)
established primarily on specimen records from the University of
Delaware Insect Reference Collection (UDCC) in Newark, DE; provide a
measure of completeness of this inventory using the Chao1
abundance-based diversity estimator (
Planthopper specimens from Delaware, Maryland, New
Jersey, and Pennsylvania in the UDCC were identified to species.
Identification of some taxa requires dissection of male genitalia, in
which case the abdomen was removed (sometimes after relaxing the
specimen overnight in high humidity) and cleared for 24 hours in 15%
potassium hydroxide (KOH), rinsed in water and transferred to glycerol
for observation and manipulation (see, e.g.,
Family-level nomenclature follows
Incidence records were listed for Maryland, New Jersey, Pennsylvania, and the District of Columbia based on literature (see below) and specimen records. Specimen records were compiled both from the UDCC and USNM collections. Specimens from Delaware were totaled by county and collection date increment. For collection date tallies, each month was divided into two increments, “early” (the 1–15th of each month), and “late” (the 16th-end of month) dates. Specimens with incomplete date information were omitted from these counts (resulting in the number of specimens tallied for seasonal data for some species to be less than the number of specimens observed). Because some species were at times found in abundance, seasonality records were tallied in two ways; complete specimen counts, and observation records where each series (all specimens recorded from a particular location and date) was tallied as a single observation.
To help assess completeness of the inventory, literature records were compiled from published sources (viz.
Photographs were taken using a Nikon SMZ-1500 Digital Imaging Workstation with Nikon DS-U1 digital Camera and NIS Elements Imaging software (version 3.0). Line drawings were made by Kimberley Shropshire (see acknowledgements) by tracing photographs and rendering detail freehand with reference to specimens.
Total planthopper species richness for Delaware was also evaluated using Chao’s (1984) abundance based estimator of species richness calculated as Schao = Sobs + F12/2F2, where Sobs = # observed species, F1 = # of species observed by exactly one specimen, F2 = # of species observed by exactly two specimens.
ResultsAmong 1, 734 specimens from Delaware we observed 62 planthopper species in 27 genera and 9 families (Table 1), including 55 new state records. Not surprisingly, specimen records were strongly biased (72% of observed specimens) toward New Castle County where the main campus of University of Delaware is located. Some females in the genera Bothriocera, Cixius, Haplaxius, Melanoliarus (all Cixiidae) and Cedusa (Derbidae), representing 88 specimens, could not be definitively identified to species and these female specimens were subsequently excluded from the species tally and the calculation of the Chao1 statistic; however, one of the female Bothriocera specimens appears to represent an additional species. Specimens of Omolicna evidently represented 2 species, but we were unable to identify them or parse the species with confidence. For this reason, we have reported the specimens identified to the generic level and included them in the species count and calculations.
County and seasonality records for Delaware planthoppers. Number of observed specimens given for county records, with distribution of records over the year provided, including earliest and latest observation. For seasonality records, records were divided into early (day 1–15 of the month) and late (remainder of month) observations, and for each observation a specimen count is followed parenthetically by number of independent collecting events (see methods). Sum of seasonality records may be less that sum of specimen records as ambiguous date records were omitted from seasonality tally. Column totals below seasonal entry is a count of the number of species observed during that time interval.
| County records | March | April | May | June | July | August | September | October | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New Castle | Kent | Sussex | Sum | Early date | Late date | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | Early | Late | |
| Acanaloniidae | ||||||||||||||||||||||
| Acanalonia bivittata | 29 | 5 | 11 | 45 | 16-May | 5-Oct | 1(1) | 5(1) | 8(4) | 6(5) | 11(6) | 9(7) | 4(3) | 1(1) | ||||||||
| Acanalonia conica | 138 | 10 | 2 | 150 | 2-Jul | 7-Oct | 16(7) | 25(17) | 14(10) | 24(15) | 49(18) | 22(8) | 22(7) | 2(2) | ||||||||
| Achilidae | ||||||||||||||||||||||
| Catonia carolina | 9 | 15 | 24 | 29-Jul | 7-Oct | 1(1) | 4(1) | 6(3) | 10(1) | 1(1) | 1(1) | |||||||||||
| Catonia cinctifrons | 1 | 1 | 26-Jul | 1(1) | ||||||||||||||||||
| Catonia nava | 10 | 10 | 3-Aug | 27-Sep | 1(1) | 8(1) | ||||||||||||||||
| Catonia picta | 1 | 1 | 7-Oct | 1(1) | ||||||||||||||||||
| Catonia pumila | 3 | 3 | 4-Aug | 8-Sep | 1(1) | 2(1) | ||||||||||||||||
| Cixidia fusca | 4 | 4 | 28-Jul | 7-Oct | 1(1) | 3(1) | ||||||||||||||||
| Cixidia opaca | 1 | 1 | 28-Aug | 1(1) | ||||||||||||||||||
| Cixidia variegata | 1 | 1 | 7-Oct | 1(1) | ||||||||||||||||||
| Caliscelidae | ||||||||||||||||||||||
| Aphelonema simplex | 15 | 141 | 9 | 165 | 30-May | 3-Oct | 8(1) | 9(1) | 4(1) | 69(1) | 70(1) | 1(1) | ||||||||||
| Bruchomorpha sp. n. | 7 | 7 | 29-Jun | 21-Aug | 2(1) | 3(2) | 2(1) | |||||||||||||||
| Bruchomorpha oculata | 53 | 1 | 54 | 22-Jun | 9-Oct | 4(1) | 6(4) | 3(2) | 15(3) | 12(5) | 12(6) | |||||||||||
| Bruchomorpha pallidipes | 2 | 2 | 29-Jun | 2(1) | ||||||||||||||||||
| Cixiidae | ||||||||||||||||||||||
| Bothriocera cognita | 9 | 3 | 34 | 46 | 22-Jun | 4-Aug | 1(1) | 4(2) | 20(5) | 17(8) | 2(1) | |||||||||||
| Bothriocera drakei | 2 | 2 | 29-Jun | 3-Jul | 1(1) | 1(1) | ||||||||||||||||
| Bothriocera maculata | 3 | 3 | 29-Jun | 1(1) | ||||||||||||||||||
| Bothriocera spp. Female | 1 | 1 | 15-Jul | 1(1) | ||||||||||||||||||
| Cixius angustatus | 1 | 1 | 9-May | 1(1) | ||||||||||||||||||
| Cixius nervosus | 45 | 45 | 8-May | 29-Jul | 10(4) | 1(1) | 5(3) | 28(6) | 1(1) | |||||||||||||
| Cixius spp. Female | 2 | 2 | 24-Apr | 2-Jun | 1(1) | 1(1) | ||||||||||||||||
| Haplaxius ovatus | 1 | 3 | 4 | 7-Jun | 29-Jul | 3(1) | 1(1) | |||||||||||||||
| Haplaxius pictifrons | 57 | 3 | 60 | 18-Jun | 29-Jul | 27(3) | 30(3) | 3(3) | ||||||||||||||
| Haplaxius radicus | 3 | 1 | 4 | 11-Jun | 29-Jun | 3(2) | 1(1) | |||||||||||||||
| Haplaxius spp. Female | 1 | 1 | 22-Jul | 1(1) | ||||||||||||||||||
| Melanoliarus chuliotus | 1 | 1 | 12-Jul | 1(1) | ||||||||||||||||||
| Melanoliarus ecologus | 90 | 90 | 16-Jun | 14-Jul | 66(4) | 24(1) | ||||||||||||||||
| Melanoliarus montanus | 1 | 1 | 19-Jun | 1(1) | ||||||||||||||||||
| Melanoliarus placitus | 265 | 2 | 47 | 314 | 22-Apr | 30-Aug | 2(1) | 10(3) | 21(2) | 38(10) | 198(18) | 35(12) | 1(1) | 3(3) | ||||||||
| Melanoliarus quinquelineatus | 10 | 1 | 11 | 24-Jun | 16-Jul | 1(1) | 9(5) | 1(1) | ||||||||||||||
| Melanoliarus sablensis | 1 | 1 | 27-Jun | 1(1) | ||||||||||||||||||
| Melanoliarus near sablensis | 6 | 6 | 16-Jun | 27-Jun | 6(3) | |||||||||||||||||
| Melanoliarus spp. Female | 59 | 4 | 63 | 23-May | 26-Jul | 1(1) | 47(6) | 12(8) | 3(3) | |||||||||||||
| Melanoliarus spp. Fem. dark wing | 4 | 1 | 5 | 29-Jun | 26-Jul | 2(2) | 3(3) | |||||||||||||||
| Oecleus productus | 2 | 2 | 11-Aug | 2(1) | ||||||||||||||||||
| Pintalia vibex | 27 | 20 | 47 | 23-May | 4(2) | 3(2) | 19(8) | 16(5) | 5(2) | |||||||||||||
| Derbidae | ||||||||||||||||||||||
| Anotia kirkaldyi | 5 | 5 | 22-Jul | 9-Sep | 1(1) | 1(1) | 1(1) | 2(1) | ||||||||||||||
| Anotia robertsonii | 5 | 3 | 1 | 9 | 19-Jun | 19-Oct | 1(1) | 3(3) | 2(2) | 3(1) | ||||||||||||
| Anotia westwoodi | 7 | 7 | 18-Jun | 1-Sep | 3(2) | 3(2) | 1(1) | |||||||||||||||
| Apache degeerii | 19 | 10 | 29 | 1-Mar | 21-Oct | 9(3) | 7(3) | 3(3) | 2(2) | 1(1) | 3(2) | 3(1) | 1(1) | |||||||||
| Cedusa carolinensis | 1 | 1 | 13-Aug | 1(1) | ||||||||||||||||||
| Cedusa near cedusa | 10 | 1 | 11 | 26-Jul | 13-Aug | 6(1) | 5(3) | |||||||||||||||
| Cedusa kedusa | 21 | 2 | 23 | 7-Jul | 30-Aug | 1(1) | 19(1) | 1(1) | 2(1) | |||||||||||||
| Cedusa redusa | 6 | 8 | 14 | 9-Jun | 8-Sep | 5(1) | 5(1) | 2(1) | 1(1) | |||||||||||||
| Cedusa mallochi | 1 | 1 | 23-Jun | 1(1) | ||||||||||||||||||
| Cedusa vulgaris | 1 | 1 | 3-Aug | 1(1) | ||||||||||||||||||
| Cedusa spp. Female | 16 | 16 | 9-Jun | 26-Jul | 1(1) | 3(1) | 12(1) | |||||||||||||||
| Neocenchrea heidemanni | 3 | 3 | 2-Sep | 6-Sep | 3(2) | |||||||||||||||||
| Omolicna spp. | 11 | 2 | 23 | 36 | 14-Jun | 7-Oct | 1(1) | 2(1) | 1(1) | 1(1) | 17(6) | 4(3) | 7(1) | |||||||||
| Otiocerus coquebertii | 1 | 1 | 2 | (22-26)-June | 12-Jul | 1(1) | 1(1) | |||||||||||||||
| Otiocerus francilloni | 1 | 1 | 15-Jul | 1(1) | ||||||||||||||||||
| Otiocerus reaumurii | 2 | 2 | 26-Jul | 9-Aug | 1(1) | 1(1) | ||||||||||||||||
| Otiocerus wolfii | 7 | 5 | 12 | (19-20)-July | 7-Sep | 2(2) | 4(3) | 6(2) | ||||||||||||||
| Patara vanduzei | 2 | 2 | 4 | 3-Jul | 4-Aug | 1(1) | 2(1) | 1(1) | ||||||||||||||
| Sikaiana harti | 1 | 1 | 1-Jul | 1(1) | ||||||||||||||||||
| Dictyopharidae | ||||||||||||||||||||||
| Rhynchomitra lingula | 14 | 14 | 21-Aug | 1-Sep | 10(2) | 4(1) | ||||||||||||||||
| Rhynchomitra microrhina | 16 | 1 | 1 | 18 | 27-Jul | 13-Sep | 3(2) | 4(3) | 11(7) | |||||||||||||
| Scolops angustatus | 8 | 8 | 18-Jul | 20-Jul | 2(2) | |||||||||||||||||
| Scolops perdix | 7 | 7 | 19-Aug | 21-Aug | 7(2) | |||||||||||||||||
| Scolops pungens | 1 | 1 | 17-Jul | 1(1) | ||||||||||||||||||
| Scolops sulcipes | 92 | 2 | 94 | 11-Jul | 1-Oct | 1(1) | 50(14) | 4(4) | 12(4) | 3(1) | 11(4) | 10(1) | ||||||||||
| Flatidae | ||||||||||||||||||||||
| Flatormenis chloris | 85 | 18 | 9 | 112 | 2-Jul | 21-Oct | 2(1) | 14(12) | 16(11) | 23(18) | 19(12) | 15(12) | 8(5) | 11(3) | ||||||||
| Metcalfa pruinosa | 58 | 12 | 13 | 83 | 22-Jun | 11-Oct | 2(2) | 6(6) | 15(11) | 20(14) | 12(7) | 14(10) | 5(5) | 4(3) | ||||||||
| Ormenoides venusta | 18 | 2 | 1 | 21 | 22-Jul | 1-Oct | 2(2) | 2(2) | 8(3) | 5(3) | 2(1) | |||||||||||
| Fulgoridae | ||||||||||||||||||||||
| Cyrpoptus belfragei | 4 | 4 | 4-Jun | 18-Jul | 2(2) | 1(1) | 1(1) | |||||||||||||||
| Issidae | ||||||||||||||||||||||
| Thionia bullata | 1 | 1 | 2 | 2-Aug | 17-Oct | 1(1) | 1(1) | |||||||||||||||
| Thionia simplex | 14 | 14 | 4-Jul | 16-Sep | 2(2) | 1(1) | 1(1) | 2(2) | 7(5) | 1(1) | ||||||||||||
| Totals | 1253 | 209 | 272 | 1734 | 1 | 0 | 0 | 3 | 2 | 6 | 9 | 24 | 28 | 36 | 27 | 19 | 17 | 13 | 14 | 5 | ||
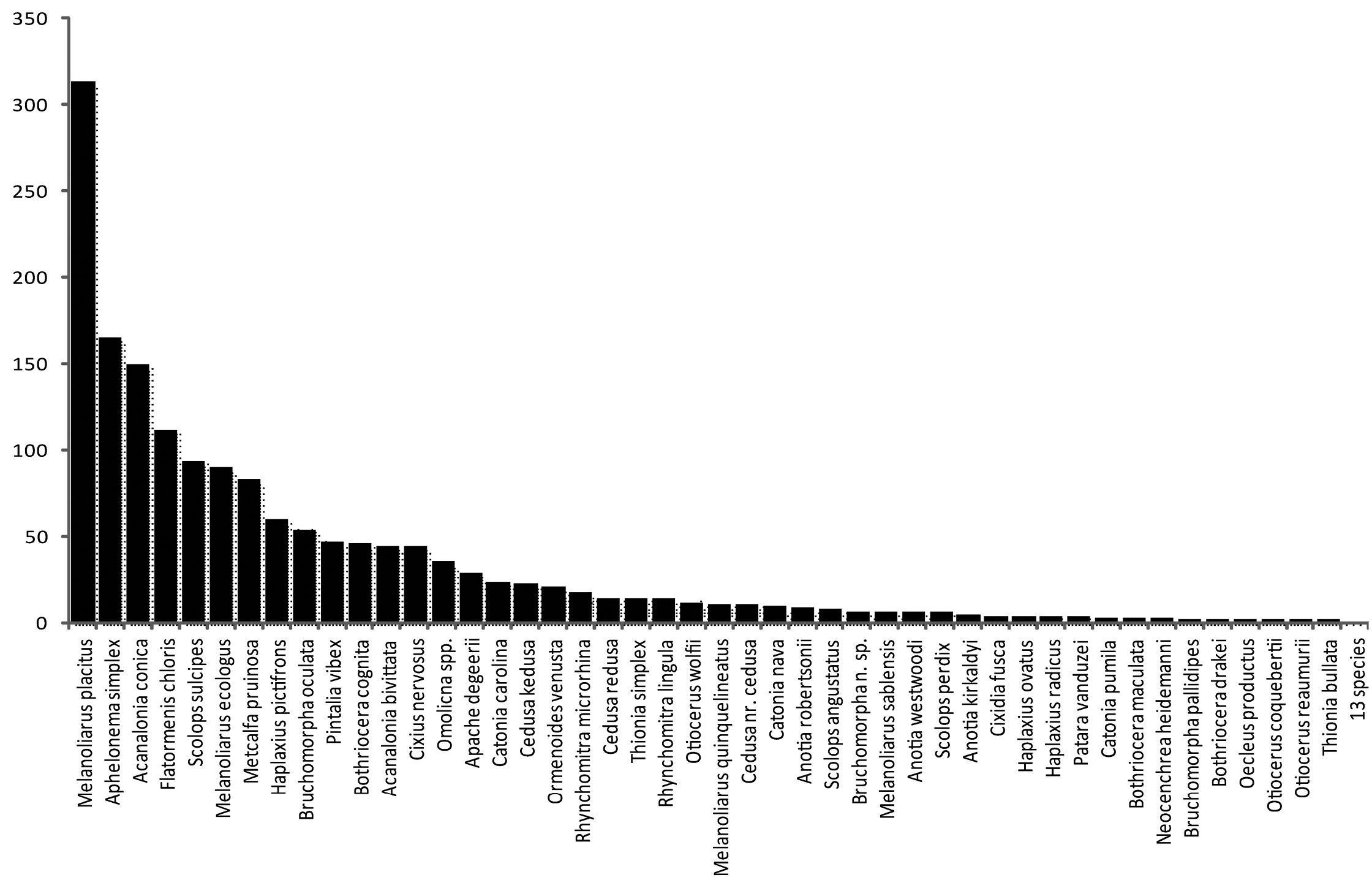
The most abundant species were Melanoliarus placitus (18% of observed specimens), Aphelonema simplex (10%), Acanalonia conica (9%), Flatormenis chloris (7%), and Scolops sulcipes (5%), collectively representing 49% of the specimens observed (Figure 1). However, for Aphelonema simplex there were only 5 collecting events, one of which comprised 70, and a second 69 specimens (out of 165 total observed specimens). In contrast, Metcalfa pruinosa (5%) and Acanalonia bivittata (3%) were both observed in many collecting events, but these frequently encountered species are readily recognized in the field and either avoided by collectors or not accessioned by the collection manager, and therefore are probably relatively underrepresented.
Rank abundance frequency distribution of planthopper species of Delaware. Number of specimens of each species found in Table 1.
The Chao1 biodiversity estimator was calculated as 74.08 species, indicating that 12 additional planthopper species are predicted to occur. The incidence list for Delaware and adjacent states (Table 2) includes 112 taxa, of which 50 species were recorded from surrounding states with no Delaware records. In addition, 22 species from MD, 5 from NJ, 8 from PA, and 21 from DC are new state records.
Planthopper incidence list for Delaware, Maryland New Jersey, Pennsylvania, and the District of Columbia. Specimen records are indicated by “S”, literature records by “L”, tentative or subsequent questioned records are annotated by “?”, and records reported as erroneous by “E”. Records from Wilson and McPherson (1980) except as noted.
| Species | DE | MD | NJ | PA | DC | References and comments |
|---|---|---|---|---|---|---|
| Acanaloniidae | ||||||
| Acanalonia bivittata (Say, 1825) | S | S, L | S, L | S, L | S, L | |
| Acanalonia conica (Say, 1830) | S, L | S, L | S, L | S, L | S | |
| Acanalonia servillei Spinola, 1839 | S | S, L | Acanalonia latifrons (Walker, 1851) synonymized with Acanalonia servillei by Fennah 1971: 334-6. | |||
Achilidae | ||||||
| Catonia carolina (Metcalf, 1923) | S | S, L | S | S | ||
| Catonia cinctifrons (Fitch, 1856) | S, L | S, L | S, L | S, L | S | |
| Catonia lunata Metcalf, 1923 | S, L | S, L | S | |||
| Catonia nava (Say, 1830) | S | S, L | S | |||
| Catonia picta Van Duzee, 1908 | S | S, L | ||||
| Catonia pumila Van Duzee, 1908 | S | S, L | S, L | S, L | S | |
| Cixidia fusca (Walker, 1852) | S | S | S, L | S | ||
| Cixidia opaca (Say, 1830) | S | L | L | S, L | ||
| Cixidia pallida (Say, 1830) | L | L | S, L | |||
| Cixidia septentrionalis (Provancher, 1889) | L | L | L | |||
| Cixidia variegata (Van Duzee, 1908) | S | S | S, L | S | ||
| Synecdoche dimidiata (Van Duzee, 1910) | S, L | S, L | S, L | |||
| Synecdoche grisea (Van Duzee, 1908) | S, L | L | ||||
| Synecdoche impunctata (Fitch, 1851) | S | L | L | S | ||
Caliscelidae | ||||||
| Aphelonema decorata (Van Duzee, 1908) | L | |||||
| Aphelonema histrionica (Stål, 1864) | S | |||||
| Aphelonema rugosa (Ball, 1932) | L? | |||||
| Aphelonema simplex Uhler, 1876 | S | S, L | S, L | |||
| Bruchomorpha dorsata Fitch, 1856 | S, L | L | ||||
| Bruchomorpha jocosa Stål, 1862 | L | L | ||||
| Bruchomorpha oculata Newman, 1838 | S | S, L | S, L | S, L | S | |
| Bruchomorpha pallidipes Stål, 1862 | S | S | S | S, L | ||
| Bruchomorpha sp. n. | S | S | S | |||
| Bruchomorpha tristis Stål, 1862 | L | L | ||||
| Fitchiella robertsonii (Fitch, 1856) | L | L | ||||
Cixiidae | ||||||
| Bothriocera bicornis (Fabricius, 1803) | E | E | Noted as error by |
|||
| Bothriocera cognita Caldwell, 1943 | S, L | S, L | L |
|
||
| Bothriocera drakei Metcalf, 1923 | S | |||||
| Bothriocera maculata Caldwell, 1943 | S | |||||
| Bothriocera signoreti Stål, 1864 | E | Noted as error by |
||||
| Cixius angustatus Caldwell, 1938 | S | S | ||||
| Cixius apicalis Metcalf, 1923 | L | Kramer 1981 | ||||
| Cixius coloepeum Fitch, 1856 | S | L | L | |||
| Cixius misellus Van Duzee, 1906 | L | L | Kramer 1981(PA record) | |||
| Cixius nervosus (Linnaeus, 1758) | S, L | S, L | S, L | S, L | Kramer 1981 | |
| Cixius nike Kramer, 1981 | S, L | Kramer 1981 | ||||
| Cixius pini Fitch, 1851 | S, L | S, L | S | Kramer 1981 | ||
| Cixius stigmatus (Say, 1825) | L | L | ||||
| Haplaxius enotatus (Van Duzee, 1909) | S, L | |||||
| Haplaxius ovatus (Ball, 1933) | S | S, L | S, L | |||
| Haplaxius pictifrons (Stål, 1862) | S | S, L | L | S, L | S | |
| Haplaxius pusillus (Van Duzee, 1909) | S, L | |||||
| Haplaxius radicus (Osborn, 1903) | S | S, L | S | |||
| Haplaxius wheeleri (Wilson, 1996) | S, L | S | ||||
| Melanoliarus chuliotus (Ball, 1934) | S | L | ||||
| Melanoliarus ecologus Caldwell, 1947 | S | S, L | S, L | S, L | S, L |
|
| Melanoliarus humilis (Say, 1830) | S | S, L | L | S, L | L |
|
| Melanoliarus montanus (Metcalf, 1923) | S | S, L | S | S, L |
|
|
| Melanoliarus placidus Van Duzee, 1912 | S | S, L | S, L | S | L |
|
| Melanoliarus quinquelineatus (Say, 1830) | S | S, L | S, L | S, L |
|
|
| Melanoliarus sablensis (Caldwell, 1951) | S | S, L | S, L | S, L | L |
|
| Melanoliarus near sablensus | S | S | S | |||
| Melanoliarus spp. females | S | S | S | |||
| Pentastiridius cinnamomeus (Provancher, 1889) | S | L | L | Kramer 1981 | ||
| Oecleus borealis Van Duzee, 1912 | S, L | S, L | S, L | S | ||
| Oecleus productus Metcalf, 1923 | S | S, L | ||||
| Pintalia delicata (Fowler, 1904) | S, L | |||||
| Pintalia vibex Kramer, 1981 | S | S, L | Kramer 1981 | |||
Derbidae | ||||||
| Anotia bonnetii Kirby, 1821 | L | |||||
| Anotia burnetii Fitch, 1856 | L | |||||
| Anotia fitchi (Van Duzee, 1893) | L | |||||
| Anotia kirkaldyi Ball, 1902 | S | S, L | ||||
| Anotia robertsonii Fitch, 1856 | S | L | L | |||
| Anotia westwoodi Fitch, 1856 | S | S | L | S, L | S | |
| Apache degeerii (Kirby, 1821) | S | S, L | S, L | S, L | ||
| Cedusa carolinensis Flynn & Kramer, 1983 | S | S, L | S |
|
||
| Cedusa cedusa McAtee, 1924 | S, L | S |
|
|||
| Cedusa chuluota Ball, 1928 | S, L |
|
||||
| Cedusa edentula (Van Duzee, 1912) | S | L | S |
|
||
| Cedusa gedusa McAtee, 1924 | S, L | S, L | S, L |
|
||
| Cedusa hedusa McAtee, 1924 | S, L |
|
||||
| Cedusa incisa (Metcalf, 1923) | S | S, L |
|
|||
| Cedusa kedusa McAtee, 1924 | S | S, L | S, L | S |
|
|
| Cedusa maculata (Van Duzee, 1912) | S, L | S, L |
|
|||
| Cedusa mallochi McAtee, 1924 | S | L |
|
|||
| Cedusa obscura(Ball, 1902) | S, L | S, L | S |
|
||
| Cedusa redusa McAtee, 1924 | S | S, L |
|
|||
| Cedusa shawi Flynn & Kramer, 1983 | S, L |
|
||||
| Cedusa vulgaris (Fitch, 1851) | S | S, L |
|
|||
| Cedusa spp. Females | S | S | S | |||
| Neocenchrea heidemanni (Ball, 1902) | S | L | L | L | ||
| Omolicna uhleri (Ball, 1902) | L | L | L | |||
| Otiocerus amyotii Fitch, 1856 | L | L | L | |||
| Otiocerus coquebertii Kirby, 1821 | S | L | L | S, L | ||
| Otiocerus francilloni Kirby, 1821 | S | L | ||||
| Otiocerus reaumurii Kirby, 1821 | S | |||||
| Otiocerus signoretii Fitch, 1856 | S | L | L | |||
| Otiocerus stollii Kirby, 1821 | S | L | L | |||
| Otiocerus wolfii Kirby, 1821 | S | S, L | L | S, L | ||
| Patara vanduzei Ball, 1902 | S | S | L | |||
| Shellenius ballii (McAtee, 1923) | S, L | |||||
| Shellenius schellenbergii (Kirby, 1821) | L | |||||
| Sikaiana harti (Metcalf, 1923) | S | S | ||||
Dictyopharidae | ||||||
| Mitrops dioxys (Walker, 1858) | L | L | ||||
| Phylloscelis atra Germar, 1839 | L | L | S, L | L | ||
| Phylloscelis pallescens Germar, 1839 | L | L | L | |||
| Phylloscelis rubra Ball, 1930 | S, L | |||||
| Rhynchomitra lingula (Van Duzee, 1908) | S | S | S, L | |||
| Rhynchomitra microrhina (Walker, 1851) | S | S | L | S | ||
| Scolops angustatus Uhler, 1929 | S | S, L | S, L | L | ||
| Scolops grossus Uhler, 1876 | L? | Record probably in error. | ||||
| Scolops perdix Uhler, 1900 | S | L | S, L | S | L | |
| Scolops pungens (Germar, 1830) | S | L | S, L | L | L | |
| Scolops sulcipes (Say, 1825) | S | S, L | S, L | S, L | L | |
Flatidae | ||||||
| Flatormenis chloris (Melichar, 1902) | S | S, L | S, L | S, L | L |
Anormenis septentrionalis auct. (nec. Spinola, 1839) synonymized with Anormenis chloris by |
| Cyarda melichari Van Duzee, 1907 | L | Species needs confirmation. | ||||
| Metcalfa pruinosa (Say, 1830) | S, L | S, L | S, L | S, L | L | |
| Ormenoides venusta (Melichar, 1902) | S | S | S | S | S | |
| Fulgoridae | ||||||
| Cyrpoptus belfragei Stål, 1869 | S | L | ||||
| Poblicia fuliginosa (Olivier, 1791) | S | |||||
| Issidae | ||||||
| Exortus punctiferus (Walker, 1851) | L | Originally reported by |
||||
| Thionia bullata (Say, 1830) | S | S | S, L | S, L | S, L | |
| Thionia elliptica (Germar, 1830) | S | L | L | |||
| Thionia simplex (Germar, 1830) | S | S, L | S, L | S | L | |
| New records* | 55 | 22 | 5 | 8 | 21 | |
| Total species* | 62 | 88 | 74 | 60 | 46 | |
* Unidentified females and errors excluded, Melanoliarus near sablensus included with Melanoliarus sablensus, 2 species of Omolicna counted for Delaware.
The seasonality data suggests that the optimal time of
year to find planthoppers in Delaware is between late June and early
August (Table 1). It appears that most species have one generation per year, although the available data is sparse for some taxa. Bruchomorpha oculata, Aphelonema simplex, and Cixius nervosus may have two generations a year. It is evident from specimens collected in logs in March that Apache degeerii
overwinters as adults (early record March 1: 9 specimens from 3
collection events), although the overwintering status of other taxa is
not clear from this data. Records of cixiids from late April may
indicate overwintering as immatures, as has been reported for cixiids
in Germany (
Specimens reported incidentally by
Planthoppers reported by
| Planthopper species | |||||
|---|---|---|---|---|---|
| Plant Family | Plant species | Melanoliarus spp. | Flatormenis chloris | Thionia simplex | |
| Native | |||||
| Aceraceae | Acer rubrum | 0 | 0 | 0 | |
| Betulaceae | Betula nigra | 0 | 2 | 0 | |
| Betulaceae | Carpinus caroliniana | 2 | 0 | 0 | |
| Cornaceae | Cornus florida | 0 | 0 | 0 | |
| Fagaceae | Fagus grandifolia | 0 | 0 | 0 | |
| Hamamelidaceae | Hamamelis virginiana | 0 | 0 | 0 | |
| Juglandaceae | Juglans nigra | 4 | 0 | 0 | |
| Moraceae | Morus rubra | 6 | 0 | 0 | |
| Rosaceae | Prunus serotina | 0 | 2 | 0 | |
| Ericaceae | Rhododendron periclymenoides | 7 | 0 | 0 | |
| Rosaceae | Rosa carolina | 2 | 0 | 0 | |
| Salicaceae | Salix nigra | 1 | 1 | 1 | |
| Tiliaceae | Tilia americana | 1 | 0 | 0 | |
| Ulmaceae | Ulmus americana | 0 | 0 | 0 | |
| Caprifoliaceae | Viburnum dentatum | 8 | 0 | 0 | |
| Subtotal | 31 | 5 | 1 | ||
Non-native congeneric plants | |||||
| Aceraceae | Acer platanoides | 0 | 2 | 0 | |
| Betulaceae | Betula pendula | 3 | 0 | 0 | |
| Betulaceae | Carpinus betulus | 0 | 0 | 0 | |
| Cornaceae | Cornus kousa | 2 | 0 | 0 | |
| Fagaceae | Fagus sylvatica | 3 | 0 | 0 | |
| Hamamelidaceae | Hamamelis mollis | 2 | 0 | 0 | |
| Juglandaceae | Juglans regia | 2 | 0 | 0 | |
| Moraceae | Morus alba | 0 | 0 | 0 | |
| Rosaceae | Prunus serrulata | 1 | 1 | 0 | |
| Ericaceae | Rhododendron mucronatum | 42 | 0 | 0 | |
| Rosaceae | Rosa multiflora | 7 | 1 | 0 | |
| Salicaceae | Salix babylonica | 0 | 0 | 0 | |
| Tiliaceae | Tilia cordata | 2 | 0 | 0 | |
| Ulmaceae | Ulmus parvifolia | 2 | 5 | 0 | |
| Caprifoliaceae | Viburnum dilatatum | 14 | 2 | 0 | |
| Subtotal | 80 | 11 | 0 | ||
Alien plants | |||||
| Lardizabalaceae | Akebia quinata | 9 | 0 | 0 | |
| Fabaceae | Albizia julibrissin | 5 | 1 | 0 | |
| Rosaceae | Cotoneaster lucidus | 16 | 2 | 0 | |
| Fabaceae | Cytisus scoparius | 9 | 1 | 0 | |
| Oleaceae | Forsythia suspensa | 10 | 0 | 0 | |
| Ginkgoaceae | Ginkgo biloba | 1 | 0 | 0 | |
| Araliaceae | Hedera helix | 6 | 0 | 0 | |
| Sapindaceae | Koelreuteria paniculata | 1 | 0 | 0 | |
| Lythraceae | Lagerstroemia indica | 5 | 0 | 0 | |
| Oleaceae | Ligustrum vulgare | 6 | 1 | 0 | |
| Scrophulariaceae | Paulownia tomentosa | 1 | 0 | 0 | |
| Rutaceae | Phellodendron amurens | 0 | 0 | 0 | |
| Rutaceae | Poncirus trifoliata | 1 | 0 | 0 | |
| Rosaceae | Pyrus pashia | 4 | 0 | 0 | |
| Oleaceae | Syringa vulgaris | 1 | 0 | 1 | |
| Subtotal | 75 | 5 | 1 | ||
| Total | 186 | 21 | 2 | ||
Artificial key to genus and select planthopper species from Delaware and vicinity.
| 1 | Hind tibiae with large movable spur at apex (Fig. 2A) | Delphacidae |
| – | Hind tibiae without movable spur at apex (e.g., Figs 2B–D) | 2 |
| 2 | Second tarsomere of hind legs with row of apical spines (Fig. 2E) | 3 |
| – | Second tarsomere of hind legs with one apical spine on each side (Fig. 2F) or spines absent | 7 |
| 3 | Larger species, greater than 10 mm, with patterned forewings (Figs 3H, I); hindwings with numerous cross veins near apex and in anal area; uncommon in study area | Fulgoridae, 71 |
| – | Mostly smaller species, forewings variable; hindwings without cross veins near apex or in anal area | 4 |
| 4 | Forewings overlapping posteriorly (Figs 4G–L, 5F–L, 6F–H), trailing margins angled; body flattened | Achilidae, 13 |
| – | Forewings not overlapping posteriorly; body variable | 5 |
| 5 | Beak with apical segment subequal in length and width (except Cedusa); forewings often with tubercles on claval veins (Figs 8G, 9B); antennae may bear projections (Figs 10E, F) or subtended by a shelf-like structure (Figs 10A–D); median carina of frons often absent; parameres of male much longer than pygofer | Derbidae (most), 41 |
| – | Beak with apical segment longer than wide; forewings without tubercles on claval veins (or with tubercles on all veins); antennae never bearing projections or subtended by a shelf-like structure; median carina of frons present; parameres of male shorter than length of pygofer | 6 |
| 6 | Frons with two or three median carinae and/or head with elongate anterior projection (Figs 13–14); median ocellus absent; wing vein tubercles usually absent | Dictyopharidae, 60 |
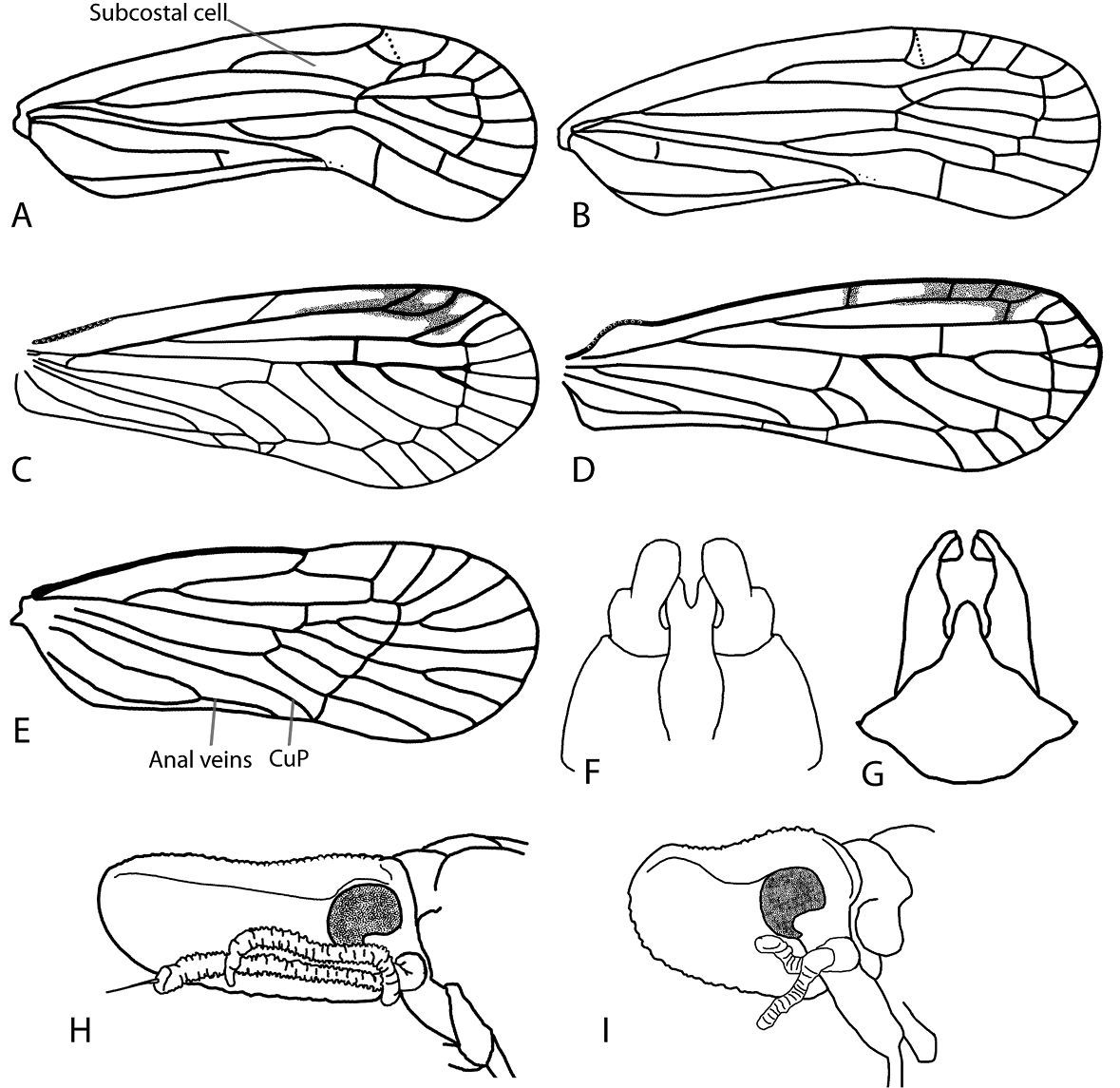
| – | Frons with one median carina; head not elongate; median ocellus usually present above frontoclypeal suture (Figs 6D–E, 7B, H); usually with tubercles on veins of wings | Cixiidae, 35 |
| 7 | Forewings with tubercles on claval veins (e.g., Figs 8G, 9B), if tubercles present in claval area (Figs 3D–G) then forewings waxy with row of many small peripheral cells; beak with apical segment subequal in length and width; frons often compressed with median carina absent (Figs 10B, D, G); parameres much longer than pygofer | Derbidae (few), 41 |
| – | Forewings without tubercles on claval veins (or with tubercles on all veins); beak with apical segment longer than wide; frons not compressed, median carina generally present (e.g., Figs 12, 15A–D); parameres shorter than length of pygofer | 8 |
| 8 | Forewings waxy, bearing tubercles between veins on clavus (Figs 3D–G) and with numerous costal crossveins | Flatidae, 68 |
| – | Forewings not waxy, without tubercles on clavus; without numerous costal crossveins (e.g., Figs 3A–C, 15E–H) | 9 |
| 9 | Hind tibiae without lateral spines (Fig. 2B); forewings with reticulate venation, usually extending to apex of abdomen (even in brachypters); usually green (occasionally pink) (Figs 3A–C) | Acanaloniidae, 11 |
| – | Hind tibiae with lateral spines (Figs 2C–D); forewing venation not reticulate (Figs 15E–H), brachypters may have forewings short (Fig. 11), exposing several segments in dorsal view; color not green, usually brown, black, or straw (pinkish in males of 1 species) | 10 |
| 10 | Usually brachypterous with forewings shorter than abdomen (Fig. 11); frons with sublateral carinae bordering a large disc-like or elongate areolet, sublateral carinae of frons meeting ventrally (or nearly so) (Fig. 12); hind tibiae with single lateral spine (Fig. 2C) | Caliscelidae, 26 |
| – | Forewings covering abdomen (both brachypters and macropters) (Figs 15E–H); frons with median carina, with or without sublateral carinae; if present, not meeting ventrally (Figs 15A–D); hind tibiae with two lateral spines (Fig. 2D) | Issidae, 72 |
Acanaloniidae | ||
| 11 | Body green (rarely pink) with conspicuous brownish to reddish marking along lateral portions of thoracic nota (Fig. 3B), continuing onto wings | Acanalonia bivittata |
| – | Body uniformly green (rarely pink) (Figs 3A, C); may have middorsal vitta on thorax | 12 |
| 12 | Head distinctly produced conically (Fig. 3A); without prominent median carina across vertex and thorax; abundant in Mid-Atlantic states | Acanalonia conica |
| – | Head not produced conically (Fig. 3C); with prominent median carina across vertex and thorax; southeastern species occasional in Mid-Atlantic States | Acanalonia servillei |
Achilidae | ||
| 13 | Head, including eyes, less than 2/3 as wide as pronotum (Figs 5F–J) (Myconini) | Cixidia, 14 |
| – | Head including eyes at least 2/3 as wide as pronotum (Figs 4G–L, 6F–H) (Plectoderini) | 18 |
| 14 | Clypeus and upper half of frons dark brown or black, strongly contrasting with pale lower half of frons (Fig. 5B) | Cixidia opaca |
| – | Frons more uniformly colored, upper half not strongly contrasting (Figs 5A, C–E) | 15 |
| 15 | Vertex short, projecting in front of eye for distance less than length of eye (Fig. 5J); frons distinctly and uniformly speckled (Fig. 5E) | Cixidia variegata |
| – | Vertex elongate, projecting in front of eye for distance equal to or greater to length of eye; frons more uniformly colored (Figs 5A, C) | 16 |
| 16 | Frons and clypeus uniformly colored (Fig. 5D) | Cixidia septentrionalis |
| – | Clypeus distinctly darker than frons (Figs 5A, C) | 17 |
| 17 | Vertex projected in front of eye for distance greater than eye length, vertex 1.3–1.5× as long as basal width (Fig. 5F); frons and clypeus about as dark as pronotum; forewings nearly uniform brown | Cixidia fusca |
| – | Vertex projected in front of eye for distance about equal to eye length, vertex length about equal (1–0.95x) to basal width (Fig. 5H); frons and clypeus paler than pronotum; forewing variegated with grayish white | Cixidia pallida |
| 18 | Subcostal cell of forewing longer than 1/3 length of forewing, narrow throughout (Fig. 16B); medioventral lobe of male pygofer entire (Fig. 16G) | Synecdoche, 19 |
| – | Subcostal cell of forewing about 1/3 length of forewing, wider before its apex (Fig. 16A); medioventral lobe of male pygofer apically bifurcate (Fig. 16F) | Catonia, 21 |
| 19 | Frons entirely pale (Fig. 6B) | Synecdoche grisea |
| – | Frons with dark transverse bands or all dark (Figs 6A, C) | 20 |
| 20 | Frons with dark bands (Fig. 6C) | Synecdoche impunctata |
| – | Frons uniformly dark, contrasting with pale clypeus (Fig. 6A) | Synecdoche dimidiata |
| 21 | Upper dark band of frons mottled, distinctly paler than lower band (Fig. 4D); larger species usually more than 5.8 mm | Catonia nava |
| – | Frons, if banded (Figs 4A–C, E), with upper dark band not mottled and not paler than lower, or frons not dark banded (Fig. 4F); size less than 6.2 mm | 22 |
| 22 | Frons with two very dark transverse bands (Figs 4B, E) | 23 |
| – | Frons pale with pale bands, or uniformly pale (Figs 4A, C, F) | 24 |
| 23 | Lower dark band distinctly paler near frontoclypeal suture giving frons a tricolored appearance (Fig. 4E); body often with orangish cast | Catonia picta |
| – | Lower dark band uniformly dark (Fig. 4B); body brown or grayish | Catonia cinctifrons |
| 24 | Pale transverse marking at frontoclypeal suture not reaching lateral margin of frons (Figs 4A, C) | 25 |
| – | Frons uniformly colored or pale transverse marking at frontoclypeal suture extending to lateral margin of frons (Fig. 4F) | Catonia pumila |
| 25 | Pale transverse marking at level of ocelli complete, reaching lateral margin of frons (Fig. 4C) | Catonia lunata |
| – | Pale transverse marking at level of ocelli incomplete, not reaching lateral margin of frons (Fig. 4A) | Catonia carolina |
Caliscelidae | ||
| 26 | Head produced into weevil-like snout (Figs 11E–H); usually black | 30 |
| – | Head not produced (Fig. 11A–D); paler | Aphelonema, 27 |
| 27 | Vertex very broad, width at least 5–6× median length (Figs 12A, D); frons greatly exposed above, fastigium rounded when viewed laterally; mostly straw to pink colored (Figs 11A, D), may have darker wings and abdomen | 28 |
| – | Vertex longer, width 2–3× median length, frons not as exposed from above (Figs 12B–C); fastigium angled when viewed laterally; mostly black and pale colored (Figs 11B–C) | 29 |
| 28 | Head and thorax orange-tan, rest of dorsum blackish brown (Fig. 11A, especially in males); central frontal tablet of frons pointed below (Fig. 12A); found mostly in the southeast, reported from NJ | Aphelonema decorata |
| – | Uniformly pale ochreous (females) to pink (most males) in color (Fig. 11D); central frontal tablet of frons almost circular (Fig 12D) | Aphelonema simplex |
| 29 | When viewed from the side, fastigium of head produced forward, frons slanted; vertex somewhat triangular (Fig. 11B) | Aphelonema histrionica |
| – | When viewed from the side, fastigium not produced, frons not slanted; vertex broadly rounded anteriorly (Fig. 11C) | Aphelonema rugosa |
| 30 | Middle and front tibiae expanded | Fitchiella robertsonii |
| – | Middle and front tibiae not expanded | Bruchomorpha, 31 |
| 31 | Dorsal light stripe broad and conspicuous, extending from near apex of face to apex of forewings or beyond | Bruchomorpha sp. n. |
| – | Dorsal light stripe not broad and conspicuous, generally of lesser extent | 32 |
| 32 | Nasal process distinctly pronounced, head concave ventrally in lateral view (Fig. 11F); in dorsal view extending anteriorly beyond eye for a distance equal or greater than length of eye | Bruchomorpha oculata |
| – | Nasal process less pronounced, head weakly convex ventrally; in dorsal view extending anteriorly beyond eye for a distance less than length of eye (Figs 11E, G–H) | 33 |
| 33 | Reddish-brown in color with a dark spot on clypeus | Bruchomorpha jocosa |
| – | Uniformly black, usually with light stripe on vertex (sometimes reaching thorax) | 34 |
| 34 | Legs pale (Fig 11G); small species, less than 2.6 mm | Bruchomorpha pallidipes |
| – | Legs dark (Fig 11H); large species, more than 2.6 mm | Bruchomorpha tristis |
Cixiidae | ||
| 35 | Antennae arising from elongated cup-like cavities anterior to eyes (Fig. 7A) | Bothriocera |
| – | Antennae not within cup-like cavities, arising below eyes (Fig. 7E, 7I) | 36 |
| 36 | Hind tibiae without spines (similar to Fig. 2B) | 37 |
| – | Hind tibiae with one or more spines along axis before apex (similar to Figs 2C–D) | 38 |
| 37 | Mesonotum with 5 carinae; crown strongly narrowed (Fig. 6J) | Oecleus |
| – | Mesonotum with 3 carinae; crown slightly narrowed (Fig. 6I) | Haplaxius |
| 38 | Mesonotum with 5 longitudinal carinae (although intermediate pair sometimes obsolete); posterior margin of crown angularly incised (Figs 7F–G) | 39 |
| – | Mesonotum with 3 carinae; posterior margin of crown quadrately or roundly incised (Figs 7D, J) | 40 |
| 39 | Apex of basitarsus of hind leg with 12 teeth | Pentastiridius |
| – | Apex of basitarsus of hind leg with no more than 10 teeth | Melanoliarus |
| 40 | Forewings roof-like in position with distal portions clearly separated (Fig. 7D); spines on hind tibiae conspicuous | Cixius |
| – | Forewings vertical in position with distal portions oppressed (Figs 7I–J); spines on hind tibiae inconspicuous | Pintalia |
Derbidae | ||
| 41 | Clavus open (Figs 16C–D; combined anal veins reaching posterior cubitus and usually curved to follow wing margin); most taxa with head projecting well beyond eyes in lateral view (e.g., Figs 10E–F); frons very narrow (Fig. 10G); forewings twice as long as body or more, delicate appearing (Otiocerinae: Otiocerini and Sikaianini) | 42 |
| – | Clavus closed (Fig. 16E; combined anal veins reaching wing margin within claval area); most taxa with head projecting only slightly beyond eyes (Figs 10A, C); frons usually not as narrow (Figs 10B, D) (except Patara, see Fig. 8G); forewings not as long, most taxa less delicate (Otiocerinae: Patarini; Cedusinae; and Derbinae: Cenchreini) | 57 |
| 42 | Antennae with 2 or 3 conspicuous appendages (Figs 10E–F) | 43 |
| – | Antennae lacking appendages (Figs 8J, 10G) | 51 |
| 43 | General color uniformly rose or reddish (Fig. 8E); head in lateral view with vertex distinctly concave in apical third and apex pointed (Fig. 10F); dorsal margin of wings in repose sharply angled upward in apical third; forewings with dusky spots in cells | Apache degeerii |
| – | General color white or yellow (e.g., Figs 9D–J), although red markings may be present; head in lateral view with vertex rounded (Fig. 9I, J, 16H, I), or nearly flat (Fig. 10E); dorsal margin of wings straight or curved slightly upward | 44 |
| 44 | In lateral view, demarcation between vertex and frons obtusely angular (Fig. 10E) | Otiocerus, 46 |
| – | In lateral view, demarcation between vertex and frons smoothly rounded (Figs 9I, J; 16H–I) | Shellenius, 45 |
| 45 | Head in lateral view 1.5× as long as broad (Figs 9J, 16I); forewing brownish apically in trailing portion of wing; red markings reduced or absent | Shellenius schellenbergii |
| – | Head in lateral view 2.0× as long as broad (Figs 9I, 16H); forewings with very pale brown markings widely distributed; with red markings on head and wing | Shellenius balli |
| 46 | Wings with conspicuous round dusky spots in cells (Figs 9E, F, H) | 47 |
| – | Wings without conspicuous round dusky spots in cells (Figs 9D, G) | 50 |
| 47 | Apical margin of forewings with a row of spots in the cells (Figs 9E, H) | 48 |
| – | Spots not in row within apical cells (Fig. 9F) | 49 |
| 48 | Apex of head with a black line laterally followed by a broader red line (Fig. 9H); forewings with spots throughout | Otiocerus wolfii |
| – | Apex of head without a black line laterally (Fig. 9E); forewings with spots mostly in proximal half | Otiocerus francilloni |
| 49 | Forewings with a large black spot on the sutural margin (in the clavus) and four smaller ones in a square, including 1 in costal cell | Otiocerus signoretii |
| – | Forewings with spots arranged differently from above (Fig. 9F) | Otiocerus reaumurii |
| 50 | Color of the wings dark, without distinct band (Fig. 9G) | Otiocerus stollii |
| – | Color of the wings pale with distinct reddish forked band (Fig. 9D) | Otiocerus coquebertii |
| 51 | In lateral view, head projecting in front of eyes for a distance of less than half width of eyes; forewings with scattered spots | Sikaiana harti |
| – | In lateral view, head projecting in front of eyes for a distance subequal to width of eyes (Fig. 8J); color mostly following veins | Anotia, 52 |
| 52 | Costa narrow; forewings with veins not crowded together to give appearance of a stigma (Figs 8A–D); some or most veins of forewings with smoky borders | 53 |
| – | Costa broader; Sc and R vein tips crowded together to give appearance of a stigma (Fig. 8I); forewings more extensively marked with fuscous | Anotia fitchi |
| 53 | First 3 segments of abdomen with middorsal black stripe | Anotia burnetii |
| – | Abdomen without middorsal black stripe | 54 |
| 54 | Forewings mostly pale with a few fuscous marked crossveins (Fig. 8C); apex of forewing without dark round spots | Anotia robertsonii |
| – | Forewings more extensively marked; most veins with smoky borders (Figs 8B, D); apex of forewing often with dark round spots | 55 |
| 55 | Head with a single marking, below antennae; apical border of forewings with four dark round spots in the cells (Fig. 8A) | Anotia bonnetii |
| – | Head with dark or red markings above and below antennae; apical border of forewings usually without round spots in the cells | 56 |
| 56 | At least some veins dark in color (Fig. 8B) | Anotia kirkaldyi |
| – | All veins pale (Fig. 8D) | Anotia westwoodi |
| 57 | Antennae terete, subtended by flattened subantennal process from gena or anterior portion of lateral margin of pronotum (Figs 10A–D), often strongly modified into a reversed “c” (in lateral view) directly behind antennae, or strongly keeled; face not strongly compressed, frons evident; clavus at least half as long as whole forewing (Derbinae: Cenchreini, and Cedusinae) | 58 |
| – | Second segment of antennae flattened (more evident in males than females), antennae not subtended by process; lateral margin of pronotum not strongly modified; face strongly compressed, frons keel-like (similar to Fig. 10G); clavus less than half as long as whole forewing (Fig. 9C) (Otiocerinae: Patarini) | Patara vanduzei |
| 58 | Subantennal process large, extending from gena, completely subtending antennae as a shelf (Fig. 10A); reduced (or absent) sensory pits on head and wings; color uniform, near black or deep grey (Fig. 9A), infrequently near white with yellowish brown patches (Cedusinae) | Cedusa |
| – | Subantennal process extending from pronotum, smaller (Fig. 10C); lateral carinae of vertex and second claval vein with sensory pits; color usually orange to pale (Figs 8F, 9B) (Derbinae: Cenchreini) | 59 |
| 59 | Media with more than two branches, connected to cubitus by crossvein; size less than 6 mm, usually distinctly orangish (Fig. 9B) | Omolicna |
| – | Media and cubitus each with two branches, not connected by crossveins; size over 7 mm; color orangish white (Fig. 8F) | Neocenchrea heidemanni |
Dictyopharidae | ||
| 60 | Head projected in front of eyes (Figs 13, 14G, H); front femora not foliaceous | 63 |
| – | Head not projected in front of eyes (Figs 14A–F); front femora foliaceous | Phylloscelis, 61 |
| 61 | Eight or fewer longitudinal veins on the forewing; color either uniformly black to dark brown in dorsal view or yellowish body with reddish-brown forewings with prominent yellow wing veins (Figs 14C, F); carinae of frons indistinct | Phylloscelis atra |
| – | With more than 8 longitudinal veins; color not as above; carinae of frons distinct (Figs 14A–B, D–E) | 62 |
| 62 | Veins concolorous with forewings; body black to light reddish brown (Fig. 14D) | Phylloscelis rubra |
| – | Veins of forewings dark mottled with pale; body light grey-brown (Fig. 14E) | Phylloscelis pallescens |
| 63 | Forewings clear, macropterous; head projection anterior to eyes subequal in width to vertex; body green (Figs 14G–H) | Rhynchomitra, 64 |
| – | Forewings patterned, usually brachypterous; head projection anterior to eyes narrower than vertex; body brownish (Fig. 13) | Scolops, 65 |
| 64 | Head projection long (Fig. 14G), in dorsal view narrowing anterior to eyes, projected in front of eyes greater than width of vertex; upcurved in lateral view | Rhynchomitra microrhina |
| – | Head projection short (Fig. 14H), in dorsal view rather quadrate, projected in front of eyes for distance about width of vertex; not distinctly upcurved in lateral view | Rhynchomitra lingula |
| 65 | Costal cell of forewing with costal vein and membrane white (Fig. 13A) | Scolops angustatus |
| – | Costal cell of forewing with costal vein variegated (Figs 13B–D) | 66 |
| 66 | Forewings reticulate over apical half (especially brachypters), veins margined with dark (Figs 13D, H) | Scolops sulcipes |
| – | Forewings not reticulate over apical half (Figs 13B–C, F–G) | 67 |
| 67 | Pronotum and usually vertex with dark markings (Fig. 13G); body with grayish cast | Scolops perdix |
| – | Pronotum and vertex without dark markings (Fig. 13F); body with brownish cast | Scolops pungens |
| Flatidae | ||
| 68 | Wings much longer than wide, distinctly narrowing caudally to caudal apex (Fig. 3E); brown | Cyarda |
| – | Wings slightly longer than wide, truncate to broadly rounded caudally (Figs 3D, F–G); green or grey | 69 |
| 69 | Body grey to blackish (Fig. 3F); forewings with single row of marginal cells along apical and trailing margin (set off by a submarginal vein) | Metcalfa pruinosa |
| – | Body green (Figs 3D, G); forewings with one or two rows of marginal cells | 70 |
| 70 | Frons broader than long; forewings with two rows of marginal cells along apical and trailing margin (set off by two submarginal veins) (Fig. 3D); wings usually rather truncate apically | Flatormenis chloris |
| – | Frons longer than broad; forewings with one row of marginal cells (Fig. 3G); wings usually rounded apically (forewings often with orangish cast along apices) | Ormenoides venusta |
| Fulgoridae | ||
| 71 | Forewings and much of body nearly black (Fig. 3I); caudal abdominal tergites red; head in lateral view with frons at acute angle from vertex; flange of head behind eye small | Poblicia fuliginosa |
| – | Forewings and body mottled (Fig. 3H), predominately reddish brown; abdomen not red; head in lateral view with frons at sharp angle from vertex; flange of head behind eye distinct | Cyrpoptus belfragei |
| Issidae | ||
| 72 | Hind wings absent or rudimentary; smaller insects, less than 4.5 mm (Figs 15A, E); southeastern species, reported from NJ, possibly in error | Exortus punctiferus |
| – | Hind wings present, entire, with strongly marked notches at the joints of the folds, anal area large; larger insects varying from 5.5 to 8.0 mm (Figs 15B–D, F–H) | Thionia, 73 |
| 73 | Uniformly colored, lacking proximal bulla (Fig. 15H); carinae of face weak (Fig. 15D) | Thionia simplex |
| – | Body patterned, wings with proximal bulla (Figs 15F–G); carinae of face conspicuous (Figs 15B–C) | 74 |
| 74 | Vertex broader than long; distinctly concave in frontal view with lateral margins elevated (Fig. 15B) | Thionia elliptica |
| – | Vertex longer than broad, slightly concave in frontal view, lateral margins not strongly elevated (Fig. 15C) | Thionia bullata |
Hind legs of planthoppers. A Delphacidae, tibia with calcar B Acanaloniidae, tibia without spines C Caliscelidae, tibia with 1 spine D Issidae, tibia with 2 spines E Dictyopharidae, second tarsal segment with row of teeth F Acanaloniidae, second tarsal segment with pair of spines.
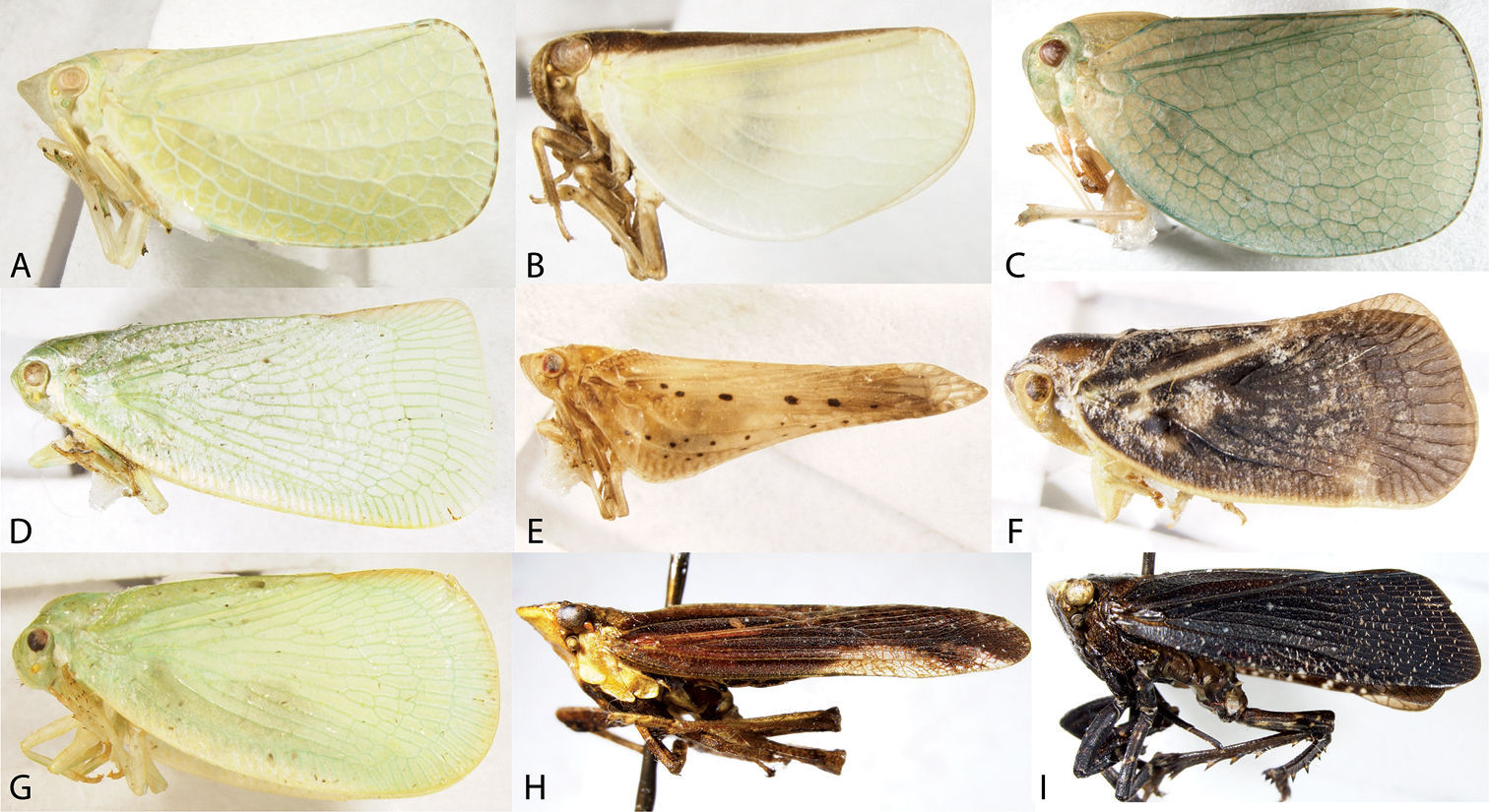
Lateral habitus of Acanaloniidae, Flatidae, and Fulgoridae. A Acanalonia conica B Acanalonia bivittata C Acanalonia servillei D Flatormenis chloris E Cyarda sp. F Metcalfa pruinosa G Ormenoides venusta H Cyrpoptus belfragei I Poblicia fuliginosa.
Habitus of Catonia (Achilidae) (A–F frons, G–K dorsal view). A, G Catonia carolina B, H Catonia cinctifrons C, I Catonia lunata D, J Catonia nava E, K Catonia picta F, L Catonia pumila.
Habitus of Cixidia (Achilidae) (A–E frons, F–J dorsal view). A, F Cixidia fusca B, G Cixidia opaca C, H Cixidiapallida D, I Cixidia septentrionalis E, J Cixidia variegata.
Habitus of Synecdoche (Achilidae), Haplaxius and Oecleus (Cixiidae) (A–F frons, F–J dorsal view). A, F Synecdoche dimidiata B, G Synecdoche grisea C, H Synecdoche impunctata D, I Haplaxius pictifrons E, I Oecleus borealis.
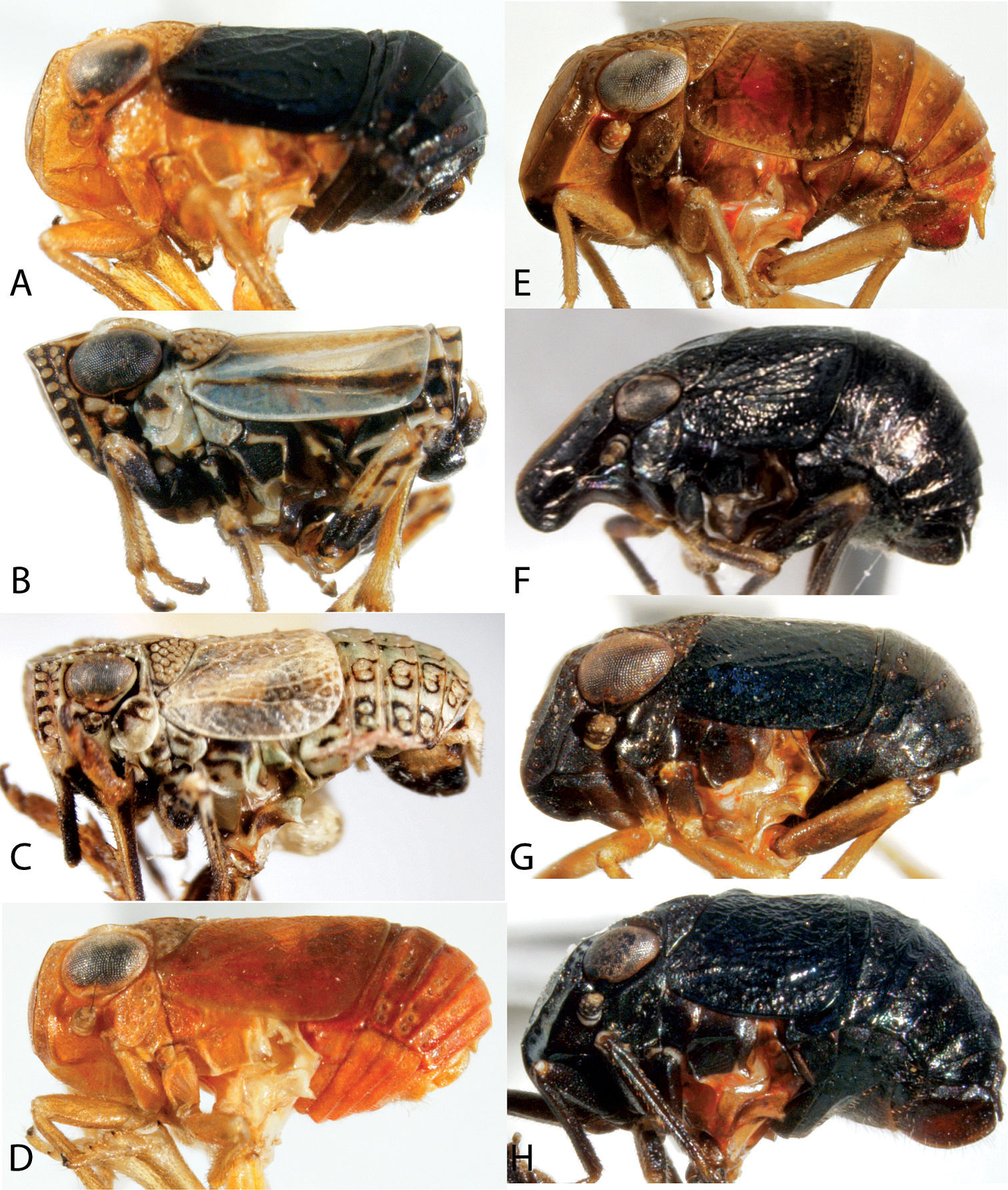
Habitus of Cixiidae. A Bothriocera cognita, head, lateral view B same, frons C same, dorsal view D Cixius pini, dorsal view E Melanoliarus placidus, head, lateral view F same, dorsal view G Pentastiridius cinnamomeus, dorsal view H Pintalia vibex, frons I same, lateral view J same, dorsal view.
Lateral habitus of Derbidae I. A Anotia bonnetii B Anotia kirkaldyi C Anotia robertsonii D Anotia westwoodi E Apache degeerii F Neocenchrea heidemanni G Patara vanduzei H Sikaiana harti I Anotia fitchi J Anotia westwoodi, head lateral view; K Sayiana sayi, head lateral view.
Lateral habitus of Derbidae II. A Cedusa sp. B Omolicna sp. C Sayiana sayi D Otiocerus coquebertii E Omolicna francilloni F Omolicna reaumurii G Omolicna stollii H Omolicna wolfii I Shellenius balli J Shellenius chellenbergii.
Heads of Derbidae. A Cedusa sp. lateral view B Cedusa sp., frontal view C Omolicna sp., lateral view D Omolicna sp., frontal view E Otiocerus wolfi, lateral view F Apache degeerii, lateral view G Anotia robertsonii, frontal view.
Lateral view of Caliscelidae. A Aphelonema decorata B phelonema histrionica C phelonema rugosa D phelonema simplex E Bruchomorpha jocosa F Bruchomorpha oculata G Bruchomorpha pallidipes H Bruchomorpha tristis.
Frontal view of Caliscelidae. A Aphelonema decorata B Aphelonemahistrionica C Aphelonemarugosa D Aphelonemasimplex E Bruchomorpha jocosa F Bruchomorpha oculata G Bruchomorpha pallidipes H Bruchomorpha tristis.
Habitus of Scolops (Dictyopharidae). A, E Scolops angustatus B, F Scolops perdix C, G Scolops pungens D, H Scolops sulcipes.
Phylloscelis and Rhynchomitra (Dictyopharidae) (A–C, G Dorsal view habitus, D–F frontal view, H Dorsal view, head and thorax). A, D Phylloscelis rubra B, E Phylloscelis pallescens C, F Phylloscelis atra G Rhynchomitra microrhina H Rhynchomitra lingula.
Issidae (A–D frontal view E–H Lateral view). A, E Exortus punctiferus B, F Thionia bullata C, G Thionia elliptica D, H Thionia simplex.
Line drawings of Achilidae and Derbidae (A–E Forewing, head left, costal margin top F–G Male genitalia, ventral view H–I Shellenius spp., head, left lateral view). A Catonia pumila; B Synecdoche rubella (Van Duzee, 1910); C Anotia fitchi; D Anotia robertsonii; E Cedusa sp.; F Catonia picta; G Synecdoche dimidiata; H Shellenius balli; I Shellenius schellenbergii (A–B Redrawn from O’Brien, 1971; E redrawn from Metcalf, 1923).
This survey brings the known diversity of Delaware planthoppers (excluding Delphacidae) from 7 to 62, plus provides new state records for MD (22), NJ (5), PA (8) and DC (21) providing species counts for those states as 88, 74, 60 and 46 respectively (Table 2). The Chao1 estimator suggests an additional 12 species may be found in the state. State-level incidence records of 112 species (Table 2) provides some basis for speculation of which species might be missing from the current inventory, and might be interpreted to suggest that the true diversity of planthoppers in Delaware may be closer to 100 species. A better understanding of the habits and finer-scale distribution patterns would be desirable in order to construct a candidate list of species not yet detected in the Delaware fauna. However, some species detected were not previously known from the region (viz. Aphelonema histrionica, Bothriocera drakei, Bothriocera maculata, Cixius angustatus, Sikaiana harti, Poblicia fuliginosa, and Otiocerus reaumurii), suggesting that the compiled species list may yet be substantially incomplete for the combined states.
In addition to the planthopper fauna reported here, a preliminary inventory of the delphacids of Delaware suggests at least 54 species in the state, although additional taxa are likely to be found before the completion of that inventory.
TaxonomyCixiidae: A number of specimens presented taxonomic difficulties. In the Cixiidae, specimens that appeared close to Melanoliarus sablensis differed from that depicted by
Achilidae: Species of Cixidia were identified primarily using features described by
Dictyopharidae: The only member of Phylloscelis collected by the authors (or the senior author’s students) was Phylloscelis rubra in New Jersey on cranberry (Vaccinium macrocarpon
Aiton). This genus is a good example of a taxon that is likely to be in
Delaware, but has not yet been found. While there are only 4 species
in the genus, and 3 in the study area (Figure 14), the species are best confirmed by genitalic features as presented in
Derbidae: A number of taxonomic issues were found among the Derbidae, including problems separating species in the genera Omolicna, Cedusa and two genera of Otiocerinae (Anotia and Otiocerus). Specimens of Omolicna (Derbidae) could not be definitively identified to species despite there being only 4 described North American species, and only 3 of these eastern - Omolicna fulva (Van Duzee, 1909), Omolicna mcateei (Dozier, 1928), and Omolicna uhleri (Ball, 1902). While literature records suggest that Omolicna uhleri (Ball, 1902) should be the only northern species, it was evident from the genitalia of Delaware specimens that at least 2 species are present. Because the original descriptions are incomplete, and at times conflicting with subsequent authors, we were unable to determine which of the specimens were Omolicna uhleri, and whether the remainder were Omolicna mcateei, Omolicna fulva or undescribed.
The derbid genus Cedusa
is diverse and its members require examination of male genitalia for
identification, and even then considerable study is required. Two
species within this genus were found to differ from the descriptions
provided by
Species in the Otiocerinae
tended to be problematic, particularly since most taxa are rare in
collections. It is also a problem that otiocerines have been described
primarily based on superficial color features whose diagnostic value has
not been verified by reference to genitalic features. While attempting
to verify our species concepts, we solicited photographs or examined
type specimens of select otiocerines. We found that many of the Fitch
types (deposited at the USNM) are in poor shape and greatly faded. It is
likely that some of the Kirby collection had been lost (see
Ten species of Anotia are reported from the United States (including species formerly in Amalopota Van Duzee, 1889, subsumed under Anotia by Fennah, 1951: 152). Of the 10 species, Anotia caliginosa Ball, 1937, and Anotia lineata Ball, 1937, are southwestern species (recorded from Arizona) and Anotia mcateei (Dozier, 1928), reported from Illinois and Mississippi, does not occur in the study area. Of the remainder, 5 (Anotia burnetii, Anotia bonnetii, Anotia kirkaldyi, Anotia robertsoni, and Anotia westwoodi) are similar in appearance in having white wings whose veins are variably bordered with dark. It is not clear how much intraspecific variation would be expected in features of wing color or pattern, and such patterns were difficult to interpret in the greatly faded Fitch type specimens (we examined types of Anotia robertsonii and Anotia burnetii). Anotia kirkaldyi and Anotia westwoodi share with Anotia bonnetii the presence of dark spots in the apical cells of the forewing, although they may be more prominent in the latter species. Anotia kirkaldyi and Anotia westwoodi can be separated with difficulty based on the presence of darkened wing veins in the former species, but these taxa are otherwise very similar and may not be distinct. Anotia robertsonii is similar to Anotia burnetii in possessing less extensive wing markings than Anotia kirkaldyi, Anotia westwoodi, and Anotia bonnetii; and in possessing dark markings on the dorsum of the abdomen, although in Anotia burnetii the markings are confined to the middorsum of segments 1–3 and in Anotia robertsonii the entire dorsum of subsequent terga (5–7 or 8).
The type specimen of Anotia bonnetii (the type species of the genus) was also sought, along with types of other otiocerines described by
Nine species of Otiocerus are reported from the north of Mexico; two species, Otiocerus abbotii Kirby, 1821, and Otiocerus kirbyii Fitch, 1851; are not reported from the study area (but see below). We examined the types of Otiocerus signoretii and Otiocerus stollii to help confirm features attributed to these species. The type specimen of Otiocerus signoretii,
at the USNM, is in rather poor condition, faded, and partially
enmeshed in mycelium, but shows the pattern of spots described by
A single specimen of Otiocerus from Maryland was not clearly associated with any of the described species. The specimen is uniformly pale, head without markings, forewings without spots and with a very faint band. A similar specimen was found among undetermined Derbidae at the USNM. It is possible that this specimen is Otiocerus kirbyii, but we were unable to confirm this identification.
The genus Cyarda is under revision by S. Wilson (S. Wilson, pers. comm.). Species in this genus are largely Caribbean. Four Cyarda have been reported from the United States: Cyarda acuminipennis (Spinola, 1839), Cyarda melichari Van Duzee, 1907, Cyarda sordida Fennah, 1965 ( = C. sp. nr. acutissima Metcalf & Bruner, 1948; see Fennah, 1965: 115) and Cyarda walkeri Metcalf, 1923. However,
Specimens reported as Bruchomorpha
sp. n.were collected at Phillips Landing, Sussex Co., DE (on 3 dates)
as well as single specimens from Medford, NJ and Baltimore, MD.
Superficially, these specimens are similar to Bruchomorpha dorsata, which has been reported in the Mid-Atlantic region by
Seasonality data were compiled from available
Delaware specimens as a way to begin to understand the life history of
local planthopper taxa. From the available seasonality information, it
appears that all non-delphacid planthoppers have a single generation a
year in Delaware, with the possible exceptions of Bruchomorpha oculata, Aphelonema simplex, and Cixius nervosus. This would be in general agreement with
A large number of Melanoliarus placitus
were collected in early July of 2002 by the senior author and several
students. The series was collected at mercury vapor lights (many
specimens landed on trees near the lights instead of at the lights).
Interestingly, this time period fell between the last quarter (July 2,
2002) and the New Moon (July 10 2002), which is similar to
observations made by Bartlett and colleagues (
While the planthoppers of the eastern United States may
be characterized as relatively well known from a taxonomic perspective,
their faunistics and ecology remain poorly understood. Although
Delaware is near the two largest insect collections in the US (the USNM
and the American Museum of Natural History, both of which employ
hemipterists), it is a testament to our inchoate understanding of US
planthopper faunistics that this study has increased our known Delaware
fauna by over 700%. The diversity of planthopper species in Delaware is
expected to be relatively modest relative to other states because it is
small and physiographically rather uniform, and because planthopper
diversity tends to generally increase inversely with latitude (and
within North America, is greatest overall in the southwest). Here we
also report totals of 88 species for Maryland, 74 for New Jersey, 60
for Pennsylvania, and 46 for the District of Columbia based on a
compilation of literature records and available specimens. The only
other state with a modern, relatively complete, survey of its
planthopper fauna is Illinois (
We are indebted to Kimberley Shropshire (University of Delaware) for the line art and most of the photography used in this work. We are grateful to Lois O’Brien for advice, support, specimens, and many helpful comments on early versions of this text; Stuart McKamey (USNM) for the loan of specimens, photographs of types, and assistance at the USNM collection; Zoë Simmons (Hope Entomological Collections, Oxford University Museum of Natural History) for locating and photographing some of the Kirby derbid types; Dmitri Logunov (Manchester Museum, University of Manchester, UK) and Mick Webb (British Natural History Museum, London) for seeking Kirby types in their collection. We are particularly grateful for past and present students whose diligent collecting has provided much of the basis for this work, especially Lawrence Barringer, Leo Donovall, Christopher Heckscher, Ashley Kennedy, Nate Nazdrowicz, Rob Snyder, and Katie Weglarz. We also thank an anonymous reviewer for helpful comments. This project was supported by the USDA Agriculture and Food Research Initiative Competitive Grants Program Grant No. 2009-55605-05006 from the National Institute of Food and Agriculture and Hatch Project W-2185 Biological Control in Pest Management Systems of Plants. Additional support was provided by the University of Delaware Department of Entomology and Wildlife Ecology.